Abstract
Wnt signalling induces a gradient of stem/progenitor cell proliferation along the crypt‐villus axis of the intestine, which becomes expanded during intestinal regeneration or tumour formation. The YAP transcriptional co‐activator is known to be required for intestinal regeneration, but its mode of regulation remains controversial. Here we show that the YAP‐TEAD transcription factor is a key downstream effector of Wnt signalling in the intestine. Loss of YAP activity by Yap/Taz conditional knockout results in sensitivity of crypt stem cells to apoptosis and reduced cell proliferation during regeneration. Gain of YAP activity by Lats1/2 conditional knockout is sufficient to drive a crypt hyperproliferation response. In particular, Wnt signalling acts transcriptionally to induce YAP and TEAD1/2/4 expression. YAP normally localises to the nucleus only in crypt base stem cells, but becomes nuclear in most intestinal epithelial cells during intestinal regeneration after irradiation, or during organoid growth, in a Src family kinase‐dependent manner. YAP‐driven crypt expansion during regeneration involves an elongation and flattening of the Wnt signalling gradient. Thus, Wnt and Src‐YAP signals cooperate to drive intestinal regeneration.
Keywords: intestine, regeneration, Src, Wnt, YAP
Subject Categories: Cancer, Development & Differentiation, Regenerative Medicine
Wnt transcriptionally controls YAP and TEAD expression to enable Src‐dependent activation of YAP‐TEAD activity during intestinal regeneration.

Introduction
The Wnt signalling pathway was discovered to signal via the beta‐catenin transcriptional co‐activator, which binds to the TCF/LEF1 family of DNA‐binding transcription factors to control nuclear gene transcription (Bienz & Clevers, 2000; MacDonald et al, 2009; Clevers, 2013; Franz et al, 2017; Gammons & Bienz, 2018). In the mammalian intestine, Wnt ligands are secreted from mesenchymal niche cells (Valenta et al, 2016; Degirmenci et al, 2018) such that Wnt signalling forms a gradient along the crypt‐villus axis, with beta‐catenin/TCF‐driven transcription strongest in the base of the crypt, where it induces expression of stem cell fate markers such as Lgr5 and Olfm4 (Bienz & Clevers, 2000; Clevers, 2013). In addition to controlling stem cell fate, one important function of the Wnt‐induced beta‐catenin/TCF activity gradient is to induce a corresponding gradient of cell proliferation to maintain normal intestinal homeostasis (Korinek et al, 1998; Ireland et al, 2004; Fevr et al, 2007; van Es et al, 2012; Valenta et al, 2016). Ectopic activation of beta‐catenin, either directly or in Apc mutant intestinal cells, is sufficient to induce expanded hypertrophic proliferation along the crypt‐villus axis or formation of adenomas (Korinek et al, 1997; Morin et al, 1997; Harada et al, 1999; Sansom et al, 2004; Andreu et al, 2005). One key target gene of beta‐catenin/TCF is Myc, encoding a transcription factor expressed in a crypt‐villus gradient that is required to promote cell proliferation during normal intestinal homeostasis and tumour formation (He et al, 1998; Muncan et al, 2006; Sansom et al, 2007; Finch et al, 2009). Another beta‐catenin/TCF target gene, Sox9, is expressed similar gradient to Myc along the crypt‐villus axis, but is also expressed in Paneth cells, where it is required for Paneth cell differentiation as marked by expression of Lysozyme (Lyz) (Bastide et al, 2007; Mori‐Akiyama et al, 2007).
A remarkable feature of the intestine is its ability to regenerate after tissue damage, a process that involves a transiently expanded gradient of cell proliferation along the crypt‐villus axis (Potten & Grant, 1998; Potten, 1998; Bach et al, 2000). Wnt/beta‐catenin signalling, Myc and Sox9 are all essential for intestinal regeneration after damage, along with additional signalling proteins such as the focal adhesion kinase (FAK), Src kinase and the YAP transcriptional co‐activator that are specifically required for regeneration (and organoid culture) but are dispensable for normal homeostasis (Ashton et al, 2010; Cai et al, 2010; Cordero et al, 2014; Gregorieff et al, 2015; Roche et al, 2015). Interestingly, FAK‐Src‐YAP form a signalling pathway that acts downstream of integrins to promote cell proliferation in skin (Kim & Gumbiner, 2015; Elbediwy et al, 2016; Li et al, 2016). During intestinal regeneration, Src phosphorylation is increased (Cordero et al, 2014) and the YAP protein becomes elevated and more strongly nuclear localised (Cai et al, 2010; Gregorieff et al, 2015). Recent work showed that FAK or Src inhibitors reduce nuclear YAP localisation during intestinal repair and confirmed the requirement for YAP in this process (Yui et al, 2018). Furthermore, activation of Src with overexpressed gp130 (IL6‐ST) causes YAP to become nuclear localised throughout the crypt‐villus axis (Taniguchi et al, 2015; Taniguchi et al, 2017).
The relationship between Wnt signalling and YAP in the intestine is a subject of some controversy. YAP protein is found at high levels in the crypts of the small intestine as well as in Apc mutant tumours or upon activation of beta‐catenin (Cai et al, 2015; Gregorieff et al, 2015). Importantly, three groups demonstrated that conditional deletion of YAP abolished adenoma formation in ApcMin mice, indicating that YAP is essential for Wnt signalling to drive tumours (Azzolin et al, 2014; Cai et al, 2015; Gregorieff et al, 2015). However, YAP was dispensable for proliferative hypertrophy upon acute Apcflox / flox homozygous deletion throughout the intestine (Gregorieff et al, 2015; Taniguchi et al, 2015; Taniguchi et al, 2017), suggesting that hypertrophic growth is simply an expansion of the normal Wnt‐dependent, YAP‐independent, homeostatic proliferation programme, while tumour formation may require additional input from other signals that induce YAP nuclear translocation.
A prominent model has been proposed for the molecular mechanism connecting Wnt signalling with YAP in the gut: that Apc directly inactivates YAP via Axin‐YAP binding to retain YAP in the cytoplasm and promote YAP degradation (Azzolin et al, 2014). This model proposes a post‐translational mechanism for Wnt‐dependent regulation of YAP subcellular localisation and stability, a notion that has been challenged by the observation that acute deletion of Apc in the intestine is not always sufficient to cause nuclear localisation of YAP, indicating that signals other than Wnt must drive YAP nuclear localisation during regeneration and tumour formation (Gregorieff et al, 2015; Gregorieff & Wrana, 2017). Alternative models suggest that YAP nuclear localisation in the intestine is inhibited by the canonical Hippo pathway (Cai et al, 2015; Gregorieff et al, 2015) and promoted by Src family kinase signalling (Rosenbluh et al, 2012; Taniguchi et al, 2015; Taniguchi et al, 2017; Yui et al, 2018).
In addition to Wnt signalling promoting YAP function, there is evidence that YAP activity can induce “negative feedback” upon expression of certain Wnt target genes, such as Lgr5, Olfm4 and Lyz—particularly during the intestinal regenerative response to irradiation (Gregorieff et al, 2015). Very recently, two groups reported that YAP activation can directly inhibit Wnt signalling in the intestine (Cheung et al, 2020; Li et al, 2020), with one report claiming that YAP therefore functions as a tumour suppressor, rather than an oncogene, in colorectal cancer (Cheung et al, 2020), building on their previous work (Barry et al, 2013), but in conflict with other reports of an oncogenic role for YAP in the intestine (Cai et al, 2015; Gregorieff et al, 2015). Thus, the role and regulation of YAP as a downstream effector of Wnt signalling is still not clearly resolved in either intestinal regeneration or cancer. We therefore sought to re‐examine the relationship between Wnt signalling and YAP‐TEAD activity in the intestine.
Results
Yap/Taz double knockouts exhibit crypt stem cell apoptosis and defects in the transverse folds of the ascending colon
We began by investigating the normal role of YAP/TAZ in the intestine by re‐examining the consequences of loss of both proteins in Villin‐CreERt Yapflox / flox Tazflox / flox double conditional knockouts (Yap/Taz dKO) treated with tamoxifen to induce deletion of both genes. Although YAP is far more strongly expressed than TAZ in the intestine (Fig EV1A–E), we induced deletion of both genes to be certain of a full loss of function. We confirm previous reports (Cai et al, 2010; Gregorieff et al, 2015) that loss of both YAP and TAZ does not strongly affect the overall morphology of the small intestine and detect only a mild increase in the rate of apoptosis in crypt base stem cells, while loss of both YAP and TAZ in the large intestine caused increased apoptosis, particularly in the transverse folds of the ascending colon (Fig 1A,B). Identical results were obtained using Yapflox / flox Tazflox / flox animals (i.e. without Villin‐CreERt expression) or Villin‐CreERt animals (i.e. without Yapflox / flox Tazflox / flox) as controls (Fig 1A–D). Notably, many Yap/Taz dKO animals showed profound defects in the transverse folds of the ascending colon (75% of n = 4 animals; Fig 1D). These transverse folds project into the lumen of the colon and are therefore highly susceptible to mechanical damage, which may explain why YAP and TAZ are specifically required in these folds (Fig 1D). We confirm previous findings that after treatment with gamma irradiation (12 Gy), Yap/Taz dKO animals exhibit defective regeneration throughout the intestine (Fig EV2A and B, and EV3A and B). These findings identify a physiological requirement for YAP‐TEAD signalling in promoting intestinal stem cell survival and in regeneration of regions susceptible to frequent tissue damage.
Figure EV1. YAP, TAZ and CTGF expression patterns in the human intestine, adenomas and invasive CRC.
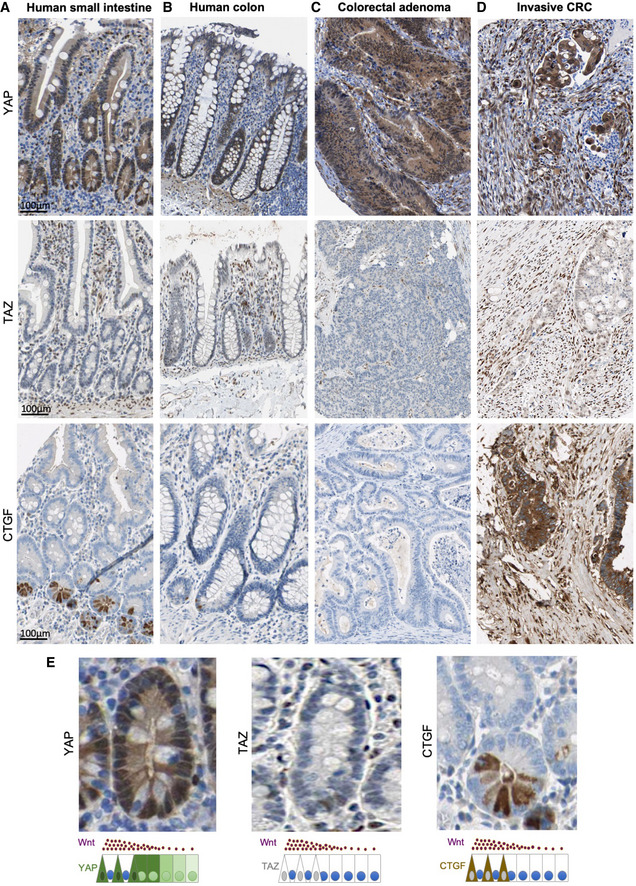
- Normal human small intestines stained for YAP, TAZ and CTGF.
