Abstract
Background:
The application of continuous passive motion (CPM) after anterior cruciate ligament reconstruction (ACLR) was popularized in the 1990s, but advancements in the understanding of ACLR rehabilitation have made the application of CPM controversial. Many sports medicine fellowship–trained surgeons report using CPM machines postoperatively.
Purpose:
To determine the efficacy of CPM use for recovery after ACLR with respect to knee range of motion (ROM), knee swelling, postoperative pain, and postoperative complications.
Study Design:
Systematic review; Level of evidence, 3.
Methods:
The PubMed (MEDLINE), EMBASE, Cochrane, Cumulative Index of Nursing, and Allied Health Literature databases were searched from inception to January 1, 2020, for studies with evidence levels 1 to 3 on the use of CPM for ACLR rehabilitation. Included studies were those that comparatively evaluated postoperative outcomes after ACLR between at least 2 groups of patients, with 1 having received CPM rehabilitation and the other not having received CPM.
Results:
A total of 12 studies from 1989 to 2019 met the inclusion criteria. These studies included 808 patients who underwent ACLR. There was no evidence of CPM improving knee stability, final postoperative ROM, or subjective pain scores. Additionally, CPM did not lead to decreased muscle atrophy or improved International Knee Documentation Committee scores. Regarding pain medication intake during postoperative hospitalization, 2 studies found that the CPM group used less pain medication, 1 study found the CPM group used more pain medication, and 1 study found that there was no difference between the 2 groups. Complications varied widely, with 2 of 12 studies reporting complications that required a return to the operating room.
Conclusion:
A clinical benefit of postoperative CPM use after ACLR was not identified in this review. While our systematic review identified a number of studies that suggest CPM use may be associated with lower usage of pain medication in hospitalized patients, this cannot be confirmed without further investigation with standardized CPM protocols and larger sample sizes. Routine CPM use after ACLR was not supported by this systematic review.
Keywords: continuous passive motion, CPM, anterior cruciate ligament repair, ACL rehabilitation
Anterior cruciate ligament (ACL) injuries are among the most frequent injuries seen by sports medicine surgeons, with more than 120,000 occurring each year. 11 Advancements in the understanding of pre- and postoperative rehabilitation have changed the way many surgeons and physical therapists achieve the ultimate goal of returning their patients to sports. Specific inhibitors to return to activity include joint stiffness and loss of neuromuscular control, including muscle strength. Most rehabilitation programs have the same general focus: early range of motion (ROM), preservation of quadriceps function, and gradual progression of sports-related activity that respects the healing of involved tissues. 24
Historically, there has been widespread use of continuous passive motion (CPM) after knee surgery. 15 Rabbit studies utilizing CPM revealed more rapid and complete cartilage, tendon, and ligament healing. 20 These animal models have shown that CPM use prevented adhesions, significantly reduced the incidence of late posttraumatic arthritis, and significantly increased the strength of medial collateral ligament repair at 6 and 12 weeks. 20 This research led to the implementation of CPM use in rehabilitation after ACL reconstruction (ACLR) and total knee arthroplasty (TKA) in humans. 15,19 However, in recent years, the TKA literature has shown no long-term differences in postoperative ROM with the use of a CPM device, and some studies have even shown a detrimental effect of CPM in terms of pain and length of hospital stay. 9,10,21,25 CPM after TKA is no longer recommended. 8
Utilization of CPM after ACLR was popularized in the 1990s, when postoperative protocols often included hospitalization and intra-articular drain placement. Many historical rehabilitation programs after ACLR included an initial period of immobilization or restricted motion that led to a higher risk of arthrofibrosis. For this reason, the use of CPM became more popular to help mitigate this risk. 22 Advancements in the understanding of ACLR postoperative rehabilitation have changed many surgeons’ protocols. However, since the 1990s, little research has been foucsed upon CPM use after ACLR. A survey of graduates from 4 Accreditation Council for Graduate Medical Education sports medicine fellowship programs completed in 2016 found that 26% utilize CPM machines postoperatively after an ACLR. 12 The purpose of this systematic review is to determine if CPM use after ACLR affects knee ROM, knee swelling, postoperative pain, and postoperative complications. This systematic review also sought to determine the quality of the available literature. The null hypotheses were that CPM after ACLR would not improve knee ROM, have no effect on postoperative swelling, have no effect on postoperative pain, and lead to an increase in postoperative complications.
