Abstract
Background
Chemotherapy resistance is an intractable problem in treating patients with epithelial ovarian cancer (EOC). Heat shock proteins (HSPs) act as apoptosis inhibitors and are highly conserved genetically. Most HSPs have strong cytoprotective effects, and their overexpression inhibits apoptosis. This has been demonstrated for HSP70. Heat shock protein 70 (HSP70) expression is abnormally upregulated in malignant cells. Furthermore, HSP70 can inhibit cell death and promote chemotherapeutic resistance. In our study, the relationship between the HSP70 gene and primary chemotherapy resistance and clinical outcome in patients with EOC was explored.
Methods
Quantitative real-time polymerase chain (qRT-PCR) was applied to determine HSP70 messenger RNA (mRNA) levels, and immunohistochemistry assay was conducted to determine HSP70 protein level. HSP70 overexpression was assessed to clarify its role on chemotherapy resistance to cisplatin in SKOV3 cell lines.
Results
RT-qPCR assay indicated a strong relationship between HSP70 expression and chemotherapy resistance in patients with EOC. In cultured SKOV3 cells, overexpression of HSP70 inhibited cell sensitivity to cisplatin. Kaplan-Meier analysis demonstrated high HSP70 expression was associated with poor outcome of EOC patients. In multivariate models, high HSP70 expression independently predicted this poor outcome.
Conclusions
HSP70 predicts the prognosis and response to chemotherapy in EOC patients.
Keywords: Heat shock protein 70 (HSP70), epithelial ovarian cancer (EOC), platinum resistance, prognosis, gene
Introduction
Epithelial ovarian cancer (EOC) has the highest incidence and mortality among all gynecologic malignancies (1). The median time to recurrence of advanced ovarian cancer is less than 2 years (2), and the 5-year overall survival (OS) rate is about 30% (3). Presently, the standard therapies for EOC patients with advanced stage consists of surgeries and platinum-based chemotherapy (4). However, primary chemotherapy is ineffective in more than 20% patients (5). After primary chemotherapy, 70% of patients can achieve complete clinical remission; however, more than 85% of them will ultimately relapse and become resistant to chemotherapy (6). Chemotherapy resistance, either primary or acquired, is the main obstacle to successful treatment and remains a major problem in the management of patients with EOC.
Heat shock proteins (HSPs) act as apoptosis inhibitors and are highly conserved genetically (7). Most HSPs have strong cytoprotective effects, and their overexpression inhibits apoptosis (8,9). This has been demonstrated for HSP70 (10,11). In addition, HSP70 expression is abnormally high in malignant cells, and overexpression of HSP70 is related to chemotherapy resistance and lymph node metastasis (12,13). Numerous studies have indicated that reduced expression of HSP70 in cells may improve the effectiveness of cancer treatment (14,15). Therefore, we hypothesized that HSP70 expression may be involved in chemotherapy resistance in patients with EOC via inhibiting ovarian cancer cell apoptosis.
In this research, the relationship between the levels of HSP70 messenger RNA (mRNA) and protein in EOC tissue with clinical prognosis was investigated. In addition, the role of HSP70 in the viability and apoptosis of cultured ovarian cancer cells was further examined. We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/atm-21-2087).
Methods
Tissue sample collection
Between December 2012 and June 2015, tumor tissue samples from EOC patients admitted to the Department of Gynecology in the Fourth Hospital of Hebei Medical University, China, were collected. The detailed clinical pathological information of EOC patients are shown in Table 1. All patients were fully informed of the study and signed informed consent. The study was approved by the Institutional Medical Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2017ME96). This study was performed according to the Declaration of Helsinki (as revised in 2013).
Table 1. Clinical characteristics of 154 EOC patients.
| Characteristics | Stage | Patients (n) | Median | Percentage/range |
|---|---|---|---|---|
| Age | <50 years | 56 | 58 years | 36.4% |
| ≥50 years | 98 | 58 years | 63.6% | |
| Histology | Serous | 96 | 62.3% | |
| Endometrioid | 35 | 22.72% | ||
| Mucinous | 9 | 5.84% | ||
| Clear cell | 5 | 3.2% | ||
| Mixed type | 9 | 5.84% | ||
| FIGO stage | I–II | 34 | 22.1% | |
| III–IV | 120 | 77.9% | ||
| Grade | 1 | 39 | 25.3% | |
| 2 | 68 | 44.2% | ||
| 3 | 47 | 30.5% | ||
| Tumor residual size | 0 | 41 | 26.6% | |
| <1 cm | 76 | 49.4% | ||
| >1 cm | 37 | 24.0% | ||
| Platinum-based | Cisplatin | 41 | 26.6% | |
| Carboplatin | 113 | 73.4% | ||
| Follow-up time | 154 | 37.2 months | 2–60 months |
EOC, epithelial ovarian cancer.
