Abstract
Objective
We aimed to compare the efficacy and risks of proton pump inhibitor (PPI) versus histamine-2 receptor blocker (H2B) use for stress ulcer prophylaxis (SUP) in critically ill patients with sepsis and risk factors for gastrointestinal bleeding (GIB).
Methods
In this retrospective cohort study, we used the Medical Information Mart for Intensive Care III Clinical Database to identify critically ill adult patients with sepsis who had at least one risk factor for GIB and received either an H2B or PPI for ≥48 hours. Propensity score matching (PSM) was conducted to balance baseline characteristics. The primary outcome was in-hospital mortality.
Results
After 1:1 PSM, 1056 patients were included in the H2B and PPI groups. The PPI group had higher in-hospital mortality (23.8% vs. 17.5%), GIB (8.9% vs. 1.6%), and pneumonia (49.6% vs. 41.6%) rates than the H2B group. After adjusting for risk factors of GIB and pneumonia, PPI use was associated with a 1.28-times increased risk of in-hospital mortality, 5.89-times increased risk of GIB, and 1.32-times increased risk of pneumonia.
Conclusions
Among critically ill adult patients with sepsis at risk for GIB, SUP with PPIs was associated with higher in-hospital mortality and higher risk of GIB and pneumonia than H2Bs.
Keywords: Stress ulcer prophylaxis, sepsis, Medical Information Mart for Intensive Care III, gastrointestinal bleeding, histamine-2 receptor blocker, proton pump inhibitor
Introduction
Critically ill patients admitted to the intensive care unit (ICU) are at risk of developing gastrointestinal (GI) bleeding owing to stress ulcers, 1 which is associated with a prolonged length of stay (LOS) in the ICU and an increased risk of death. 2 To prevent GI bleeding in these critically ill patients, ICU doctors frequently prescribe stress ulcers prophylaxis (SUP). 3 Proton pump inhibitors (PPIs) and histamine type 2 receptor blockers (H2Bs) are the most commonly used SUP agents.4,5
Sepsis has been considered a risk factor for the development of stress ulceration and GI bleeding for many years, 6 and SUP is recommended by the Surviving Sepsis Campaign guidelines for patients with sepsis or septic shock who have risk factors for GI bleeding. 7 Although these guidelines further recommend either H2Bs or PPIs when SUP is indicated, there is limited evidence of the effects of H2Bs in comparison with PPIs when used as SUP in critically ill patients with sepsis.
A recent meta-analysis of a general population of patients in the ICU suggested that PPIs are the most effective agents in preventing clinically important GI bleeding. 8 However, two retrospective studies found that PPIs were associated with a higher rate of GI bleeding than H2Bs.9,10 In clinical practice, PPIs are more commonly prescribed by critical care providers, possibly because of their superior acid suppression capability in comparison with H2Bs. 11 However, PPI use is not without risk. Some studies have shown that patients receiving PPIs have a higher risk of nosocomial pneumonia, 12 Clostridium difficile infection (CDI), 13 and myocardial infarction. 14
Accordingly, it is reasonable to evaluate the comparative effects of SUP using PPIs and H2Bs on important outcomes in patients with severe sepsis or septic shock. We conducted a retrospective cohort study to compare two strategies for SUP among adult patients with sepsis or septic shock who have risk factors for GI bleeding. Specifically, we hypothesized that PPIs are associated with a lower occurrence of GI bleeding and in-hospital mortality than H2Bs but may increase the risk of pneumonia and CDI.
Methods
Data source
The study data were extracted from the Medical Information Mart for Intensive Care III (MIMIC-III) Clinical Database. Briefly, this is a large database containing data from 53,423 distinct hospital admissions of adult patients admitted to the ICU of Beth Israel Deaconess Medical Center (BIDMC) in Boston in the United States between 2001 and 2012. 15 The database is freely available online. We used the most recent version (version 1.4) in this study.