Methods
Search Strategy
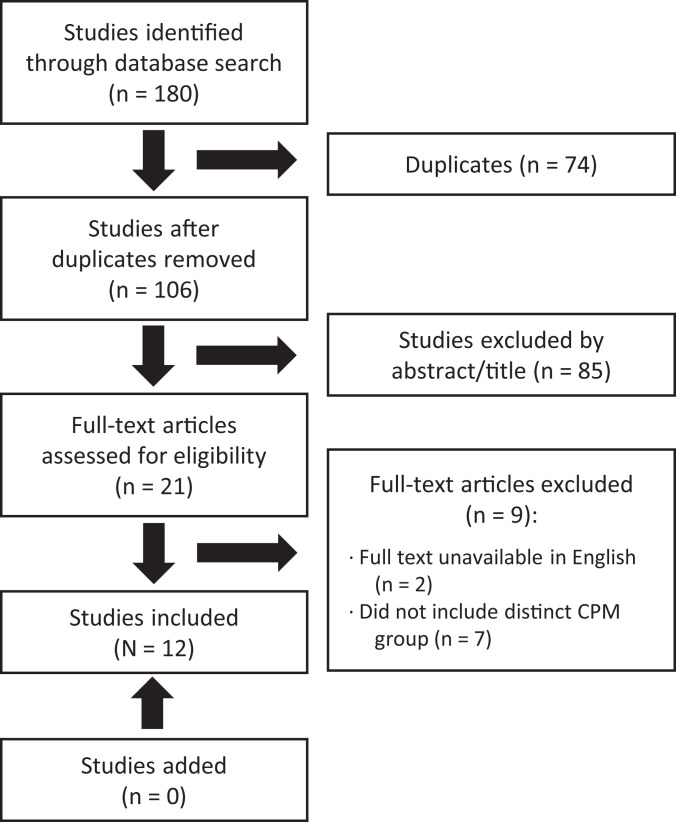
We performed a systematic review of studies with evidence levels 1 to 3 conducted on the use of CPM in the rehabilitation of ACLR. The investigation was completed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Figure 1). The literature search was completed using the PubMed (MEDLINE), EMBASE, Cochrane, and Cumulative Index of Nursing and Allied Health Literature databases from inception to January 1, 2020. The following search criteria was used: ((“ACL”) OR (“anterior cruciate ligament”)) AND ((“CPM” OR (“continuous passive motion”)). The search results were then compiled and duplicates removed.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) chart outlining the review of articles from the search. CPM, continuous passive motion.
Three independent reviewers (T.D., J.C., S.R.) performed a title and abstract review of the articles under the direct supervision of a sports medicine–trained clinical fellow (R.A.J.) to determine eligibility for inclusion in this investigation. Full-text articles were then obtained and reviewed in a similar fashion to determine final inclusion. An additional search through the references of all full-text articles was performed to ensure that any other relevant articles were not missed. Any discrepancies among the reviewers were resolved by the sports medicine fellow and the senior author (K.B.F.).
Inclusion/Exclusion Criteria
Articles that investigated the influence of CPM versus no CPM on ACLR and subsequent outcomes were of primary interest. Studies were required to nclude patients who underwent ACLR and directly compare their postoperative outcomes between at least 2 groups, with 1 having received CPM rehabilitation and the other having not received CPM rehabilitation. Literature review articles, case reports, technique articles, or articles that were not available in a peer-reviewed journal were excluded. The search resulted in 12 articles for inclusion. The search process is detailed in the PRISMA flowchart (Figure 1).
Data Extraction, Quality Assessment, and Analysis
From each study, the extracted details included the following, when available: journal, year, study design, number of patients, patient characteristics (age and sex), surgical details, graft use, CPM protocols used, ROM, patient-reported outcome measures, complications, and return to sport. The extracted data were used to investigate whether CPM provides any additional benefit in the postoperative rehabilitation after ACLR.
To assess the methodological quality of articles, the modified Coleman Methodology Score (CMS) was used to rate the articles. The CMS is composed of 2 sections and 10 criteria developed to yield a score from 1 to 100, with a higher score indicating better quality of evidence. Scores were calculated by 2 independent reviewers (T.D. and J.C.). Data were collected and analyzed using Microsoft Excel. A meta-analysis was not performed because of the heterogeneous nature of the collected data.
Results
Article Quality Assessment
The 12 included studies were published between 1989 and 2019; of these, 9 were published more than 20 years ago. Table 1 shows a summary of the modified CMS results for the 12 papers that were reviewed. The mean CMS for the studies was 64.4. The scores suggest that the methodological quality of the evidence base was poor. Engström et al, 4 Rosen et al, 18 Witherow et al, 23 and Yates et al 26 scored the highest of the group, with scores above 70, while Gáspár et al 7 and Ravan et al 16 showed the lowest scores of 54 and 36, respectively. Notable limitations included the follow-up period, with none of the papers exceeding 18 months of follow-up; study size (with only Witherow et al and Bram et al 2 having sample sizes over 100 patients); and description of the patient selection process, with a mean of 5.4 out of 10 for the papers. The low scores for patient selection process were largely due to not reporting recruitment rate. Only Anderson and Lipscomb 1 and Bram et al reported recruitment rates more than 90% and scored a 10 out of 10 in the description of patient selection process category. Another notable weakness was the diagnostic certainty of the ACL injury, with a mean score of 1.3 out of 5 points. Only Friemert et al, 6 Bram et al, and Rosen et al mention the use of history, physical examination, or manual chart review to confirm diagnosis.
Table 1.