- Normal human colons stained for YAP, TAZ and CTGF.
- Human colorectal adenomas stained for YAP, TAZ and CTGF.
- Human invasive colorectal carcinomas stained for YAP, TAZ and CTGF.
- Magnified view of (A) showing nuclear YAP and CTGF expression in crypt base stem cells.
Figure 1. YAP/TAZ double knockouts exhibit defects in the transverse folds of the ascending colon.
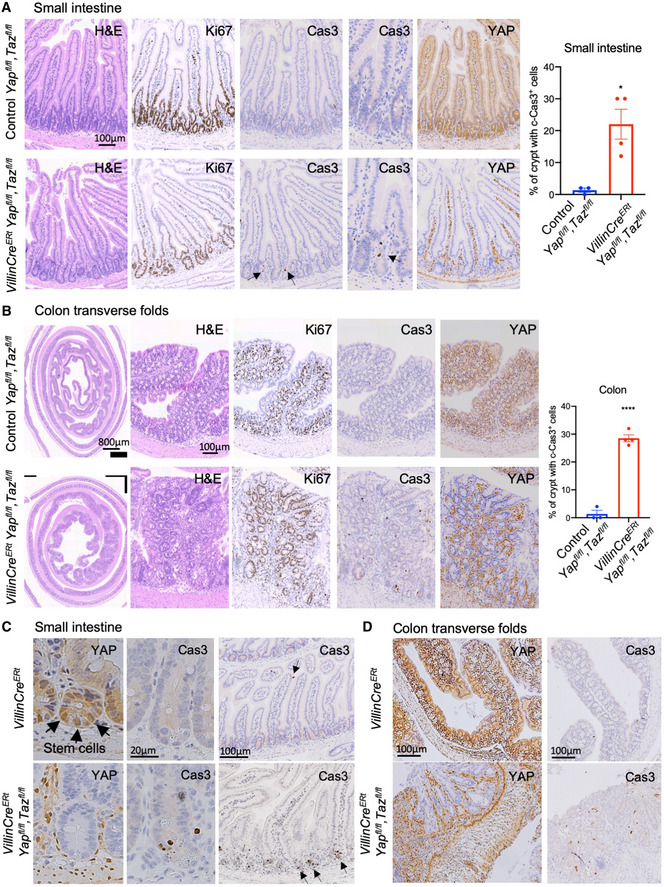
- Murine small intestines immunostained for the proliferation marker Ki67, apoptosis marker cleaved caspase 3 (Cas3) or YAP from four control (Yapfl / fl Tazfl / fl) and four YAP/TAZ double conditional knockout (Villin‐CreERt Yapfl / fl Tazfl / fl) animals treated with tamoxifen. Tissues were harvested and analysed at day 7 after first tamoxifen injection. Loss of YAP/TAZ leads to a moderate increase in apoptosis (Cas3+) in crypt base stem cells, quantified on the right, but no overall effect on the morphology of the small intestine. Arrows point to apoptotic cells. *P < 0.05.
- Murine large intestines immunostained for proliferation marker Ki67, apoptosis marker cleaved caspase 3 (Cas3) or YAP from control (Yapfl / fl Tazfl / fl) and YAP/TAZ double conditional knockout (Villin‐CreERt Yapfl / fl Tazfl / fl) animals treated with tamoxifen. Tissues were harvested and analysed at day 7 after first tamoxifen injection. Loss of YAP/TAZ leads to an increased number of apoptotic (Cas3+) cells within the transverse folds of the ascending colon. ****P < 0.0001.
- Murine small intestines immunostained for YAP or cleaved caspase 3 (Cas3) from control (Villin‐CreERt) and YAP/TAZ double conditional knockout (Villin‐CreERt Yapfl / fl Tazfl / fl) animals treated with tamoxifen. Tissues were harvested and analysed at day 7 after first tamoxifen injection. Loss of YAP/TAZ leads to a mild increase in apoptosis (Cas3+) in crypt base stem cells, but no overall effect on the morphology of the small intestine. Arrows point to stem cells (YAP nuclear staining), while arrows point to apoptotic cells (Cas3 staining). N > 10 animals per genotype.
- Murine large intestines immunostained for YAP or cleaved caspase 3 (Cas3) from control (Villin‐CreERt) and YAP/TAZ double conditional knockout (Villin‐CreERt Yapfl / fl Tazfl / fl) animals treated with tamoxifen. Tissues were harvested and analysed at day 7 after first tamoxifen injection. Loss of YAP/TAZ leads to increased apoptosis (Cas3+) and severe morphological defects in the transverse folds of the ascending colon, but does not affect morphology of the remaining colon regions. Quantitatively, 75% of n = 4 animals showed this severe phenotype.
Figure EV2. Yap/Taz double knockouts exhibit reduced proliferation and abnormal regeneration after gamma irradiation in the small intestine.
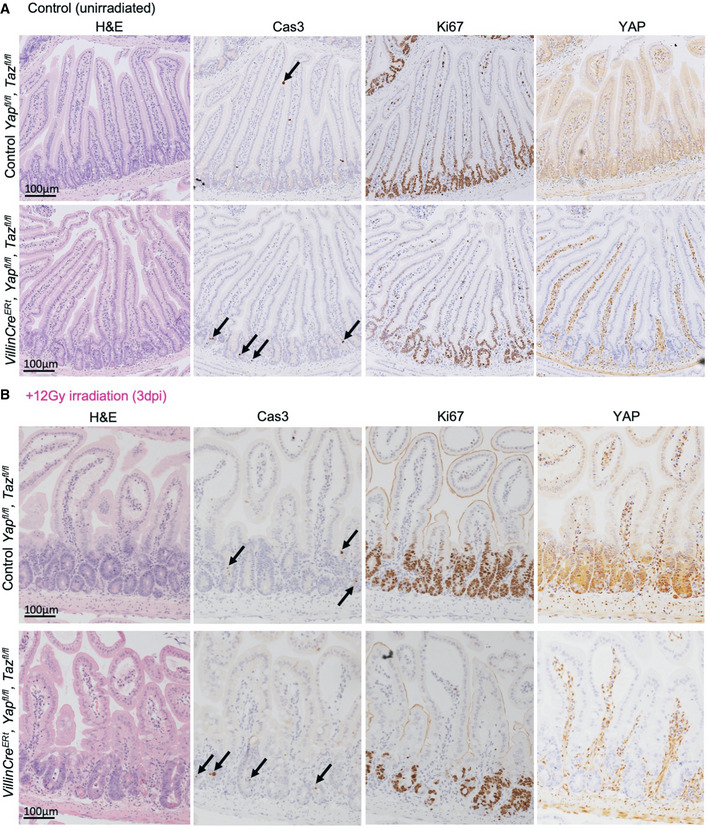
- Murine small intestines from (Cre negative) and Villin‐CreERt Yapfl / fl Tazfl / fl animals (Yap/Taz dKO) display a mildly increased rate of crypt cell apoptosis, marked by cleaved caspase 3 immunostaining, but no decrease in cell proliferation, marked by Ki67 immunostaining. Note that the images shown in this control are identical to those shown in Fig 1A.
- Murine small intestines isolated 3 days after treatment with 12 Gy irradiation (3 dpi) upon Yap/Yap dKO show both increased apoptosis, marked by cleaved caspase 3 immunostaining, and reduced cell proliferation, marked by Ki67 immunostaining. n = 5 animals at 3 dpi.
Figure EV3. Yap/Taz double knockouts exhibit abnormal regeneration after gamma irradiation in the large intestine.
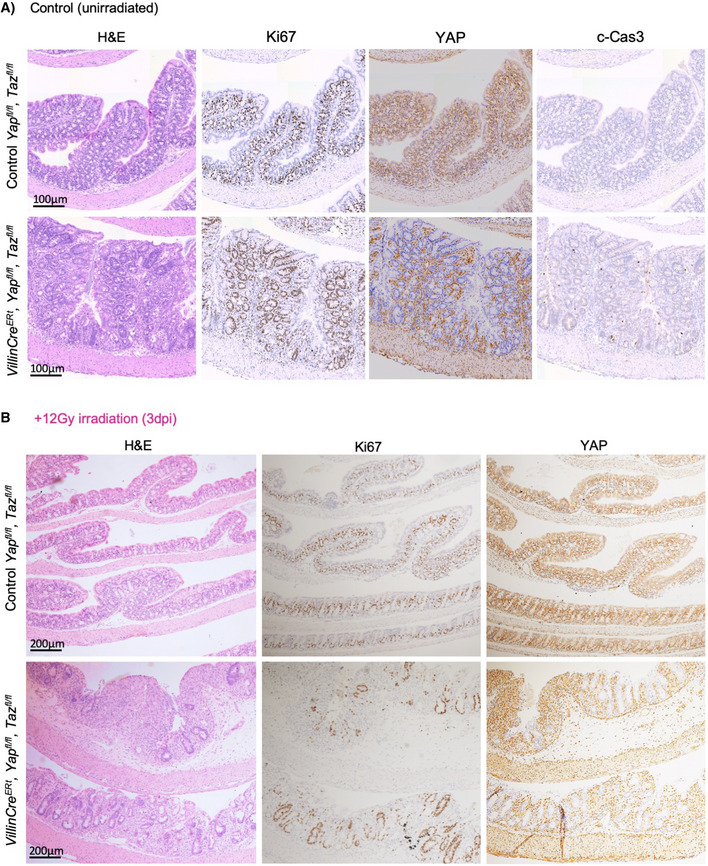
- Murine colon from (Cre negative) and Villin‐CreERt Yapfl / fl Tazfl / fl animals (Yap/Taz dKO) display a mildly increased rate of crypt cell apoptosis, marked by cleaved caspase 3 immunostaining, but no decrease in cell proliferation, marked by Ki67 immunostaining. These figure panels are identical to those shown in Fig 1B and are shown for comparison only.
- Murine colon isolated 3 days after treatment with 12 Gy irradiation (3 dpi) upon Yap/Taz dKO show both increased apoptosis, marked by cleaved caspase 3 immunostaining, and reduced cell proliferation, marked by Ki67 immunostaining. n = 5 animals at 3 dpi.
Lats1/2 dKO drives YAP nuclear localisation, crypt hyperplasia and a long/flat Wnt signalling gradient
We next examined the consequence of constitutively activating YAP‐TEAD signalling in the intestine in Villin‐CreERt Lats1flox / flox Lats2flox / flox dKOs (Lats1/2 dKO) treated with tamoxifen to induce deletion of both genes. We find that YAP becomes strongly nuclear localised throughout the intestinal epithelium, as expected, and that cell proliferation is strongly upregulated in crypts of both the proximal small and large intestines (Figs 2A and EV4A and B). Ectopic expression of nlsYAP5SA causes a similar phenotype to Lats1/2 dKO, mimicking an irradiation‐induced regenerative state (Fig EV5A and B). Analysis of the Wnt target genes Sox9 (detected by immunostaining) and Axin2 (detected by RNA scope in situ hybridisation) reveals an expansion along the crypt‐villus axis in Lats1/2 dKO animals (Fig 2A–C), in contrast to recent reports that Wnt signalling is inactivated in these mutants (Cheung et al, 2020). The normally steep gradient of Sox9 and Axin2 expression is elongated and flattened in the Lats1/2 dKO animals, indicating that the shape of the Wnt signalling gradient has been altered (Fig 2A–C). Expression of the high‐threshold Wnt target gene and Paneth cell marker Lyz is also lost from the base of the crypts in Lats1/2 dKO small intestines (Fig 2A). qPCR analysis confirms that high‐threshold Wnt target genes such as Lgr5 and Olfm4 are reduced in Lats1/2 dKO small intestines, while YAP targets such as Ctgf and Cyr61 are strongly induced and the common Wnt and YAP target gene CyclinD1 is also moderately induced (Fig 2D). These results indicate that nuclear localisation of YAP is sufficient to drive proliferation specifically in intestinal crypts, consistent with the notion that YAP must cooperate with Wnt signalling to drive the expression of crypt proliferation markers such as Sox9 and CyclinD1 (Fig 2A–C). These findings agree with recent reports that Myc is also a common target gene of both YAP and Wnt signalling (Choi et al, 2018; Li et al, 2020). That the gradient of Wnt signalling undergoes elongation and flattening during YAP‐driven crypt expansion (Fig 2C) explains the upregulation of transit‐amplifying cell proliferation (and expansion of medium‐threshold Wnt targets such as Sox9, Myc and CyclinD1) at the expense of crypt base stem cells (and expression of high‐threshold Wnt targets such as Lgr5 and Olfm4).