The study was exempt from institutional review board approval and the requirement for informed consent because any investigator who completes the required training course online receives approval to access and use data from the MIMIC-III database from the Institutional Review Board of BIDMC and the Massachusetts Institute of Technology.
Our study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. 16
Patient inclusion and exclusion criteria
We included adult patients (aged 16 years and over) with sepsis or septic shock who were admitted to the BIDMC ICU between 2001 and 2012. Only those who received SUP and had risk factors for GI bleeding were included in the analysis.
The use of SUP was defined as the administration of either an H2B or PPI for 48 hours or more during the ICU stay. Patients with risk factors for GI bleeding were defined as having one or more of the following conditions: mechanical ventilation for more than 48 hours, renal replacement therapy (RRT), coagulopathy on the first day of ICU admission, higher organ failure scores, or pre-existing liver disease. 7 Only data for each patient’s first ICU admission were used in this study. In this study, the definition of sepsis was in agreement with the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), which is defined as infection plus sequential organ failure assessment (SOFA) score ≥2 points. 17
Patients were excluded if any of the following conditions were present: (1) age over 89 years; (2) GI bleeding listed as a primary diagnosis (defined using International Classification of Diseases, Ninth Revision [ICD-9] codes) or diagnosis on hospital admission (diagnosis query strings used); (3) variceal bleeding listed as a primary or secondary diagnosis; (4) a history of peptic ulcer disease; (5) length of ICU stay <2 days or >100 days; (6) administration of both an H2B and PPI (concomitantly or consecutively) during the ICU stay; or (7) missing data for analysis.
Variables and outcomes
We collected demographic (age, sex, body mass index), disease severity, and intervention information from the MIMIC-III database. We also collected information on the following confounding covariates that may affect the use of PPIs or H2Bs or increase the risk of GI bleeding: coagulopathy, thrombocytopenia, acute kidney injury, chronic hepatic disease, corticosteroid use, vasopressor use mechanical ventilation >48 hours, RRT during the ICU stay, Elixhauser comorbidity index, 18 Simplified Acute Physiology Score II (SAPS II), SOFA score at ICU admission, and use of enteral nutrition.
The primary outcome was in-hospital mortality. The secondary outcomes were a diagnosis of secondary GI bleeding, diagnosis of secondary pneumonia, CDI, and hospital LOS. GI bleeding, pneumonia, and CDI were defined using ICD-9 codes. We further examined whether blood transfusion was received in patients diagnosed with secondary GI bleeding; clinically important and overt bleeding was defined if these patients received transfusion.
Missing and extreme values
For all selected baseline variables, we determined whether there were any missing values and their patterns. If a missing variable had a pattern of being missing completely at random or missing at random, the characteristics between non-missing and missing groups were compared. If important baseline characteristics showed no statistical significance between the non-missing and missing groups, the mean of the non-missing group was imputed for the missed variable. If significant differences were present in some critical baseline variables, the stratified mean was imputed. Extreme values for continuous variables were also examined. An extreme value was defined as exceeding 1.5 times the interquartile range (IQR); if present, the value was changed to the value nearest 1.5 times the IQR value.
Propensity score matching (PSM) and balance diagnosis
Similarity between the groups was described using the standardized mean difference (SMD). An important imbalance was defined as SMD larger than 0.1. After checking the balance of the baseline variables, we built a propensity score model using the unbalanced variables, described in Table 1 . We performed 1:1 PSM using the nearest neighbor method and caliper width of 0.02. After matching, a PSM density plot of the PPI and H2B groups was drawn before and after matching. The imbalance was checked using the SMD.
Table 1.