Modified Coleman Methodology Score for the Included Studies
| Study (Year) | Study Size (10) | Mean Follow-up (10) | Surgical Approach (10) | Study Type (15) | Diagnostic Certainty (5) | Description of Surgical Procedure Given (10) | Description of Postoperative Rehabilitation (5) | Outcome Criteria (10) | Procedures for Assessing Clinical Outcomes (15) | Description of Patient Selection Process (10) | Total Score (100) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderson (1989) 1 | 4 | 4 | 10 | 15 | 0 | 5 | 5 | 7 | 9 | 10 | 69 |
| Bram (2019) 2 | 10 | 0 | 7 | 0 | 5 | 0 | 5 | 9 | 15 | 10 | 61 |
| Engström (1995) 4 | 4 | 0 | 10 | 15 | 0 | 10 | 5 | 10 | 15 | 5 | 74 |
| Friemert (2006) 6 | 7 | 0 | 0 | 15 | 5 | 5 | 5 | 7 | 15 | 5 | 64 |
| McCarthy (1993) 13 | 0 | 4 | 5 | 15 | 0 | 10 | 5 | 7 | 12 | 5 | 63 |
| McCarthy (1993) 14 | 4 | 0 | 10 | 15 | 0 | 5 | 5 | 7 | 15 | 5 | 66 |
| Rosen (1992) 18 | 7 | 0 | 10 | 15 | 5 | 5 | 5 | 10 | 15 | 5 | 77 |
| Witherow (1993) 23 | 10 | 0 | 10 | 10 | 0 | 10 | 5 | 5 | 15 | 5 | 70 |
| Gáspár (1997) 7 | 4 | 0 | 10 | 10 | 0 | 5 | 5 | 5 | 10 | 5 | 54 |
| Yates (1992) 26 | 4 | 0 | 10 | 15 | 0 | 10 | 5 | 10 | 12 | 5 | 71 |
| Rigon (1993) 17 | 4 | 0 | 10 | 15 | 0 | 10 | 5 | 7 | 12 | 5 | 68 |
| Ravan (2019) 16 | 7 | 0 | 0 | 15 | 0 | 0 | 5 | 2 | 7 | 0 | 36 |
| Mean score | 64.42 |
Population and Follow-up
The 12 reviewed studies included 808 patients who underwent ACLR. All but 1 of the studies reported patient sex, which totaled 38% women. The mean age of the patients was 24.21 years, with age not reported by 2 studies. 16,17 Of the included patients, 568 underwent reconstruction using bone–patellar tendon–bone autograft, 4,6,7,13,14,16 –18,23,26 77 had reconstruction with semitendinosus and gracilis tendon, 1,6 and the type of reconstruction was not listed for 163 patients. 2 Seven of the 12 papers included concomitant meniscectomies, 1,2,4,7,18,23,26 3 included patients with MCL injuries, 4,14,23 and 2 papers noted concomitant chondral injuries. 23,26 Five of the 12 papers excluded other injuries. 6,13,14,16,17 The time from injury to surgery was variable and was not reported in 5 studies. 2,13,14,16,17 Table 2 contains information on time to surgery for the 7 papers that included this information.
Table 2.
Chronicity of ACL Tears a
| Study | Acute | Chronic | Other |
|---|---|---|---|
| Anderson (1989) 1 | Non-CPM: 13; CPM: 7 | Non-CPM: 3; CPM: 6 | Non-CPM: 4 subacute; CPM: 6 subacute |
| Engström (1995) 4 | Defined: <3 mo CPM group: 3; AM group: 9 | Defined: >3 mo 22 patients | |
| Friemert (2006) 6 | CPM group: 6.9 ± 9.1 mo Non-CPM: 7.9 ± 9.4 mo |
||
| Rosen (1992) 18 | Defined:<3 wk 23 patients | Defined: >3 wk 52 patients | |
| Witherow (1993) 23 | Defined:<3 wk CPM group: 20; Non-CPM: 23 | Defined: >3 wk CPM: 83; Non-CPM:85 | Groups in study were “remarkably similar” in terms of acute vs chronic injury |
| Gáspár (1997) 7 | Defined: NA 41 patients | ||
| Yates (1992) 26 | Defined: <6 wk 10 patients | Defined: >6 wk 20 patients | No significant difference between acute and chronic injuries in CPM and non-CPM groups |
a AM, active motion; CPM, continuous passive motion; NA, not applicable.
CPM Protocol
The CPM protocol used by each of the authors varied among studies. The duration of use varied from 1 day to 4 weeks postoperatively, Witherow et al 23 reported CPM use for the first 24 hours postoperatively, while Rosen et al 18 had their patients use the CPM machine up to 4 weeks postoperatively. An exact breakdown of each CPM protocol can be seen in Table 3. Ravan et al 16 did not report the details of their tailor-made rehabilitation program, which included CPM use.
Table 3.
Postoperative Rehabilitation Protocols a
| Study | LOE | Intervention |
|---|---|---|
| Anderson (1989) 1 | 1 | · Non-CPM (n = 20): placed in a hinged knee brace at 60° of flexion. At 2 wk postop, limited passive ROM from 35° to 70° began, gradually increasing to unlimited ROM at 3 mo. Full WB began at 3 mo and immobilization was discontinued at that time. · CPM (n = 19): treated similar to those in non-CPM group except CPM began in the recovery room and continued through hospital stay. Two cycles/min, with range of 35°-70°. |
| Bram (2019) 2 | 3 | · Non-CPM (n = 66): mean of 28 PT sessions. Exact protocol not listed. · CPM (n = 97): prescribed 2 h 3 times a day for 3 wk, starting at a flexion endpoint of 30° and increased 10° per day as tolerated. Mean of 31 PT sessions. |
| Engström (1995) 4 | 1 | All patients were fitted with a locked brace with 10° of flexion and crutches for PWB. · Non-CPM (n = 17): early active ROM training 3 times/d for 6 d. Emphasis was put on dynamic active knee flexion, and passive full extension was allowed. · CPM (n = 17): same as non-CPM in addition to 6 h of passive training using a CPM device for the first 6 d. |
| Friemert (2006) 6 | 1 | On postop day 1, CPM (n = 30) and CAM (n = 30) were instructed to exercise 3 h/d for 1 wk using CPM and CAM devices, respectively. They were allowed to bend knee joints as far as possible, as pain allowed. Lymphatic drainage was performed on a daily basis and isometric strengthening exercises using both legs. All patients were allowed to perform PWB exercises during the first 2 wk postop. |
| McCarthy (1993) 13 | 1 | · Non-CPM (n = 10): fitted with a hinge-type brace with motion between 10° and 90°. Began active exercises with 2 PT sessions/d for 3 d postop, then progressed to an outpatient rehabilitation program. Mobilized PWB permitted. · CPM (n = 10): CPM started immediately for 16 h/d, starting at 0°-60°. On postop day 4, reduced to 6 h/d for 11 d until postop day 14, increasing range to 0-90°. Brace removed in both groups for CPM use or PT. |