Figure 2. LATS1/2 double knockout drives YAP nuclear localisation, crypt hyperplasia and a long/flat Wnt gradient.
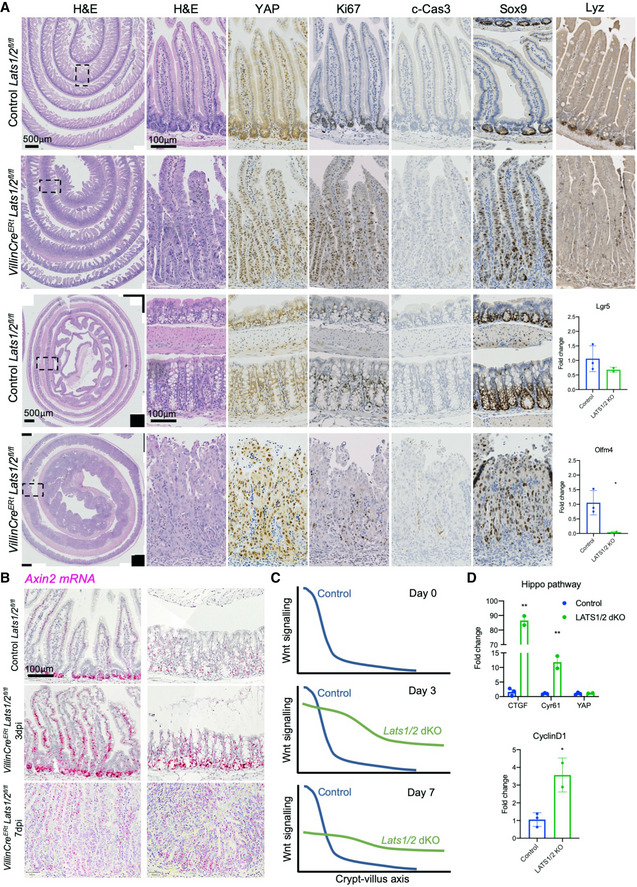
- Murine small (top) and large (bottom) intestines isolated from control (Cre negative) Lats1flox / flox Lats2flox / flox animals and Villin‐CreERt Lats1flox / flox Lats2flox / flox animals treated with tamoxifen to induce homozygous deletion of Lats1/2 (dKO). Immunostaining for YAP and Ki67 shows a gradient of YAP expression along the crypt‐villus axis in controls, with nuclear YAP‐ and Ki67‐positive cells restricted to the crypt base (representative images from n = 5 mice). Tamoxifen‐treated (3 days i.p.) Villin‐CreERt Lats1flox / flox Lats2flox / flox double homozygous mouse intestines show an enlarged crypt compartment after 7 days with strongly nuclear YAP immunostaining in all epithelial cells and an expanded proliferative zone marked by Ki67‐positive cells. Note the gradient of YAP expression levels is maintained along the crypt‐villus axis (representative images from n = 5 mice for each genotype). (Right) Immunostaining for the Paneth cell marker Lyz reveals loss of this marker from the crypt base. (Bottom right) qPCR analysis of Wnt pathway target genes reveal that Lats1/2 dKO causes a mild reduction in Lgr5 expression, with complete loss of Olfm4. *P < 0.05.
- Axin2 mRNA expression was measured by RNAscope (red) and found to be increased in both intensity and uniformity of staining along the crypt‐villus axis in Lats1/2 dKO after 3 days post‐i.p. injection (dpi) with tamoxifen to induce the homozygous deletion of Lats1 and Lats2. At 7 dpi, Axin2 mRNA levels remain uniform along the crypt‐villus axis in Lats1/2 dKO animals, although their total level has declined compared to 3 dpi.
- Schematic diagram showing alteration of the Wnt signalling gradient upon Lats1/2 dKO in the intestine, with crypt expansion being accompanied by an elongation/flattening of the Wnt signalling gradient, whose elevated level gradually declines over time.
- qPCR analysis of YAP‐TEAD and their target genes Ctgf and Cyr61 reveal that Lats1/2 dKO causes a strong induction of Ctgf and Cyr61 expression, as expected, as well as a strong induction of the common Wnt and YAP target gene CyclinD1. *P < 0.05; **P < 0.01.
Figure EV4. Lats1/2 double knockouts exhibit increased proliferation in both the small and large intestine, which can become ulcerated.

- Murine small intestines isolated from control (Villin‐CreERt) animals and Villin‐CreERt Lats1fl / fl Lats2fl / fl double knockout (Lats1/2 dKO) animals immunostained for YAP and proliferation marker Ki67.
- Murine colons isolated from control (Villin‐CreERt) animals and Villin‐CreERt Lats1fl / fl Lats2fl / fl double knockout (Lats1/2 dKO) animals immunostained for YAP and proliferation marker Ki67. Note region of colonic ulceration (red dotted line) arising at 7 days after 3× tamoxifen i.p. treatment. Inset shows quantification of the number of Ki67‐positive cells per crypt in control and dKO animals. n = 5 animals for each genotypes.
Figure EV5. Irradiation induces apoptosis followed by regenerative crypt hyperproliferation, a phenotype mimicked by ectopic expression of nuclear YAP.
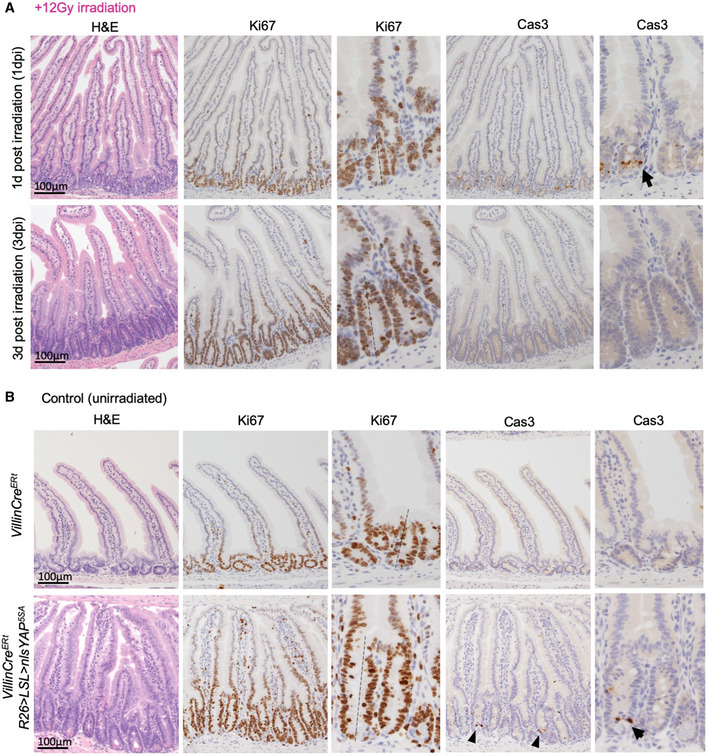
- Murine small intestines treated with 12 Gy irradiation and isolated 1 day post‐irradiation (dpi) and 3 dpi. Immunostaining for proliferation marker Ki67 and apoptosis marker cleaved caspase 3 (Cas3) is shown. n = 4 animals for each time point.
- Murine small intestines from control (Villin‐CreERt) and Villin‐CreERt Rosa26>loxSTOPlox>nlsYAP5SA transgenic animals treated with 3× tamoxifen i.p. show a phenotype identical to that induced by irradiation in (A) after 5 days. n = 4 animals for each genotype.
In the foregoing qPCR analysis, we noticed that expression of TEAD1/4 mRNA (but not TEAD2/3) was dramatically induced in Lats1/2 dKO small intestines, which we then confirmed by RNAscope in situ hybridisation to visualise single molecules in both the small intestine and colon (Fig 3A and B). Temporal analysis at 0, 3 and 7 days post‐tamoxifen‐induction (dpi) of Lats1/2 dKO reveals the progressive increase in TEAD1/4 mRNA expression, similar to that of the classical YAP target gene Ctgf (Fig 3C), while TEAD2 exhibits a mild downregulation similar to the classical Wnt target gene Axin2 (Fig 3C). These results show that TEAD1 and TEAD4 behave as classical YAP target genes, while TEAD2 appears to behave as a classical Wnt target gene in the intestine.
Figure 3. TEAD1/4 mRNA expression is progressively expanded in Lats1/2 dKO intestines.
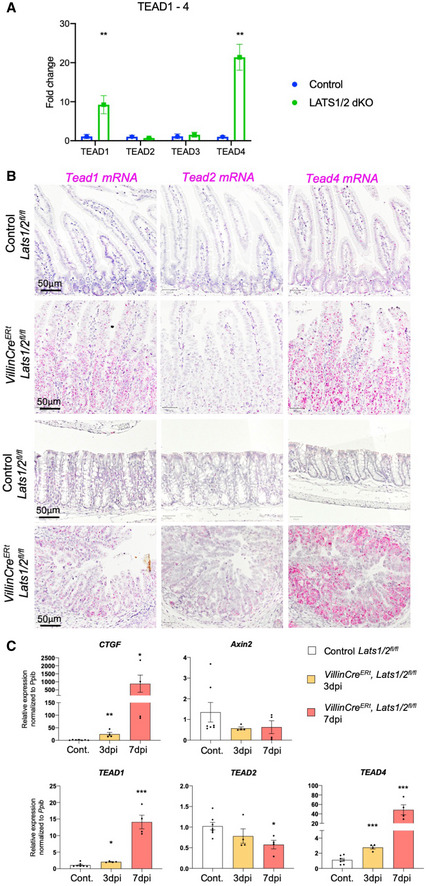
- qPCR analysis of TEAD1‐4 mRNA expression in control versus Lats1/2 dKO intestines at 7 days post‐i.p. injection with tamoxifen. Note the strong increase in TEAD1 and TEAD4 mRNA levels in particular, which indicates that these are both target genes of YAP signalling. **P < 0.01.
- RNAscope in situ hybridisation analysis of TEAD1/2/4 mRNA expression from control (Cre negative) Lats1flox / flox Lats2flox / flox animals and Villin‐CreERt Lats1flox / flox Lats2flox / flox animals treated with tamoxifen to induce homozygous deletion of Lats1/2 (dKO). Tamoxifen‐treated (3 days i.p.) Villin‐CreERt Lats1flox / flox Lats2flox / flox double homozygous mouse intestines show elevated expression of TEAD1 and TEAD4 in both the small intestine (top) and colon (bottom). Tissues were harvested and analysed at day 7 after first tamoxifen injection. (n = 5 animals for each genotype).