Baseline characteristics before and after matching.
|
Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| H2B | PPI | p valuea | H2B | PPI | p valuea | |
| Variable | (N=1111) | (N=3569) | (N=1056) | (N=1056) | ||
| Demographic characteristics | ||||||
| Age (years), median [IQR] | 66.33 [53.20, 76.94] | 66.63 [55.21, 77.08] | 0.205 | 66.99 [54.18, 77.18] | 66.39 [54.81, 77.09] | 0.666 |
| Male sex, n (%) | 652 (58.7) | 1987 (55.7) | 0.083 | 608 (57.6) | 616 (58.3) | 0.758 |
| Illness severity scores and Elixhauser comorbidity score on first day of ICU admission | ||||||
| SAPS II, median [IQR] | 42.0 [33.0, 51.0] | 44.0 [36.0, 54.0] | <0.001 | 42.0 [34.0, 52.0] | 43.0 [34.0, 52.0] | 0.272 |
| SOFA score, median [IQR] | 6.0 [4.0, 8.0] | 7.0 [4.0, 9.0] | <0.001 | 6.0 [4.0, 8.3] | 6.0 [4.0, 8.0] | 0.811 |
| Elixhauser comorbidity index, median [IQR] | 11.0 [5.0, 17.0] | 13.0 [7.0, 19.0] | <0.001 | 11.0 [5.0, 17.0] | 11.0 [6.0, 17.0] | 0.717 |
| Confounding covariates | ||||||
| Coagulopathy, n (%) | 219 (19.7) | 954 (26.7) | <0.001 | 213 (20.2) | 206 (19.5) | 0.743 |
| Chronic kidney injury, n (%) | 207 (18.6) | 823 (23.1) | 0.002 | 205 (19.4) | 214 (20.3) | 0.662 |
| Acute kidney injury, n (%) | 410 (36.9) | 1819 (51.0) | <0.001 | 408 (38.6) | 421 (39.9) | 0.593 |
| Chronic hepatic injury, n (%) | 72 (6.5) | 372 (10.4) | <0.001 | 71 (6.7) | 86 (8.1) | 0.246 |
| Acute hepatic injury, n (%) | 81 (7.3) | 481 (13.5) | <0.001 | 80 (7.6) | 101 (9.6) | 0.120 |
| Thrombocytopenia, n (%) | 162 (14.6) | 649 (18.2) | 0.006 | 159 (15.1) | 137 (13.0) | 0.188 |
| Head injury, n (%) | 35 (3.2) | 15 (0.4) | <0.001 | 7 (0.7) | 5 (0.5) | 0.772 |
| Oral feeding, n (%) | 700 (63.0) | 2515 (70.5) | <0.001 | 681 (64.5) | 669 (63.4) | 0.618 |
| Enteral nutrition, n (%) | 623 (56.1) | 1831 (51.3) | 0.006 | 574 (54.4) | 600 (56.8) | 0.274 |
| Parenteral nutrition, n (%) | 75 (6.8) | 522 (14.6) | <0.001 | 75 (7.1) | 74 (7.0) | 1.000 |
| MV ≥48 hours, n (%) | 671 (60.4) | 1921 (53.8) | <0.001 | 618 (58.5) | 618 (58.5) | 1.000 |
| RRT during ICU stay, n (%) | 116 (10.4) | 775 (21.7) | <0.001 | 112 (10.6) | 128 (12.1) | 0.090 |
| Vasopressor use ≥24 hours, n (%) | 420 (37.8) | 1435 (40.2) | 0.163 | 403 (38.2) | 390 (36.9) | 0.590 |
| Corticosteroid use (≥250 mg hydrocortisone or equivalent), n (%) | 90 (8.1) | 459 (12.9) | <0.001 | 87 (8.2) | 91 (8.6) | 0.814 |
| Therapeutic anticoagulation use, n (%) | 251 (22.6) | 884 (24.8) | 0.15 | 242 (22.9) | 298 (28.2) | 0.006 |
| Platelet inhibitor use, n (%) | 463 (41.7) | 1136 (31.8) | <0.001 | 438 (41.5) | 445 (42.1) | 0.791 |
IQR, interquartile range; MV, mechanical ventilation; PSM, propensity score matching; SAPS II, Simplified Acute Physiology Score II; SOFA, sequential organ failure assessment, RRT, renal replacement therapy; H2B, histamine type 2 receptor blocker; PPI, proton pump inhibitor; ICU, intensive care unit.
aTwo-way comparison between H2RA and PPI groups.