| McCarthy (1993) 14 | 1 | · Non-CPM (n = 15): fitted with a hinge-type brace with motion between 10° and 90°. Active exercises started on postop day 1. PWB was permitted using crutches from postop day 1. · CPM (n = 15): identical to non-CPM group but CPM applied immediately postop. For postop days 1-3, a minimum of 16 h of CPM a day at a rate of 15 cycles/h with ROM ranging from 0° extension to 60° of flexion. Patients gradually increased ROM to 90°. |
| Rosen (1992) 18 | 1 | On postop day 1, all patients were placed in a hinged brace locked at 10° of flexion, which was removed for PT. Toe-touch WB was allowed, progressing to full WB at 4 wk. · Active-motion non-CPM (n = 25): brace was worn when sleeping and walking for 4 wk, only removed for active ROM exercise as directed by a physical therapist. PT was continued for 3 times/wk after discharge, with the same emphasis as CPM groups. · CPM (n = 25): same as active motion non-CPM group but with CPM immediately postop. CPM involved initial cycling between 0° and 30° for 20 h/d and increased as tolerated to a range of 0° to 90° (frequency of 0.5 cycles/min) for 1 wk. After discharge, they were instructed to use the CPM for 6 h/d for 4 wk at a range of 0°-90° with the same rate of cycling, 0.5 cycles/min. · CPM with delayed PT (n = 25): same as CPM group except they did not start outpatient PT until 1 mo postop. |
| Witherow (1993) 23 | 2 | · Non-CPM (n = 108): on postop day 1, inner-range quadriceps and hamstring exercises were started as well as active flexion, aiming for 90°. Patients were mobilized 24 h postop, WBAT, and using crutches if needed. Drains removed at 24 h. · CPM (n = 108): same as non-CPM but with 24-h CPM started in the recovery room with ROM from 0° to 60°. |
| Gáspár (1997) 7 | 2 | · Non-CPM (n = 13): underwent a rehabilitation program. · CPM (n = 28): treated similarly in addition to CPM. CPM 6 h/d was used for 5 d from full extension to the painful flexion position of the knee (mean, 70°-80°). |
| Yates (1992) 26 | 1 | · Non-CPM (n = 15): placed in a hinged knee brace (10°-90° of flexion) worn for 2 wk postop for protection during ambulation. WBAT and PT. · CPM (n = 15): same treatment as non-CPM, but CPM at 0°-60° used immediately postop. CPM was used for 16 h/d during postop days 1-3, and patients were encouraged to increase their flexion to 90° as tolerated. After postop day 3, CPM was reduced to 6 h/d and continued until postop day 14. |
| Rigon (1993) 17 | 1 | Postop immobilization in a knee brace at 0° for 24 h, then: · Non-CPM (n = 20): active mobilization started postop day 1, with active and assisted knee flexion and extension. · CPM (n = 20): from postop day 1, CPM performed for 1 h 3 times/d at a range of 0°-60°, increasing by 10° each day (maximum speed of 10 cycles/min). Used for 3 wk. All patients begin WB after 1 wk with limb protected by knee brace in extension. Crutches were abandoned after 2 wk in both groups, and from postop wk 3 onward the rehabilitation protocols were identical in both groups. |
| Ravan (2019) 16 | 3 | · Non-CPM (n = 30): Patients were given only conventional PT protocol. PT protocol was divided into 3 phases, and each phase included various exercises given to patients. · CPM (n = 30): received a tailor-made PT program that included CPM. |
a CAM, continuous active motion; CPM, continuous passive motion; LOE, level of evidence; postop, postoperatively; PT, physical therapy; PWB, partial weightbearing; ROM, range of motion; WB, weightbearing; WBAT, weightbearing as tolerated.
Postoperative ROM
Postoperative ROM was reported in 9 studies. 1,2,4,6,7,17,18,23,26 Seven of these 9 studies found no difference between patients treated with CPM and those without. 1,2,4,6,17,18,23 None of the 9 studies showed any long-term ROM difference between the 2 groups. Gáspár et al 7 found a significant difference in flexion-extension of the CPM group compared with the non-CPM group on the day of discharge, but it was not a statistically significant difference at 6 months of follow-up. Yates et al 26 found increased active and passive flexion on postoperative days 3, 7, and 21 before physical therapy (PT), but this difference was no longer seen at the completion of the PT session. It should be noted that in the Rosen et al 18 study, the CPM group appeared to have increased flexion compared with the non-CPM group at 1 month, 2 months, and 6 months, but this difference was negated when data from a control knee were included in the analysis. The time point for ROM measurement between the CPM group and the non-CPM group ranged from 1 week 2 to 18 months. 1
Postoperative Pain
Eight studies measured postoperative pain. All 6 studies that included a subjective pain measurement found no difference in pain between the CPM and non-CPM groups. 2,6,14,16,17,26 Two studies that looked at pain medication intake during postoperative hospitalization found that the CPM group used less pain medication, 1 study found that the CPM group used more pain medication, and 1 study found that there was no difference in total intake of pain medication between the 2 groups. 7,14,23,26 Bram et al 2 measured postoperative pain on a 0 to 10 scale and found no significant difference between pain in the CPM versus non-CPM groups at 1 week, 1 month, 3 months, or 6 months. Using a visual analog score (VAS), Friemert et al 6 found no difference in postoperative pain between their CPM and continuous active motion groups through the first postoperative week. Ravan et al 16 used the Numeric Pain Rating Scale, a segment version of the VAS, to measure pain and found that the experimental and control groups both had a decrease in pain from the preoperative to postoperative time, but it is unknown if one decreased more than the other. Rigon et al 17 used a scale of 1 through 4 to measure pain on the fifth postoperative day. There was no difference in pain in patients receiving CPM and those not receiving CPM.
Witherow et al 23 recorded the postoperative injectable and oral analgesia given to patients. The study showed that the non-CPM group had a significantly lower mean injectable and oral analgesia requirement when compared with the group using CPM. Gáspár et al 7 measured total intake of pain medication during hospitalization and found no significant difference between the CPM and non-CPM groups.
McCarthy et al 14 used narcotic delivery from a patient-controlled analgesia (PCA) pump to measure postoperative pain in the CPM compared with non-CPM groups on postoperative day 1. They found that patients in the CPM group used a significantly smaller amount of narcotic pain medication and pushed the PCA pump button significantly fewer times compared with the non-CPM group through the first 24 hours after surgery. Yates et al 26 found that the CPM group used significantly less morphine in the first 24 hours postoperatively compared with the non-CPM group (46.2 mg vs 69.6 mg morphine). McCarthy et al 14 continued to measure pain on postoperative days 2 and 3 using oral analgesia intake. Similar to postoperative day 1, patients in the CPM group used significantly less oral analgesia through the third postoperative day compared with the non-CPM group. This was similar to Yates et al, who also found that the CPM group required less oral analgesia on postoperative days 2 and 3 compared with the non-CPM group (46 mg vs 70.0 mg of hydrocodone). Additionally, McCarthy et al 14 used a graphic pain scale to measure the patients’ perceived pain on postoperative days 1 and 2 and before discharge on day 3. No significant difference was found between the CPM and non-CPM groups in terms of perceived pain. As with McCarthy et al, 14 there was not a significant difference in patient-perceived pain between the 2 groups in the Yates et al study.
Postoperative Swelling
Four papers measured postoperative swelling in patients using CPM compared with those who did not use CPM. One group found that the non-CPM group had significantly more swelling in the first 3 postoperative days, 26 1 group found no long-term difference in swelling between the 2 groups but a faster rate of decrease in swelling in the CPM group, 17 1 group did not state whether there was a significant difference in swelling between the CPM and non-CPM groups, 6 and 1 group had a significant difference in swelling preoperatively between the CPM and non-CPM groups so they could not appropriately compare them postoperatively. 4 Engström et al 4 measured knee circumference at the midpatellar position and at the base of the patella to determine degree of joint swelling. It was determined that the non-CPM group had more pronounced swelling preoperatively and at 6 weeks of follow-up. Actual numbers for what counted as more pronounced was not reported. 4 Friemert et al 6 performed an ultrasound on the day of discharge to evaluate for the presence or absence of a joint effusion. Moreover, 24 of 30 patients in the CPM group and 20 of 30 patients in the non-CPM group had an effusion present, and whether this was a significant difference or not was not stated. 6 Yates et al 26 measured swelling by calculating the cross-sectional area of the knee at 4 different places and found that the non-CPM group had significantly more swelling at each time point (postoperative days 1-3). Rigon et al 17 found that at 45 days postoperatively, swelling at the middle third of the patella and tibial tuberosity had stabilized to the same extent in both the non-CPM and the CPM groups. However, the decrease in size occurred at a faster rate in the CPM group.
Muscle Atrophy
Two papers evaluated postoperative muscle atrophy using volumetric thigh measurements. 1,4 Neither study found any differences between CPM and no CPM use. Anderson and Lipscomb 1 calculated volumetric thigh measurements and found no difference between the non-CPM group and the CPM group. These measurements were taken at 6 weeks, 3 months, 7 months, 12 months, and 18 months. Engström et al 4 measured thigh circumference at set distances (7.5 and 15 cm) above the patella to determine the degree of muscle atrophy. No significant difference was found between the CPM and non-CPM groups at 6 weeks of follow-up.
Duration of Stay
Three studies commented on the length of hospitalization between patients receiving CPM and those not receiving CPM. Witherow et al 23 found that patients using CPM had a significantly shorter length of hospital stay (2.42 days) compared with the non-CPM group (2.94 days). Two groups found no significant difference in length of hospital stay. 7,18 Five other studies included information on hospitalization after the ACLR, with patients being discharged on postoperative day 7 in 1 study, postoperative day 5 in 1 study, and postoperative day 3 in 3 studies. 1,6,13,14,26 Another study had patients use the CPM machine in the hospital during the first 6 postoperative days. 4
Functional Outcome
Two papers used the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form to assess postoperative outcomes. Both of these groups reported no significant difference in IKDC scores between patients who used CPM postoperatively and those who did not at 6 months after surgery. 7,18
Blood Loss
Five studies commented on postoperative drains or bleeding. 7,17,18,23,26 Witherow et al 23 found a significantly increased amount of postoperative drainage in their group receiving CPM (233.7 mL) compared with the non-CPM group (187.1 mL). Two groups found there to be no significant difference in postoperative bleeding. Gáspár et al 7 found that the CPM group required 5 aspirations of the hemarthrosis, while the non-CPM group required 2 aspirations postoperatively. It was not reported if this was statistically significant.
Joint Laxity
Three studies investigated the effects CPM had on joint laxity, with all 3 finding no difference in joint laxity between patients who received CPM and those who did not. 1,13,18 Anderson and Lipscomb 1 performed stress radiographs with the knee in 15° of flexion and 15 pounds of force applied both medially and laterally. They found no significant difference in stability of the knee in the group receiving CPM compared with the non-CPM group. McCarthy et al 13 measured anterior tibial translation 1 year postoperatively using a KT-1000 instrumented arthrometer for a 30-pound Lachman test. Additionally, they performed a manual Lachman test and lateral pivot-shift test to evaluate for subjective laxity and graded each on a 0 to 4 scale. As with Anderson and Lipscomb, 1 they found that CPM use did not lead to any increased laxity on any of their measurements compared with patients who did not use CPM. 13 Rosen et al 18 also used the KT-1000 to measure anterior laxity at 2 and 6 months postoperatively and found that CPM use did not have a deleterious effect on joint laxity measurements.
Complications
Six papers discussed postoperative complications, with Bram et al 2 finding more complications in the non-CPM group and Witherow et al 23 finding more complications in the CPM group. The other 4 studies did not have a significant difference in complications. 1,7,18,26 Anderson and Lipscomb 1 reported 1 case of hematoma and staple removal in the non-CPM group. Bram et al 2 found a statistically significant need for manipulation under anesthesia (MUA) in the non-CPM group (4 patients) compared with the group that received CPM (0 patients). Rosen et al 18 reported that 4 patients needed to return to the operating room for MUA. These patients were split relatively evenly across their 3 groups. One patient each in the non-CPM group and the CPM plus delayed PT group had a lack of extension. A patient in the non-CPM group developed lack of flexion, while a patient in the CPM and early PT groups developed a cyclops lesion. After their study period was over, it was reported that 3 additional patients required surgery, 2 for cyclops lesions (1 each from the non-CPM and CPM plus early PT groups) and 1 for loss of motion (CPM and early PT group). 18 Witherow et al reported 3 of 108 reruptures within 6 months in the group receiving CPM and none in the non-CPM cohort. Gáspár et al 7 indicated wound-healing complications but did not provide any details. Yates et al 26 reported 1 temporary sensory palsy, thought to be secondary to tourniquet placement, that completely resolved by 3 months postoperatively. Whether this complication occurred in the CPM or the non-CPM group was not specified.
None of the 12 papers commented on preoperative athletic involvement or postoperative return to activity and sports. Additionally, none of the papers described any type of preoperative rehabilitation before the index surgery.
Discussion
We hypothesized that the literature surrounding CPM use after ACLR would initially improve knee ROM, have no effect on postoperative swelling, have no effect on postoperative pain, and lead to an increase in postoperative complications. While the literature was not definitive, the data suggest no improvement in knee ROM, postoperative swelling, or subjective postoperative pain in patients receiving CPM after ACLR compared with those who did not receive CPM treatment. In 2 studies, postoperative pain was initially improved as measured by use of intravenous pain medication, but this information is outdated, as patients are no longer admitted to the hospital and placed on postoperative PCA after ACLR. 14,26 Additionally, 1 study 23 found the opposite, with CPM use associated with a greater intake of pain medication, and a fourth study 7 found no difference in analgesic intake. Specific postoperative complications differed between patients who received CPM and those who did not. Patients utilizing CPM were at greater risk for ACL rerupture, and patients who did not use CPM were at risk of return to the operating room for MUA, likely secondary to postoperative immobilization and the prevention of terminal extension, which was common practice in the 1990s. The methodological assessment showed that the quality of the available studies was poor.
Only 1 study found a significant improvement in ROM in patients receiving CPM compared with those who did not. 7 While 1 study found significantly increased active and passive flexion in the first 21 days after surgery before PT, the increased ROM was negated when flexion and extension were measured at the end of the therapy session. As both groups underwent therapy, CPM use did not lead to an overall improvement in early postoperative ROM. 26 All but 1 study found no improvement in early postoperative ROM in patients utilizing CPM, and no studies showed long-term improvement in ROM in patients who received CPM compared with those who did not.
CPM use may decrease the total amount of pain medication used by a hospitalized patient in the immediate postoperative period, as found in 2 studies, but a third study found the opposite, and a fourth found no difference. However, all 6 studies that measure pain subjectively supported the fact that CPM has no effect on the perceived patient pain. Given the concern with opioid use, especially in young patients such as those frequently seen with ACL injuries, this is an important consideration. Two studies showed that patients who received CPM used significantly less intravenous pain medication in the immediate 24 hours postoperatively and that the patients receiving CPM required less oral analgesia through postoperative day 3. 14,26 Both of these studies showed no difference in perceived patient pain between the 2 groups, which fits with 3 of the 4 other studies that measured pain. Studies that used a numeric score (eg, 1-10) or a graphic pain scale (eg, VAS) did not find a difference in pain between the CPM and non-CPM groups. The effect of CPM on modern pain control practices, including blocks and multimodal pain therapy, is not known, and the use of postoperative intravenous pain medication has largely been abandoned.
Based on the available literature, the effect of CPM use on postoperative swelling has had mixed results. A possible reason for this may be the variation in when the swelling was measured. Immediately postoperatively (postoperative days 1-3), 1 group found more swelling in the non-CPM group, while another group reported more swelling in the CPM group on the day of discharge (the mean length of hospital stay was not reported). 6,26 The third group that reported this outcome found that at 45 days postoperatively, the swelling was the same but the decrease had occurred faster in the CPM group. 17 While Engström et al 4 reported on swelling over a month after surgery, the non-CPM group had significantly increased swelling preoperatively compared with the CPM group, so it is unclear what the actual effect of CPM was on swelling. Overall, there are no data to support a clinically significant decrease in postoperative swelling with CPM use.
While the reported complications vary widely between the studies, 2 deserve additional attention because they required the patient to return to the operating room. One study reported that 3 of their patients in the CPM group reruptured their ACL within 6 months of the index surgery, while this complication was not seen in any patients who did not receive CPM. 23 On the other hand, a different study found that 4 patients not receiving CPM required surgery for MUA, while no patients in the CPM group required a return trip to the operating room. 2 The increased risk of rerupture when using CPM must be weighed against a possible decrease in need for MUA when considering whether to prescribe CPM. No other studies found a significant difference in postoperative complications between groups.
It is important to note that most of the literature examined was published in the 1990s, with only 3 of the 12 studies published since 2000. 2,6,16 The technique for ACLR has changed greatly since then. An operation that postoperatively required at least 1 night’s stay in the hospital with restriction of terminal extension is now commonly performed in outpatient surgical centers with immediate postoperative motion encouraged. Additionally, most patients now participate in some form of preoperative rehabilitation program before surgery, as this has been shown to improve postoperative outcomes. 3,5 Preoperative rehabilitation was not mentioned in any of the included studies. The methodological review of the papers using the validated CMS showed that the quality of evidence was low.
The question remains why 26% of sports medicine surgeons still use CPM after all ACLRs and an additional 8% use CPM after ACLR with meniscal or cartilage repair. Part of this could be explained by the sample pool, as the surveys were only sent to graduates of 4 programs. It may be a result of a surgeon’s training continuing to have a significant influence on practice years later, despite the existence of data that may contradict routine practice.
In studies included in this systematic review, the meantime from injury to surgery varied, with one group reporting two-thirds of their patients having surgery 6 or more weeks after injury and another group reporting the average time from injury to surgery to be greater than 6 months. 6,26 Additionally, as return to athletic activity may be one of the most important postoperative outcomes in this patient population, there was no comment on this in any of the 12 papers. Rosen et al 18 did report that the cost for PT over the course of a month was $840 ($70 per session × 4 weeks), while the cost of a CPM machine was $1800, there was not a formal cost analysis of CPM versus PT found during this review.
This study has several strengths. It examined only comparative literature on the use of CPM after ACLR. This allows a comprehensive, up-to-date review of the published literature on this topic. This study also has several limitations. The quality of a systematic review is based off of the quality of the studies included. Many of these studies examining CPM use outdated surgical and rehabilitation techniques and are of low quality overall. Most included studies contained small sample sizes in which patients were not randomized for risk factors such as sex and age. Additionally, the heterogeneity of CPM protocols made it difficult to compare all of the papers (see Table 3), and because of this, we were unable to perform a meta-analysis.
Conclusion
The results of this systematic review do not identify a clinical benefit of postoperative CPM use after ACLR. While our systematic review identified a number of studies that suggest CPM use may be associated with lower usage of pain medication in hospitalized patients, this cannot be confirmed without further investigation with standardized CPM protocols and larger sample sizes. Routine CPM use after ACLR is not supported by this systematic review.
Footnotes
Final revision submitted December 29, 2020; accepted February 15, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: T.D. has received education payments from Liberty Surgical and Medical Device Business Services. R.A.J. has received education payments from Liberty Surgical and hospitality payments from Pacira Pharmaceuticals. F.P.T. has received nonconsulting fees from Medtronic and Smith & Nephew and hospitality payments from Stryker, DePuy, Ferring, FX Shoulder USA, Wright Medical Technology, Horizon Therapeutics, Flexion Therapeutics, Arthrex, Lilly USA, and OrthogenRx and has stock/stock options in Franklin/Keystone Biosciences and Trice Medical. M.G.C. has received research support from DJO, education payments from Liberty Surgical, and hospitality payments from Zimmer Biomet, Stryker, DePuy, Arthrex, Tornier, and Cayenne Medical. K.B.F. has received consulting fees from DePuy, Vericel, and Medical Device Business Services; education payments from Liberty Surgical; honoraria and nonconsulting fees from Vericel; and hospitality payments from Smith & Nephew, Ferring Pharmaceuticals, Arthrex, Cumberland Pharmaceuticals, and Flexion Therapeutics. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Anderson AF, Lipscomb AB. Analysis of rehabilitation techniques after anterior cruciate reconstruction. Am J Sports Med. 1989;17(2):154–160. [DOI] [PubMed] [Google Scholar]
