Abstract
Background:
Caesarean skin scars (CSS; hypertrophic scars and keloids) are very stressful for women and treatment strategies vary. However, there is a lack of knowledge about the outcome of surgical excision of CSS during caesarean section (CS). The study aims to determine the rate of recurrence and risk factors of recurrence for surgically removed CSS.
Method:
This is a retrospective cohort study that used STROBE guidelines. Pfannenstiel incisions of 145 patients were evaluated. Patients were divided into two groups: recurred (group 1, n = 19) and non-recurred group (group 2, n = 126). The groups were compared.
Results:
The rate of recurrence of CSS was 13% in the total cohort (19/145), one of the main outcomes of the study. While emergency CS was performed for 12 patients in group 1 (63%), CS was carried out in 25 patients in group 2 (20%); this difference was significant (P = 0.001). Before surgery, white blood cell and neutrophil counts were significantly higher in group 1 (P = 0.014 and P = 0.023, respectively). There were 11 dark-skinned women (26%; Fitzpatrick type 4) in group 1 and 31 (74%) in group 2. This difference was statistically significant (P = 0.031). As the other main outcome, emergency CS could be accepted as a risk factor for recurrence in the multivariate regression analysis (P = 0.060; odds ratio = 5.07; 95% confidence interval = 0.93–17.51).
Conclusion:
The rate of recurrence of surgically removed previous CSS at CS is promising without adjunct therapy. Emergency CS was found to be a risk factor for recurrence.
Lay Summary
Background
Caesarean skin scars (CSS; hypertrophic scars and keloids) are very stressful and are generally itchy and painful for women. Treatment strategies vary. However, there is a lack of knowledge about the outcome of only surgical excision of CSS scars during caesarean section (CS).
The issue being explored
There are few data in the literature for CSS in the lower abdomen. These scars can be removed during the second or third CS, but the results are not known exactly.
How was the work conducted?
In our clinic, 145 patients with CSS were given a CS and their scars were removed at the same time. While most of these scars were reported as hypertrophic by pathological examination, some were reported as keloid. At the earliest, one year after surgery, the rate of recurrence was found to be 13%.
What we learned from the study
Asymptomatic patients who are planning another pregnancy and do not want to receive any other radiotherapy or steroid injection therapy can wait to remove their CSS at the next CS, especially elective CS with or without adjunct therapy. Emergency CS was found to be a risk factor for the recurrence of these scars.
Keywords: caesarean section, caesarean skin scar, hypertrophic scars, keloids
Introduction
Abnormal skin scars (hypertrophic scars and keloids) are a reaction to any injury such as surgical incisions, piercings, burns, even insect bites. These pathologies present as pruritic and painful symptoms and an unattractive appearance for women. Exaggerated wound healing leads to hypertrophic scars or keloids seen in 10%–15% of all wounds. 1 Keloids are clinically different from hypertrophic scars and it is known that keloids extend beyond the boundaries of the original wound; however, frequently it can be difficult to distinguish between the two in a clinical setting. 2 We decided to use the term caesarean skin scars (CSS). Patients aged < 30 years and have dark skin are at risk for the development of skin scars. 3 Caesarean section (CS) is one of the most common surgeries in the world and the incidence of primary CS is up to 32%, even in some high-income countries. 4 Therefore, these high rates will increase the likelihood of encountering CSS in young women. Corticosteroid injection is accepted as a first-line therapy for CSS; it needs several sessions and can be done alone or combined with radiotherapy, surgical excision or other modalities. 5
Surgically removing the scar is another suggested treatment of CSS. However, surgical excision of the keloid usually provides temporary cosmetic relief only and is followed by even more aggressive regrowth of scar tissue in 50%–100% of cases. 6 For hypertrophic scars, the rate of recurrence after surgical excision is stated as 10% in a review article. 7 As we know, keloids and hypertrophic scars frequently are found in taut areas such as the skin of the ear, shoulder and chest wall, 8 but the suprapubic area where CS is performed is generally loose. These high rates of recurrence after surgical excision may be related to other areas of the human body except the Pfannenstiel incision area. Our literature search has shown a lack of research on the only surgical excision of CSS and no study has the exact rate of recurrence of excised CSS. The aim of the present study was to determine the rate of recurrence of surgically removed previous CSS during CS, in addition to comparing the risk factors for recurred and non-recurred patients.