Logistic regression and survival analysis
The primary outcome of our study was in-hospital mortality; therefore, Kaplan–Meier survival curves were used to compare in-hospital mortality between PPI and H2B groups after matching. A Cox proportional hazards model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for in-hospital death and PPI or H2B use. We performed a log-rank test to compare differences in the survival curves. For the secondary outcome, we performed multivariate logistic regression for GI bleeding and pneumonia.
Statistical analysis
Categorical variables are presented as frequency and percentage and were compared using the chi-square test. If the expected cell number were less than 5, Fisher's exact test was used. Normality was checked for all continuous variables using the Kolmogorov–Smirnov test. Continuous variables with a non-normal distribution are presented as median and IQR, and the Mann–Whitney U test was used for comparisons.
All analyses were completed using R version 3.6.2 (The R Project for Statistical Computing, Vienna, Austria). PSM and survival analysis were conducted using the R packages “Matchit”, “survival” and “survminer”. A two-sided p value <0.05 was considered statistically significant.
Results
Study population
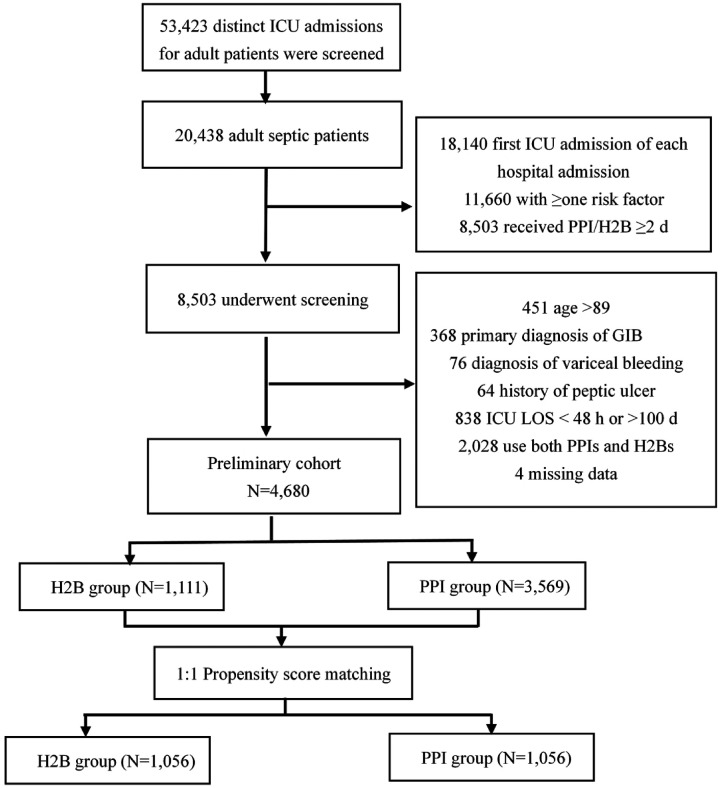
After screening 53,423 distinct ICU admissions for adult patients (aged 16 years or above) admitted to the BIDMC ICU from 1 June 2001 to 31 October 2012, we identified 20,438 adult patients who fit the Sepsis-3 definition. Additionally, 8503 patients with at least one GI bleeding risk factor and H2B or PPI use were eligible for further exclusion. Our final study cohort comprised 1111 (23.7%) patients exposed to an H2B more than 48 hours after ICU admission and 3569 (76.3%) patients who received PPIs for more than 48 hours. The total 4680 patients had an average age of 66.56 (53.20–77.08) years and 56.39% were men (Figure 1).
Figure 1.
Flowchart of variables included in the study.
GIB, gastrointestinal bleeding; H2B, histamine type 2 receptor blocker; PPI, proton pump inhibitor; ICU, intensive care unit; LOS, length of stay.