- qPCR analysis reveals a progressive increase in the YAP‐TEAD target gene Ctgf in Lats1/2 dKO intestines at 3 and 7 days post‐i.p. injection with tamoxifen, as well as similarly increased TEAD1 and TEAD4 expression, confirming that TEAD1 and TEAD4 are YAP‐responsive genes. Notably, Axin2 and TEAD2 exhibit a mild but progressive decline over 3–7 days after Lats1/2 deletion, consistent with Axin2 being a known Wnt‐specific target gene and with Wnt signalling remaining active in Lats1/2 dKO intestines. *P < 0.05; **P < 0.01; ***P < 0.001.
Wnt signalling induces YAP & TEAD1/2/4 mRNA expression in the intestine
We next sought to directly test whether TEAD1/4 and TEAD2 are induced upon activation of Wnt signalling in ApcMin mutant intestinal tumours. We find strong induction of all three genes in ApcMin mutant tumours of both the small intestine and colon (Fig 4A). We confirmed induction of TEAD1/2/4, but not TEAD3, by qPCR analysis in Apc mutant intestinal organoids in vitro, ApcMin mutant tumours in vivo and in Apcfl / fl knockout intestinal crypts in vivo (Fig 4B). We further note that immunostaining for the TEAD4 protein in the Human Protein Atlas database reveals a gradient of expression along the crypt‐villus axis in the human small intestine and colon, and is uniformly induced in human colorectal adenomas, similar to Sox9 (Fig 4C). Together, our results show that TEAD1/4 are common Wnt and YAP target genes, while TEAD2 is a Wnt‐only target gene and TEAD3 is regulated by neither pathway.
Figure 4. Wnt signalling induces TEAD1/2/4 mRNA expression in the intestine.
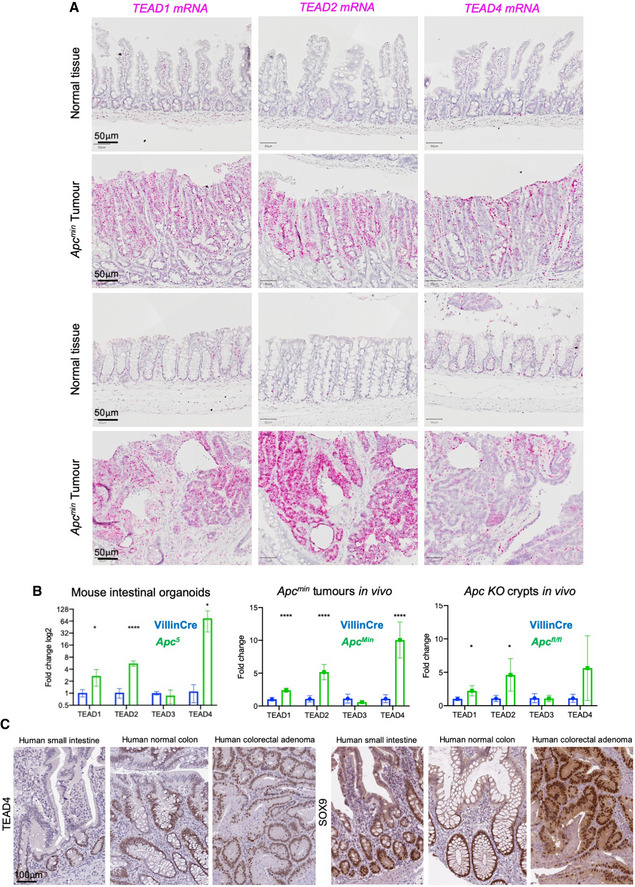
- RNAscope in situ hybridisation analysis of TEAD1/2/4 mRNA expression from control (wild type) and ApcMin mutant tumours from the small intestine (top) and colon (bottom). All three genes were strongly induced within the ApcMin mutant tumours, with TEAD2 being most strongly induced in colon ApcMin tumours (n = 3 animals with multiple tumours within the small and large intestines).
- qPCR analysis of control and Apc5 mutant murine intestinal organoids in vitro, control and ApcMin mutant tumour samples in vivo, and control and Apcfl / fl knockout hyperplastic crypts in vivo. In all cases, TEAD1/2/4 mRNA expression was strongly elevated upon loss of Apc function, while TEAD3 mRNA expression was unaffected. (RNA was isolated from six individual tumours). *P < 0.05; ****P < 0.0001.
- Antibody staining for TEAD4 reveals a gradient of expression along the crypt‐villus axis in both the small and large intestine, with uniformly high expression in colorectal adenomas, similar to SOX9.
The above results raise the question of whether the YAP gene is also a target of Wnt signals in the intestine. We compared immunostaining with anti‐YAP antibodies with in situ hybridisation for YAP mRNA in mouse small intestine (Appendix Fig S1A). We again took advantage of the high sensitivity and specificity of the RNAscope technology to visualise single molecules of YAP mRNA. We find a correlation between the gradients of YAP mRNA and YAP protein level along the crypt‐villus axis, with YAP most strongly expressed in the crypt (Appendix Fig S1A). These findings suggest that regulation of YAP gene expression is sufficient to explain the regulation of YAP protein levels by Wnt signalling. In support of this notion, activation of Wnt signalling in Apcfl / fl mutant intestinal adenomas is sufficient to induce both YAP mRNA and YAP protein throughout the tumour (Appendix Fig S1B). Notably, most nuclei in the Apc mutant adenomas are negative for YAP protein, which remains largely cytoplasmic (Fig EV1C and Appendix Fig S1B). The subtle Wnt‐induced increase in YAP mRNA expression between crypt and villus can be explained by beta‐catenin/TCF4 activation of transcription from a single previously identified site in the YAP promoter (Konsavage et al, 2012) (Appendix Fig S1C–F). These results show that Wnt signalling can drive a subtle increase in the transcription of the YAP gene in the intestine, in addition to strong induction of TEAD1/2/4 gene expression, indicating that the primary mechanism by which Wnt induces YAP‐TEAD activity is transcriptional in nature.
To further test this hypothesis, we sought to examine YAP in mouse intestinal organoids upon Wnt pathway modulations. Inhibition of Wnt signalling by addition of Porcupine (Porc) inhibitor LGK974 or activation of Wnt signalling in Apc homozygous mutant (Apc5) organoids (Novellasdemunt et al, 2017) results in corresponding changes in YAP mRNA and protein levels (Fig 5A–C; Appendix Fig S1G and H). Notably, the magnitude of YAP mRNA regulation (Appendix Fig S1G and H) was very mild compared with the magnitude of Ctgf or TEAD1/2/4 mRNA regulation in these experiments (Fig 4A–D, Appendix Figs S1G and H, and S2A), suggesting that strong upregulation of TEAD1/2/4 mRNA levels by Wnt signalling may be of primary importance in determining YAP‐TEAD activity. Notably, we confirm that expression of YAP is essential in either Apcfl / fl or Apcfl / fl, p53fl / fl mutant tumour‐derived organoids, which cannot grow when mutant for YAP/TAZ (Appendix Fig S1I). These results indicate that Wnt signalling acts transcriptionally to regulate expression of the YAP and TEAD1/2/4 genes in the intestine, rather than acting post‐translationally to regulate YAP localisation.
Figure 5. YAP integrates Wnt signalling and mechanical stimuli in intestinal organoids.

- YAP and Ezrin immunostaining in control murine intestinal organoids cultured for 1–5 days as indicated. Note the initially cytoplasmic localisation of YAP, which becomes nuclear localised after 3 days.
- YAP immunostaining of 3‐day‐old wild‐type organoids treated with DMSO (Control) or Porcupine inhibitor LGK974 at 5 µM. Wnt signalling inhibition upon Porcupine inhibitor treatment decreases YAP levels without affecting YAP subcellular localisation. Quantification of YAP protein levels is shown below, with statistical significance in an unpaired two‐tailed t‐test. ****P < 0.0001.
- Wnt activation in Apc5 homozygous mutant organoids drives a drastic shift in organoid morphology from budding structures to cystic spheres and increases the YAP protein level. Scale bar is 250 µm. n > 10 organoids in each experiment. Quantification of YAP protein levels from crypt and villus regions of control organoids as well as from the regions bounded by rectangles in Apc5 organoids are shown on the right, with statistical significance in an unpaired two‐tailed t‐test. ****P < 0.0001.
- Wild‐type organoids, at early stages of development (1–2 days) prior to crypt budding, form small spheroids that also exhibit either a columnar or a stretched epithelium where YAP is cytoplasmic or nuclear, respectively.
- Apc5 organoids (5 days) exhibit either a columnar or a stretched epithelium where YAP is cytoplasmic or nuclear, depending on the shape of the cells.
YAP integrates Wnt signalling and mechanical stimuli in intestinal organoids
Recent reports indicate that YAP can become nuclear localised in organoids cultured in mechanically stiff matrix (Gjorevski et al, 2016), collagen matrix (Yui et al, 2018) or after 3 days of culture in soft Matrigel (Gregorieff et al, 2015). Accordingly, we find that YAP is generally initially cytoplasmic and then translocates to the nucleus in organoids after 3–5 days in Matrigel, when crypt buds form (Fig 5A). The inhibition of Wnt signalling by addition of Porcupine (Porc) inhibitor reduces YAP protein levels in crypt buds without having a strong effect on YAP nuclear localisation, presumably owing to continued expression of TEAD3 (Fig 5B, Appendix Fig S2B–E). Activation of Wnt signalling in Apc5 organoids is associated with generally increased nuclear YAP levels and consequent induction of Ctgf mRNA (Appendix Fig S2A), but also with a drastic change in morphology, such that the organoids grow to become highly cystic (Fig 5C) (Novellasdemunt et al, 2017). It is likely that mechanical stretching of the epithelium (stress and/or strain) contributes to the difference in YAP subcellular localisation in organoids, as in other epithelia (Zhao et al, 2007; Dupont et al, 2011; Wada et al, 2011; Benham‐Pyle et al, 2015; Fletcher et al, 2018; Meng et al, 2018). In agreement with this view, in Apc5 organoids, YAP is strongly nuclear in stretched cells yet cytoplasmic in cuboidal cells and the same regulation is observable in early wild‐type organoids, prior to crypt budding, where small spheres have either a cuboidal or a stretched epithelium (Fig 5D and E). Thus, Wnt signalling transcriptionally controls YAP and TEAD levels, while YAP subcellular localisation is primarily regulated in a Wnt‐independent fashion in intestinal organoids.
YAP nuclear localisation is regulated by Src family kinase activity
We next sought to clarify which alternative signalling mechanisms might drive translocation of YAP from the cytoplasm to the nucleus upon organoid culture. One of the main candidates promoting YAP nuclear localisation is the Src family kinases (Cordero et al, 2014; Kim & Gumbiner, 2015; Elbediwy et al, 2016; Li et al, 2016; Si et al, 2017), which can also become activated in wild‐type or Apc mutant intestine (Cordero et al, 2014) via inflammatory cytokine signalling or experimentally induced colitis (Taniguchi et al, 2015; Taniguchi et al, 2017; Yui et al, 2018). We therefore treated intestinal organoids featuring nuclear YAP, with the Src family kinase inhibitor Dasatinib. We find that Dasatinib efficiently blocks the normal nuclear localisation of YAP in organoid buds, without affecting the gradient of YAP expression (Fig 6A and B). Comparably, YAP becomes cytoplasmic (and less active) throughout the whole epithelium in all Apc5 organoids treated with Dasatinib, or with the more specific Src inhibitor eCF506, indicating that YAP nuclear localisation is Src‐dependent even in Wnt active conditions (Fig 6A and C). These results indicate that Src family kinase activity is required for nuclear localisation of YAP in organoids in response to mechanical cues arising during growth of organoids into spherical cysts or during crypt budding.
Figure 6. In both wild‐type and Apc5 organoids, YAP nuclear localisation is prevented by a Src family kinase inhibitor.
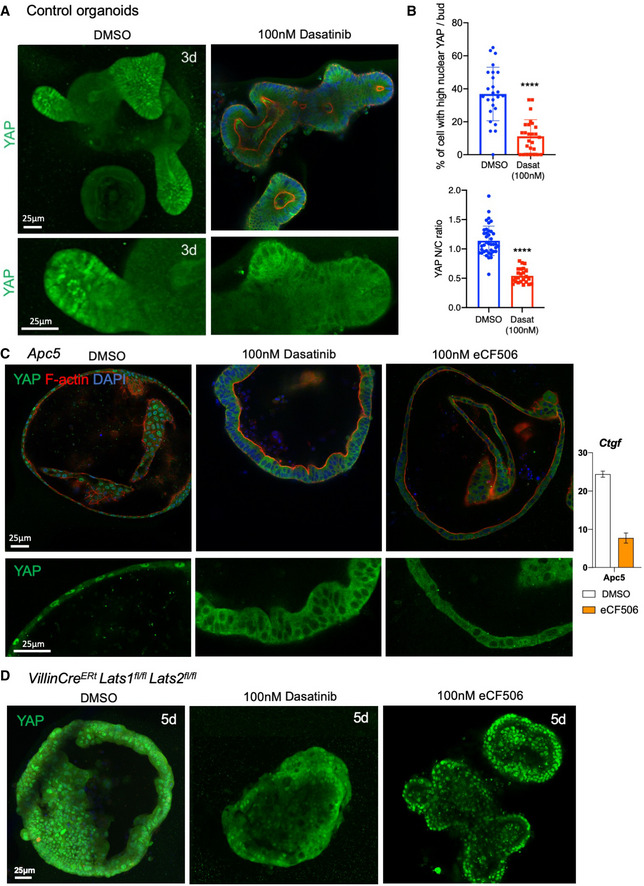
- Src family kinase inhibition via Dasatinib treatment in control organoids enforces YAP cytoplasmic localisation after 4 h of treatment in organoids cultured for 3 days. n > 10 organoids in each experiment.
- Quantification of the percentage of nuclear YAP cells per control crypt bud as shown in panel (A), error bars show 1 SD (****P < 0.0001). n > 10 organoids per condition (top). Quantification of the nuclear/cytoplasmic ratio of YAP per cell in Apc5 mutant organoids as shown in panel (C). n > 10 organoids per condition (bottom). Statistical analysis was performed with an unpaired Student's t‐test.
- Src family kinase inhibition via Dasatinib or eCF506 treatment in Apc5 organoids causes relocalisation of YAP to the cytoplasm. n > 10 organoids in each experiment. qPCR analysis of the YAP‐TEAD target gene Ctgf reveals a strong inhibition of mRNA expression upon treatment with eCF506 (relative expression, error bars = 1 SD). Statistical analysis was performed with an unpaired Student's t‐test.
- Src family kinase inhibition via Dasatinib or eCF506 treatment in Villin‐CreERt Lats1/2 dKO organoids (induced in vitro with 4‐OHT) fails to cause relocalisation of YAP to the cytoplasm after 4 h treatment in culture. n > 10 organoids in each experiment.
Source data are available online for this figure.
Previous work in cell culture has produced two different models for how Src family kinases might regulate YAP. Src activation has been reported to drive YAP to the nucleus either via direct tyrosine phosphorylation of YAP or via phosphorylation of LATS1 (Li et al, 2016; Si et al, 2017). In support of the latter hypothesis, knockouts for MST1/2, Sav1 or LATS1/2 in the intestine are sufficient to induce YAP nuclear localisation (Cai et al, 2010; Zhou et al, 2011; Cai et al, 2015; Gregorieff et al, 2015) and we find that Lats1/2 dKO intestines exhibit a crypt expansion phenotype with elevated cell proliferation (Fig 2A). We therefore tested whether Src inhibition with Dasatinib would have any effect in Lats1/2 dKO organoids. We find that YAP becomes strongly nuclear in all cells of the Lats1/2 dKO intestines and organoids, as expected, and that treatment with Dasatinib can only partially reduce YAP nuclear localisation in this context, with eCF506 having no effect (Fig 6D). These results support the notion that Src family kinases act primarily via inhibition of LATS1/2 (Si et al, 2017) rather than via a LATS‐independent mechanism, such as direct tyrosine phosphorylation of YAP (Taniguchi et al, 2015; Li et al, 2016; Elbediwy et al, 2018). Thus, our findings support the view that YAP subcellular localisation is primarily controlled by the opposing action of LATS1/2 and Src family kinase signalling, independently of the Wnt pathway.
Irradiation‐induced nuclear localisation of YAP requires Src family kinase signalling
Interestingly, Src was recently shown to become strongly active after irradiation in the intestinal epithelium where it is required for a proper regeneration (Cordero et al, 2014). Since the intestinal‐specific Yap single knockout and Yap, Taz double knockout each impair intestinal regeneration (Cai et al, 2010; Gregorieff et al, 2015; Gregorieff & Wrana, 2017), we wondered whether activated Src could be required to drive nuclear YAP during irradiation‐induced regeneration. We find that Src family kinase activity is also required for the strong nuclear localisation of YAP that occurs following irradiation both in organoids and in vivo (Fig 7A and B) (Elbediwy et al, 2016). Notably, the nuclear translocation of YAP occurs within 4 h of irradiation in freshly cultured organoids, which are cultured in the absence of inflammatory immune cells or stromal tissue, suggesting that Src family kinases and YAP can act directly within the epithelium itself as sensors of DNA damage, in addition to their established roles as sensors of mechanical forces (Kim & Gumbiner, 2015; Elbediwy et al, 2016; Si et al, 2017; Elbediwy et al, 2018) or cytokine/prostaglandin signalling (Taniguchi et al, 2015; Taniguchi et al, 2017; Roulis et al, 2020) (Fig 7A).
Figure 7. Irradiation‐induced nuclear localisation of YAP involves Src family kinase signalling.
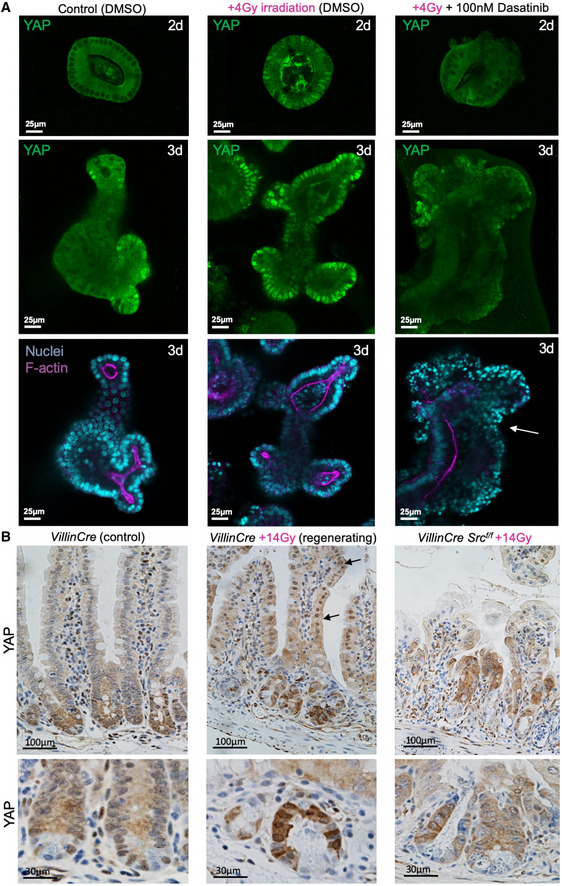
- Mouse intestinal organoids immunostained for YAP (green). Note the mostly cytoplasmic localisation of YAP at 48 h in the organoid. Irradiation with 4 Gy of X‐rays drives strong nuclear localisation of YAP in many cells, which is reversible by treatment with the Src family kinase inhibitor Dasatinib. The Dasatinib‐treated organoids exhibit increased cell death after irradiation, as indicated by pyknotic nuclei (white arrow). n > 10 organoids in each experiment. This experiment employs the same control batch of 2–3 days organoids also shown in Fig 6A.
- Control Villin‐CreERt mouse small intestine immunostained for YAP shows a gradient of expression with mostly cytoplasmic localisation. Irradiation with 14 Gy of X‐rays drives strong nuclear localisation of YAP along the entire crypt‐villus axis. Deletion of Src within Villin‐CreERt Srcflox / flox intestines prevents YAP nuclear localisation and normal regeneration after 14 Gy irradiation. Arrows point to villar cells with nuclear YAP localisation. Lower panels show high‐mag views of a different example than those shown in upper panels.
Source data are available online for this figure.
Irradiation‐induced YAP‐TEAD activity and crypt regeneration is Wnt‐dependent
Given that Src‐YAP signalling becomes strongly active upon irradiation of the intestinal epithelium, we sought to examine the consequences for YAP and Wnt target gene expression and the role of Wnt signals. We find that, as expected, expression of both Sox9 (analysed by immunohistochemistry) and Myc (analysed by RNAscope in situ hybridisation) becomes expanded after irradiation, consistent with the notion that Wnt signalling is still active (analysed by nuclear beta‐catenin staining) and that Sox9 & Myc are common YAP and Wnt target genes (Fig 8A). qPCR analysis confirms that YAP‐only target genes (Ctgf & Cyr61) are strongly induced upon irradiation, while a Wnt‐only target gene (Axin2) is mildly reduced. Other common YAP and Wnt targets show intermediate effects, with TEAD4 being strongly induced while TEAD1 and Sox9 levels being only moderately altered (Fig 8B). Importantly, the expansion of the Sox9 expression domain upon irradiation does require active Wnt signalling, as it is strongly reduced in size, and Sox9 mRNA levels decrease, upon treatment with Porc inhibitor (Fig 8C and D). Interestingly, p‐Src levels are also reduced upon treatment with Porc inhibitor (Fig 8C), possibly reflecting a role of the Wnt target gene CD44 in promoting Src activation and LATS1/2 inhibition in crypts, similar to its function in other cell and tumour types (Bourguignon et al, 2001; Li et al, 2001; Xu et al, 2010; Nam et al, 2015; Pastushenko et al, 2021). Indeed, intestinal knockouts of CD44 cause a phenotype similar to the Src KO or Yap/Taz dKO, featuring increased crypt apoptosis and prevention of ApcMin tumour formation (Zeilstra et al, 2008). These findings are consistent with an elongation and flattening of the Wnt gradient as the crypt expands upon irradiation, with an essential role of the re‐shaped Wnt signalling gradient in driving regeneration in combination with YAP‐TEAD activation. The essential role of Wnt signalling in supporting YAP‐TEAD expression and target gene activation is also evident by qPCR analysis of crypts in vivo and in organoids after irradiation, where expression of both Wnt and YAP target genes is strongly affected by treatment with Porc inhibitor (Appendix Fig S2E and F). Together, these results show that intestinal regeneration is orchestrated by the combined action of YAP‐TEAD signalling with a re‐shaped Wnt signalling gradient.
Figure 8. Irradiation induces YAP‐TEAD activity to drive crypt expansion, which also requires Wnt signalling.
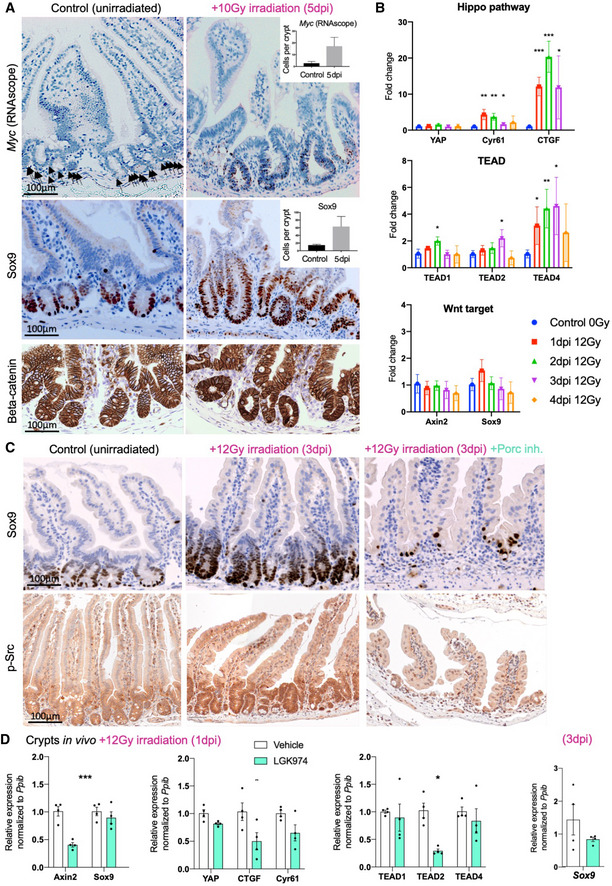
- Control and irradiated murine small intestines analysed with RNAscope in situ hybridisation for Myc mRNA (black arrows point to mRNA granules in the control) and by immunostaining for Sox9 protein reveals an expansion in the expression domain of both genes. Nuclear localisation of beta‐catenin is readily detectable after irradiation. n = 3 samples per condition, quantification shown in the insets, error bars = 1 SD. Statistical analysis was performed with an unpaired Student's t‐test. Similar results were obtained at 3 and 5 dpi.
- qPCR analysis of YAP, TEADs and their target genes Ctgf and Cyr61 over a 4 days post‐irradiation time course at 12 Gy. Note the strong upregulation of TEAD4, Ctgf and Cyr61. In contrast, Axin2, TEAD2 and Sox9 mRNA expression levels are only moderately altered, confirming that Wnt signalling remains active upon irradiation‐induced YAP‐TEAD activation. n = 4 biological replicates per time point. Statistical analysis was performed with unpaired t‐test compared to the control 0 Gy condition, *P < 0.05, **P < 0.01, ***P < 0.001.
- Control and irradiated murine small intestines analysed by immunostaining for Sox9 protein reveals that treatment with Porcupine inhibitor LGK974 strongly reduces the Sox9 expression domain induced by 3 days after 12 Gy irradiation. Phosphorylated Src immunostaining reveals an upregulation by 3 days after irradiation which is also lost upon treatment with Porcupine inhibitor LGK974 (n = 4 animals in each condition).
- qPCR analysis of Wnt and YAP target gene expression in intestinal crypts isolated at 1 day after 12 Gy irradiation. Note the inhibition of Axin2, Sox9, YAP, Ctgf, Cyr61 and TEAD family expression upon treatment with Porcupine inhibitor LGK974. The mild reduction of Sox9 mRNA expression by LGK974 at 1 day after irradiation (1 dpi) becomes more pronounced at 3 days after irradiation (3 dpi). n = 4 biological replicates per condition, statistical analysis was performed with Student's two‐tailed t‐test, error bars = 1 SD, *P < 0.05, **P < 0.01, ***P < 0.001.
Our results argue against the notion that YAP‐TEAD is a direct inhibitor of Wnt signalling in the intestine (Cheung et al, 2020; Li et al, 2020), which led one group to propose that YAP function as a tumour suppressor (Cheung et al, 2020), building on their previous findings that activation of a tetO‐YAPS127A rtTA transgene inhibits intestinal proliferation (Barry et al, 2013). We find that YAP activation (with either Lats1/2 dKO or transgenic nlsYAP5SA) does not inhibit cell proliferation or abolish Wnt signalling in vivo (Figs 2, 3 and EV3, EV4, EV5). Furthermore, Lats1/2 dKO organoids grow normally and express Sox9 (Appendix Fig S3), while expression of transgenic nlsYAP5SA does not impair growth of Apcfl / fl p53fl / fl knockout organoids in vitro (Appendix Fig S4) or formation of tumours after subcutaneous implantation into nude mice (Appendix Fig S5). These results support the notion that YAP acts as a pro‐proliferative and oncogenic factor during intestinal regeneration and tumour progression.
Discussion
Our findings confirm that YAP is normally nuclear localised in crypt base stem cells and show that Yap/Taz dKOs (Yap/Taz dKO) render stem cells susceptible to apoptosis. In addition, Yap/Taz dKO intestines revealed defects in the transverse folds of the ascending colon, a region exposed to a higher likelihood of tissue damage. These findings confirm a physiological requirement for the role of YAP in maintaining cell survival and promoting proliferative regeneration—a function previously observed after experimentally induced intestinal damage by irradiation or dextran sodium sulphate (DSS) treatment, which drives strong YAP nuclear localisation throughout the intestine (Cai et al, 2010; Gregorieff et al, 2015), which we also confirm. Ectopic induction of YAP nuclear localisation in Lats1/2 dKO intestines was sufficient to drive crypt expansion via hyperproliferation of transit‐amplifying cells, in agreement with recent findings (Li et al, 2020). The Lats1/2 dKO phenotype is highly similar to that caused by conditional expression of nlsYAP5SA, or that occurring during regeneration after irradiation, indicating that YAP activation is both necessary and sufficient to drive the regenerative response.
Our results also show that the YAP and TEAD1/2/4 genes are regulated transcriptionally by Wnt/beta‐catenin signalling in the intestine, while nuclear translocation of YAP upon tissue damage depends on Src family kinase signalling. The Wnt‐induced transcriptional gradient of YAP mRNA expression is sufficient to explain the resulting gradient of YAP protein along the crypt‐villus axis, as well as the elevation of YAP protein observed in Apc mutant tumours, while the Wnt‐induced TEAD1/2/4 mRNA expression is even more potent. Src‐dependent YAP nuclear localisation is then necessary for activation of YAP‐TEAD transcriptional targets and transit‐amplifying cell proliferation (see graphical abstract for a schematic model). Consequently, YAP remains mostly cytoplasmic until the tissue undergoes a regenerative response, when Src family kinases inhibit LATS1/2 and thereby drive YAP to the nucleus to enable it to activate YAP‐TEAD‐mediated transcription.
These results explain why Src‐YAP signalling is largely dispensable for normal intestinal development but essential to prime the intestinal regeneration response (Ashton et al, 2010; Cai et al, 2010; Cordero et al, 2014; Gregorieff et al, 2015; Taniguchi et al, 2015). Notably, the phenotype of Lats1/2 dKO, or nlsYAP5SA expression, appears to be a chronic regenerative proliferation response, which we observe induces diarrhoea, strongly reminiscent of inflammatory colitis—a key factor predisposing patients to colorectal cancer. Importantly, uniform nuclear YAP does not drive proliferation in differentiated cells of the villus, presumably because these villar cells lack sufficient Wnt signalling to induce TEAD1/2/4 expression, or to induce common Wnt and YAP target genes such as Sox9 and Myc, which explains why regenerative proliferation is normally limited to transit‐amplifying cells of the intestinal crypt. In contrast, in Apc mutant tumour cells, uniformly elevated Wnt signalling induces YAP, TEAD1/2/4, Sox9 and Myc expression throughout the tissue, enabling all cells to proliferate in an unlimited fashion in response to conditions that activate YAP, which include various stimuli such as DNA damage, mechanical stress or inflammatory signals (Thompson, 2020). Thus, all of our results are consistent with a pro‐proliferative, pro‐inflammatory and oncogenic role for YAP in both regeneration and in Apc mutant tumours, in agreement with several other reports (Cai et al, 2010; Cai et al, 2015; Gregorieff et al, 2015; Taniguchi et al, 2015; Taniguchi et al, 2017; Roulis et al, 2020).
Very recently, Li et al (2020) and Cheung et al (2020) reported that activation of YAP directly inhibits Wnt signalling in the intestinal epithelium, with one of these reports claiming that YAP therefore functions as a “tumour suppressor” in colorectal cancer (Cheung et al, 2020), in line with previous work from the same group (Barry et al, 2013). Our findings are only partly consistent with these reports. Since YAP is normally localised to the nucleus in crypt base stem cells, all groups agree that physiological YAP activity does not normally interfere with intestinal homeostasis, Wnt signalling or expression of high‐threshold Wnt target genes: crypt stem cell markers (Lgr5, Olfm4) or Paneth cell markers (Lyz) (Cai et al, 2010; Gregorieff et al, 2015; Cheung et al, 2020; Li et al, 2020). Furthermore, in Yap/Taz dKO, there is no observable increase in Wnt target gene expression in the intestinal epithelium (Cai et al, 2010; Gregorieff et al, 2015; Li et al, 2020). Thus, any potential role of YAP as an inhibitor of Wnt signalling does not manifest during normal stem cell proliferation or intestinal homeostasis—arguing against any general function for YAP as a Wnt signalling inhibitor in the intestine, as variously proposed to occur via inhibition of Dishevelled (DVL) function (Varelas et al, 2010; Barry et al, 2013), direct binding and retention of beta‐catenin in the cytoplasm (Imajo et al, 2012) or incorporation of YAP into the beta‐catenin destruction complex (Azzolin et al, 2014).
In contrast, the Wnt signalling gradient could be indirectly affected by the YAP‐driven crypt hyperproliferation phenotype during regeneration. Wnt gradients are known to “expand” to adjust to the size of the tissue over which they spread (Capek & Muller, 2019). Indeed, the rapid proliferation, migration (Krndija et al, 2019) and turnover of cells along the crypt‐villus axis during regeneration would be expected to carry secreted Wnt molecules across a greater distance than in normal homeostasis, elongating and flattening the Wnt signalling gradient. According to this “gradient scaling” model, expression of high‐threshold Wnt target genes (crypt stem cell markers) would be lost while expression of medium‐threshold Wnt target genes would actually be expanded along the crypt‐villus axis. Consistent with this model, Li et al and Cheung et al show that expression of high‐threshold Wnt target genes Lgr5, Olfm4 & Lyz is reduced in Lats1/2 cKO intestines, owing to activation of YAP (Cheung et al, 2020; Li et al, 2020), results supported by our own analysis in these mutants (Fig 2A–D). Importantly, medium‐threshold Wnt target genes, such as Axin2, Sox9 and Myc (Li et al, 2020), are actually induced and expanded upon YAP activation in Lats1/2 dKO intestines (Fig 2A), with Sox9 and Myc (common Wnt and YAP target genes) being more highly expressed than Axin2 (a Wnt‐only target gene), whose expression gradually declines. Thus, our results support an elongation/flattening of the Wnt signalling gradient upon YAP activation in the intestine, rather than a direct inhibition of Wnt signalling by YAP in this tissue.