Methods
This was a retrospective cohort study. Excisions were performed during CS in 260 patients with CSS between September 2016 and September 2019, in a tertiary hospital in Diyarbakır, Turkey. Approval from the local ethic committee (14.02.2020/426) was taken and the study followed the Declaration of Helsinki. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist was used to develop the study. Among the 260 patients, the phone numbers of 221 patients were obtained. A total of 191 patients agreed to participate and were recruited into the study; 30 women did not agree to participate. In total, 30 patients were excluded from the study because they used traditional or modern medical implementations for their scars after CS (silicone gel sheeting, etc.), six patients were excluded for receiving treatment for surgical site infection and skin hematoma, and 10 women were excluded due to maternal disease such as gestational diabetes mellitus. The Pfannenstiel incisions of the remaining 145 women were evaluated in our hospital; there were no median infraumbilical abdominal incisions. The Japan Scar Workshop (JSW) scar scale (JSS) was used to define recurrence. A score above 3 according to JSS (Figure 1) was accepted as recurrence. 9 We found that the specimens of 79 of 145 patients were not pathologically examined after excision. Unfortunately, most of our surgeons do not need a pathological examination of skin scars and dispose of them. Therefore, an image of a patient with CSS was shown to 79 participants who were not pathologically examined for their excised scar to confirm if they had a similar lesion before CS (Figure 2). All of those stated that they had similar or worse lesions before CS. Demographic features and the time between the examination and CS with the excision of CSS were noted for every participant. When the medical records of the patients were reviewed, the same skin closure technique (subcuticularly and continuous) using 3/0 absorbable sutures was seen in every patient. All pathological examinations that were sent to the pathology department for investigation were reviewed.
Figure 1.

JSW Scar Scale (JSS) scoring system for evaluation of skin scars. 9
Figure 2.
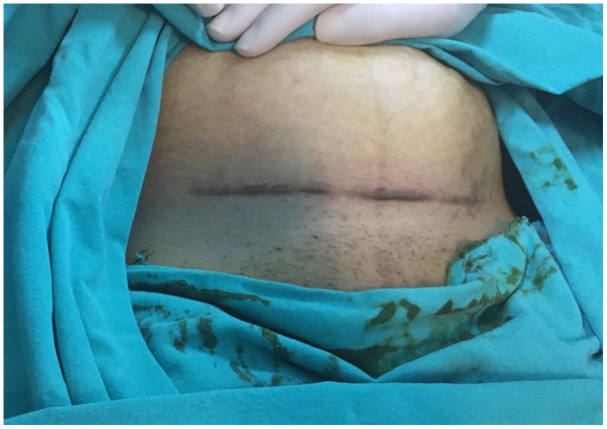
A patient was prepared for caesarean section; after complete excision of the caesarean skin scar, pathological examination revealed a hypertrophic scar.
Patients were divided into two groups: recurred (group 1, n = 19) and non-recurred (group 2, n = 126). The features and laboratory findings in both groups were compared.
Statistical analysis
Statistical analysis was performed using SPSS version 20, and P values < 0.05 were considered statistically significant. The required size of the study population was calculated to be 143 participants (alpha = 0.05 and study power = 0.80).
For the descriptive statistics, the mean, standard deviation, median, range and frequency were used according to the normality distribution. Different categorical variables were made using the chi-square and Fisher exact tests. The Student’s t test and Mann–Whitney U test were used to compare the parametric and non-parametric data, respectively. Univariate logistic regression was used to predict factors affecting recurrence. Variables that were significant in the univariate analysis underwent multivariate logistic regression analysis to find an independent factor. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for the predictors of recurrence of surgically excised skin scar as the main outcomes of the study.
Results
Descriptive and comparative characteristics are shown in Table 1. The mean time between the surgical excision and the study was 18 ± 7.2 months in group 1 and 23.1 ± 9.3 months in group 2. The duration of surgery was not different between the recurred (group 1) and non-recurred groups (group 2) (P = 0.061). All scars were located at the Pfannenstiel incision. We found that the specimens of 79 of the 145 patients had not been pathologically examined, while 50 (76%) were reported as hypertrophic scars, 12 (18%) results were reported as hypertrophic scars combined with keloid and 4 (6%) resulted in keloid scars.
Table 1.
Comparison of demographic and laboratory findings between the groups.
| Group 1 (n = 19) | Group 2 (n = 126) | P | |
|---|---|---|---|
| Age (years) | 26.5 ± 4.1 | 25.0 ± 4.5 | 0.103 |
| BMI (kg/m2) | 25.6 ± 4.6 | 26.8 ± 3.7 | 0.131 |
| Time between surgery and examination for study (months) | 18.0 ± 7.2 | 23.1 ± 9.3 | 0.022* |
| Duration of surgery (min) | 42.0 ± 6.0 | 40.0 ± 8.0 | 0.061 |
| Week of gestation | 37.6 ± 1.3 | 38.3 ± 1.0 | 0.023* |
| WBC count (×103) | 11.63 ± 3.22 | 8.59 ± 1.65 | 0.014* |
| Neutrophil count (×103) | 9.35 ± 2.89 | 7.12 ± 2.01 | 0.023* |
| JSS † | 12.0 ± 2.0 | 1±1 | 0.009* |
| Skin colour | 0.031* | ||
| Open | 8 (42) | 95 (75) | |
| Dark | 11 (58) | 31 (25) | |
| Condition of CS | 0.001* | ||
| Emergency CS | 12 (63) | 25 (20) | |
| Elective CS | 7 (37) | 101 (80) | |
| Number of CS | 0.462 | ||
| 2 | 14 (74) | 106 (84) | |
| >2 | 5 (26) | 20 (16) |
Values are given as n (%) or mean ± SD.
P < 0.05 was considered to be significant.
JSS > 3 is accepted as recurrence.
BMI, body mass index; CS, caesarean section; JSS, Japan Scar Workshop scale; SD, standard deviation; WBC, white blood cell.
Nineteen women were detected as having developed recurrent scar using the JSS. As a main outcome of the study, the rate of recurrence of surgically excised skin scars was found to be 13% (19/145). There were 11 dark-skinned women (26%; Fitzpatrick type 4) in group 1 and 31 (74%) in group 2; this difference was statistically significant (P = 0.031) (Table 1). A total of 108 CS were performed electively, while 37 were emergencies. While emergency CS was performed for 12 women (63%) in group 1, it was performed in 25 women (20%) in group 2; this difference was statistically significant (P = 0.001) (Table 1).
Respective WBC and neutrophil counts were 11.63 ± 3.22 and 9.35 ± 2.89 in group 1 and 8.59 ± 1.65 and 7.12 ± 2.01 in group 2. These differences were considered significant (P = 0.014 for WBC and P = 0.023 for neutrophil levels) (Table 1). The other main outcomes of the study—emergent CS, dark skin, elevated WBC and neutrophil counts—were found to be risk factors for developing recurrence of CSS in univariate logistic regression analysis and only emergent CS from those can be accepted as a main risk factor for the recurrence in multivariate regression analysis (P = 0.060; OR = 5.07; 95% CI = 0.93–17.51). The other OR values with 95% CIs are shown in Table 2.
Table 2.
Univariate and multivariate logistic regression analysis of predictors of scar recurrence.
| Univariate | P value | Multivariate | P value* | |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Age | 1.07 (0.97–1.19) | 0.162 | ||
| BMI | 0.921 (0.80–1.05) | 0.218 | ||
| Weeks of gestation | 0.623 (0.42–0.91) | 0.015 | 1.15 (0.64–2.06 | 0.624 |
| Skin colour | 0.005 | 0.459 | ||
| Open | 1 | 1 | ||
| Dark | 4.21 (1.55–11.41) | 1.60 (0.45–5.65) | ||
| CS | 0.0001 | 0.060 | ||
| Elective | 1 | 1 | ||
| Emergency | 6.92 (2.47–17.23) | 5.07 (0.93–17.51) | ||
| WBC count | 0.023 | |||
| >8.65 | 1 | |||
| <8.65 | 3.29 (1.17–9.23) | |||
| Neutrophil count | 0.003 | 0.086 | ||
| >7.35 | 1 | 1 | ||
| <7.35 | 4.29 (1.69–12.50) | 2.61 (0.87–7.86) |
P < 0.05 was considered to be significant.
BMI, body mass index; CI, confidence interval; CS, caesarean section; OR, odds ratio; WBC, white blood cell.
Discussion
CSS (hypertrophic scars and keloids) is one of the major problems for women after CS. These are generally symptomatic and are pruritic and/or painful. Skin scars also are disturbing aesthetically and also often result in severe emotional distress among women. 10 In our hospital, located in the southeast of Turkey, there are 25,000 births per year, and there are many young patients aged < 30 years. Fortunately, the rate of primary CS is around 15% at our hospital. We do not encounter large caesarean keloids in black women, as presented by Lutgendorf et al. 2 As a Middle East population, our skin colour is between open (Fitzpatrick skin type 2–3) and dark (Fitzpatrick skin type 4). Another complication is the difficulty in distinguishing between hypertrophic scars and keloids, despite the pathological discrimination being clear. 7 It could be seen that the majority of CSS (76%) at the Pfannenstiel incision in our study are hypertrophic scars. For instance, while the histopathological examination of Figure 2 in our study revealed a hypertrophic scar, Chua et al. 11 conducted a study that included 150 pregnant women who all had caesarean keloid scars that were only diagnosed according to the appearance of the scars. On the other hand, Tsai et al. 9 treated 40 midline infraumbilical CSS and defined all scars as hypertrophic scars, unlike Chua et al. As can be seen from the study Bayat et al., 12 hypertrophic scars and keloids can co-exist in the same incision line. In our study, we found that 18% of these lesions can co-exist as a result of pathological examination. Therefore, all scars were called CSS.
A high rate of recurrence of 40%–90% of keloids in women who underwent only surgical excision is circulating in the literature.2,13 Even if high rates of recurrence of keloid excisions are stated in the literature, there is no one study that has found rates of recurrence of only surgically removed CSS during CS. Speranza et al. 14 found that the abdominal wall was more responsive than other areas while the ear was a more common non-responder region. Compared to areas such as the ear and the shoulder, the suprapubic region is a more flexible area in terms of tissue tension. Therefore, the recurrence rate of CSS in the suprapubic region is expected to be lower than the other areas. In addition, Ogawa et al. 15 concluded that some anatomic sites with increased tautness were associated with statistically higher rates of recurrence rather than CSS. In one study, 14 after surgical excision of the primary keloid, orthovoltage-based radiotherapy was shown to be a good method for preventing further recurrence; only 14.6% of patients needed additional adjuvant treatment. We reached a lower rate of recurrence (13%) with only surgical excision without adjuvant therapy.
In another study protocol, the physicians performed intralesional steroid injections after the excision of keloids at the time of suture removal and then every two weeks for a total of five treatment sessions, with concomitant application of a steroid ointment twice a day from the time of suture removal for a total of six months. In that study, 16 only 14.3% of keloids recurred; recurrence was lower in the abdominal area, but the sample size in the study by Hayashi et al. was very small (18 patients with abdominal midline keloids and six with abdominal midline hypertrophic scar).
There has not been enough investigation of CSS in the obstetric population; there are limited numbers and studies with small sample sizes have been published. For example, Karmisholt et al. 17 discussed the effect of fractional CO2 laser on the treatment of CSS (hypertrophic scars) on 11 patients; after six months they were able to get a satisfying result. The images were called hypertrophic scars in the study by Karmisholt et al., whereas Park et al. 18 named theirs keloids. However, it was clearly seen that both studies had similar images of CSS. Ogawa et al. 15 evaluated postoperative electron-beam irradiation therapy for keloids and hypertrophic scars in 147 cases; however, 11 of them had suprapubic scars, in whom four recurred (36% rate of recurrence for suprapubic scars). In addition, Ogawa et al. also had some patients who had combined hypertrophic and keloid scars pathologically and had some difficulties distinguishing between these scars; therefore, they also decided to put them in the same category.
According to the review article by Berman et al., 5 the primary treatment to consider is the application of silicone gel/sheeting with intralesional corticosteroids, followed by fractional or pulsed-dye laser; if the lesions remain refractory, surgical excision should be considered with adjuvant treatments. However, this treatment algorithm was not designed for Pfannenstiel caesarean incision skars. To our knowledge, there are no exact rates of recurrence after only surgical excisions of CSS as we mentioned above. Therefore, the present study sheds light on the literature.
In one of the few studies on caesarean keloids, total excision was performed during 26 CS, followed by radiotherapy, and the rate of recurrence was 23%. In addition, ovarian radiation exposure must be considered. 19
According to Berman et al., 5 general principles for minimising the risk of recurrence during the surgical excision of scars include gentle handling techniques at primary wound repair sites, an appropriately planned closure so as to minimise tension on the wound bed, closure within relaxed skin tension lines and the use of buried sutures when needed to reduce tension on the wound closure. However, in an emergency CS, this patience and diligence may be reduced. This may indicate that the frequency of recurrence increases in patients undergoing emergency CS. The present study supports the increase in recurrence in patients undergoing emergency CS. However, a major limitation of the present study is its retrospective nature.
Similar to Ogawa et al., 20 who touched on the role of chronic inflammation in the development of keloids and hypertrophic scars, we found a high WBC and neutrophil count preoperatively in the recurred group (group 1). When we investigated whether these patients had mostly undergone emergency CS due to some of them having pain or amnion fluid drainage before surgery, these emergent conditions probably led to an increase in the WBC and neutrophil counts. Thus, a normal range of preoperative inflammatory cell counts may indicate a low risk of CSS recurrence.
In an ongoing study aiming to prevent CSS with mesenchymal stem cell injection, it is stated that the treatment of hypertrophic scars is expensive, painful and rates of recurrence are high. 21 The present study shows that CS and scar removal can be done easily in the same session at no extra cost and without extra pain for scar treatment.
Conclusion
When only surgical excision of CSS is compared with radiotherapy and steroid injection is combined with surgical excision, the rate of recurrence is reasonable, especially in non-emergent surgery. Asymptomatic patients who are planning another pregnancy and do not want to receive any other radiotherapy or steroid injection therapy can wait before removing their CSS in the next elective CS with or without adjuvant therapy.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: İhsan Bağlı  https://orcid.org/0000-0002-3195-9164
https://orcid.org/0000-0002-3195-9164
Rei Ogawa  https://orcid.org/0000-0003-3658-555X
https://orcid.org/0000-0003-3658-555X
References
- 1. Berman B, Perez O, Konda S, et al. A review of the biologic effects, clinical efficacy, and safety of silicone elastomer sheeting for hypertrophic and keloid scar treatment and management. Dermatol Surg 2007; 33(11): 1291–1303. [DOI] [PubMed] [Google Scholar]
- 2. Lutgendorf MA, Adriano EM, Taylor BJ. Prevention and management of keloid scars. Obstet Gynecol 2011; 118(2 Pt 1): 351–356. [DOI] [PubMed] [Google Scholar]
- 3. Bock O, Schmid-Ott G, Malewski P, et al. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 2006; 297(10): 433–438. [DOI] [PubMed] [Google Scholar]
- 4. Montoya-Williams D, Lemas DJ, Spiryda L, et al. What are optimal cesarean section rates in the U.S. and how do we get there? A review of evidence-based recommendations and interventions. J Womens Health (Larchmt) 2017; 26(12): 1285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg 2017; 43 Suppl 1: S3–S18. [DOI] [PubMed] [Google Scholar]
- 6. Kelly A. Medical and surgical therapies for keloids. Dermatol Ther 2004; 17(2): 212–218. [DOI] [PubMed] [Google Scholar]
- 7. Verhaegen PD, van Zuijlen PP, Pennings NM, et al. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: An objective histopathological analysis. Wound Repair Regen 2009; 17(5): 649–656. [DOI] [PubMed] [Google Scholar]
- 8. Renz P, Hasan S, Gresswell S, et al. Dose effect in adjuvant radiation therapy for the treatment of resected keloids. Int J Radiat Oncol Biol Phys 2018; 102(1): 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsai CH, Kao HK, Akaishi S, et al. Combination of 1,064- nm neodymium- doped yttrium aluminum garnet laser and steroid tape decreases the total treatment time of hypertrophic scars: an analysis of 40 cases of cesarean-section scars. Dermatol Surg 2020; 46(8): 1062–1067. [DOI] [PubMed] [Google Scholar]
- 10. Kelly A. Keloids and hypertrophic scars. In: Parish L, Lask G, editors. Aesthetic Dermatology. New York: McGraw-Hill, 1991, pp.8–69. [Google Scholar]
- 11. Chua SC, Gidaszewski B, Khajehei M. Efficacy of surgical excision and sub-dermal injection of triamcinolone acetonide for treatment of keloid scars after caesarean section: a single blind randomised controlled trial protocol. Trials 2019; 20(1): 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ 2003; 326(7380): 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shridharani SM, Magarakis M, Manson PN, et al. The emerging role of antineoplastic agents in the treatment of keloids and hypertrophic scars: a review. Ann Plast Surg 2010; 64(3): 355–361. [DOI] [PubMed] [Google Scholar]
- 14. Speranza G, Sultanem K, Muanza T. Descriptive study of patients receiving excision and radiotherapy for keloids. Int J Radiat Oncol Biol Phys 2008; 71(5): 1465–1469. [DOI] [PubMed] [Google Scholar]
- 15. Ogawa R, Mitsuhashi K, Hyakusoku H, et al. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg 2003; 111: 547–553. [DOI] [PubMed] [Google Scholar]
- 16. Hayashi T, Furukawa H, Oyama A, et al. A new uniform protocol of combined corticosteroid injections and ointment application reduces recurrence rates after surgical keloid/hypertrophic scar excision. Dermatol Surg 2012; 38: 893–897. [DOI] [PubMed] [Google Scholar]
- 17. Karmisholt KE, Taudorf EH, Wulff CB, et al. Fractional CO2 laser treatment of caesarean section scars-A randomized controlled split-scar trial with long term follow-up assessment. Lasers Surg Med 2017; 49(2): 189–197. [DOI] [PubMed] [Google Scholar]
- 18. Park TH, Chang CH. Keloid recurrence in pregnancy. Aesthetic Plast Surg 2012; 36(5): 1271–1272. [DOI] [PubMed] [Google Scholar]
- 19. Kim J, Lee SH. Therapeutic results and safety of postoperative radiotherapy for keloid after repeated Cesarean section in immediate postpartum period. Radiat Oncol J 2012; 30(2): 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci 2017; 18(3): E606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fan D, Xia Q, Wu S, et al. Mesenchymal stem cells in the treatment of Cesarean section skin scars: study protocol for a randomized, controlled trial. Trials 2018; 19(1): 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
How to cite this article
- Bağlı İ, Ogawa R, Bakır S, Taşın C, Yıldırım A, Öcal E, Yavuz M, Bala M, Turan G. The predictors of recurrence of surgically removed previous caesarean skin scars at caesarean section: A retrospective cohort study. Scars, Burns & Healing, Volume 7, 2021. DOI: 10.1177/ 20595131211023388. [DOI] [PMC free article] [PubMed] [Google Scholar]