Baseline characteristics
Baseline characteristics of the H2B and PPI groups are shown in Table 1. Before PSM, patients in the PPI group had higher median [IQR] SOFA scores (7.0 [4.0, 9.0] vs. 6.0 [4.00, 8.0]; p < 0.001), SAPS II (44.0 [36.0, 54.0] vs. 42.0 [33.0, 51.0]; p < 0.001), and Elixhauser comorbidity scores (13.0 [7.0, 19.0] vs. 11.0 [5.0, 17.0]; p < 0.001) than patients in the H2B group. The PPI and H2B groups also showed statistically significant differences in most baseline variables, such as coagulopathy and acute or chronic kidney injury. Using PSM, 1056 patients who received H2Bs were matched with those who received PPIs. Age, sex, SAPS II, SOFA score, Elixhauser comorbidity score, and other confounding variables were well balanced between the two groups.
Outcomes
Before PSM, patients in the PPI group had significantly higher in-hospital mortality (27.1% vs. 17.3%, p < 0.001), GI bleeding rates (9.2% vs. 1.6%, p < 0.001), and clinically important and overt bleeding (6.9% vs. 1.0%, p < 0.001) than patients in the H2B group. The rate of CDI and pneumonia and hospital LOS were not significantly different between the two groups.
After PSM, in-hospital mortality (23.8% vs. 17.5%, p < 0.001), rate of GI bleeding (8.9% vs. 1.6%, p < 0.001), clinically important and overt bleeding (6.3% vs. 1.0%, p < 0.001), and pneumonia (49.6% vs. 41.6%, p < 0.001) were higher in the PPI group than in the H2B group. The rate of CDI and hospital LOS were similar between the two groups (Table 2).
Table 2.
Outcomes before and after matching.
|
Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| H2B | PPI | p valuea | H2B | PPI | p valuea | |
| Outcome | (N=1111) | (N=3569) | (N=1056) | (N=1056) | ||
| Primary outcome | ||||||
| In-hospital mortality, n (%) | 192 (17.3) | 968 (27.1) | <0.001 | 185 (17.5) | 251 (23.8) | <0.001 |
| Secondary outcomes | ||||||
| GI bleeding, n (%) | 18 (1.6) | 330 (9.2) | <0.001 | 17 (1.6) | 94 (8.9) | <0.001 |
| Clinically important and overt bleeding, n (%) | 11 (1.0) | 265 (6.9) | <0.001 | 11 (1.0) | 71 (6.3) | <0.001 |
| CDI, n (%) | 70 (6.3) | 276 (7.7) | 0.127 | 66 (6.2) | 81 (7.7) | 0.231 |
| Pneumonia, n (%) | 471 (42.4) | 1628 (45.6) | 0.064 | 439 (41.6) | 524 (49.6) | <0.001 |
| Hospital LOS, median [IQR] | 13.08 [8.42, 20.42] | 13.38 [8.26, 22.04] | 0.361 | 12.99 [8.26, 19.76] | 12.52 [7.96, 20.54] | 0.540 |
CDI, Clostridium difficile infection; GI, gastrointestinal; LOS, length of stay; IQR, interquartile range. SAPS II, Simplified Acute Physiology Score II; H2B, histamine type 2 assessment receptor blocker; PPI, proton pump inhibitor.
aTwo-way comparison between H2B and PPI groups.
Survival analysis
We further explored the risk factors for GI bleeding and pneumonia, which were the two secondary outcomes that showed statistically significant differences between the PPI and H2B groups. The results are presented in Tables S1 and S2. After adjusting for baseline characteristics, such as congestive heart failure, hypertension, diabetes, anticoagulation, body mass index, Elixhauser comorbidity score, SOFA score, SAPS II, and vasopressors, we determined that PPI use was associated with a 5.89-times higher risk of GI bleeding and a 1.32-times higher risk of pneumonia.
