Abstract
Background:
Hip arthroscopy is a rapidly growing surgical approach to treat femoroacetabular impingement (FAI) syndrome with a significant learning curve pertaining to complication risk, reoperation rate, and total hip arthroplasty conversion. Hip arthroscopy is more frequently being taught in residency and fellowship training. The key, or critical, parts of the technique have not yet been defined.
Purpose:
To identify the key components required to perform arthroscopic treatment of FAI syndrome.
Study Design:
Consensus statement.
Methods:
A 3-question survey comprising questions on hip arthroscopy for FAI was sent to a convenience sample of 101 high-volume arthroscopic hip surgeons in the United States. Surgeon career length (years) and maintenance volume (cases per year) were queried. Hip arthroscopy was divided into 10 steps using a Delphi technique to achieve a convergence of expert opinion. A step was considered “key” if it could (1) avoid complications, (2) reduce risk of revision arthroscopy, (3) reduce risk of total hip arthroplasty conversion, or (4) optimize patient-reported outcomes. Based on previous literature, steps with >90% of participants were defined as key. Descriptive and correlation statistics were calculated.
Results:
A total of 64 surgeons (63% response rate) reported 5.6 ± 2.1 steps as key (median, 6; range, 1-9). Most surgeons (56.3%) had been performing hip arthroscopy for >5 years. Most surgeons (71.9%) had performed >100 hip arthroscopy procedures per year. Labral treatment (97% agreement) and cam correction (91% agreement) were the 2 key steps of hip arthroscopy for FAI. Pincer/subspine correction (86% agreement), dynamic examination before capsular closure (63% agreement), and capsular management/closure (63% agreement) were selected by a majority of respondents but did not meet the study definition of key. There was no significant correlation between surgeon experience and designation of certain steps as key.
Conclusion:
Based on a Delphi technique and expert opinion survey of high-volume surgeons, labral treatment and cam correction are the 2 key parts of hip arthroscopy for FAI syndrome.
Keywords: hip arthroscopy, FAI, surgical education, learning curve, critical steps
Hip arthroscopy is an increasingly common, minimally invasive procedure for the treatment of femoroacetabular impingement (FAI) syndrome. 19 Between 2006 and 2013, the number of hip arthroscopies performed increased 25-fold. 10 The learning curve of arthroscopic hip preservation surgery is significant. Career hip arthroscopy volume greatly affects patient outcomes, including reoperation rate and total hip arthroplasty (THA) conversion rate. 32 In a statewide database study of >8000 hip arthroscopies, low-volume surgeons (0-97 career cases) had a 17% reoperation rate (12% for THA; 5% for arthroscopy) versus high-volume surgeons (>518 career cases) who had a 2.5% reoperation rate (1.5% for THA; 1% for arthroscopy). Similarly, using the same statewide database, higher maintenance annual volume (>164 cases per year) resulted in a significantly lower reoperation risk versus low volume (<102 cases per year). 13 No current evidence exists on the effect of patient selection, preoperative treatments, or postoperative management on the outcome of hip arthroscopy. Thus, given the importance of surgeon experience (ie, case volume, patient selection skills [knowledge, decision making, meeting of expectations]) for improved patient outcomes, early and frequent exposure to hip arthroscopy through dedicated fellowship training is vital in an attempt to lower the learning curve.
The contribution of each step of a procedure to the learning curve of a procedure is unknown. Only anecdotal reports have speculated on which steps of hip arthroscopy are key or critical in determining success. 18,23,25 Given the importance of cam correction in reducing risk of reoperation, 11 capsular management, 33 and good patient selection (relative contraindications include arthritis and dysplasia), 8 these issues have been suggested to be critical in determining outcome.
Defining the critical portions of an operation has recently come under much scrutiny from multiple governing bodies in surgery, including the American College of Surgeons (ACS) and the American Academy of Orthopaedic Surgeons. 5 ACS guidelines allow the attending surgeon to determine what steps are “critical.” 2 Although this may permit system abuse because critical steps may be used for medical billing and determining which Current Procedural Terminology codes are used, it is necessary if no consensus exists on defined key parts of a surgical procedure.
While recent high-quality level 1 randomized controlled evidence has shown statistically significant and clinically relevant subjective patient-reported and economic outcome improvements using hip arthroscopy for FAI syndrome, 20,31,34 no current evidence exists to define the key or critical parts of the procedure that affect those outcomes. This has great potential to aid in teaching trainees (residents, fellows) and surgeons. It is unknown if surgical training (more specifically, hip arthroscopy) follows a Dunning-Kruger type skill-learning model—meaning that trainees receive just enough knowledge to gain sufficient confidence to perform a procedure that, in reality, they are insufficiently skilled to competently perform (“unskilled and unaware”). 26 This may roughly equate to unconscious incompetence on the conscious competence model (Figure 1). 1,7,9 If the key steps can be identified, improved methods of training could be developed to facilitate more efficient learning (ie, competence to proficiency to mastery) of hip arthroscopy.
Figure 1.
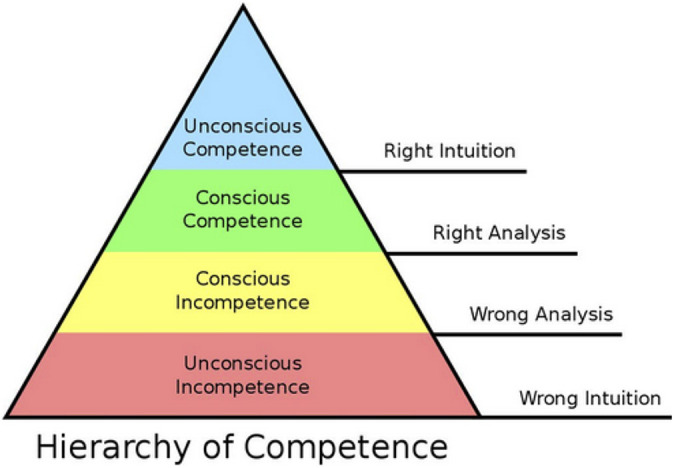
Hierarchy of competence illustrating the 4 states involved in progressing from incompetence to competence in a skill. Copyright Gordon Training International (www.gordontraining.com). Reproduced with permission.
The primary purpose of this study was to identify the key components required to perform arthroscopic treatment of FAI syndrome. The authors hypothesized that the majority of parts (>50%) of hip arthroscopy for FAI syndrome, including labral treatment, femoral treatment, dynamic examination, and capsular management, would be key.
Methods
A 3-question survey comprising questions on technical aspects of hip arthroscopy was sent to a convenience sample of 101 arthroscopic hip surgeons in the United States. The survey was sent via email using SurveyMonkey (http://www.surveymonkey.com). The survey was Health Insurance Portability and Accountability Act–compliant and was exempt from institutional review board approval. Medical students, residents, and fellows were not eligible for participation. The survey was sent to surgeons in the United States (because of the role of the ACS guidelines in the United States health care system) who were members of ≥1 of the following groups: International Society of Hip Arthroscopy, The Hip Preservation Society, Academic Network for Conservative Hip Outcome Research, and Multicenter Arthroscopy of the Hip Outcomes Research Network, Multicenter Arthroscopic Study of the Hip. We also conducted a PubMed search of “hip arthroscopy” and sent the survey to authors of the first 200 citations whom we had not previously identified. No criteria were used to exclude surgeons based on years of experience, American Board of Orthopaedic Surgery certification status, or fellowship experience in order to ensure a wide variety of surgeons performing hip arthroscopy. If no response to the initial survey email, which was sent on March 19, 2018, was received, then a second email was sent 2 weeks later. If necessary, a third and final email was sent in 2 additional weeks. Each participant was only allowed to complete the survey once. The survey was not endorsed by any international, national, or regional medical, surgical, or hip preservation society.
Hip arthroscopy was divided into 10 distinct steps using a Delphi technique to achieve a convergence of expert opinion on what steps compose the procedure. 14,24 A select group of 6 sports medicine fellowship–trained hip arthroscopic surgeons (including J.D.H.) were involved in an iterative 3-round process via email to determine the number and composition of key or critical steps of hip arthroscopy. As used in a previous investigation creating best practice guidelines for hip arthroscopy in FAI, 30 a nominal group technique was used. The latter requires an unbiased nonparticipant moderator, nonleading impartial declarative statements, and questions designed to elicit answers from the participants, expert participants, and anonymous voting. In order to ensure anonymity via email, all responses were collected and copied into a Microsoft Word (Microsoft Corp) document as deidentified responses. All 6 surgeons participated in all 3 rounds. Each surgeon was chosen based on years of experience in postfellowship practice, career number of hip arthroscopy procedures performed, and number of publications on hip arthroscopy available on PubMed. In the event of lack of consensus at the conclusion of 3 rounds, additional rounds would be instituted until consensus was reached and the final list of critical steps of hip arthroscopy was created. Items in each round are shown in Appendix Table A1.
Round 1 commenced January 4, 2018. Items in round 1 were generated from a routine literature review, especially synthetic reviews (eg, systematic review, meta-analysis, scoping review). Item retention was assessed for inclusion based on all 6 surgeons’ responses via 4-item Likert-style evaluation (strongly agree, agree, disagree, strongly disagree). Consensus was achieved for item retention if >80% of respondents agreed or strongly agreed. Given that there were 6 participants, this required an agree/strongly agree decision by either 5 or all 6 participants. Based on responses to items in round 1, revisions to proposed items were made in rounds 2 and 3. After reaching consensus for the proposed critical steps items to be distributed to the convenience sample queried, verbiage/wording/syntax/terminology revisions were suggested or made via emailed comments. In order to ensure that the convenience sample of surveyed surgeons paid attention to each question, a quasi-negative control item (portal closure, item 10) was added to the final list to ensure fidelity of responses.
Surveyed surgeons were asked to report a step as “key” or “critical” if they deemed this step was essential to (1) avoid a complication, (2) reduce the risk of revision arthroscopy, (3) reduce the risk of THA conversion, or (4) optimize patient-reported outcomes. Based on a similar study defining key parts of hip and knee arthroplasty, 5 we considered a step to be key if >90% of participants designated the step as key. The 2 other questions of the survey queried how many years that individual had been performing hip arthroscopy (0-5, 6-10, 11-15, 16-20, >20 years) and how many annual hip arthroscopy procedures that individual performed (0-50, 51-100, 101-150, 151-200, >200).
Descriptive statistics were calculated. Continuous data were presented as mean ± standard deviation. Categorical data were presented as frequencies with percentages. Normality of the number of key steps reported was assessed using the Kolmogorov-Smirnov test of normality. This indicated a normal distribution (D = 0.109; P = .41). Spearman rank correlation was used to compare the number of items deemed key and the years in practice and to compare the number of items deemed key and the number of cases performed per year. Statistical analysis was performed using MedCalc statistical software Version 19.3 (MedCalc Software Ltd; https://www.medcalc.org). Statistical significance was defined as an alpha level of < .05.
Results
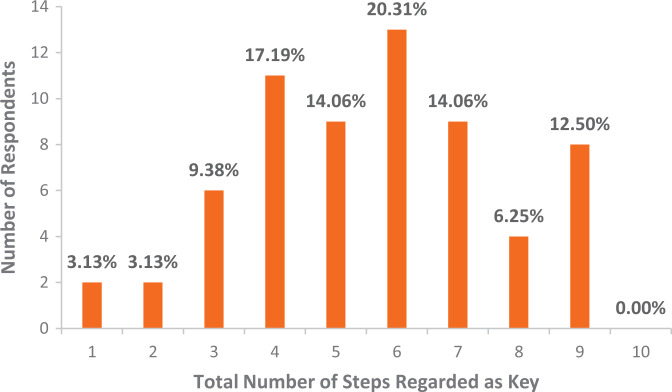
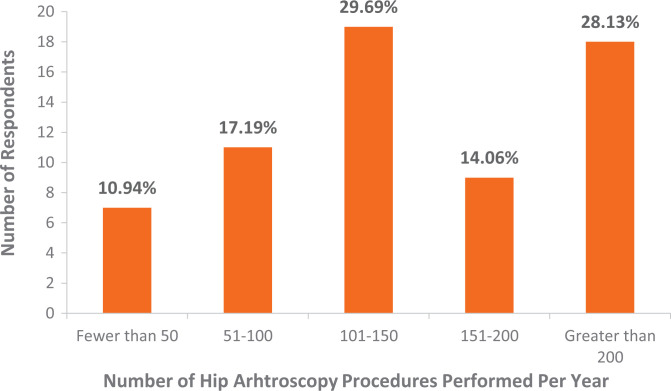
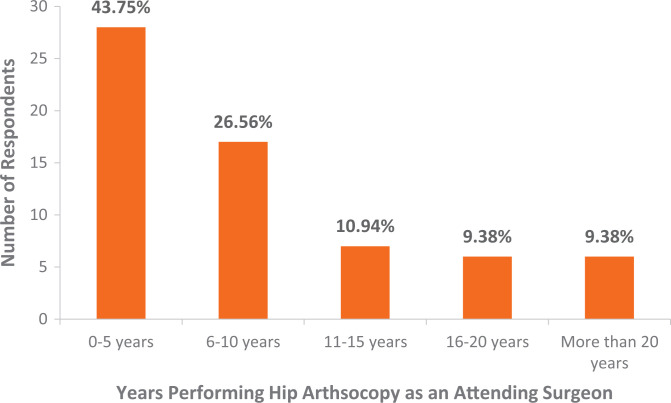
A total of 64 surgeons responded and answered all 3 questions of the survey (64% response rate). Surgeons reported 5.6 ± 2.1 steps as key (Figure 2). Most surgeons (71.9%) had been performing >100 hip arthroscopy procedures per year (Figure 3). Most surgeons (56.3%) had been performing hip arthroscopy for >5 years (Figure 4). Most surgeons designated cam correction (91%) and labral treatment (debridement, repair, or reconstruction including anchor placement, suture passage, and knot tying; 97%) as key steps (Table 1).
Figure 2.
The sum total of steps designated as key by respondents (x-axis) and the number of respondents with each total (y-axis). Percentage represents the percentage of total respondents.
Figure 3.
Results of survey question 1 regarding number of hip arthroscopy procedures performed per year (x-axis) and the number of respondents in each range (y-axis). Percentage represents the percentage of total respondents.
Figure 4.
Results of survey question 2 regarding career length performing hip arthroscopy (x-axis) and the number of respondents with each response (y-axis). Percentage represents the percentage of total respondents.
Table 1.
The 10 Distinct Steps of Hip Arthroscopy for FAI That Could Be Regarded as Critical and the Results of the Respondents (N = 64) a
| Which of the Following Are “Key” or “Critical” Steps of Hip Arthroscopy for FAI? | No. of Respondents (%) |
|---|---|
| Patient positioning | 17 (27) |
| Pulling traction | 19 (30) |
| Skin incision and portal placement | 31 (48) |
| Capsulotomy | 36 (56) |
| Acetabular treatment (pincer or subspine) | 55 (86) |
| Labral treatment (debridement, repair, or reconstruction, including anchor placement, suture passing, and knot tying) | 62 (97) |
| Femoral treatment (femoroplasty for cam morphology) | 58 (91) |
| Dynamic examination of impingement correction (arthroscopic and/or fluoroscopic), assessment before capsular closure | 40 (63) |
| Capsular management and closure | 40 (63) |
| Portal closure | 0 (0) |
| Other | 3 (5) |
| Total respondents | 64 (100) |
a FAI, femoroacetabular impingement.
There was no significant correlation between the number of items deemed key per surgeon and the years performing hip arthroscopy (ρ = –0.139; P = .27) or the annual number of hip arthroscopy procedures performed (ρ = 0.180; P = .15). There was no significant correlation between the number of years performing hip arthroscopy and the designation of the labrum as a key step (ρ = –0.172; P = .17), the designation of the cam correction as a key step (ρ = –0.219; P = .08), or the designation of capsular management as a key step (ρ = –0.047; P = .71). There was no significant correlation between the annual number of hip arthroscopy procedures performed and the designation of the labrum as a key step (ρ = –0.018; P = .89), the designation of the cam correction as a key step (ρ = –0.076; P = .55), or the designation of capsular management as a key step (ρ = 0.157; P = .21).
Discussion
Based on a Delphi technique and expert opinion survey of high-volume arthroscopic hip preservation surgeons, labral treatment (97%) and cam correction (91%) are the 2 key parts of hip arthroscopy for FAI syndrome, with >90% of survey participants reporting these 2 steps as a key part of the procedure. Although only 2 steps obtained sufficient generalized consensus to be designated as key, the average number of total steps reported as key by the surveyed hip arthroscopists was 5.6 ± 2.1 (out of 10). There was no significant correlation between surgeon experience and designation of certain steps as key. While they did not meet the threshold of key (>90%), acetabular treatment (pincer or subspine; 86%), capsular management and closure (63%), and dynamic arthroscopic and/or fluroscopic evaluation of impingement correction (63%) were regarded as key by a majority of respondents.
Hip arthroscopy is a technically challenging procedure and requires great skill to achieve optimal outcomes and reduce the risk of complications, such as traction and positioning injuries, iatrogenic chondrolabral damage, inadequate (or overcorrected) osseous reshaping, or misplaced suture anchors. 21 Despite the known potential implications of capsular management, only 35% of large-scale hip arthroscopy outcome studies have reported the use or type of capsular repair. 21 A large systematic review (>35,000 participants from >200 papers) compared original research publications (assumed to be published by leaders in hip preservation surgery with presumed low complication rates) and “big data” database studies (which includes all surgeons, good and bad, with anticipated higher complication rates). 41 The big data study showed significantly higher complication rates related to technique (femoral neck fracture, relative risk [RR] = 8.0; hip dislocation, RR = 2.2; reoperations, RR = 1.2; THA conversion, RR = 2.2). Although big database studies do not elucidate the root causes of complications, risks such as chondral damage, labral injury, femoral neck fracture, and avascular necrosis can be technique-dependent complications associated with femoral osteoplasty and labral repair.
The amount of hip arthroscopy exposure a resident or fellow receives during training is largely dependent on the volume performed at one’s respective institution, which varies widely among programs. There is currently no Accreditation Council for Graduate Medical Education (ACGME) case log requirement for hip arthroscopy for orthopaedic surgery residents or sports medicine fellows. The fact that respondents reported a mean of 5.6 steps to be key indicates that many steps of hip arthroscopy are potentially viewed as key and have direct implications on patient morbidity. Consequently, residents and fellows may have difficulty in gaining enough surgical repetitions without dedicated hip arthroscopy training. 12,23 A key element in learning is a definition of what is to be learned and how it will be evaluated. Although defining critical steps has not been directly correlated with the learning curve, previous studies have stressed the importance of standardized steps and outcome measures to depict the learning curve. 6,43 A better understanding of the surgical steps in hip arthroscopy is needed to teach the techniques required for the procedure in a more efficient manner.
When learning a new procedure, performance typically improves with experience. The idea that there is a positive relationship between clinical outcomes and volume of procedures was one of the first applications of a surgical learning curve in 1979. 29 One of the greatest challenges of residency and fellowship training in orthopaedics is exposure to a sufficient number of cases to overcome the learning curve for difficult procedures and techniques, which may be affected by changes to resident work-hours and supervision. 22,40 In 2014, Leopold 28 described the transition from resident to attending surgeon as a binary model, in which a resident right before graduation must be supervised in all clinical duties but he or she immediately after graduation can perform any surgery with little or no oversight. Although the transition is not as abrupt as a binary model, this idea highlights that learning curves must be understood to ensure sufficient resident case exposure and volume.
Surgeons who are implementing a new procedure or skill must define potential learning curves in order to suggest ways to optimize surgical outcomes and minimize patient morbidity. Critical portions of a procedure are viewed to have a greater influence on surgical outcomes, and understanding this allows a surgeon to focus one’s training in residency, fellowship, and practice to move beyond competence to proficiency and mastery (Figure 1). The learning curve can be divided into 3 phrases: learning, consolidation, and plateau. 42 The learning phase has the lowest success rates and highest complication rates. As trainees gain experience, they progress to the consolidation phase, and their complication and failure rates decrease. However, relevant to hip arthroscopy, as experience is gained, more challenging procedures may be attempted/performed, including revision arthroscopy, which introduces several aspects of surgical skill not encountered in primary arthroscopy. 11 Eventually, trainees are proficient enough at the procedure that they progress to the plateau phase, where the success rates are high and complication rates are very low. Continued maintenance of skills is necessary to prevent loss of skill and regression down the learning curve. While the exact number of cases needed to establish proficiency has not been established by the ACGME, it is clear that a larger volume of surgical repetition leads to more efficient surgery and fewer patient complications. Relevant to hip arthroscopy, it is unknown when trainees and surgeons progress (and possibly even regress) across these phases.
The establishment of key steps also applies to the 5-stage model of mental stages in skill acquisition by Dreyfus and Dreyfus. 15 In this model, acquisition, competency, and proficiency are mutually exclusively different—competence can be defined as the lowest suitable level of performance, while proficiency represents greater consistency and responsibility, albeit not at “expert” levels yet. 3,15,35 Competence develops with experience; proficiency develops when one uses intuition in decision making and plan formulation. 15 Expertise is demonstrated with fluid performance that happens subconsciously, automatically, and no longer depends on explicit knowledge. Improved training methods that focus on mastering each step could ultimately reduce complications and reoperations while improving patient outcomes.
The Dunning-Kruger effect describes a cognitive bias whereby people are incompetent because they have a metacognitive inability to recognize their incompetence or are underperforming because they have not seen good performance (Figure 5). 26 To optimize the surgical learning curve in their favor and not just accept poor outcomes as part of learning, surgeons need an opportunity for a learning apprenticeship, in either residency or fellowship, to exit the novice stage and progress to competence. Simulation training, cadaver-based courses, and operating with preceptors are the safest modern approaches to learn novel and challenging techniques. 27 These methods help smooth the transition to operating alone by allowing trainees to understand learning curves and have specified operative objectives.
Figure 5.
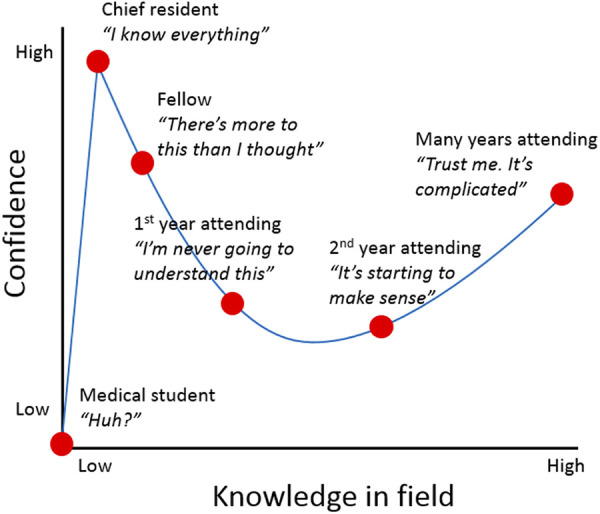
Dunning-Kruger effect curve applied to surgical training.
As orthopaedic surgery residency and fellowship move toward a competency-based framework with less hands-on experience, trainees may not achieve adequate repetitions for this highly specialized procedure. 28 Simulation-based training has become increasingly used in medical education, from standardized patient interactions in medical school to advanced computer-based platforms that allow trainees to develop the psychomotor skills necessary to perform a procedure without placing real patients at risk. 36,38 Unfortunately, simulator fidelity translation in medicine and surgery has not yielded the high quality that the aerospace industry has achieved. 17 Relative to hip arthroscopy, although simulator use is in its infancy, this finding illustrates a similar conclusion to that of clinical outcome studies regarding a substantial learning curve—610 previous arthroscopic procedures (all joints) are necessary to achieve an expert simulator score, and 78 procedures are necessary to achieve competence. 16
Previous studies have demonstrated that for those with minimal training performing hip arthroscopy, skills are improved when using a hip simulator. 37 The ACGME is an advocate for simulation-based education, as this learning model is associated with improved patient safety outcomes and better patient care. 39 Through simulation, critical skills can be taught to residents and fellows in a low-risk environment. This enables a training environment that follows a method of learning, seeing, and proving that the trainee understands a surgical concept before he or she completes this step in the operating room. It has been well established that training on simulators improves performance on simulators, but current literature provides very limited evidence on simulation training and its ability to improve basic diagnostic (knee, shoulder) arthroscopy skills in vivo. 17 Nonetheless, training using simulators provides experience practicing all steps of hip arthroscopy to gain hands-on experience and to become more comfortable with individual steps and technical aspects. 4
Limitations of this study include that the learning curve and critical step designation do not account for patient selection, a vital component in optimizing outcomes for treatment of FAI. This small pilot investigation survey had insufficient responses to determine if specific critical parts change with a greater number of years (or case volume) experience in practice. Similarly, there was no comparison between surgeons performing arthroscopy only (no open surgery, such as periacetabular osteotomy or surgical hip dislocation) and both arthroscopic and open surgery. While an impetus for this investigation surrounds the issue of “overlapping surgeries,” an entity primarily problematic in the United States, another limitation is the exclusion of surgeons outside the United States (homogeneity, study internal validity). Research has already begun to assess a complete international cohort of surgeons performing arthroscopic and open surgery (heterogeneity, study external validity, and generalizability). Surgeons were queried to estimate years of experience and annual maintenance volume, which may be subject to recall bias. Total career volume of surgeons was not included in the analysis. Surgeons were not queried about hip arthroscopy training location and duration (residency or fellowships in sports medicine, adult reconstruction, pediatrics, hip preservation).
Further limitations include that a 90% threshold of surgeons designating a part as “key” or “critical” was arbitrary and based on 1 similar previous study. 5 It is unknown if 90% should be the threshold or if the threshold should be lower (or higher). The 10 steps analyzed were possibly too broad and insufficiently specific. For labral preservation, further granularity could be obtained via implant selection (eg, all-suture vs polyetheretherketone vs metal, 1.4 mm vs 1.8 mm vs 2.0 mm, knotless vs tying knots, deployment mechanism), drill guide technique (straight, curved), and repair (eg, looped circumferential suture vs pierced labral base mattress refixation) versus reconstruction (eg, graft type, segmental vs circumferential). This study analyzed intraoperative surgical technique skills only, without consideration of the effect of patient selection in the key steps of the procedure, any preoperative treatments (eg, physical therapy, injections), or postoperative recovery (eg, physical therapy, restrictions or precautions, return to sports). Future research should be done to investigate these limitations and include responses from residents and fellows to see if their responses are concordant with those of high-volume hip arthroscopic surgeons.
Conclusion
Based on a Delphi technique and expert opinion survey of high-volume surgeons, labral treatment and cam correction are the 2 key parts of hip arthroscopy for FAI syndrome.
APPENDIX
Table A1.
Three Rounds of Delphi Technique to Determine 10 Key Parts of Hip Arthroscopy. a
| Round 1 items |
|---|
| 1. Anesthesia type (general, neuraxial, regional, local) |
| 2. Arthroscope lens angle (70, 30, other, multiple) |
| 3. Arthroscopy fluid type (normal saline, lactated Ringer, other) |
| 4. Bed type (Smith & Nephew, Arthrex, Stryker, Hana, fracture table, other) |
| 5. Patient positioning (Trendelenburg angle) |
| 6. Pulling traction (post-assisted vs postless) |
| 7. Joint entry (arthrogram to break seal before distraction) |
| 8. Skin incision (vertical, transverse) |
| 9. Portal placement (number) |
| 10. Portal placement (location) |
| 11. Capsulotomy type (interportal, T, H, periportal) |
| 12. Capsulotomy size (interportal) |
| 13. Capsulotomy size (T) |
| 14. Capsulotomy size (H) |
| 15. Capsulotomy size (periportal) |
| 16. Acetabular treatment (labral takedown/separation) |
| 17. Acetabuloplasty (burr from single or multiple portals) |
| 18. Acetabuloplasty (on/off traction) |
| 19. Acetabuloplasty (focal/global) |
| 20. Subspine decompression (intra-articular vs capsular side) |
| 21. Subspine decompression (performance [preoperative decision based on imaging/examination vs intraoperative decision based on arthroscopic appearance]) |
| 22. Iliopsoas treatment (release, tenotomy, fractional lengthening, other) |
| 23. Labral treatment (seal evaluation pretreatment [repair, reconstruction, debridement]) |
| 24. Labral treatment (seal evaluation posttreatment [repair, reconstruction, debridement]) |
| 25. Labral treatment (repair, suture configuration [loop, mattress, other]) |
| 26. Labral treatment (repair, suture type [suture, tape, other]) |
| 27. Labral treatment (repair, anchor type [all-suture, PEEK, bioabsorbable, metal, other]) |
| 28. Labral treatment (repair, anchor drilling [straight guide, curved guide, other]) |
| 29. Labral treatment (repair, knot type [sliding, nonsliding, locking, nonlocking, knotless, other]) |
| 30. Labral treatment (reconstruction [graft type—autograft, allograft, synthetic, other]) |
| 31. Labral treatment (reconstruction [graft type—iliotibial band, peroneus longus, tibialis anterior, tibialis posterior, other]) |
| 32. Labral treatment (reconstruction [segmental, circumferential, other]) |
| 33. Articular cartilage treatment (debridement, marrow stimulation, cartilage restoration, other) |
| 34. Fovea management (ligamentum teres, pulvinar, transverse acetabular ligament) |
| 35. Femoral treatment (cam osteoplasty correction) |
| 36. Dynamic arthroscopic examination |
| 37. Dynamic fluoroscopic evaluation |
| 38. Capsular closure (complete, partial, capsulotomy, other) |
| 39. Capsular closure (plication degree [capsular shift, plication, capsular subtraction, other]) |
| 40. Capsular closure (suture type [absorbable, nonabsorbable]) |
| 41. Capsular closure (suture type [suture, tape, other]) |
| 42. Portal closure (close, open) |
| 43. Other |
| Round 2 items |
| 1. Patient positioning |
| 2. Pulling traction |
| 3. Skin incision (portal placement) |
| 4. Capsulotomy type |
| 5. Capsulotomy size |
| 6. Acetabular treatment (pincer) |
| 7. Acetabular treatment (subspine) |
| 8. Iliopsoas treatment |
| 9. Labral treatment (seal evaluation) |
| 10. Labral repair (anchor placement) |
| 11. Labral repair (suture passage) |
| 12. Femoral treatment (cam osteoplasty correction) |
| 13. Dynamic arthroscopic (fluoroscopic evaluation) |
| 14. Capsular closure |
| 15. Portal closure |
| 16. Other |
| Round 3 items |
| 1. Patient positioning |
| 2. Pulling traction |
| 3. Skin incision (portal placement) |
| 4. Capsulotomy |
| 5. Acetabular treatment (pincer, subspine) |
| 6. Labral treatment (debridement, repair, reconstruction, including anchor placement, suture passing, knot tying) |
| 7. Femoral treatment (femoroplasty for cam morphology) |
| 8. Dynamic examination of impingement correction (arthroscopic, fluoroscopic) assessment before capsular closure |
| 9. Capsular management (closure) |
| 10. Portal closure |
| 11. Other |
a PEEK, polyetheretherketone.
Footnotes
Final revision submitted January 5, 2021; accepted February 18, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: T.J.E. has received research support from Medacta, consulting fees from Medacta and Stryker, educational support from Medacta, and royalties from Acute Innovations. S.J.N. has received research support from Allosource, Arthrex, Athletico, DJO, Linvatec, Miomed, Smith & Nephew, and Stryker; consulting fees from Ossur and Stryker; royalties from Ossur and Springer; and educational support from Elite Orthopedics. J.D.H. has received research support from DePuy and Smith & Nephew, consulting fees from Smith & Nephew, speaking fees from Smith & Nephew and Xodus Medical, educational support from Arthrex, and royalties from Slack. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Adams L. Learning a new skill is easier said than done. Gordon Training International. Accessed May 25, 2020. https://www.gordontraining.com/free-workplace-articles/learning-a-new-skill-is-easier-said-than-done
- 2. American College of Surgeons statements on principles. Bull Am Coll Surg. 2016;101(9):19-34. [PubMed] [Google Scholar]
- 3. Azari D, Greenberg C, Pugh C, Wiegmann D, Radwin R. In search of characterizing surgical skill. J Surg Educ. 2019;76(5):1348–1363. [DOI] [PubMed] [Google Scholar]
- 4. Bartlett JD, