Abstract
Identifying a hypercoagulable state in patients with COVID-19 may help identify those at risk for virus–induced thromboembolic events and improve clinical outcomes using personalized therapeutic approaches. Herein, we aimed to perform a global assessment of the patients’ hemostatic system with COVID-19 using rotational thromboelastometry (ROTEM) and to describe whether patients with different disease severities present different coagulation profiles. Together with 37 healthy volunteers, a total of 65 patients were included and then classified as having mild, moderate, and severe disease depending on clinical severity. Peripheral blood samples were collected and analyzed using a ROTEM Coagulation Analyzer. Also, complete blood count and coagulation parameters including prothrombin time, activated partial thromboplastin time, fibrinogen levels, and D-dimer levels were measured at admission. EXTEM and INTEM MCF (P < 0.001) values were significantly higher and the EXTEM CFT (P = 0.002) value was significantly lower in patients with COVID-19 when compared with controls. In particular, patients with the severe disease showed a significant decrease in CFT (P < 0.001) and an increase in MCF (P < 0.001) in both INTEM and EXTEM assays compared with patients with the non-severe disease. Correlation analysis revealed significant correlations between ROTEM parameters and other coagulation parameters. There were significant positive correlations between fibrinogen, D-dimer, platelet count, and MCF in both EXTEM and INTEM assays. Our data demonstrate thromboelastographic signs of hypercoagulability in patients with COVID-19, which is more pronounced in patients with increased disease severity. Therefore, ROTEM analysis can classify subsets of patients with COVID-19 at significant thrombotic risk and assist in clinical decisions.
Keywords: COVID-19, coagulopathy, thromboelastometry
Introduction
Coronavirus disease 2019 (COVID-19) is associated with the development of a hypercoagulable state leading to an increased incidence of venous and arterial thrombotic events. Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is common in patients with COVID-19, seen in up to one-third of patients in the intensive care unit (ICU), even when prophylactic anticoagulation is used. 1 –6
Current evidence suggests that COVID-19–associated coagulopathy is a combination of endothelial injury, stasis, and hypercoagulable state, described as 3 major components of Virchow’s triad involved in clot formation. 7 Endothelial damage (endotheliitis) is caused by direct invasion of endothelial cells by the SARS-CoV-2 virus via ACE-2 receptors, the formation of neutrophil extracellular traps (NETs), the release of inflammatory cytokines such as interleukin (IL)-6, acute-phase reactants, and complement activation, and direct injury from intravascular catheters. 8 –12 Stasis occurs due to immobilization in all hospitalized patients. A hypercoagulable state is seen due to several changes in circulating prothrombotic factors such as elevated factor VIII, fibrinogen, D-dimer, von Willebrand factor (vWF), prothrombotic microparticles, and anionic phospholipids. 13
Viscoelastic methods (VMs) such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are used to evaluate the complete process of blood coagulation from the activation of clotting factors to fibrin formation, clot stabilization, and, finally, clot lysis. Thromboelastogram can reveal coagulopathy, even when this is not detected in routine coagulation tests. 14
Therefore, we conducted this prospective study to perform a global assessment of the hemostatic system of patients’ with COVID-19 using ROTEM and to describe whether patients with different disease severities presented different coagulation profiles.
Materials and Methods
Patients
Sixty-five (41 men and 24 women, mean age 49.3 ± 17.9 years) patients with COVID-19 who presented to our institution between May 4th and December 12th, 2020, were included in the study. The diagnosis of SARS-CoV-2 was made using reverse transcription-polymerase chain reaction (RT-PCR) from the throat or nasal swab. Patients were classified as having mild, moderate, and severe disease using the Novel Coronavirus Pneumonia Diagnosis and Treatment Guideline 15 according to their clinical features. Mild and moderate outpatients were considered as a single group and were compared with the patients in the severe group who were hospitalized. Patients with known preexisting congenital bleeding or thrombotic disorders and/or preexisting acquired coagulopathies, active cancer and/or chemotherapy, pregnancy, and ongoing anticoagulant therapy were excluded. Thirty-seven age-matched healthy volunteers over the age of 18 and having no disease that could cause coagulopathy comprised the control group.
Blood samples from each patient were explicitly obtained at the time of admission before thromboprophylaxis was initiated. Venous blood for ROTEM and conventional coagulation tests were drawn into 4.5-mL vacutainers (Becton Dickinson) containing 3.2% trisodium citrate with a citrate/blood ratio of 1:9. With the same procedure, a 4-mL blood sample was drawn for complete blood count (CBC) into EDTA K2 anticoagulated tubes (Becton Dickinson).
The Ethics Commission of Koç University (Istanbul, Turkey) approved this prospective study (IRB No: 2020.182.IRB1.050) and written informed consent was obtained from each participant.
Methods
Thromboelastometry
Whole blood thromboelastometry profiles were obtained using a ROTEM delta device (Tem Innovations GmBH, Munich, Germany), as previously described. 14 Extrinsic and intrinsic coagulation pathways were evaluated using extrinsic and intrinsic thromboelastometry (EXTEM, INTEM, respectively), according to the manufacturer’s recommendations. The following ROTEM parameters were analyzed: (1) clotting time (CT) is the time from when the test starts until an amplitude of 2 mm is reached, which reflects the initiation of the clotting process; (2) clot formation time (CFT) is the time between 2 mm amplitude and 20 mm amplitude, which reflects the propagation phase of whole blood clot formation; (3) maximum clot firmness (MCF) is the maximum amplitude in millimeters reached in the thromboelastogram, corresponding to clot stability. A “hypercoagulable profile” was defined as a shorter CT, shorter CFT, and/or higher MCF than the corresponding values of the healthy controls.
Blood Count and Conventional Coagulation Tests
To evaluate the CBC parameters, we used a Sysmex XN-3100 hematology analyzer (Sysmex, Kobe, Japan). The conventional coagulation parameters prothrombin time (PT), activated partial thromboplastin time (aPTT), D-dimer, and fibrinogen were analyzed using a Sysmex-Cs 2000i (Sysmex, Kobe, Japan).
Statistical Analysis
Data are given as mean, standard deviation, median, and interquartile range (25%-75% percentile) in descriptive statistics. Conformity to normal distribution was evaluated using the Shapiro-Wilk test. One-way analysis of variance (ANOVA) and the Kruskal-Wallis test were used to compare continuous data in independent groups. The post hoc Tukey test after ANOVA and the Mann-Whitney U test with Bonferroni correction after the Kruskal-Wallis test was used for post hoc comparisons of paired groups. The T-test and Mann-Whitney U test were used to compare values for the patient and control groups. Co-variation of continuous data was evaluated using Spearman correlation analysis. The cut-off values of fibrinogen, D-dimer, and platelet values according to ROTEM parameters were determined using receiver operating characteristics (ROC) curves. For statistical significance, a P-value below 0.05 at a 95% confidence interval was considered significant. SPSS v. 21.0 program was used for statistical analysis.
Results
The entire cohort of 65 patients with COVID-19 was divided into 2 groups: the first group exhibiting mild (n = 17, 26%) and moderate (n = 20, 31%) phenotypes, and the second group indicating the severe (n = 28, 43%) phenotype.
The laboratory data of the patients with COVID-19 and the control group are shown in Table 1. ROTEM parameters are presented in Table 2.
Table 1.
Values of Laboratory Parameters in Patients and Healthy Volunteers.a
| Healthy group (n = 37) | Patient group (n = 65) | P-value | |
|---|---|---|---|
| Hemoglobin (g/dL) | 14.9 (14.4/15.9) | 13.3 (12.2/14.3) | <0.001 |
| Platelet count (109/L) | 246 (194/276) | 218 (179/272) | 0.071 |
| Ferritin (ng/mL) | 75 (57/91) | 343 (133/759) | <0.001 |
| PT (%) | 12.3 (11.5/12.6) | 10.5 (9.8/11.0) | <0.001 |
| PTT (sec.) | 31.7 (29.9/34.9) | 28.5 (26.0/30.0) | <0.001 |
| Fibrinogen (g/L) | 2.9 (2.6/4.1) | 4.4 (3.4/5.2) | <0.001 |
| D-Dimer (µg/L) | 410 (275/640) | 550 (280/1130) | 0.08 |
Abbreviations: PT, prothrombin time; APTT, activated partial thromboplastin time.
a Data are given as median values (25th/75th percentiles).
Table 2.
Values of ROTEM Parameters in Patients and Healthy Volunteers.a
| Healthy group (n = 37) | Patient group (n = 65) | P-value | |
|---|---|---|---|
| Extem-CT (s) | 73 (58/76) | 69 (62/77) | 0.974 |
| Extem-CFT (s) | 103 (91/129) | 77 (60/94) | 0.002 |
| Extem-MCF (mm) | 60 (55/61) | 66 (61/70) | <0.001 |
| Intem-CT (s) | 177.0 (163.0/192.0) | 163.5 (143.0/186.5) | 0.164 |
| Intem-CFT (s) | 87.0 (75.0/99.0) | 72,0 (60.5/86.5) | 0.210 |
| Intem-MCF (mm) | 57.0 (54.0/62.0) | 63.5 (58.0/68.5) | <0.001 |
Abbreviations: INTEM, contact activated TEG; EXTEM, tissue factor activated TEG; CT, clotting time (s); CFT, clot formation time (s); MCF, maximum clot firmness (mm).
a Data are given as median values (25th/75th percentiles).
Fibrinogen levels were significantly higher in patients when compared to healthy controls (P < 0.01). Although higher values were observed in D-dimer values of the patient group than healthy ones, this difference was not statistically significant (P = 0.08). When evaluated between healthy, mild/moderate, and severe disease groups, the D-dimer values of those with severe disease were higher than the other 2 groups (P < 0.001). There was no significant difference between patients and the control group regarding platelet counts (P = 0.071) (Table 1).
Rotational thromboelastometry parameters were compared between COVID-19 patients and controls after then subgroup analysis was performed. EXTEM MCF and INTEM MCF values were significantly higher in the patients when compared with the healthy controls (P < 0.001). In contrast, the EXTEM CFT value was significantly lower in the patients when compared with the controls (P = 0.002) (Table 2). Subgroup analysis yielded significant differences for ROTEM parameters in the mild/moderate and severe disease groups. Patients with the severe disease showed a significant decrease in CFT (P < 0.001) and an increase in MCF (P < 0.001) in both INTEM and EXTEM assays compared with patients with milder disease.
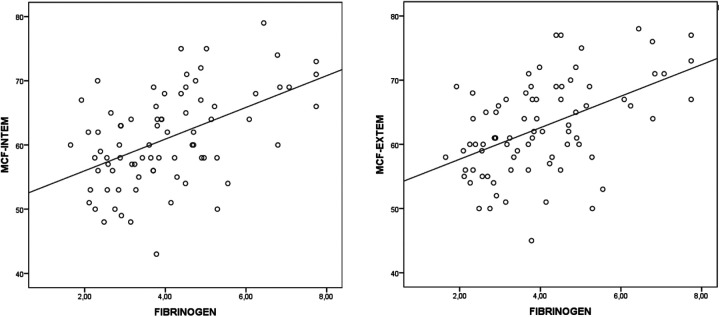
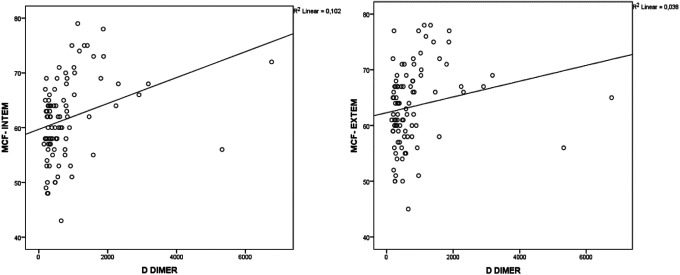
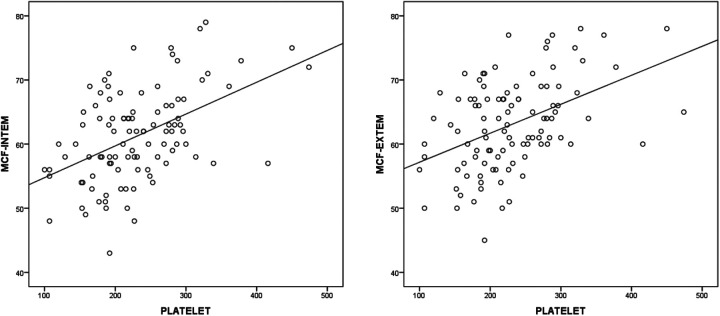
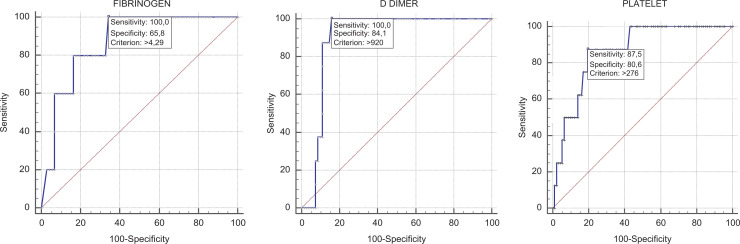
Correlation analysis showed significant correlations between ROTEM parameters and some laboratory parameters in patients with COVID-19 (Table 3). Fibrinogen was positively correlated with MCF in INTEM (r = 0.471, P < 0.001) and MCF in EXTEM (r = 0.474, P < 0.001) (Figure 1); D-dimer was positively correlated with MCF in INTEM (r = 0.413, P < 0.01) and MCF in EXTEM (r = 0.346, P = 0.001) (Figure 2) and the platelet count was positively correlated with MCF in INTEM (r = 0.432, P < 0.001) and MCF in EXTEM (r = 0.417, P < 0.001) (Figure 3). ROC curves describing the critical fibrinogen level (4.29 g/L as a cut-off value), D-dimer level (920 µg/L as a cut-off value) and platelet count (276 x 103/ µl as a cut-off value) that affect MCF are shown in Figure 4.
Table 3.
Correlation Coefficients (r) Between ROTEM and Other Laboratory Parameters.
| Platelet | D-dimer | Fibrinogen | |
|---|---|---|---|
| Extem-CTF (s) | −0.393 | −0.362 | −0.423 |
| Extem-MCF (mm) | 0.417 | 0.346 | 0.474 |
| Intem-CFT (s) | −0.442 | −0.408 | −0.400 |
| İntem-MCF (mm) | 0.432 | 0.413 | 0.471 |
Abbreviations: INTEM, contact activated TEG; EXTEM, tissue factor activated TEG; CFT, clot formation time (s); MCF, maximum clot firmness (mm).
Figure 1.
The correlation between fibrinogen level and MCF INTEM (r = 0.471, P < 0.001) and MCF EXTEM (r = 0.474, P < 0.001).
Figure 2.
The correlation between D-dimer level and MCF INTEM (r = 0.413, P < 0.001) and MCF EXTEM (r = 0.346, P = 0.001).
Figure 3.
The correlation between platelet number and MCF INTEM (r = 0,432 P < 0.001) and MCF EXTEM (r = 0.417, P < 0.001).
Figure 4.
ROC curves describing the relationship between INTEM MCF and fibrinogen, D-dimer, and platelet count. ROC curve INTEM MCF—fibrinogen cut-off 4.29 AUC = 0.87 (95% CI: 0.775-0.935) P < 0.001. ROC curve INTEM MCF—D-dimer cut-off 920 AUC = 0.897 (95% CI: 0.815-0.951) P < 0.001. ROC curve INTEM MCF—platelet cut-off 276 AUC = 0.866 (95% CI: 0.783-0.925) P < 0.001.
Discussion
The current study confirmed significant hypercoagulability in patients with COVID-19 by the presence of accelerated clot formation, as shown by the shortening of CFT in EXTEM assay and an increase of clot strength, as demonstrated by the increasing of MCF in both INTEM and EXTEM assays. Moreover, this hypercoagulable pattern was more pronounced in patients with severe disease. ROTEM was also able to identify fibrinogen, D-dimer, and platelet contributions to clot strength in patients with COVID-19.
Significant alterations in blood coagulation parameters have been implicated in patients with COVID-19, consisting of increases in fibrinogen and D-dimer levels, slight prolongation of PT and aPTT, and mild thrombocytosis or thrombocytopenia. 16,17 Several reports have defined the clinical and prognostic values of these blood coagulation disarrangements in patients with COVID-19. Compared with survivors, non-survivors showed significantly higher D-dimer levels, more prolonged PT and aPTT levels, and low platelet counts at admission. 18 –21 Given the general limitations of standard coagulation tests, which assess the isolated portions of the coagulation system and give no information on important interactions essential for the evaluation of hemostatic disorders, with this study, we aimed to perform a global assessment of the hemostatic function of patients with COVID-19 using ROTEM analysis and to clarify whether the hemostatic changes predicted the severity of the disease.
The application of ROTEM technology to demonstrate hypercoagulability has been well documented in various clinical settings, including major surgery, cancer, Behcet’s disease, chronic myeloproliferative neoplasms, and apheresis. 22,23 The results of these studies yielded significant hypercoagulability as detected by shorter CT and/or CFT values and higher MCF values in study groups compared with healthy controls. MCF appears to be the most crucial ROTEM parameter to identify the persistence of a hypercoagulable state, which makes ROTEM technology superior to other hemostatic tests. Relevant studies assessing ROTEM in critically ill patients with COVID-19 treated in intensive care units (ICUs) have shown high MCF values. Spiezia et al evaluated coagulation abnormalities using ROTEM profiles in a group of 22 patients with COVID-19 in the ICU with acute respiratory failure. 24 Forty-four healthy subjects served as controls. The patients showed markedly hypercoagulable profiles, as reflected by the significantly shorter CFT in INTEM and EXTEM and higher MCF in INTEM, EXTEM, and fibrinogen thromboelastometry (FIBTEM) assays compared with the 44 healthy subjects.
Similarly, Pavoni et al, performing a ROTEM analysis, documented that patients with severe COVID-19 pneumonia had a hypercoagulation that persisted over time. Forty patients demonstrated an accelerated propagation phase of blood clot formation (shortening of CFT in the INTEM and EXTEM) and a significantly higher clot strength (increasing of MCF in the INTEM, EXTEM, and FIBTEM) as compared with the normal range limits. 25 In another study, Hoechter et al found that 22 patients with COVID-19 acute respiratory distress syndrome (ARDS) had higher FIBTEM MCF than 14 patients with non-COVID-19 ARDS, suggesting a higher procoagulant potential in the case of COVID-19 critical illness. 26
All the above studies identified a procoagulant profile in critically ill patients with COVID-19 on ICU admission. However, limited ROTEM data at earlier stages of the disease have been reported so far. Almskog et al performed ROTEM analyses in COVID-19 patients divided into 2 groups according to their care level, hospitalized either in regular wards or wards with specialized ventilation support. All hospitalized patients with COVID-19 showed elevated values of EXTEM-MCF and FIBTEM-MCF at admission to the hospital, and this hypercoagulable pattern was prominent in severely ill patients compared with patients on regular wards. 27 However, 60% of the included patients had received anticoagulant treatment before ROTEM analysis, which might have counteracted the hypercoagulation found in the present study. Likewise, Boscolo et al investigated the difference in MCF values between internal medicine wards (IMW) and ICU patients, excluding patients on anticoagulant or antiplatelet therapies, as in our study. The authors found that FIBTEM-MCF was significantly higher in patients in the ICU than IMWs and concluded that patients with COVID-19 with mild respiratory failure admitted to IMWs had less severe hypercoagulability and a lower incidence of symptomatic VTE as compared with more critical patients. 28 Finally, ROTEM parameters were analyzed in a group of patients with COVID-19 with different severities of pneumonia (moderate, severe, and critical) by Mitrovic et al. The authors showed that a hypercoagulable ROTEM pattern characterized by acceleration of blood clot formation, high clot strength, and reduced fibrinolysis was more frequent in advanced disease groups and patients with high IL6 levels. 29
Recent guidelines recommend a standard dose of VTE prophylaxis for all hospitalized patients with COVID-19, regardless of the severity level. 30,31 However, venous thromboembolic events are reported frequently despite the use of thromboprophylaxis, especially in ICU patients. Therefore, we aimed to assess the hypercoagulability level of patients with COVID-19 in different severity groups and observed that patients with severe COVID-19 had more severe hypercoagulability as compared with patients with mild disease. We also identified the value of fibrinogen, D-dimer, and platelets in COVID-19–induced hypercoagulability. We determined cut-off values for these conventional coagulation parameters which is a significant strength of our study, extending the available data. In addition to ROTEM parameters, we think that levels of fibrinogen, D-dimer, and platelets on admission might be helpful, early parameters to improve anticoagulant management strategies. A second strength of our study is that all included patients were tested prior to initiation of thromboprophylaxis which provided accurate and reliable test results.
