Abstract
Introduction
Alzheimer’s disease (AD) is one of the most common causes of dementia. Pathogenic variants in the presenilin 1 (PSEN1) gene are the most frequent cause of early-onset AD. Medications for patients with AD bearing PSEN1 mutation (PSEN1-AD) are limited to symptomatic therapies and no established radical treatments are available. Induced pluripotent stem cell (iPSC)-based drug repurposing identified bromocriptine as a therapeutic candidate for PSEN1-AD. In this study, we used an enrichment strategy with iPSCs to select the study population, and we will investigate the safety and efficacy of an orally administered dose of bromocriptine in patients with PSEN1-AD.
Methods and analysis
This is a multicentre, randomised, placebo-controlled trial. AD patients with PSEN1 mutations and a Mini Mental State Examination-Japanese score of ≤25 will be randomly assigned, at a 2:1 ratio, to the trial drug or placebo group (≥4 patients in TW-012R and ≥2 patients in placebo). This clinical trial consists of a screening period, double-blind phase (9 months) and extension phase (3 months). The double-blind phase for evaluating the efficacy and safety is composed of the low-dose maintenance period (10 mg/day), high-dose maintenance period (22.5 mg/day) and tapering period of the trial drug. Additionally, there is an open-labelled active drug extension period for evaluating long-term safety. Primary outcomes are safety and efficacy in cognitive and psychological function. Also, exploratory investigations for the efficacy of bromocriptine by neurological scores and biomarkers will be conducted.
Ethics and dissemination
The proposed trial is conducted according to the Declaration of Helsinki, and was approved by the Institutional Review Board (K070). The study results are expected to be disseminated at international or national conferences and published in international journals following the peer-review process.
Trial registration number
jRCT2041200008, NCT04413344.
Keywords: dementia, clinical trials, neurogenetics, neurology, neurobiology
Strengths and limitations of this study.
This trial will provide the safety and efficacy data of bromocriptine for Alzheimer’s disease patients with mutations in the presenilin 1 (PSEN1) gene (PSEN1-AD).
Bromocriptine is an approved drug with safety information accumulated over decades.
The trial design includes enrichment of the patient population by induced pluripotent stem cell technology.
Minimally invasive biomarkers, using plasma samples, positron emission tomography and digital tools, will be investigated in addition to amyloid β-protein and tau in cerebrospinal fluid samples.
A possible limitation may be a difficulty in recruiting a large-enough number of patients due to the rarity of PSEN1-AD.
Introduction
Alzheimer’s disease (AD) was first reported by Dr Alios Alzheimer in 1906, and it is now regarded as the most prevalent neurodegenerative disease, accounting for 60%–80% of dementia cases. Over 50 million people are estimated to live with dementia globally, a figure set to increase to 152 million by 2050.1 The number of AD patients is also expected to increase explosively in Japan in line with our super-ageing society. Although this disease should be treated promptly, available treatments are limited to several symptomatic therapies.2
AD is a neurodegenerative disease that presents clinical symptoms, primarily including progressive decline in memory. Elucidation of the central pathology of AD began in the 1980s with the identification of amyloid β-protein (Aβ) by biochemical methods. Aβ is a major component of senile plaques, which represent a pathological characteristic of AD. Subsequently, in the 1990s, genetic approaches identified the Aβ precursor protein (APP), presenilin 1 (PSEN1) and presenilin 2 as causative genes for familial AD, thereby rapidly advancing the understanding of such condition.3 Notably, AD patients with mutations in the PSEN1 gene (PSEN1-AD) experience onset in their 20s–50s, and their cognitive function deteriorates rapidly, leading to death within several years, as curative therapies are unavailable.4 Thus, the development of new drugs for PSEN1-AD is eagerly awaited. Converging basic and clinical evidence suggests that mutant PSEN1 could affect the function of γ-secretases in neurons and increase the Aβ42 levels in plasma of FAD patients, transfected cells and transgenic mice.5–7 This abnormal production of Aβ was estimated to originate from the altered conformation of the γ-secretase complex and changes in the active site of the cleavage process.8 9
To develop therapeutic compounds for PSEN1-AD, we previously established induced pluripotent stem cells (iPSCs) from patients with PSEN1-AD. iPSCs were established by introducing a small number of genes into patients’ cells. Established iPSCs can differentiate into any type of cell in the body and proliferate indefinitely. iPSC technology provided in vitro models of inaccessible human cell types and impacted investigations of disease mechanisms especially in brain disorders. We established an iPSC-based screening system by modelling Aβ phenotypes of PSEN-1AD.10 We utilised an existing drug library the safety and pharmacokinetic profile of which had already been confirmed clinically and approved for use in humans.10 After the screening, we found bromocriptine to be the most potent modifier of Aβ production for PSEN1-AD neurons among existing drugs. Dose-dependency assay showed that bromocriptine reduced the Aβ42 dose and Aβ42/40 ratio by up to ~50% and~40%, respectively. Furthermore, we prepared cortical neurons of several patients with PSEN1-AD and sporadic AD to evaluate the specificity of bromocriptine for PSEN1-AD. Bromocriptine reduced the Aβ42 dose and Aβ42/40 ratio of PSEN1-AD neurons more strongly than those of sporadic AD neurons. From these results, we selected patients with PSEN1-AD as a bromocriptine-responsive subgroup in AD.11 12
Bromocriptine is an already approved drug with few safety concerns and a long history of usage in clinical settings. Bromocriptine was approved for the treatment of Parkinson’s disease (approved dosage 22.5 mg/day),13 pituitary tumour and hyperprolactinaemia, and was proven to penetrate the blood–brain barrier. Based on iPSC studies, as we hypothesised that bromocriptine might attenuate the clinical symptoms of PSEN1-AD patients, we decided to conduct a clinical trial to evaluate the safety and efficacy of the first-time administration to PSEN1-AD patients as an investigator-initiated clinical trial (exploratory study), which will confirm its safety and provide evidence of its efficacy.
Objectives
The aim of this study is to investigate the safety and efficacy of an orally administered dose of bromocriptine in patients with PSEN1-AD using a placebo group as control. In addition, long-term safety will be examined in an open-label extension trial.
Methods and analysis
Trial population and rationale for selecting participants
The following patients will be included in this study. Detailed eligibility criteria are presented in ‘box 1’. This ongoing clinical trial started to enrol participants in June 2020. The study is planned to end in March 2022.
Box 1. Eligibility criteria of REBRAnD study.
Eligibility criteria
Patients who meet all the following inclusion criteria and do not meet any of the exclusion criteria will be enrolled as eligible participants.
Inclusion criteria
Alzheimer’s disease (AD) patients with presenilin 1 (PSEN1) mutations.
Patients diagnosed with ‘probable AD’ according to the diagnostic guideline of National Institute on Aging-Alzheimer’s Association (NIA-AA) or ‘probable Alzheimer-type dementia’ according to the diagnostic criteria for AD specified in Diagnostic and Statistical Manual of Mental Disorders (DSM-5).
An Mini Mental State Examination-Japanese score of ≤25.
Patients whose cognitive function and every-day function are obviously impaired based on their medical record or information provided by a person well acquainted with the patient.
Patients for whom intellectual disability and mental disorders other than dementia can be ruled out based on their academic background, work history and life history.
Patients with a reliable and close relationship with a partner/caregiver.
Age ≥20 years at the time of providing informed consent.
Written informed consent has been obtained from the patient or his/her legally acceptable representative to participate in this trial.
Exclusion criteria
Difficulty with the oral intake of tablets.
Patients receiving anti-dementia drugs who have changed the dosing regimen during the 2 months prior to providing informed consent.
Patients with dementia due to a pathology other than AD (eg, vascular dementia, frontotemporal dementia, Lewy body dementia, progressive supranuclear palsy, corticobasal degeneration, Huntington’s disease, prion disease).
Presence of clinically relevant or unstable mental disorders. Patients with major depression in remission can be enrolled.
Imminent risk of self-harm or harm to others.
Body mass index of ≤17 or ≥35.
Patients with a history of alcohol dependence, drug dependence or drug abuse within 5 years before providing informed consent.
HBs antigen positive.
Anti-HIV antibody positive.
Anti-human T-lymphotropic virus type 1 (HTLV-1) antibody positive.
Patients with an active infection such as hepatitis C and syphilis (positive serological test for syphilis and T. pallidum haemagglutination assay (STS/TPHA)).
-
Patients with the following liver function values by testing before enrolment.
Aspartate aminotransferase (AST/GOT)>4.0 × upper limit of institutional reference range or
Alanine aminotransferase (ALT/GPT) >4.0 × upper limit of institutional reference range.
Patients with uncontrolled, clinically significant medical conditions (eg, diabetes mellitus, hypertension, thyroid/endocrine disease, congestive cardiac failure, angina pectoris, cardiac/gastrointestinal disease, dialysis and abnormal renal function with an estimated creatinine clearance (CLCr) <30 mL/min) within 3 months prior to providing informed consent in addition to the underlying disease to be investigated in the trial and for whom the investigator or subinvestigator considers that there is a significant medical risk in the patient’s participation in the trial.
Patients with long QT syndrome or tendency towards prolonged corrected QT (QTc) interval (male: ≥470 ms, female: ≥480 ms), or patients with a history/complication of torsades de pointes.
-
Patients with a history of malignancies within 5 years prior to providing informed consent. However, patients with the following diseases can be enrolled if they are treated appropriately:
Skin cancer (basal cell, squamous cell).
Cervical carcinoma in situ.
Localised prostate cancer.
Malignancies that have not recurred for at least 3 years since surgery and the patient’s physician has determined that the risk of recurrence is low.
Patients with clinically significant vitamin B1/B12 deficiency or folic acid deficiency within 6 months prior to providing informed consent.
Patients who participated in other clinical research/trials involving interventions within 3 months prior to providing informed consent.
Patients who previously received bromocriptine or TW-012R.
Patients with a history of hypersensitivity to bromocriptine or ergot alkaloids.
Patients with current or a history of thickened heart valve cusps, restricted heart valve motion and associated heart valve lesions, such as stenosis, confirmed by echocardiography.
Pregnant females, lactating females and females who may be pregnant (pregnancy test will be performed to confirm this status), and females who wish to become pregnant.
Other patients who are considered inappropriate for participating in this trial at the discretion of the investigator or subinvestigator.
*CYP3A4 inhibitors/inducers and dopamine antagonists are contraindicated during the trial period except for domperidone and quetiapine in emergent settings.
Alzheimer’s disease patients with PSEN1 mutations (PSEN1-AD).
Patients diagnosed with ‘probable AD’ according to the diagnostic guideline of National Institute on Aging-Alzheimer’s Association (NIA-AA)14 or ‘probable Alzheimer-type dementia’ according to the diagnostic criteria for AD specified in Diagnostic and Statistical Manual of Mental Disorders (DSM-5).15
Mini Mental State Examination-Japanese (MMSE-J) score of ≤25.
PSEN1-AD is the target disease. The MMSE-J score will be specified at 25 points or lower to enrol patients with dementia and mild cognitive impairment (MCI) and to exclude those with normal cognitive function. We will exclude MCI participants with MMSE-J scores of 26 or 27, who are reported to have lower risk and longer duration of MCI conversion to AD.16 The concomitant use of existing drugs for the treatment of AD (donepezil, galantamine, rivastigmine and memantine) will be allowed to evaluate the safety and efficacy of future practical bromocriptine use on approval, but patients who have changed their regimen within 2 months before providing informed consent will be excluded because the efficacy of bromocriptine alone will be difficult to evaluate. Patients taking oral donepezil hydrochloride will be carefully monitored because the blood concentration may be increased by the additional oral administration of the trial drug. Recruiting will be done by neurologists who treat PSEN1-AD patients and are blinded to their allocation. All participants in the study need to be confirmed for the presence of PSEN-1 mutation by genetic analyses. Given that PSEN1-AD patients are very few, the planned feasible recruitment duration will be 10 months.
Sample size
The number of PSEN1-AD patients confirmed by genetic diagnosis in Japan is approximately 100 according to the Japanese Familial Alzheimer’s Disease (JFAD) database17 and a report of a research project on the support system for individuals with familial AD and their families in Japan.18 This number corresponds to the global data of reported cases from Asia, the UK and the USA.3 19 20 In addition, compared with patients with sporadic AD without PSEN1 mutations, PSEN1-AD patients have a more rapid progression of cognitive decline and a more substantial decrease in the ability to perform daily living activities (ADLs) owing to the overlap of various neurologic symptoms, including spastic paralysis and extrapyramidal symptoms. Hence, the number of patients is more limited to those who meet the inclusion criteria (box 1) in the limited trial duration. Given such rarity, the patient accumulation is extremely low and the targeted sample size for this randomised trial is ≥4 patients in TW-012R and ≥2 patients in placebo based on feasibility and the aforementioned validity. Unequal randomisation will be employed because of ethical considerations. There is no formal hypothesis or power sample size calculation due to the exploratory nature of this study. Thus, we did not perform sample size calculation. To ensure that sufficient participants complete the trial, clinical research coordinators will communicate well and frequently both with the central coordinating centre and the participants/care givers.
Intervention and control
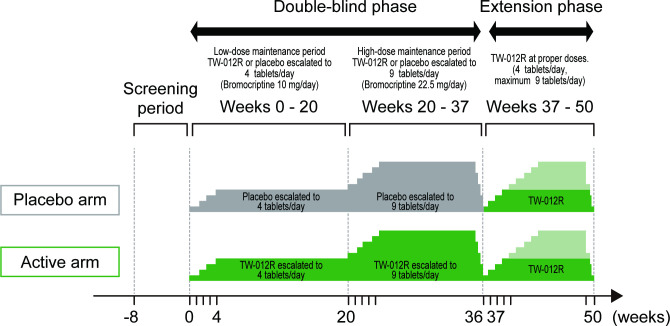
This clinical trial consists of the screening period (8 weeks), double-blind phase (37 weeks) and extension phase (13 weeks). The double-blind phase for evaluating the efficacy and safety is composed of the low-dose maintenance period (20 weeks), high-dose maintenance period (16 weeks) and tapering period of the trial drug (1 week). Additionally, there is an open-labelled active drug extension period for evaluating long-term safety (figure 1). Participants who are screened during the screening period and assessed as eligible will be randomly assigned to the bromocriptine and placebo groups.
Figure 1.
Design of REBRAnD study. The study consists of the screening period (8 weeks), double-blind phase (37 weeks) and extension phase (13 weeks). The double-blind phase for evaluating the efficacy and safety is composed of the low-dose maintenance period (up to 10 mg/day), high-dose maintenance period (up to 22.5 mg/day) and tapering period of the trial drug. Additionally, there is an open-labelled active drug extension period (up to 10 or 22.5 mg/day) for evaluating long-term safety.
Intervention—bromocriptine (TW-012R, each tablet contains 2.5 mg of bromocriptine, Towa Pharmaceutical)13
Control—placebo (tablets identical in appearance, smell and taste)
Participants will receive the trial drug in the double-blind phase, during which the low-dose maintenance period (from the start of trial treatment until week 20) will be started with 1 tablet of TW-012R or placebo administered orally once daily. The daily dose will be increased by one tablet per week up to four tablets (10 mg) daily until week 20 as the maintenance dose. The safety and efficacy of bromocriptine orally administered at 10 mg/day will be evaluated in comparison with the placebo. Then, the high-dose maintenance period (weeks 20–36) will be started with five tablets of TW-012R or placebo daily (further increased by one tablet from the maintenance dose of the low-dose maintenance period). The daily dose will be increased by one tablet per week, and a maximum of nine tablets (22.5 mg) daily will be continued as the maintenance dose until week 36. The safety and efficacy of bromocriptine orally administered at a dose of 22.5 mg/day will be evaluated in comparison with the placebo. During the tapering period of the trial drug (weeks 36–37), the dose of bromocriptine or placebo will be tapered to one tablet from the previous dose during 1 week.
In the extension phase (weeks 37–50), the participants in both groups will start oral administration of 1 tablet of TW-012R (2.5 mg) once daily. The daily dose will be increased by one tablet per week, and the administration will be continued until week 48 at a standard dose of four tablets daily, with a maximum of nine tablets, as maintenance dose to evaluate the safety of the long-term administration of bromocriptine. Then, the dose will be tapered during about 1–2 weeks to complete the treatment.
In all phases, ‘not increasing the dose’ or ‘decreasing the dose’ is allowed at the discretion of the investigator from the viewpoint of safety of the participants. Adherence will be monitored by counting empty PTP (press through pack) sheets of study drugs. Due to the COVID-19 pandemic in 2020, we amended our protocol to make it possible to deliver study drugs if a participant cannot visit a medical facility. For participant retention and safety reasons, a participant will have 10 phone-call-visits in addition to monthly onsite-visits.
As for drug interactions, CYP3A4 inhibitors/inducers and dopamine antagonists are prohibited because bromocriptine is primarily metabolised by CYP3A4 and drug interaction is anticipated. Benzodiazepines are also prohibited because they might result in reduced efficacy in the clinical phenotype, especially cognitive function.
This study will be conducted at six academic hospitals and one community hospital in Japan.
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT checklist) was followed in designing the study protocol.
Randomisation, allocation, and blinding
Patients meeting all the inclusion criteria and not meeting any of the exclusion criteria (box 1) at the time of enrolment in the trial will be randomly assigned, at a 2:1 ratio, to receive either TW-012R or placebo. Owing to the ethical issue for patients assigned to the placebo group, unequal randomisation will be employed. Randomisation will be performed using permuted blocks and stratified based on the important confounding variable of the baseline MMSE-J score (<13 or ≥13). The allocation sequence will be prepared by an external contract research organisation, and trial participants, care givers, outcome assessors and investigators will be blinded. Registration will be done by investigators, etc., using the web enrolment system. When a patient is determined to be eligible by the web enrolment system, the allocated drug number will be displayed as the enrolment result. Under emergency circumstances such as severe adverse events (AEs), unblinding is permissible by the discretion of the coordinating investigators.
Outcomes
Primary endpoint (1) safety
As the primary analysis, safety will be evaluated by collecting incidence information and severity of AEs or adverse reactions from the starting time of the administration of the trial drug to the end of the follow-up period (table 1). Known frequent adverse effects of bromocriptine include nausea and vomiting (8.3%), anorexia (2.5%) and gastric discomfort, based on 5212 cases with Parkinson’s syndrome.21
Table 1.
Trial schedule
| Screening period | Double-blind phase | |||||||||||||||||||
| Escalation to four tablets/day | Escalation to nine tablets/day | Taper | ||||||||||||||||||
| Timing | Before enrolment | Before start of trial treatment | At start of administration | Week 1 |
Week 2 |
Week 3 |
Week 4 |
Week 8 |
Week 12 |
Week 16 |
Week 20 |
Week 21 |
Week 22 |
Week 23 |
Week 24 |
Week 28 |
Week 32 |
Week 36 |
Week 37 |
|
| Visit/medical examination | ◯ | ◯ | ◯ | ◯ | Phone | Phone | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | Phone | Phone | ◯ | ◯ | ◯ | ◯ | ◯ | |
| Informed consent | ◯ | |||||||||||||||||||
| Neuropsychological/ motor assessment |
ADAS-J cog | ◯ | ||||||||||||||||||
| SIB-J | ◯ | ◯ | ◯ | ◯ | ||||||||||||||||
| NPI | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | |||||||||
| MENFIS | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | |||||||||
| MMSE-J | ◯ | ◯ | ◯ | ◯ | ◯ | |||||||||||||||
| DAD | ◯ | ◯ | ◯ | ◯ | ||||||||||||||||
| UPDRS part III | ◯ | ◯ | ◯ | ◯ | ||||||||||||||||
| Apathy Scale |
◯ | ◯ | ◯ | ◯ | ||||||||||||||||
| UMNB | ◯ | ◯ | ◯ | ◯ | ||||||||||||||||
| Laboratory biomarkers | Plasma biomarkers |
◯ | ◯ | ◯ | ◯ | ◯ | ◯ | |||||||||||||
| CSF biomarkers | ◯ | ◯ | ||||||||||||||||||
| Digital biomarkers | Wearable physical activity | → | → | → | → | → | → | → | → | → | → | → | → | → | → | → | → | → | ||
| Finger tapping | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | |||||||||
| PET | Brain amyloid PET | ◯ | ◯ | |||||||||||||||||
| Brain tau PET | ◯ | ◯ | ||||||||||||||||||
| Safety assessment | Cardiac ultrasound | ◯ | ◯ | ◯ | ||||||||||||||||
| Chest X-ray | ◯ | ◯ | ◯ | ◯ | ◯ | |||||||||||||||
| ECG | ◯ | ▲ | ◯ | ▲ | ▲ | ▲ | ◯ | ▲ | ◯ | ▲ | ▲ | ◯ | ▲ | |||||||
| Head MRI | ◯ | ◯ | ||||||||||||||||||
| Blood bromocriptine concentration | ◯ * | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | |||||||||||||
| Laboratory tests | ◯ | ◯ | ◯ | ◯ | ◯ | |||||||||||||||
| AEs | → | → | → | → | → | → | → | → | → | → | → | → | → | → | → | → | → | → | ||
| Extension phase | ||||||||||||||||||||
| Escalation to 4–9 tablets/day† | End of trial | |||||||||||||||||||
| Timing | Week 37 |
Week 38 |
Week 39 |
Week 40 |
Week 41 |
Week 42 |
Week 43 |
Week 44 |
Week 45 |
Week 48 |
Week 50 |
|||||||||
| Visit/medical examination | ◯ | Phone | Phone | ◯ | Phone | Phone | Phone | ◯ | Phone | ◯ | ◯ | |||||||||
| Informed consent | ||||||||||||||||||||
| Neuropsychological/ motor assessment |
ADAS-J cog |
|||||||||||||||||||
| SIB-J | ||||||||||||||||||||
| NPI | ||||||||||||||||||||
| MENFIS | ||||||||||||||||||||
| MMSE-J | ||||||||||||||||||||
| DAD | ||||||||||||||||||||
| UPDRS part III |
||||||||||||||||||||
| Apathy scale |
||||||||||||||||||||
| UMNB | ||||||||||||||||||||
| Laboratory biomarkers | Plasma biomarkers |
|||||||||||||||||||
| CSF biomarkers |
||||||||||||||||||||
| Digital biomarkers | Wearable physical activity | |||||||||||||||||||
| Finger tapping |
||||||||||||||||||||
| PET | Brain amyloid PET | |||||||||||||||||||
| Brain tau PET | ||||||||||||||||||||
| Safety assessment | Cardiac ultrasound |
◯ | ||||||||||||||||||
| Chest X-ray |
◯ | |||||||||||||||||||
| ECG | ▲ | ▲ | ▲ | ▲ | ◯ | |||||||||||||||
| Head MRI |
||||||||||||||||||||
| Blood bromocriptine concentration | ||||||||||||||||||||
| Laboratory tests |
◯ | ◯ | ||||||||||||||||||
| AEs | → | → | → | → | → | → | → | → | → | → | → | |||||||||
| Time after first administration of trial drug (hour) | Before administration | 1 | 2 | 3 | 4 | 6 | ||||||||||||||
| Time window (in principle) | −2 hours | ±5 min | ±5 min | ±5 min | ±5 min | ±5 min | ||||||||||||||
| Plasma bromocriptine | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ |
◯ To be performed.
▲ To be performed if donepezil hydrochloride is coadministered.
· After the final visit of the last participant at week 37, the blind will be broken following data lock to start analyses.
*Schedule for measuring blood bromocriptine concentration on day 1 on the bottom of Table 1.
†Standard dose is four tablets/day, up to nine tablets/day at maximum.
ADAS-J cog, Alzheimer’s Disease Assessment Scale-Cognitive Subscale Japanese Version; AEs, adverse events; CSF, cerebrospinal fluid; DAD, disability assessment for dementia; MENFIS, Mental Function Impairment Scale; MMSE-J, Mini Mental State Examination-Japanese; NPI, Neuro Psychiatric Inventory; PET, positron emission tomography; SIB-J, severe Impairment Battery-Japanese; UMNB, Upper Motor Neuron Burden Score; UPDRS part III, movement disorder society-sponsored revision of the Unified Parkinson’s Disease Rating Scale part III.
Primary endpoint (2) Severe Impairment Battery-Japanese (SIB-J)
Severe Impairment Battery-Japanese (SIB-J) is validated in the Japanese version and will be used to evaluate cognitive function.22–24 Higher scores indicate better cognitive function (range 0–100). Participants will be questioned and assessed by the investigator, subinvestigator, clinical psychologist or speech therapist. Individual time courses of observed values and changes from baseline in SIB-J score up to week 20 and week 36 after trial drug administration will be shown (table 1).
Primary endpoint (3) Neuropsychiatric Inventory (NPI)
Neuropsychiatric Inventory (NPI) (Japanese version) is a validated clinical instrument for evaluating psychopathology in dementia.25–27 Higher scores indicate more severe neuropsychiatric derangements (range 10–120). Caregivers will be interviewed and assessed by the investigator, subinvestigator, clinical psychologist or speech therapist. Individual time courses of observed values and changes from baseline in NPI score up to weeks 20 and 36 after trial drug administration will be shown (table 1).
Secondary endpoints
Changes in the following (1)–(11) will be shown individually. For the blood bromocriptine concentration on day 1, key pharmacokinetic parameters (Cmax, Tmax) will be calculated (table 1).
Mental Function Impairment Scale (MENFIS).28
MMSE-J.29–31
Disability Assessment for Dementia (DAD).32
Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale Part III.33
Apathy Evaluation Scale Informant Version.34 35
Plasma Aβ protein.36
Plasma neurofilament light chain protein (NfL).37
Plasma total tau and plasma phosphorylated tau protein.36 38–40
Cerebrospinal fluid Aβ (CSF Aβ).41 42
Cerebrospinal fluid total tau and phosphorylated tau protein (CSF total/p-tau).41 42
Blood bromocriptine concentration.
Reference endpoints
For exploratory purposes, additional measurement with wearable physical activity metre (SilmeeW20, TDK Corporation, Tokyo, Japan),43 measurement with magnetic sensing finger tap device (UB-2, Maxell, Ltd., Tokyo, Japan),44 45 brain amyloid positron emission tomography (PET),46 brain tau PET,47 Upper Motor Neuron Burden Score (UMNB)48 and plasma Aβ-related peptides49 will be assessed as reference endpoints (table 1). The wearable physical activity metre continuously monitors activity, sleep habits, amount of speech, time of physical movement and heart rate variability. Finger tapping is correlated with cognitive function and Parkinsonian signs, and it allows obtaining parameters directly related to ADLs not limited to cognitive function evaluated by questionnaires.
All neuropsychological testing will be conducted by certified physicians or psychologists who completed the assessment training of this trial. Learning effect cannot be negligible, but this trial has a placebo arm, and previous trials using SIB-J, NPI, MENFIS and DAD adopted similar measuring time frames and no obvious learning effects were reported.50 51
Data management, monitoring, and auditing
The Data Centre will manage the data using the participant identification code or enrolment number. In direct access to source data related to the conduct of this trial, the participants’ informed consent forms, and publication of the trial results, full consideration should be given to the protection of privacy and personal information, such as the participants’ names and diseases. The full data set without personal identifiable information will be accessed by data managers, statisticians and investigators. An independent data monitoring committee has been established to assess the safety data.
Trial auditing will be conducted multiple times by an outside contract research organisation. Biological specimens will be preserved for future use in ancillary studies with the participants’ consent.
Statistical analysis
Safety analyses will be performed for patients who undergo at least part of the trial treatment. Efficacy analyses will be performed among patients who undergo at least part of the trial treatment, provide efficacy information and satisfy study inclusion criteria. Descriptive statistics will be used to analyse safety data; results will be summarised as counts and percentages. For efficacy endpoints, changes from baseline will be summarised and compared at weeks 20 and 36 using descriptive statistics including mean differences with 95% CIs and p values derived from Student’s t test with the assumption of normality. If the assumption is violated, non-parametric tests will be considered. No imputation will be performed for missing data. Owing to the exploratory nature of this study, no adjustments will be made for multiple testing. To support the robustness of the results, a sensitivity analysis with and without non-compliant patients will be conducted. A subgroup analysis in accordance with the baseline MMSE-J score (<13 or≥13) will be performed to investigate the patient response to bromocriptine. All statistical analyses will be performed using SAS software, V.9.4 or higher (SAS Institute, Cary, NC). The statistical analysis plan will be developed prior to database lock.
Patient and public involvement
No patient or public were involved in this study design. However, in the design of this study, patients and their family burdens were assessed with the help of neurologists and clinical trial coordinators of Kyoto University Hospital.
Ethics and dissemination
This trial will be conducted in compliance with the ethical principles that have their origin in the Declaration of Helsinki (1964) and its revisions, namely, ‘Act on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices’, ‘Ministerial Ordinance on Good Clinical Practice for Drugs,’ and its related notifications, written procedures and this protocol. This trial was approved by the Institutional Review Board (K070, Kyoto University Hospital; F-2020-016, Mie University Hospital; 209001-A, Osaka University Hospital; 2105 (02-5), Tokushima University Hospital; T19-04 Tokyo Metropolitan Geriatric Hospital; 20200421, Kawasaki Medical School Hospital; 20200624, Asakayama Hospital).
The investigator will prepare the written information for participants and the informed consent form and will obtain approval from the Institutional Review Board in advance. Items to be described in the written information shall be prepared based on Article 51 of the ‘Ministerial Ordinance on Good Clinical Practice for Drugs’. If the investigator, etc., obtains any information that may affect the intention of the participant or the participant’s legally acceptable representative to continue to participate in the trial, he/she will promptly provide the information to the participant or the participant’s legally acceptable representative to confirm the intention of whether to continue to participate in the trial. The investigator will also promptly revise the written information, submit it to the head of the trial site and obtain approval from the Institutional Review Board. The investigator will provide another explanation to the participant who has already been participating in the trial or his/her legally acceptable representative using the revised written information, confirm the participant’s will as to whether or not to continue participation in the trial and obtain written consent from the participant. If an adverse event occurs due to the conduct of the trial, leading to health injury in participants, the investigator will immediately provide the participant with appropriate diagnosis, treatment and necessary measures including compensation insurance.
Persons involved in this trial shall comply with applicable laws and ordinances, shall exert their utmost efforts to protect personal information and privacy of the participants and shall not leak personal information obtained in the conduct of the trial without justifiable reasons. The same shall apply after their retirement. When submitting the patient enrolment forms and case report forms, the investigator, subinvestigator and clinical trial collaborator will use the participant identification code and will not enter information that allows persons outside the trial site to identify the participant (eg, name, address and telephone number). The central trial office, data centre and statisticians will use only the participant identification codes and cannot access the participants’ personal information.
The proposed study will evaluate both the safety and efficacy of bromocriptine in PSEN1-AD, and the study results are expected to be disseminated at international and/or national conferences and published in international journals following the peer-review process.
Discussion
This study evaluates the safety and explores the efficacy of bromocriptine for patients with PSEN1-AD. The potential efficacy of bromocriptine for PSEN1-AD patients has already been identified by an approach applying iPSC-based drug repurposing.10 We previously developed a phenotypic screening system to evaluate compounds with a readout of Aβ42 reduction using familial and sporadic AD patient-iPSCs. Our high-throughput screening system revealed that bromocriptine, among 1258 pharmaceutical compounds, reduced the Aβ42 level most effectively. This drug reduced Aβ42 by 50% in PSEN1-AD iPSCs, and by 20%–30% in sporadic patient-iPSC models.10 A recent report implies that inhibition of less than 50% of Aβ might preserve neuronal signalling because the APP family plays an important role in synaptic plasticity underlying learning and memory.52 Thus, we designed the clinical trial for PSEN1-AD with the expectation that bromocriptine will become a candidate as a molecularly targeted drug for AD, and especially for PSEN1-AD.
Bromocriptine is a therapeutic agent used for the treatment of patients with Parkinson’s disease, and the accumulated data of long usage verifies its safety for patients with Parkinson’s disease. However, evaluation of the safety of bromocriptine in AD patients is required in order to support its development for the treatment of AD. This clinical trial consists of a 20-week low-dose maintenance period followed by a 16-week high-dose maintenance period to assess its safety. For recruitment and ethical considerations, we also added a 13-week open extension period to continue to follow the safety of the trial drug.
Safety information has been accumulated from long usage of bromocriptine since the 1970s. While nausea and vomiting are the most commonly reported AEs, it is generally manageable by slow titration of the medication and use of antiemetics. While the occurrence and management of AEs in AD patients seem to be comparable to those with Parkinson’s disease, this study was designed to evaluate any unacceptable AEs that specifically occur in PSEN1-AD patients. During the recruiting period of this study, the COVID-19 pandemic is influencing study recruitment, onsite monitoring and participants’ visit schedules all over the world. Our study protocol enabled the delivery of the study drugs. Furthermore, remote informed consent and cognitive/neuropsychiatric evaluation by tablet or digital devices can be used in future trials for AD to reduce the study burden for participants and their caregivers.53
In this study, we will limit the included patients to those with PSEN1-AD, because bromocriptine is most effective in PSEN1-AD patient-derived iPSCs compared with other types of familial AD and sporadic AD patients’ iPSCs.11 12 This enrichment will make it difficult to recruit many patients for this study, considering the rarity of such patients in Japan. Although the variety of clinical severities among patients is one of the reasons that cause difficulty for clinical trial evaluation, our inclusion criteria admit a broad disease-stage as we consider that PSEN1-AD patients would be the best candidates for bromocriptine administration. To explore the efficacy of the trial drug, various promising biomarkers in AD, plasma Aβ, plasma Aβ-related peptide, plasma NfL, plasma total/p-tau, CSF Aβ, CSF total/p-tau, amyloid/tau PET and digital biomarkers will be evaluated in this study. These biomarkers might indicate the target engagement, and its alteration might reflect the effect of bromocriptine treatment. Of note, digital biomarkers will potentially reduce participants’ and caregivers’ burden in future trials. Strengths of our study include participant enrichment using iPSC technology and exploratory biomarker investigation. A possible limitation of this study may be the difficulty in recruiting large numbers of patients due to the rarity of PSEN1-AD.
This study is the first to use bromocriptine for PSEN1-AD patients, and it presents both a low-dose and a high-dose safety verification as well as cognitive, neuropsychiatric and biomarker-based efficacy detections. This is a phase I/IIa study for the safety and early efficacy evaluation with a small number of participants. Therefore, a large-scale study will be needed in the following phases. This study may pave the way for a new and practical disease-modifying AD therapy.
Supplementary Material
Acknowledgments
We would like to acknowledge and thank the members of the institute and hospitals in this study. Patients and their family burdens were assessed with the help of neurologists and clinical trial coordinators of Kyoto University Hospital. We appreciate Tetsujiro Ihara at London Iryo Centre, and Arthur Valentine and Randy Trask of Pearson Clinical Assessments (San Antonio, Texas) for providing access to the Severe Impairment Battery (SIB), which provided valuable measurement regarding the effectiveness of this protocol. We would like to express our sincere gratitude to all our coworkers and collaborators, to Atsushi Onodera and Yuji Arakawa for PMDA negotiation and to Noriko Ito, Mikie Iijima and Nozomi Kawabata for their administrative support.
Footnotes
TK and HB contributed equally.
HTo and HIn contributed equally.
Contributors: TK, HB, TO, YA, HE, AN, RU, AK, HTa, SM, SS, TW and HIn conceived and designed the study. AN and RU will conduct the statistical analysis. HIs, AS, TM, KY, YT, KM, TS, MI, KF, YI, KK, KI, KS, YK, YS, SK, OU, RT and HTo provided critical advice on the study design. All authors critically revised the draft and approved the final version of this manuscript.
Funding: This trial will be conducted with funding from Time Therapeutics (award/grant number is not applicable) and a grant from the invited project at iACT, Kyoto University Hospital (0709992110). The trial drugs will be provided by Towa Pharmaceutical Co.
Competing interests: TK has a patent, agent for preventing and/or treating Alzheimer’s disease, licensed to HIn and TK; HB reports funding for this clinical trial from Time Therapeutics, trial drugs from Towa Pharmaceutical Co., during the conduct of the study; personal fees from Sumitomo Dainippon Pharma Co., outside the submitted work; RU reports personal fees from Eisai, Sawai Pharmaceutical Co. and CAC Croit, outside the submitted work; SM reports personal fees from AstraZeneca KK, Bristol-Myers Squibb Company, Chugai Pharmaceutical Co. Eli Lilly Japan KK, MSD KK, Nippon Boehringer Ingelheim Co., Ono Pharmaceutical Co., Pfizer Japan and Taiho Pharmaceutical Co.; YT reports personal fees from Sumitomo Dainippon Pharma Co., Otsuka Pharmaceutical Co., AbbVie GK, Kyowa Kirin Co., Takeda Pharmaceutical Company, Tsumura & Co., Eisai Co., Sanofi KK, Mylan EPD GK and Ono Pharmaceutical Co., outside the submitted work; TM reports personal fees from Bayer Yakuhin and Otsuka Pharmaceutical Co., outside the submitted work; MI reports grants and personal fees from Eisai Co., Sumitomo Dainippon Pharma Co., Otsuka Pharmaceutical Co., MSD KK, Daiichi Sankyo Co. and Takeda Pharmaceutical Company, grants from Mitsubishi Tanabe Pharma Corporation, personal fees from Janssen Pharmaceutical KK, Nihon Medi-Physics Co., Fujifilm, Novartis Japan, Meiji Seika Pharma Co., Nippon Chemiphar Co., Eli Lily Japan KK and Chugai Pharmaceutical Co., outside the submitted work; KF reports grants from Novartis, outside the submitted work; YI reports grants from Sumitomo Dainippon Pharma Co., Eisai Co., Japan Blood Products Organisation, Otsuka Pharmaceutical Co., Kyowa Kirin Co., Teijin Pharma, Nihon Pharmaceutical Co. and FP Pharmaceutical Corporation, outside the submitted work; KI reports grants, personal fees and other from GE Healthcare, during the conduct of the study; grants and personal fees from Nihon Medi-Physics Co., and Eli Lilly Japan KK, personal fees and other from Eisai Co. and Chugai Pharmaceutical Co., other from Biogen, personal fees from Novartis, outside the submitted work; YK reports personal fees from Tsumura & Co., Novartis Japan, UCB Japan Co. and Janssen Pharmaceutical KK, outside the submitted work; YS reports grants from Nippon Shinyaku Co. and The Nakatomi Foundation, personal fees from FP Pharmaceutical Corporation, Sumitomo Dainippon Pharma Co., and Novartis Japan, outside the submitted work; SK reports grants and personal fees from Eisai Co., personal fees from Janssen Pharmaceutical KK, Novartis Japan, Daiichi-Sankyo, Sumitomo Dainippon Pharma Co., Fujifilm Toyama Chemical Co., Nippon Chemiphar, Nihon Medi-Physics Co., Tsumura & Co. and Eli Lily Japan KK, outside the submitted work; SS is an employee of Time Therapeutics; TW is an employee of Time Therapeutics, during the conduct of the study; TW reports personal fees from KanonCure, Tsubota Laboratory, Dompé Farmaceutici S.p.A., and Novaliq GmbH, outside the submitted work; OU is an employee of Towa Pharmaceutical Co.; RT reports grants and personal fees from Takeda Pharmaceutical Co., Nippon Boehringer Ingelheim Co., Sumitomo Dainippon Pharma Co., Eisai Co., Kyowa Kirin Co., Otsuka Pharmaceutical Co. and Sanofi KK, grants from Astellas Pharma, Novartis Japan, and Nihon Medi-Physics Co., personal fees from AbbVie GK, Mitsubishi Tanabe Pharma Corporation, Mylan NV, Japan Blood Products Organization, Sanwa Kagaku Kenkyusho Co., FP Pharmaceutical Corporation, Tsumura & Co., KAN Research Institute, Kissei Pharmaceutical Co., Chugai Pharmaceutical Co., and Biogen, outside the submitted work; HTo reports personal fees from Daiichi Sankyo Co., outside the submitted work; HIn reports grants and personal fees from Takeda Pharmaceutical Co., Eisai Co., Suntory Wellness, Institute for Health Care Science, and Mitsubishi Tanabe Pharma Corporation, grants from Taisho Pharmaceutical Co., Toray Industries, KAN Research Institute, Shimadzu Corporation, MicroBiopharm Japan Co., Kaneka Corporation, Panasonic Corporation, Biogen and Stem Cell & Device Laboratory (SCAD), personal fees from Nomura Securities Co., FP Pharmaceutical Corporation, Nippon Chemiphar Co., Kansai Pharmaceutical Industries Association, Otsuka Pharmaceutical Co., Kyowa Kirin Co., outside the submitted work. HIn possesses unlisted stocks of Time Therapeutics. In addition, Kyoto University grants an exclusive license to Time Therapeutics through iPS Academia Japan regarding the invention of the trial drug (intellectual property) which was discovered through drug screening by the principal investigator (HIn). Thereby, Kyoto University and the principal investigator obtain a patent income from Time Therapeutics. HIn does not engage in data management, monitoring and statistical analyses. The coordinating investigators (HTo and HB) and Time Therapeutics will conduct the trial under the investigator-initiated clinical trial agreement. Prior to the trial, the principal investigator and the coordinating investigators underwent a review and received approval by the Conflict of Interest Review Committee based on the conflict of interest management policy at each site. All remaining authors have declared no conflicts of interest.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Alzheimer’s Disease International . World Alzheimer report 2019: attitudes to dementia. London: Alzheimer’s Disease International, 2019. [Google Scholar]
- 2.Cummings J, Lee G, Ritter A, et al. Alzheimer’s disease drug development pipeline: 2020. Alzheimers Dement 2020;65:e12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan NS, Nicholas JM, Weston PSJ, et al. Clinical phenotype and genetic associations in autosomal dominant familial Alzheimer’s disease: a case series. Lancet Neurol 2016;15:1326–35. 10.1016/S1474-4422(16)30193-4 [DOI] [PubMed] [Google Scholar]
- 4.Larner AJ, Doran M. Clinical phenotypic heterogeneity of Alzheimer's disease associated with mutations of the presenilin-1 gene. J Neurol 2006;253:139–58. 10.1007/s00415-005-0019-5 [DOI] [PubMed] [Google Scholar]
- 5.Duff K, Eckman C, Zehr C, et al. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature 1996;383:710–3. 10.1038/383710a0 [DOI] [PubMed] [Google Scholar]
- 6.Borchelt DR, Thinakaran G, Eckman CB, et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron 1996;17:1005–13. 10.1016/S0896-6273(00)80230-5 [DOI] [PubMed] [Google Scholar]
- 7.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002;297:353–6. 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- 8.Bentahir M, Nyabi O, Verhamme J, et al. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J Neurochem 2006;96:732–42. 10.1111/j.1471-4159.2005.03578.x [DOI] [PubMed] [Google Scholar]
- 9.De Strooper B, Iwatsubo T, Wolfe MS. Presenilins and γ-secretase: structure, function, and role in Alzheimer disease. Cold Spring Harb Perspect Med 2012;2:a006304. 10.1101/cshperspect.a006304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondo T, Imamura K, Funayama M, et al. iPSC-Based Compound Screening and In Vitro Trials Identify a Synergistic Anti-amyloid β Combination for Alzheimer's Disease. Cell Rep 2017;21:2304–12. 10.1016/j.celrep.2017.10.109 [DOI] [PubMed] [Google Scholar]
- 11.WIPO|PCT . WIPO PCT/JP2016/089217, 2020. Available: http://ips-cell.net/e/patent/docs/AJ139.pdf
- 12.United States Patent Application Publication, INOUE . US20190008860A1, 2020. Available: https://patents.google.com/patent/US20190008860A1/en?q=KONDO+Inoue+Bromocriptine+alzheimer&oq=KONDO+Inoue+Bromocriptine+alzheimer
- 13.Towa Pharmaceutical Co., Ltd . Interview form bromocriptine tablets 2.5 mg “Towa”, 20. Available: https://med.towayakuhin.co.jp/medical/product/product.php?id=T001706201703011557043R
- 14.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer's & Dementia 2011;7:263–9. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, fifth edition: DSM-5. Washington, DC: American Psychiatric Publishing, 2013: 611–4. [Google Scholar]
- 16.Tokuchi R, Hishikawa N, Kurata T, et al. Clinical and demographic predictors of mild cognitive impairment for converting to Alzheimer's disease and reverting to normal cognition. J Neurol Sci 2014;346:288–92. 10.1016/j.jns.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 17.JFAD . Japanese Familial Alzheimer’s Disease (JFAD) database, 2020. Available: http://www.alzdb.org/jfad/
- 18.FY 2013 subsidy for geriatric health promotion, project for geriatric health enhancement: report of research project on the support system for individuals with familial alzheimer’s disease and their families. 2013. https://www.med.osaka-cu.ac.jp/other/doc/project-20140331a.pdf https://www.med.osaka-cu.ac.jp/other/doc/project-20140331a.pdf
- 19.Shea Y-F, Chu L-W, Chan AO-K, et al. A systematic review of familial Alzheimer's disease: differences in presentation of clinical features among three mutated genes and potential ethnic differences. J Formos Med Assoc 2016;115:67–75. 10.1016/j.jfma.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 20.Tang M, Ryman DC, McDade E, et al. Neurological manifestations of autosomal dominant familial Alzheimer's disease: a comparison of the published literature with the dominantly inherited Alzheimer network observational study (DIAN-OBS). Lancet Neurol 2016;15:1317–25. 10.1016/S1474-4422(16)30229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Pharma Japan Ltd . Interview form Parlodel tablets 2.5 Mg, 2021. Available: https://medical.mt-pharma.co.jp/di/file/dc/plo.pdf
- 22.Saxton J, McGonigle-Gibson KL, Swihart AA, et al. Assessment of the severely impaired patient: description and validation of a new neuropsychological test battery. Psychol Assess 1990;2:298–303. 10.1037/1040-3590.2.3.298 [DOI] [Google Scholar]
- 23.Saxton J, McGonigle K, Swihart A. Severe impairment battery (SIB). London, UK: Pearson Assessment, 1993. [Google Scholar]
- 24.Niina R, Homma A, Sugai Y. Reliability, validity and clinical availability of a Japanese version of Severe Impairment Battery (SIB) and a Japanese version of modified Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory (ADCS-ADL). Jpn J Geriatr Psychiatry 2005;16:683–91. [Google Scholar]
- 25.Cummings JL, Mega M, Gray K, et al. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–14. 10.1212/WNL.44.12.2308 [DOI] [PubMed] [Google Scholar]
- 26.Cummings JL. The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology 1997;48:10S–16. 10.1212/WNL.48.5_Suppl_6.10S [DOI] [PubMed] [Google Scholar]
- 27.Hirono N, Mori E, Ikejiri Y, et al. [Japanese version of the Neuropsychiatric Inventory--a scoring system for neuropsychiatric disturbance in dementia patients]. No To Shinkei 1997;49:266–71. [PubMed] [Google Scholar]
- 28.Homma A, Niina R, Ishii T. Development of a new rating scale for dementia in the elderly: mental function impairment scale (MENFIS) (in Japanese). Jpn J Geriatr Psychiatry 1991;2:1217–22. [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 30.Sugishita M, Hemmi I, Takeuchi T. Reexamination of the validity and reliability of the Japanese version of the Mini-Mental state examination (MMSE-J). Japanese Journal of Cognitive Neuroscience 2016;18:168–83. [Google Scholar]
- 31.Sugishita M, Koshizuka Y, Sudou S. The validity and reliability of the Japanese version of the Mini- Mental State Examination(MMSE-J) with the original procedure of the attention and calculation task(2001). Japanese Journal of Cognitive Neuroscience 2018;20:91–110. [Google Scholar]
- 32.Galasko D, Bennett D, Sano M. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 2002;11:S33–9. [PubMed] [Google Scholar]
- 33.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–70. 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 34.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res 1991;38:143–62. 10.1016/0165-1781(91)90040-V [DOI] [PubMed] [Google Scholar]
- 35.Kasai M, Meguro K, Nakamura K. [Reliability and validity of the Japanese version of the Apathy Evaluation Scale]. Nihon Ronen Igakkai Zasshi 2014;51:445–52. 10.3143/geriatrics.51.445 [DOI] [PubMed] [Google Scholar]
- 36.Palmqvist S, Janelidze S, Stomrud E, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related β-amyloid status. JAMA Neurol 2019;76:1060–9. 10.1001/jamaneurol.2019.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz SA, Strain JF, Adedokun A, et al. Serum neurofilament light chain levels are associated with white matter integrity in autosomal dominant Alzheimer's disease. Neurobiol Dis 2020;142:104960. 10.1016/j.nbd.2020.104960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mielke MM, Hagen CE, Wennberg AMV, et al. Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the Mayo clinic study on aging. JAMA Neurol 2017;74:1073–80. 10.1001/jamaneurol.2017.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 2020;19:422–33. 10.1016/S1474-4422(20)30071-5 [DOI] [PubMed] [Google Scholar]
- 40.Tatebe H, Kasai T, Ohmichi T, et al. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer's disease and Down syndrome. Mol Neurodegener 2017;12:63. 10.1186/s13024-017-0206-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med 2012;367:795–804. 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 2014;13:614–29. 10.1016/S1474-4422(14)70090-0 [DOI] [PubMed] [Google Scholar]
- 43.Kimura N, Aso Y, Yabuuchi K, et al. Association between objectively measured walking steps and sleep in community-dwelling older adults: a prospective cohort study. PLoS One 2020;15:e0243910. 10.1371/journal.pone.0243910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kandori A, Yokoe M, Sakoda S, et al. Quantitative magnetic detection of finger movements in patients with Parkinson's disease. Neurosci Res 2004;49:253–60. 10.1016/j.neures.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 45.Suzumura S, Osawa A, Nagahama T, et al. Assessment of finger motor skills in individuals with mild cognitive impairment and patients with Alzheimer’s disease: Relationship between finger-to-thumb tapping and cognitive function. Japanese J Compr Rehabil Sci 2016;7:19–28. [Google Scholar]
- 46.Scheinin NM, Tolvanen TK, Wilson IA, et al. Biodistribution and radiation dosimetry of the amyloid imaging agent 11C-PiB in humans. J Nucl Med 2007;48:128–33. [PubMed] [Google Scholar]
- 47.Koole M, Lohith TG, Valentine JL, et al. Preclinical safety evaluation and human dosimetry of [18F]MK-6240, a Novel PET tracer for imaging neurofibrillary tangles. Mol Imaging Biol 2020;22:173–80. 10.1007/s11307-019-01367-w [DOI] [PubMed] [Google Scholar]
- 48.Brettschneider J, Toledo JB, Van Deerlin VM, et al. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS One 2012;7:e39216. 10.1371/journal.pone.0039216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature 2018;554:249–54. 10.1038/nature25456 [DOI] [PubMed] [Google Scholar]
- 50.Watanabe M, Nakamura Y, Yoshiyama Y, et al. Analyses of natural courses of Japanese patients with Alzheimer's disease using placebo data from placebo-controlled, randomized clinical trials: Japanese study on the estimation of clinical course of Alzheimer's disease. Alzheimers Dement 2019;5:398–408. 10.1016/j.trci.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noguchi-Shinohara M, Ono K, Hamaguchi T, et al. Safety and efficacy of Melissa officinalis extract containing rosmarinic acid in the prevention of Alzheimer's disease progression. Sci Rep 2020;10:18627. 10.1038/s41598-020-73729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satir TM, Agholme L, Karlsson A, et al. Partial reduction of amyloid β production by β-secretase inhibitors does not decrease synaptic transmission. Alzheimers Res Ther 2020;12:63. 10.1186/s13195-020-00635-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinberg MS, Patrick RE, Schwab NA, et al. Clinical trials and tribulations in the COVID-19 era. Am J Geriatr Psychiatry 2020;28:913–20. 10.1016/j.jagp.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.