Nevertheless, we do not rule out an eventual “negative feedback” role for YAP on Wnt signalling that emerges gradually during the intestinal regenerative response, which could contribute to the loss of high‐threshold Wnt target genes such as Lgr5, Olfm4 and Lyz (Gregorieff et al, 2015) and to the gradual decline in Axin2 levels observed in Lats1/2 dKO intestines. Klf6 (Cheung et al, 2020) or Clu (Ayyaz et al, 2019) are examples of regeneration‐specific YAP‐TEAD target genes, although whether they can inhibit Wnt signalling remains unknown. Nkd, a gene induced during intestinal regeneration (Van Landeghem et al, 2012), is a well‐known inhibitor of Wnt signalling that interferes with DVL function (Zeng et al, 2000; Gammons et al, 2020) and could provide a more plausible mechanism if found to be a YAP target gene in this tissue. Dkk1, also induced upon intestinal inflammation, is another well‐known inhibitor of Wnt signalling and could conceivably be induced downstream of YAP (Pinto et al, 2003; Kuhnert et al, 2004; Nava et al, 2010; Koch et al, 2011).
In summary, our findings help resolve conflicting models of how regenerative proliferation of intestinal crypts occurs after tissue damage and of the role of YAP‐TEAD and their regulation by Wnt signalling during this process. Our findings show that in addition to the well‐known function of Wnt signalling in maintaining homeostatic stem/progenitor cell proliferation and controlling cell fate along crypt‐villus axis, the Wnt pathway also primes the intestinal crypt for regeneration via inducing a gradient of YAP, TEAD1/2/4 and CD44 (which recruits Src). Upon tissue damage, strong Src activation and consequent YAP nuclear translocation drive YAP‐TEAD‐mediated transcription to increase cell proliferation to promote rapid regeneration. The crypt expansion phenotype that arises in response to YAP‐TEAD activation involves an elongation/flattening of the Wnt gradient, such that transit‐amplifying cells are expanded at the expense of stem cells. Synergy between Wnt and YAP signalling appears necessary for expansion of the Sox9 and Myc expression domain in the regenerating crypt. Around a week later, a negative feedback process appears to act to reduce Wnt signalling and then return the crypt to its homeostatic state. In Apc mutant tumours, Wnt signalling is permanently active in all cells, and the progression of such tumours is known to be accelerated by chronic tissue damage responses that can activate YAP‐TEAD.
Materials and Methods
Mouse strains
All experiments were carried out in accordance with the United Kingdom Animal Scientific Procedures Act (1986) and UK home office regulations under project license numbers 70/7926 and PDCC6E810. Villin‐CreERt2 mice were obtained from Ian Rosewell (The Francis Crick Institute). Wild‐type mice were used in C57/Bl(6) background. Src floxed and Fyn −/− , Yes −/− mice were obtained from Val Brunton (Edinburgh).
ApcMin mice were provided by Axel Behrens (The Francis Crick Institute) and Lats1/2 floxed mice were provided by Randy Johnson (MD Anderson Cancer Centre).
Inducible Cre activation
For in vivo experiments, Tamoxifen (Sigma, 25 mg/ml in corn oil) was injected intraperitoneally (IP; 8 μl/g body weight, or 4 µl/g for the LATS dKO experiments) for 3 or 5 consecutive days into 6‐ to 12‐week‐old controls or transgenic animals and analysed by immunohistochemistry several days thereafter. Intestinal regeneration was induced by irradiating mice with 10 to 14 Gy gamma irradiation four days after recombinase induction. Mice were sacrificed 1–5 days post‐irradiation. For transgenic cultured organoids, the Cre recombination was induced by addition of 4‐OHT (Sigma, H7904) at 1 μM in the culture media for 24 h after the first passage of the organoids.
Immunohistochemistry
Control and transgenic mouse gut were harvested and cut in four sections, which were flushed with cold PBS. A metal rod was inserted into each segment and placed in a holder to cut them longitudinally to open them flat on Whatman filters. The segments were then fixed in 10% neutral‐buffered formaldehyde for 24 h before being embedded in paraffin blocks. 4 μm thick sections were cut, deparrafinised and rehydrated using standard methods. After an antigen retrieval step, sections were stained with haematoxylin and eosin (H&E) solution or with primary antibody followed by a nuclear counterstaining. Additional images of human samples were obtained by data‐mining the www.proteinatlas.org database.
In situ hybridisation and quantification
Similar to immunohistochemistry, paraffined sections were rehydrated and subjected to the RNAscope protocol according to manufacturer's instructions. Hybridisation with PPIB (ACD, 313911) and DapB (ACD, 310043) probes as positive and negative control, respectively, was performed in parallel with the mouse Yap1 (316601, ACD), Tead1 (ACD, 457371), Tead2 (ACD, 420281), Tead4 (ACD, 312921), Axin2 (ACD, 400331) and Myc (LS, 413458 2.5) probes. The brown detection kit (ACD, 322300) was used to label the targeted Yap1 and Myc mRNA together with nuclear counterstaining, while the red detection kit (ACD, 322360) was used for Tead1, Tead2, Tead4 and Axin2.
To quantify YAP mRNA expression levels, YAP RNAscope stained slides were scanned with the Zeiss scanner and then analysed with the StrataQuest software from TissueGnostics. 10 ROIs were manually drawn on the first segments of the gut of three mice. Within each ROI, the villi were automatically detected, while the crypts were manually drawn. In each compartment, the individual brown dots corresponding to a single mRNA molecule were detected and quantified. The quantification is the number of brown punctae per μm2 in each compartment in every ROIs.
Organoid experiments
Intestinal crypts were isolated from 6‐ to 10‐week‐old C57B/l6 or transgenic mice, following the published protocol from Mahe et al (2013). Briefly, the whole gut was harvested and washed in cold DPBS (Gibco, 14190250). The most proximal 5 cm were cut open, and the villi were removed with a coverslip. The remaining tissue was washed and incubated in 2 mM EDTA for 30 min at 4°C. Two crypt fractions were then mechanically extracted, the first one being filtered through a 70 μM cell strainer before pooling both fractions together. After several low speed washes in ADF‐12 (Gibco, 12634‐010), isolated crypts were resuspended and plated in Matrigel (Corning, 354230). Organoids were cultured in IntestiCult media (Stemcell technologies, 06005) complemented with Primocin antibiotic (Invitrogen, ant‐pm‐05). Organoids were cultured in 24‐well plates for maintaining the cultures and then cultured in 8‐well chambers for drug incubations and immunostaining (Ibidi, 80827). The Porcupine inhibitor LGK974 (Selleck chemicals, S7143) was used at 5 μM for 24 h. For Src inhibition experiments, organoids were treated with 100 nM of Dasatinib (Selleck chemicals, S1021) or 100 nM eCF506 for a period of 4 h. Organoid microscopy was performed with either a Leica SP5 or a Leica SP8 laser‐scanning confocal microscope.
Antibodies
Primary antibodies used include the following: Rabbit YAP H‐125 (Santa Cruz Biotechnology sc‐15407), Mouse YAP 63.7 (Santa Cruz, sc‐101199), Rabbit pY418 Src (Life technologies, 44660G), Mouse Ezrin (Santa Cruz, sc‐58758), Rabbit Ki67 (Genetex, GTX16667) and Rabbit Cas3 (Cell signalling, 9661). Dilutions used are available from the first author upon request. For immunofluorescence secondary antibodies, Alexa‐488 (Invitrogen, A32723), Alexa‐568 (Invitrogen, A‐11011), along with Phalloidin‐647 (Invitrogen, A22287) and DAPI were used.
RT‐qPCR
Extraction of total RNA from intestinal crypts and organoids was homogenised and extracted using a RNeasy Mini Kit (Qiagen, 74106). cDNA synthesis for WT or KO mice was performed using Superscript II (Invitrogen, 18064022) or High‐Capacity cDNA Reverse Transcription Kit (Applied Biosystems, 4368813). Gene samples were run in triplicates with PowerUp SYBR Green (Applied Biosystems, A25778) on a Quantstudio 12 Flex Thermocycler. Expression values and quantitation was calculated using the ΔΔCT method relative to the housekeeping gene (PPIB). S.E.M was used for the error bars. Yap, Tead1, Tead2, Tead3, Tead4, Cyr6, Ctgf and Lgr5 primers were purchased as QuantiTect Primers (Qiagen).
Statistical analysis
Numerical data were plotted using Prism 9 software, and the mean and standard deviations were calculated in order to plot graphs and error bars. Student's t‐test was performed to determine statistical significance. *P < 0.05, **P < 0.01, ***P < 0.001.
Author contributions
OG, NA, CMS, RR, AB, AK, PA and MRR performed the experiments and prepared the figures. OG, JC, VSWL and BJT conceived the experiments and wrote the manuscript with input from NA. OS, JC, VSLW and BJT obtained research funding and supervised the project.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Source Data for Figure 6
Source Data for Figure 7
Acknowledgements
This work was funded by the Francis Crick Institute as well as by the Cancer Research UK Beatson Institute and the Australian National University. VSWL laboratory is funded by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001105), the UK Medical Research Council (FC001105) and the Wellcome Trust (FC001105). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
The EMBO Journal (2021) 40: e105770.
Data Availability
This study does not include data deposited in external repositories.
References
- Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa‐Kawakita M, Laurent‐Puig P, Kahn A, Robine S, Perret C et al (2005) Crypt‐restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development 132: 1443–1451 [DOI] [PubMed] [Google Scholar]
- Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, Wilkins JA, Athineos D, Muncan V, Kemp R et al (2010) Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c‐Myc signaling. Dev Cell 19: 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyaz A, Kumar S, Sangiorgi B, Ghoshal B, Gosio J, Ouladan S, Fink M, Barutcu S, Trcka D, Shen J et al (2019) Single‐cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569: 121–125 [DOI] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V et al (2014) YAP/TAZ incorporation in the beta‐catenin destruction complex orchestrates the Wnt response. Cell 158: 157–170 [DOI] [PubMed] [Google Scholar]
- Bach SP, Renehan AG, Potten CS (2000) Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis 21: 469–476 [DOI] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S et al (2013) Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493: 106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty‐Double C, Bibeau F, Scherer G, Joubert D, Hollande F et al (2007) Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol 178: 635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham‐Pyle BW, Pruitt BL, Nelson WJ (2015) Cell adhesion. Mechanical strain induces E‐cadherin‐dependent Yap1 and beta‐catenin activation to drive cell cycle entry. Science 348: 1024–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M, Clevers H (2000) Linking colorectal cancer to Wnt signaling. Cell 103: 311–320 [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu H, Shao L, Chen YW (2001) CD44 interaction with c‐Src kinase promotes cortactin‐mediated cytoskeleton function and hyaluronic acid‐dependent ovarian tumor cell migration. J Biol Chem 276: 7327–7336 [DOI] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D (2010) The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev 24: 2383–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Maitra A, Anders RA, Taketo MM, Pan D (2015) beta‐Catenin destruction complex‐independent regulation of Hippo‐YAP signaling by APC in intestinal tumorigenesis. Genes Dev 29: 1493–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capek D, Muller P (2019) Positional information and tissue scaling during development and regeneration. Development 146: dev177709 [DOI] [PubMed] [Google Scholar]
- Cheung P, Xiol J, Dill MT, Yuan W‐C, Panero R, Roper J, Osorio FG, Maglic D, Li Qi, Gurung B et al (2020) Regenerative reprogramming of the intestinal stem cell state via hippo signaling suppresses metastatic colorectal cancer. Cell Stem Cell 27: 590–604.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Kim J, Park J, Lee D‐H, Hwang D, Kim J‐H, Ashktorab H, Smoot D, Kim S‐Y, Choi C et al (2018) YAP/TAZ initiates gastric tumorigenesis via upregulation of MYC. Cancer Res 78: 3306–3320 [DOI] [PubMed] [Google Scholar]
- Clevers H (2013) The intestinal crypt, a prototype stem cell compartment. Cell 154: 274–284 [DOI] [PubMed] [Google Scholar]
- Cordero JB, Ridgway RA, Valeri N, Nixon C, Frame MC, Muller WJ, Vidal M, Sansom OJ (2014) c‐Src drives intestinal regeneration and transformation. EMBO J 33: 1474–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, Basler K (2018) GLI1‐expressing mesenchymal cells form the essential Wnt‐secreting niche for colon stem cells. Nature 558: 449–453 [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S et al (2011) Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183 [DOI] [PubMed] [Google Scholar]
- Elbediwy A, Vincent‐Mistiaen ZI, Spencer‐Dene B, Stone RK, Boeing S, Wculek SK, Cordero J, Tan EH, Ridgway R, Brunton VG et al (2016) Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 143: 1674–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbediwy A, Vanyai H, Diaz‐de‐la‐Loza MD, Frith D, Snijders AP, Thompson BJ (2018) Enigma proteins regulate YAP mechanotransduction. J Cell Sci 131: jcs221788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevr T, Robine S, Louvard D, Huelsken J (2007) Wnt/beta‐catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 27: 7551–7559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch AJ, Soucek L, Junttila MR, Swigart LB, Evan GI (2009) Acute overexpression of Myc in intestinal epithelium recapitulates some but not all the changes elicited by Wnt/beta‐catenin pathway activation. Mol Cell Biol 29: 5306–5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GC, Diaz‐de‐la‐Loza MD, Borreguero‐Munoz N, Holder M, Aguilar‐Aragon M, Thompson BJ (2018) Mechanical strain regulates the Hippo pathway in Drosophila . Development 145: dev159467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz A, Shlyueva D, Brunner E, Stark A, Basler K (2017) Probing the canonicity of the Wnt/Wingless signaling pathway. PLoS Genet 13: e1006700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammons M, Bienz M (2018) Multiprotein complexes governing Wnt signal transduction. Curr Opin Cell Biol 51: 42–49 [DOI] [PubMed] [Google Scholar]
- Gammons M, Renko M, Flack JE, Mieszczanek J, Bienz M (2020) Feedback control of Wnt signaling based on ultrastable histidine cluster co‐aggregation between Naked/NKD and Axin. eLife 9: e59879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordonez‐Moran P, Clevers H, Lutolf MP (2016) Designer matrices for intestinal stem cell and organoid culture. Nature 539: 560–564 [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL (2015) Yap‐dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature 526: 715–718 [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Wrana JL (2017) Hippo signalling in intestinal regeneration and cancer. Curr Opin Cell Biol 48: 17–25 [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM (1999) Intestinal polyposis in mice with a dominant stable mutation of the beta‐catenin gene. EMBO J 18: 5931–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c‐MYC as a target of the APC pathway. Science 281: 1509–1512 [DOI] [PubMed] [Google Scholar]
- Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E (2012) A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta‐catenin signalling. EMBO J 31: 1109–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, Winton DJ (2004) Inducible Cre‐mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta‐catenin. Gastroenterology 126: 1236–1246 [DOI] [PubMed] [Google Scholar]
- Kim NG, Gumbiner BM (2015) Adhesion to fibronectin regulates Hippo signaling via the FAK‐Src‐PI3K pathway. J Cell Biol 210: 503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Nava P, Addis C, Kim W, Denning TL, Li L, Parkos CA, Nusrat A (2011) The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology 141: 259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsavage WM Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS (2012) Wnt/beta‐catenin signaling regulates Yes‐associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem 287: 11730–11739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta‐catenin‐Tcf complex in APC‐/‐ colon carcinoma. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H (1998) Depletion of epithelial stem‐cell compartments in the small intestine of mice lacking Tcf‐4. Nat Genet 19: 379–383 [DOI] [PubMed] [Google Scholar]
- Krndija D, El Marjou F, Guirao B, Richon S, Leroy O, Bellaiche Y, Hannezo E, Matic VD (2019) Active cell migration is critical for steady‐state epithelial turnover in the gut. Science 365: 705–710 [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ (2004) Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf‐1. Proc Natl Acad Sci USA 101: 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wong N, Jabali MD, Johnson P (2001) CD44‐initiated cell spreading induces Pyk2 phosphorylation, is mediated by Src family kinases, and is negatively regulated by CD45. J Biol Chem 276: 28767–28773 [DOI] [PubMed] [Google Scholar]
- Li P, Silvis MR, Honaker Y, Lien WH, Arron ST, Vasioukhin V (2016) alphaE‐catenin inhibits a Src‐YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes Dev 30: 798–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Qi, Sun Y, Jarugumilli GK, Liu S, Dang K, Cotton JL, Xiol J, Chan PY, DeRan M, Ma L et al (2020) Lats1/2 sustain intestinal stem cells and Wnt activation through TEAD‐dependent and independent transcription. Cell Stem Cell 26: 675–692.e678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X (2009) Wnt/beta‐catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe MM, Aihara E, Schumacher MA, Zavros Y, Montrose MH, Helmrath MA, Sato T, Shroyer NF (2013) Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol 3: 217–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Qiu Y, Lin KC, Kumar A, Placone JK, Fang C, Wang K‐C, Lu S, Pan M, Hong AW et al (2018) RAP2 mediates mechanoresponses of the Hippo pathway. Nature 560: 655–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori‐Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B (2007) SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology 133: 539–546 [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW (1997) Activation of beta‐catenin‐Tcf signaling in colon cancer by mutations in beta‐catenin or APC. Science 275: 1787–1790 [DOI] [PubMed] [Google Scholar]
- Muncan V, Sansom OJ, Tertoolen L, Phesse TJ, Begthel H, Sancho E, Cole AM, Gregorieff A, de Alboran IM, Clevers H et al (2006) Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf‐4 target gene c‐Myc. Mol Cell Biol 26: 8418–8426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam K, Oh S, Lee KM, Yoo SA, Shin I (2015) CD44 regulates cell proliferation, migration, and invasion via modulation of c‐Src transcription in human breast cancer cells. Cell Signal 27: 1882–1894 [DOI] [PubMed] [Google Scholar]
- Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, Beeman N, Addis C, Gerner‐Smidt K, Neumaier I et al (2010) Interferon‐gamma regulates intestinal epithelial homeostasis through converging beta‐catenin signaling pathways. Immunity 32: 392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novellasdemunt L, Foglizzo V, Cuadrado L, Antas P, Kucharska A, Encheva V, Snijders AP, Li VSW (2017) USP7 is a tumor‐specific WNT activator for APC‐mutated colorectal cancer by mediating beta‐catenin deubiquitination. Cell Rep 21: 612–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastushenko I, Mauri F, Song Y, de Cock F, Meeusen B, Swedlund B, Impens F, Van Haver D, Opitz M, Thery M et al (2021) Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature 589: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H (2003) Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS (1998) Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci 353: 821–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Grant HK (1998) The relationship between ionizing radiation‐induced apoptosis and stem cells in the small and large intestine. Br J Cancer 78: 993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KC, Gracz AD, Liu XF, Newton V, Akiyama H, Magness ST (2015) SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology 149: 1553–1563.e1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC et al (2012) beta‐Catenin‐driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151: 1457–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulis M, Kaklamanos A, Schernthanner M, Bielecki P, Zhao J, Kaffe E, Frommelt L‐S, Qu R, Knapp MS, Henriques A et al (2020) Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature 580: 524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon‐Assmann P, Clevers H, Nathke IS et al (2004) Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 18: 1385–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR (2007) Myc deletion rescues Apc deficiency in the small intestine. Nature 446: 676–679 [DOI] [PubMed] [Google Scholar]
- Si Y, Ji X, Cao X, Dai X, Xu L, Zhao H, Guo X, Yan H, Zhang H, Zhu C et al (2017) Src inhibits the hippo tumor suppressor pathway through tyrosine phosphorylation of Lats1. Cancer Res 77: 4868–4880 [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Wu L‐W, Grivennikov SI, de Jong PR, Lian I, Yu F‐X, Wang K, Ho SB, Boland BS, Chang JT et al (2015) A gp130‐Src‐YAP module links inflammation to epithelial regeneration. Nature 519: 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, Moroishi T, de Jong PR, Krawczyk M, Grebbin BM, Luo H, Xu R‐H, Golob‐Schwarzl N, Schweiger C, Wang K et al (2017) YAP‐IL‐6ST autoregulatory loop activated on APC loss controls colonic tumorigenesis. Proc Natl Acad Sci USA 114: 1643–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ (2020) YAP/TAZ: drivers of tumor growth, metastasis, and resistance to therapy. BioEssays 42: e1900162 [DOI] [PubMed] [Google Scholar]
- Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, Moor MB, Hausmann G, Cantu C, Aguet M, Basler K (2016) Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep 15: 911–918 [DOI] [PubMed] [Google Scholar]
- van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo B‐K, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S et al (2012) A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self‐renewal. Mol Cell Biol 32: 1918–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST, Lund PK (2012) Activation of two distinct Sox9‐EGFP‐expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol 302: G1111–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H et al (2010) The Hippo pathway regulates Wnt/beta‐catenin signaling. Dev Cell 18: 579–591 [DOI] [PubMed] [Google Scholar]
- Wada K, Itoga K, Okano T, Yonemura S, Sasaki H (2011) Hippo pathway regulation by cell morphology and stress fibers. Development 138: 3907–3914 [DOI] [PubMed] [Google Scholar]
- Xu Y, Stamenkovic I, Yu Q (2010) CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res 70: 2455–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S, Azzolin L, Maimets M, Pedersen MT, Fordham RP, Hansen SL, Larsen HL, Guiu J, Alves MRP, Rundsten CF et al (2018) YAP/TAZ‐dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 22: 35–49.e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilstra J, Joosten SP, Dokter M, Verwiel E, Spaargaren M, Pals ST (2008) Deletion of the WNT target and cancer stem cell marker CD44 in Apc(Min/+) mice attenuates intestinal tumorigenesis. Cancer Res 68: 3655–3661 [DOI] [PubMed] [Google Scholar]
- Zeng W, Wharton KA Jr, Mack JA, Wang K, Gadbaw M, Suyama K, Klein PS, Scott MP (2000) naked cuticle encodes an inducible antagonist of Wnt signalling. Nature 403: 789–795 [DOI] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L et al (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD et al (2011) Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes‐associated protein (Yap) overabundance. Proc Natl Acad Sci USA 108: E1312–E1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File
Source Data for Figure 6
Source Data for Figure 7
Data Availability Statement
This study does not include data deposited in external repositories.