Conclusion
ROTEM analysis demonstrated a significant hypercoagulability in patients with COVID-19 and revealed distinct coagulation profiles in different severity subgroups. Therefore, ROTEM parameters and a combination of cut-off values for traditional coagulation parameters should be considered to define the potential level of anticoagulant prophylaxis and achieve improved clinical outcomes in patients with COVID-19.
Footnotes
Authors’ Note: Conception and design of study: GG, ZK, SI, OY, OMA; acquisition of data: GG, ZK, SI; analysis and/or interpretation of data: GG, OMA; drafting the manuscript: GG, OMA; revising the manuscript critically for important intellectual content: GG, OMA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mehmet Gökhan Gönenli  https://orcid.org/0000-0003-2925-7048
https://orcid.org/0000-0003-2925-7048
References
- 1. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klok FA, Kruip M, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA. 2020;324(8):799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with Covid-19: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. [DOI] [PubMed] [Google Scholar]
- 5. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singhania N, Bansal S, Nimmatoori DP, Ejaz AA, McCullough PA, Singhania G. Current overview on hypercoagulability in COVID-19. Am J Cardiovasc Drugs. 2020;20(5):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Middleton EA, He XY, Denorme F, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hidalgo A. A NET-thrombosis axis in COVID-19. Blood. 2020;136(10):1118–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Begbie M, Notley C, Tinlin S, Sawyer L, Lillicrap D. The factor VIII acute phase response requires the participation of NFkappaB and C/EBP. Thromb Haemost. 2000;84(2):216–222. [PubMed] [Google Scholar]
- 12. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akay OM, Ustuner Z, Canturk Z, Mutlu FS, Gulbas Z. Laboratory investigation of hypercoagulability in cancer patients using rotation thrombelastography. Med Oncol. 2009;26(3):358–364. [DOI] [PubMed] [Google Scholar]
- 15. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]. National Institutes of Health; 2019. Accessed May, 2020. https://www.covid19treatmentguidelines.nih.gov/ [PubMed] [Google Scholar]
- 16. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. [DOI] [PubMed] [Google Scholar]
- 17. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akay OM. The double hazard of bleeding and thrombosis in hemostasis from a clinical point of view: a Global Assessment by Rotational Thromboelastometry (ROTEM). Clin Appl Thromb Hemost. 2018;24(6):850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sahin DG, Akay OM, Uskudar Teke H, Andic N, Gunduz E. Use of rotational thromboelastometry for a global screening of coagulation profile in patients of myeloproliferative neoplasms. Platelets. 2021;32(2):280–283. [DOI] [PubMed] [Google Scholar]
- 24. Spiezia L, Boscolo A, Poletto F, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020;50(2):281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoechter DJ, Becker-Pennrich A, Langrehr J, et al. Higher procoagulatory potential but lower DIC score in COVID-19 ARDS patients compared to non-COVID-19 ARDS patients. Thromb Res. 2020;196:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Almskog LM, Wikman A, Svensson J, et al. Rotational thromboelastometry results are associated with care level in COVID-19. J Thromb Thrombolysis. 2021;51(2):437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boscolo A, Spiezia L, Correale C, et al. Different hypercoagulable profiles in patients with COVID-19 admitted to the internal medicine ward and the intensive care unit. Thromb Haemost. 2020;120(10):1474–1477. [DOI] [PubMed] [Google Scholar]
- 29. Mitrovic M, Sabljic N, Cvetkovic Z, et al. Rotational Thromboelastometry (ROTEM) profiling of COVID-19 Patients. Platelets. 2021:1–7. [DOI] [PubMed] [Google Scholar]
- 30. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]