Kaplan–Meier curves for the PPI and H2B groups are shown in Figure S1. We found that the use of H2Bs was associated with better survival than the use of PPIs (log-rank test, p = 0.018). Figure S2 presents the forest plot for the Cox model. This plot shows that after controlling for other factors, use of PPI was associated with a 1.28-times increased risk of in-hospital death. The use of vasopressors (HR = 2.16, 95% CI 1.77–2.63, p < 0.001), congestive heart failure (HR = 1.26, 95% CI 1.04–1.54, p = 0.02) and presence of GI bleeding (HR = 1.49. 95% CI 1.07–2.08, p = 0.018) were also associated with an increased risk of hospital mortality. Ampicillin use was associated with a decreased risk of in-hospital death (HR = 0.53, 95% CI 0.34–0.81, p = 0.004).
Discussion
The findings of this retrospective study demonstrated that compared with H2Bs, the use of PPIs in adult patients with sepsis who have risk factors for GI bleeding increases the risks of in-hospital mortality, GI bleeding, and pneumonia. The results of this study provide a basis for drug selection when SUP is indicated in patients with sepsis or septic shock. Further randomized studies are needed to confirm these results.
To our knowledge, there are quite a few data available in the literature regarding the efficacy of PPIs versus H2Bs in prophylaxis of GI bleeding among a heterogeneous population in the ICU. However, our study specifically focused on critically ill patients with sepsis who have risk factors for GI bleeding. We found that in-hospital mortality was higher among these patients when PPIs were used for prophylaxis, as compared with H2Bs (23.8% vs. 17.5%). Our finding is similar to a recent clinical trial (PEPTIC), which showed that the in-hospital mortality rates of using PPIs versus H2Bs for SUP in mechanically ventilated patients in the ICU were 18.3% versus 17.5%. However, the reported differences were not significant. 19 The results of the present study were also consistent with a previous cost-effectiveness study, which suggested that the use of H2Bs for prophylaxis in critically ill patients may increase survival, reduce costs, and avoid complications more than the use of PPIs. 20 However, our results are inconsistent with a recent study in patients receiving extracorporeal membrane oxygenation (ECMO), which found no significant differences in terms of endoscopic hemostasis and in-hospital mortality between the PPI and H2B groups. One reason for this contradiction is that in-hospital mortality is much higher in patients receiving ECMO (≥53%) than in our study population; therefore, PPIs or H2Bs may not have an effect on mortality in the former population. Additionally, the definition of GI bleeding in that study differed from that in our study. 21
The greater effectiveness of H2Bs observed in the present study was also supported by two previous respective studies.9,10 Several case reports have demonstrated that PPIs may induce thrombocytopenia, which potentially explains the association between the greater risk of bleeding and PPI use. 22 Furthermore, studies using animal models indicate that H2Bs can reduce reperfusion injury, which may alleviate oxidative stress injury and be associated with less severe mucosal damage. 23 However, those findings obtained from clinical practice are contrary to the findings of a recent meta-analysis, which indicated greater efficacy of PPIs than H2Bs for GI bleeding prophylaxis in critically ill patients. A possible reason for this difference may be the heterogeneity of the study population and methodological limitations.11,24 Another possible explanation is that compared with those larger retrospective studies, the size of this meta-analysis of clinical trials was relatively small.
It remains controversial whether PPIs for SUP increase the risk of hospital-acquired pneumonia. 4 PPI use may decrease gastric acidity, altering normal intestinal flora and leading to the overgrowth of gastric bacteria. 25 The results of the present study are similar to those of previous studies, 8 which demonstrated that PPI use was associated with an increased risk of pneumonia.
Limitations
The current study has several limitations. First, GI bleeding and some covariates were defined using ICD-9 codes. Limitations to ICD-9 coding have been previously reported, but these remain the main approach for extracting data from large database studies. Another limitation of using ICD-9 codes is the inability to establish any temporal relationships between covariates and outcomes. 26 Second, we did not determine whether patients had received acid suppressants, enteral nutrition, anticoagulation, or other drugs before ICU admission, which may be associated with an increased risk of GI bleeding. Third, we did not assess the source of bleeding and cannot differentiate between stress ulcers and other causes of GI bleeding. Therefore, the proportion of patients with GI bleeding may be overestimated. Finally, a pivotal limitation is that the choice of PPIs or H2Bs for each patient was not randomly assigned. The data in the MIMIC-III database are recorded during routine clinical care and are not explicitly for retrospective data analysis. We conducted PSM to reduce selection bias and confounding; however, some unknown factors may influence the outcomes. For example, prophylaxis-prescribing habits may not be captured in PSM. Therefore, future well-designed and randomized clinical trials to assess the comparative effectiveness of PPIs versus H2Bs among patients with sepsis are needed to confirm our findings.
Conclusion
Among critically ill adult patients with sepsis who are at risk for GI bleeding, PPI use was found to be associated with higher risks of in-hospital mortality, GI bleeding, and pneumonia than H2B use. This result supports the use of an H2B over a PPI for SUP in critically ill adult patients with sepsis and GI risk. Additional randomized clinical trials are needed to confirm these results.
Appendix
Figure S1. Kaplan–Meier curve comparing survival between PPI and H2B groups.
H2B, histamine type 2 receptor blocker; PPI, proton pump inhibitor.
Figure S2. Forest plot for Cox proportional hazards model of in-hospital mortality.
GI, gastrointestinal; H2B, histamine type 2 receptor blocker; PPI, proton pump inhibitor.
Table S1.
Logistic regression for gastrointestinal bleeding.
| Gastrointestinal bleeding | Odds ratio (95% confidence interval) | p valuea |
|---|---|---|
| Drug (PPI vs. H2B) | 5.89 (3.57–10.31) | <0.0001 |
| Hypertension | 0.57 (0.38–0.85) | 0.02 |
Adjusted for congestive heart failure, hypertension, diabetes complications, anticoagulation, body mass index, SOFA, SAPS II, and vasopressors.
aTwo-way comparison between H2B and PPI groups.
H2B, histamine type 2 assessment receptor blocker; PPI, proton pump inhibitor; SAPS II, Simplified Acute Physiology Score II; SOFA, sequential organ failure; odds ratio; CI, confidence interval.
Table S2.
Logistic regression for pneumonia.
| Pneumonia | OR (95% CI) | p valuea |
|---|---|---|
| Drug (PPI vs. H2B) | 1.32 (1.11–1.57) | 0.002 |
| Anticoagulation | 1.47 (1.21–1.80) | 0.0001 |
aTwo-way comparison between H2B and PPI groups.
H2B, histamine type 2 receptor blocker; PPI, proton pump inhibitor; OR, odds ratio; CI, confidence interval.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This study was funded by the Natural Science Foundation of Shenzhen University General Hospital (grant no: SUGH2019QD010, Grant Recipient: Minqiang Huang. M.D.)
ORCID iD: Lei Kuang https://orcid.org/0000-0002-0671-9855
References
- 1.Granholm A, Zeng L, Dionne JC, et al. Predictors of gastrointestinal bleeding in adult ICU patients: a systematic review and meta-analysis. Intensive Care Med 2019; 45: 1347–1359. [DOI] [PubMed] [Google Scholar]
- 2.Cook DJ, Griffith LE, Walter SD, et al . The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care 2001; 5: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krag M, Perner A, Wetterslev J, et al. Stress ulcer prophylaxis in the intensive care unit: an international survey of 97 units in 11 countries. Acta Anaesthesiol Scand 2015; 59: 576–585. [DOI] [PubMed] [Google Scholar]
- 4.Barbateskovic M, Marker S, Granholm A, et al. Stress ulcer prophylaxis with proton pump inhibitors or histamin-2 receptor antagonists in adult intensive care patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2019; 45: 143–158. [DOI] [PubMed] [Google Scholar]
- 5.Ye Z, Blaser AR, Lytvyn L, et al. Gastrointestinal bleeding prophylaxis for critically ill patients: a clinical practice guideline. BMJ 2020; 368: l6722. [DOI] [PubMed] [Google Scholar]
- 6.Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330: 377–381. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017; 43: 304–377. [DOI] [PubMed] [Google Scholar]
- 8.Alhazzani W, Alshamsi F, Belley-Cote E, et al. Efficacy and safety of stress ulcer prophylaxis in critically ill patients: a network meta-analysis of randomized trials. Intensive Care Med 2018; 44: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLaren R, Reynolds PM, Allen RR. Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med 2014; 174: 564–574. [DOI] [PubMed] [Google Scholar]
- 10.Lilly CM, Aljawadi M, Badawi O, et al. Comparative Effectiveness of Proton Pump Inhibitors vs Histamine Type 2 Receptor Blockers for Preventing Clinically Important Gastrointestinal Bleeding During Intensive Care: A Population-Based Study. Chest 2018; 154: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alhazzani W, Alenezi F, Jaeschke RZ, et al. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med 2013; 41: 693–705. [DOI] [PubMed] [Google Scholar]
- 12.Lewis SC, Li L, Murphy MV, et al. Risk factors for ventilator-associated events: a case-control multivariable analysis. Crit Care Med 2014; 42: 1839–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvanderan SP, Summers MJ, Finnis ME, et al. Pantoprazole or Placebo for Stress Ulcer Prophylaxis (POP-UP): Randomized Double-Blind Exploratory Study. Crit Care Med 2016; 44: 1842–1850. [DOI] [PubMed] [Google Scholar]
- 14.Charlot M, Ahlehoff O, Norgaard ML, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med 2010; 153: 378–386. [DOI] [PubMed] [Google Scholar]
- 15.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016; 3: 160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009; 47: 626–633. [DOI] [PubMed] [Google Scholar]
- 19.Young PJ, Bagshaw SM, Forbes AB, et al. Effect of Stress Ulcer Prophylaxis With Proton Pump Inhibitors vs Histamine-2 Receptor Blockers on In-Hospital Mortality Among ICU Patients Receiving Invasive Mechanical Ventilation: The PEPTIC Randomized Clinical Trial. JAMA 2020; 323: 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammond DA, Kathe N, Shah A, et al. Cost-Effectiveness of Histamine 2 Receptor Antagonists Versus Proton Pump Inhibitors for Stress Ulcer Prophylaxis in Critically Ill Patients. Pharmacotherapy 2017; 37: 43–53. [DOI] [PubMed] [Google Scholar]
- 21.Kondo Y, Ohbe H, Matsui H, et al. Proton pump inhibitors versus histamine-2 receptor antagonists for stress ulcer prophylaxis during extracorporeal membrane oxygenation: a propensity score-matched analysis. BMJ Open 2020; 10: e037534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korkmaz U, Alcelik A, Eroglu M, et al. Pantoprazole-induced thrombocytopenia in a patient with upper gastrointestinal bleeding. Blood Coagul Fibrinolysis 2013; 24: 352–353. [DOI] [PubMed] [Google Scholar]
- 23.Alquraini M, Alshamsi F, Møller MH, et al. Sucralfate versus histamine 2 receptor antagonists for stress ulcer prophylaxis in adult critically ill patients: A meta-analysis and trial sequential analysis of randomized trials. J Crit Care 2017; 40: 21–30. [DOI] [PubMed] [Google Scholar]
- 24.Toews I, George AT, Peter JV, et al. Interventions for preventing upper gastrointestinal bleeding in people admitted to intensive care units. Cochrane Database Syst Rev 2018; 6: CD008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaezi MF, Yang YX, Howden CW. Complications of Proton Pump Inhibitor Therapy. Gastroenterology 2017; 153: 35–48. [DOI] [PubMed] [Google Scholar]
- 26.Barletta JF. Histamine-2-receptor antagonist administration and gastrointestinal bleeding when used for stress ulcer prophylaxis in patients with severe sepsis or septic shock. Ann Pharmacother 2014; 48: 1276–1281. [DOI] [PubMed] [Google Scholar]