- 2. Bram JT, Gambone AJ, DeFrancesco CJ, Striano BM, Ganley TJ. Use of continuous passive motion reduces rates of arthrofibrosis after anterior cruciate ligament reconstruction in a pediatric population. Orthopedics. 2019;42(1):e81–e85. [DOI] [PubMed] [Google Scholar]
- 3. Eitzen I, Holm I, Risberg MA. Preoperative quadriceps strength is a significant predictor of knee function two years after anterior cruciate ligament reconstruction. Br J Sports Med. 2009;43(5):371–376. [DOI] [PubMed] [Google Scholar]
- 4. Engström B, Sperber A, Wredmark T. Continuous passive motion in rehabilitation after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1995;3(1):18–20. [DOI] [PubMed] [Google Scholar]
- 5. Failla MJ, Logerstedt DS, Grindem H, et al. Does extended preoperative rehabilitation influence outcomes 2 years after ACL reconstruction? A comparative effectiveness study between the MOON and Delaware-Oslo ACL cohorts. Am J Sports Med. 2016;44(10):2608–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friemert B, Bach C, Schwarz W, Gerngross H, Schmidt R. Benefits of active motion for joint position sense. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):564–570. [DOI] [PubMed] [Google Scholar]
- 7. Gáspár L, Farkas C, Szepesi K, Csernatony Z. Therapeutic value of continuous passive motion after anterior cruciate replacement. Acta Chir Hung. 1997;36(1-4):104–105. [PubMed] [Google Scholar]
- 8. Harvey LA, Brosseau L, Herbert RD. Continuous passive motion following total knee arthroplasty in people with arthritis. Cochrane Database Syst Rev. 2014(2):CD004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herbold JA, Bonistall K, Blackburn M, et al. Randomized controlled trial of the effectiveness of continuous passive motion after total knee replacement. Arch Phys Med Rehabil. 2014;95(7):1240–1245. [DOI] [PubMed] [Google Scholar]
- 10. Joshi RN, White PB, Murray-Weir M, Alexiades MM, Sculco TP, Ranawat AS. Prospective randomized trial of the efficacy of continuous passive motion post total knee arthroplasty: experience of the hospital for special surgery. J Arthroplasty. 2015;30(12):2364–2369. [DOI] [PubMed] [Google Scholar]
- 11. Kaeding CC, Leger-St-Jean B, Magnussen RA. Epidemiology and diagnosis of anterior cruciate ligament injuries. Clin Sports Med. 2017;36(1):1–8. [DOI] [PubMed] [Google Scholar]
- 12. Marshall NE, Keller RA, Dines J, Bush-Joseph C, Limpisvasti O. Current practice: postoperative and return to play trends after ACL reconstruction by fellowship-trained sports surgeons. Musculoskelet Surg. 2019;103(1):55–61. [DOI] [PubMed] [Google Scholar]
- 13. McCarthy MR, Buxton BP, Yates CK. Effects of continuous passive motion on anterior laxity following ACL reconstruction with autogenous patellar tendon grafts. J Sport Rehabil. 1993;2(3):171. [Google Scholar]
- 14. McCarthy MR, Yates CK, Anderson MA, Yates-McCarthy JL. The effects of immediate continuous passive motion on pain during the inflammatory phase of soft tissue healing following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1993;17(2):96–101. [DOI] [PubMed] [Google Scholar]
- 15. Noyes FR, Mangine RE, Barber S. Early knee motion after open and arthroscopic anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15(2):149–160. [DOI] [PubMed] [Google Scholar]
- 16. Ravan AD, Varadharajulu G, Shinde S. Effect of tailor made exercise programe in ACL reconstuction of knee joint. Indian J Public Health Res Develop. 2019;10(8):6–11. [Google Scholar]
- 17. Rigon A, Viola R, Lonedo F. Continuous passive motion in reconstruction of the anterior cruciate ligament. J Sports Traumatol Relat Res. 1993;15:187–192. [Google Scholar]
- 18. Rosen MA, Jackson DW, Atwell EA. The efficacy of continuous passive motion in the rehabilitation of anterior cruciate ligament reconstructions. Am J Sports Med. 1992;20(2):122–127. [DOI] [PubMed] [Google Scholar]
- 19. Salter RB. The biologic concept of continuous passive motion of synovial joints. The first 18 years of basic research and its clinical application. Clin Orthop Relat Res. 1989;242:12–25. [PubMed] [Google Scholar]
- 20. Salter RB, Hamilton HW, Wedge JH, et al. Clinical application of basic research on continuous passive motion for disorders and injuries of synovial joints: a preliminary report of a feasibility study. J Orthop Res. 1984;1(3):325–342. [DOI] [PubMed] [Google Scholar]
- 21. Schulz M, Krohne B, Roder W, Sander K. Randomized, prospective, monocentric study to compare the outcome of continuous passive motion and controlled active motion after total knee arthroplasty. Technol Health Care. 2018;26(3):499–506. [DOI] [PubMed] [Google Scholar]
- 22. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292–299. [DOI] [PubMed] [Google Scholar]
- 23. Witherow GE, Bollen SR, Pinczewski LA. The use of continuous passive motion after arthroscopically assisted anterior cruciate ligament reconstruction: help or hindrance? Knee Surg Sports Traumatol Arthrosc. 1993;1(2):68–70. [DOI] [PubMed] [Google Scholar]
- 24. Yabroudi MA, Irrgang JJ. Rehabilitation and return to play after anatomic anterior cruciate ligament reconstruction. Clin Sports Med. 2013;32(1):165–175. [DOI] [PubMed] [Google Scholar]
- 25. Yang X, Li GH, Wang HJ, Wang CY. Continuous passive motion after total knee arthroplasty: a systematic review and meta-analysis of associated effects on clinical outcomes. Arch Phys Med Rehabil. 2019;100(9):1763–1778. [DOI] [PubMed] [Google Scholar]
- 26. Yates CK, McCarthy MR, Hirsch HS, Pascale MS. Effects of continuous passive motion following ACL reconstruction with autogenous patellar tendon grafts. J Sport Rehabil. 1992;1(2):121. [Google Scholar]