Based on their platinum-free interval (PFI), the patients were separated into a chemotherapy-resistant group (n=64) and a chemotherapy-sensitive group (n=90). A PFI of <6 months was considered to indicate chemotherapy resistance, whereas a PFI of >6 months was considered to indicate chemotherapy sensitivity (16). The participants were followed up regularly for more than 5 years. Progression-free survival (PFS) and OS were used to analyze the survival status of EOC patients.
RNA extraction and quantitative real-time polymerase chain (qRT-PCR)
TRIzol reagent (Generay Biotech, Shanghai, Co., Ltd., China) was obtained to extract total RNA of tissue and cell samples. Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA) was applied to synthesize complement DNA (cDNA). For qRT-PCR assay, the primers of HSP70 and GAPDH were obtained from Sangon Biotech Co., Ltd. (Shanghai, China). The internal reference was set as GAPDH. QuantiNova TMSYBR® Green PCR Kit (Qiagen, Hilden, Germany) was used to conduct qRT-PCR reactions in a Mx3005P instrument. All detection experiments were conducted 3 times. The 2−ΔCt method was used to determine the relative expression of mRNA.
HSP70 immunohistochemistry study of clinical samples
EOC tissue samples were embedded in paraffin in the pathology department of the Fourth Hospital of Hebei Medical University. The expression of HSP70 was evaluated with immunohistochemical (IHC) staining. Rabbit antihuman HSP70 (SA0379, 1:1,000 dilution; Hangzhou HuaAn Biotechnology Co., Ltd., China) was applied to detect HSP70. Immunoreactivity for HSP70 was considered positive in tumor cells showing cytoplasmic staining without nuclear staining. Positive staining area and intensity reflected HSP70 expression level. IHC scores <4 indicated low HSP70 expression, while IHC ≥4 indicated high HSP70 expression (17).
Cell culture
To detect the role of HSP70 on EOC cells, SKOV3 cell lines were purchased from Icell Bioscience Inc., (Shanghai, China). Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Thermo Fisher Scientific, Inc.) and 10% fetal bovine serum (FBS; Invitrogen Gibco, NY, USA) was applied to culture SKOV3 cells. The cells were maintained in an incubator (37 °C, humidified, constant humidity, 5% CO2). All detections were conducted 3 times.
Transient transfection
The pCMV6-HSP70 vector and empty vector (OriGene Technology, Beijing, China) were transfected into SKOV3 cells with Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. At indicated time points, the cells were collected, and the expression of HSP70 mRNA was detected by qRT-PCR. Thus, the transfection efficacy could be determined.
Cell viability detection
The cell viability of cultured SKOV3 was analyzed by Cell Counting Kit-8 (CCK-8) assay. These cells were seeded in 96-well-plates. The seeding cell density was set at 15% confluence. The cells were cultured in fresh DMEM medium mixed with 10% FBS, penicillin, and streptomycin. At the end of cell viability assay, the cultured cells were in the growth phase. The day after seeding, different concentrations of cisplatin were applied to treat cultured SKOV3 cells; 24 hours after treatment, CCK-8 was used to detect the cell viability of cultured SKOV3 cells. In this process, 10 µL of CCK-8 was added to each well; 3 hours after adding CCK-8, the absorbance of each well at 492 nM was detected by a microplate reader. Each detection was conducted in triplicate.
Cell apoptosis detection
The SKOV3 cells at the logarithmic growth phase were seeded into 6-well plates at a density of 3×105/mL and 2 mL per well. The cultured cells were randomly separated into the control group and experimental group. Next, 48 hours after transfection, cisplatin was applied to treat cultured cells for 24 hours. Cold phosphate-buffered saline (PBS) was applied 3 times to wash cultured cells, and the rinsed cells were collected for flow cytometry assay. This was followed by 10 minutes of double staining with Annexin V-PE in a dark room. The percentage of apoptotic cells was detected by flow cytometry assay.
Statistical analysis
The collected data in this study were analyzed with SPSS 21.0 (IBM Corp., Armonk, NY, USA). A P value <0.05 was considered statistically significant. Wilcoxon rank-sum test was applied to analyze the difference of HSP70 mRNA expression between the 2 groups. The difference in HSP70 protein expression between the groups was analyzed with χ2 test. The relationship between HSP70 mRNA expression and the prognosis of EOC patients was analyzed with Kaplan-Meier analysis and the Cox proportional hazards model. t test was applied to compare the data from in vitro experiments.
Results
Association between HSP70 expression and chemotherapy resistance of EOC patients
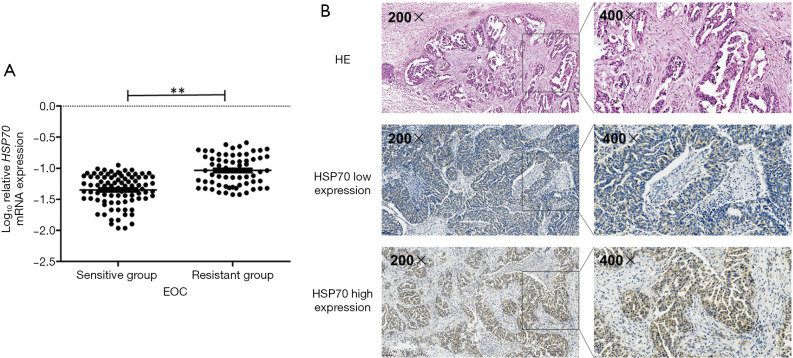
The results of qRT-PCR confirmed HSP70 mRNA expression was significantly higher in chemotherapy-resistant patients than in chemotherapy-sensitive patients (P=0.01, Figure 1A). IHC assay showed HSP70 was mostly located in the cytoplasm of EOC tumor tissue (Figure 1B). Positive expression of HSP70 occurred significantly more often in the 36 chemotherapy-resistant patients than in the 50 chemotherapy-sensitive patients (P=0.02, Table 2).
Figure 1.
Expression of HSP70 in patients with EOC. (A) HSP70 mRNA expression was higher in chemotherapy-resistant patients compared with chemotherapy-sensitive patients. (B) Representative IHC staining of HSP70 expression in EOC tissues. HE: representative hematoxylin and eosin staining in EOC tissues. **P<0.01. HSP70, heat shock protein; EOC, epithelial ovarian cancer; mRNA, messenger RNA.
Table 2. HSP70 protein expression differences between the platinum-resistant group and the platinum-sensitive group.
| HSP70 expression | Resistant group n (%) | Sensitive group n (%) | P value |
|---|---|---|---|
| High | 28 (77.55) | 27 (56.75) | 0.02 |
| Low | 8 (22.45) | 22 (43.24) |
HSP70, heat shock protein.
Correlation of high expression of HSP70 with prognosis in patients with serous ovarian cancer
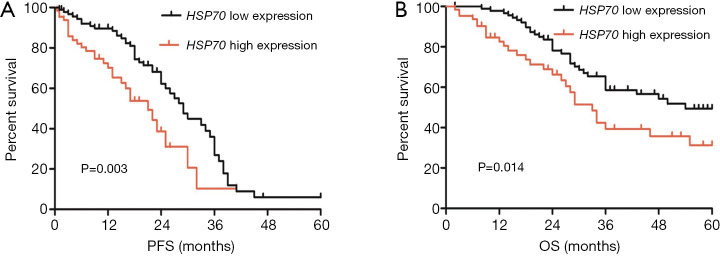
According to the median value of HSP70 mRNA expression, the patients were separated into a low-expression group and a high-expression group. Kaplan-Meier analysis showed that high HSP70 expression was associated with significantly lower PFS and OS compared as compared to low HSP70 expression (P=0.003, Figure 2A; P=0.014, Figure 2B). After adjusting for other prognostic factors (age, stage, grade, and tumor residual size), high HSP70 expression was also significantly associated with shorter PFS and OS (P=0.014, P=0.023; Table 3), demonstrating that HSP70 expression is an independent predictor of poorer clinical outcome in patients with EOC.
Figure 2.
The association between high expression of HSP70 and survival in 154 EOC patients. (A) Kaplan-Meier analysis of PFS according to HSP70 mRNA expression. (B) Kaplan-Meier analysis of OS according to HSP70 mRNA expression. HSP70, heat shock protein; EOC, epithelial ovarian cancer; PFS, progression-free survival; OS, overall survival.
Table 3. The association between clinical characteristics and treatment outcome in EOC patients treated with platinum-based chemotherapy.
| Characteristics | Recurrence | P value | Survival | P value | ||
|---|---|---|---|---|---|---|
| HR | 95% CIa | HR | 95% CIa | |||
| Age | ||||||
| <50 vs. ≥50 years | 1.06 | 0.64–1.77 | 0.820 | 1.23 | 0.73–2.10 | 0.440 |
| FIGO stage | ||||||
| I–II vs. III–IV | 9.99 | 1.93–35.05 | 0.024 | 9.66 | 1.73–40.05 | 0.026 |
| Grade | ||||||
| G1–2 vs. G3 | 5.29 | 1.73–17.05 | 0.013 | 7.40 | 0.20–14.80 | 0.019 |
| Tumor residual size | ||||||
| 0 vs. ≤1 cm | 4.39 | 1.75–10.93 | 0.011 | 5.40 | 0.20–11.80 | 0.017 |
| 0 vs. >1 cm | 3.09 | 1.05–7.93 | <0.01 | 3.40 | 1.20–6.80 | <0.01 |
| HSP70 expression | ||||||
| Low vs. high | 1.48 | 1.27–2.33 | 0.014 | 1.62 | 1.31–2.31 | 0.023 |
a, adjusted for age, stage, grade, tumor residue, and MGRN1 expression. EOC, epithelial ovarian cancer.
HSP70 overexpression in ovarian cancer cells by pCMV6-HSP70 vector
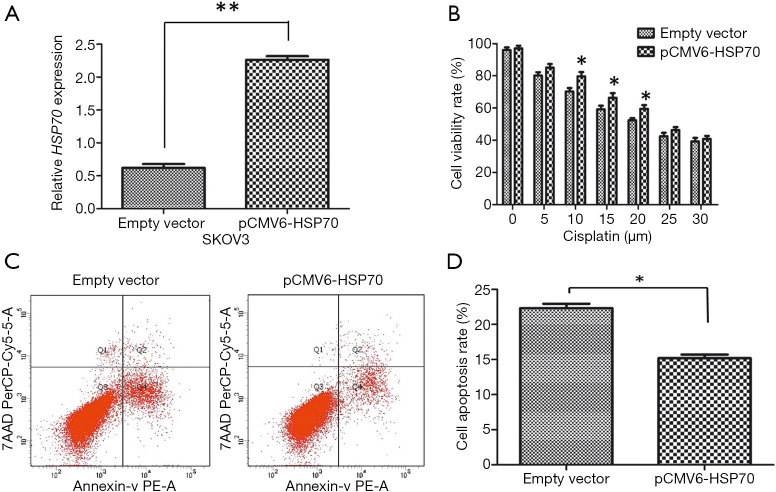
The overexpression efficiency of pCMV6-HSP70 was detected by qRT-PCR. The expression of HSP70 mRNA was significantly higher in the pCMV6-HSP70 group than the empty vector transfection group (P<0.001; Figure 3A).
Figure 3.
HSP70 overexpression enhanced the sensitivity to cisplatin in SKOV3 cells. (A) The expression of HSP70 in the pCMV6-HSP70 transfection group and the empty vector transfection group as detected by qRT-PCR. (B) The proliferative ability of SKOV3 cells in the pCMV6-HSP70 transfection group and empty vector transfection group detected by CCK-8 assays. (C,D) The apoptotic rate of SKOV3 cells in the pCMV6-HSP70 transfection group and empty vector transfection group detected by flow cytometry. *P<0.05; **P<0.01. HSP70, heat shock protein; qRT-PCR, quantitative real-time polymerase chain; CCK-8, Cell Counting Kit-8.
Effect of HSP70 overexpression on cellular response to cisplatin
As confirmed by CCK-8 assays, SKOV3 cell viability in the pCMV6-HSP70 transfection group was evidently higher than that in empty vector transfection group at different concentrations of the cisplatin condition (P<0.05; Figure 3B). This indicates that upregulation of HSP70 enhanced SKOV3 cell viability. In addition, flow cytometry confirmed that there were fewer apoptotic cells in the pCMV6-HSP70 transfection group compared with the empty vector transfection group in the 10 µM cisplatin treatment condition (P=0.025; Figure 3C,D).
Discussion
Our results confirm that, in patients with EOC, the expression of HSP70 is significantly higher in chemotherapy-resistant tissue compared with chemotherapy-sensitive tissue. Moreover, higher expression of HSP70 indicates poor clinical outcome in EOC patients, and overexpression of HSP70 may desensitize SKOV3 ovarian cancer cells to cisplatin.
Recent evidence has indicated that high expression of HSP70 may be associated with metastasis, poor prognosis, and resistance to chemotherapy (18,19). However, this study was first to report the association between HSP70 expression and chemotherapy resistance in EOC patients. Our results show that mRNA levels and protein expression of HSP70 are significantly higher in chemotherapy-resistant tissue than in chemotherapy-sensitive tissue. These results provide strong evidence that high expression of HSP70 may have a critical role in the chemotherapy resistance in EOC. Furthermore, patients with high expression of HSP70 had a poorer prognosis than those with low expression. Thus, high expression of HSP70 may significantly increase patients’ drug resistance to first-line platinum chemotherapy, resulting in a poorer prognosis. We also demonstrated, for the first time, that high expression of HSP70 mRNA is associated with a poor survival rate in EOC patients. These results provide strong evidence that high expression of HSP70 could regulate chemotherapy resistance in EOC.
HSP70 is a cell-protective protein that enhances cells, allowing them to survive in lethal conditions, and is highly conserved in evolution (10,11,20). Many models have shown that overexpression of HSP70 inhibits apoptosis following a variety of cellular stresses, including hyperthermia, oxidative stress, or cytotoxic drugs (21-23). There is evidence showing that the overexpression of HSP70 can induce cisplatin resistance in human ovarian cancer cells by blocking Bax mitochondrial translocation (24). Therefore, we speculated that upregulation of HSP70 in ovarian cancer cells may suppress apoptosis by inhibiting the Bax signaling pathway, thus facilitating chemotherapy resistance. To further confirm the effect of HSP70 on chemotherapy resistance in EOC, we investigated HSP70 expression in SKOV3 cells. As confirmed by flow cytometry analysis, the percentage of apoptotic SKOV3 cells decreased 1.3-fold after pCMV6-HSP70 transfection. Furthermore, the viability of ovarian cancer cells increased after pCMV6-HSP70 transfection. These data suggest HSP70 can inhibit the apoptosis of ovarian cancer cells.
In summary, our study suggests that high expression of HSP70 could induce chemotherapy resistance through inhibiting ovarian cancer cell apoptosis. However, further studies are necessary to confirm our findings.
Supplementary
The article’s supplementary files as
Acknowledgments
We would greatly acknowledge several doctors in Department of Obstetrics and Gynecology, the Fourth Hospital of Hebei Medical University, China, for their assistance in recruiting study participants.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Medical Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2017ME96). This study was performed according to the Declaration of Helsinki (as revised in 2013). All patients were fully informed of the study and signed informed consent.
Footnotes
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/atm-21-2087
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-21-2087
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-2087). The authors have no conflicts of interest to declare.
References
- 1.Amutha P, Rajkumar T. Role of Insulin-like Growth Factor, Insulin-like Growth Factor Receptors, and Insulin-like Growth Factor-binding Proteins in Ovarian Cancer. Indian J Med Paediatr Oncol 2017;38:198-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rochet N, Kieser M, Sterzing F, et al. Phase II study evaluating consolidation whole abdominal intensity-modulated radiotherapy (IMRT) in patients with advanced ovarian cancer stage FIGO III--the OVAR-IMRT-02 Study. BMC Cancer 2011;11:41. 10.1186/1471-2407-11-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura K, SawaDa K, Yoshimura A, et al. Abstract 3436: MiR-194 modulates paclitaxel resistance in ovarian cancer cells through the regulation of MDM-2 expression. Cancer Res 2017;77:abstr 3436.
- 4.Borley J, Wilhelm-Benartzi C, Brown R, et al. Does tumour biology determine surgical success in the treatment of epithelial ovarian cancer? A systematic literature review. Br J Cancer 2012;107:1069-74. 10.1038/bjc.2012.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantia-Smaldone GM, Edwards RP, Vlad AM. Targeted treatment of recurrent platinum-resistant ovarian cancer: current and emerging therapies. Cancer Manag Res 2011;3:25-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Yuan Z. Gambogic acid sensitizes ovarian cancer cells to doxorubicin through ROS-mediated apoptosis. Cell Biochem Biophys 2013;67:199-206. 10.1007/s12013-013-9534-7 [DOI] [PubMed] [Google Scholar]
- 7.Sichting M, Mokranjac D, Azem A, et al. Maintenance of structure and function of mitochondrial Hsp70 chaperones requires the chaperone Hep1. EMBO J 2005;24:1046-56. 10.1038/sj.emboj.7600580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musiał K, Zwolińska D. Heat shock proteins in chronic kidney disease. Pediatr Nephrol 2011;26:1031-7. 10.1007/s00467-010-1709-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goloudina AR, Demidov ON, Garrido C. Inhibition of HSP70: a challenging anti-cancer strategy. Cancer Lett 2012;325:117-24. 10.1016/j.canlet.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 10.Schmitt E, Parcellier A, Gurbuxani S, et al. Chemosensitization by a non-apoptogenic heat shock protein 70-binding apoptosis-inducing factor mutant. Cancer Res 2003;63:8233-40. [PubMed] [Google Scholar]
- 11.Mosser DD, Caron AW, Bourget L, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol 2000;20:7146-59. 10.1128/MCB.20.19.7146-7159.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrido C, Schmitt E, Candé C, et al. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle 2003;2:579-84. 10.4161/cc.2.6.521 [DOI] [PubMed] [Google Scholar]
- 13.Jego G, Hazoumé A, Seigneuric R, et al. Targeting heat shock proteins in cancer. Cancer Lett 2013;332:275-85. 10.1016/j.canlet.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 14.Saenz-Santamaría MC, McNutt NS, Bogdany JK, et al. p53 expression is rare in cutaneous melanomas. Am J Dermatopathol 1995;17:344-9. 10.1097/00000372-199508000-00007 [DOI] [PubMed] [Google Scholar]
- 15.Iersel ML, Ploemen JP, Struik I, et al. Inhibition of glutathione S-transferase activity in human melanoma cells by alpha,beta-unsaturated carbonyl derivatives. Effects of acrolein, cinnamaldehyde, citral, crotonaldehyde, curcumin, ethacrynic acid, and trans-2-hexenal. Chem Biol Interact 1996;102:117-32. 10.1016/S0009-2797(96)03739-8 [DOI] [PubMed] [Google Scholar]
- 16.Bookman MA, Tyczynski JE, Espirito JL, et al. Impact of primary platinum-free interval and BRCA1/2 mutation status on treatment and survival in patients with recurrent ovarian cancer. Gynecol Oncol 2017;146:58-63. 10.1016/j.ygyno.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 17.Liu YB, Mei Y, Tian ZW, et al. Downregulation of RIF1 Enhances Sensitivity to Platinum-Based Chemotherapy in Epithelial Ovarian Cancer (EOC) by Regulating Nucleotide Excision Repair (NER) Pathway. Cell Physiol Biochem 2018;46:1971-84. 10.1159/000489418 [DOI] [PubMed] [Google Scholar]
- 18.Nylandsted J. Extracellular heat shock protein 70: a potential prognostic marker for chronic myeloid leukemia. Leuk Res 2009;33:205-6. 10.1016/j.leukres.2008.07.020 [DOI] [PubMed] [Google Scholar]
- 19.Garrido C, Brunet M, Didelot C, et al. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle 2006;5:2592-601. 10.4161/cc.5.22.3448 [DOI] [PubMed] [Google Scholar]
- 20.Aghdassi A, Phillips P, Dudeja V, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res 2007;67:616-25. 10.1158/0008-5472.CAN-06-1567 [DOI] [PubMed] [Google Scholar]
- 21.Parcellier A, Gurbuxani S, Schmitt E, et al. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun 2003;304:505-12. 10.1016/S0006-291X(03)00623-5 [DOI] [PubMed] [Google Scholar]
- 22.Park HS, Lee JS, Huh SH, et al. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J 2001;20:446-56. 10.1093/emboj/20.3.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian SB, McDonough H, Boellmann F, et al. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature 2006;440:551-5. 10.1038/nature04600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Wang J, Zhou Y, et al. Hsp70 promotes chemoresistance by blocking Bax mitochondrial translocation in ovarian cancer cells. Cancer Lett 2012;321:137-43. 10.1016/j.canlet.2012.01.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as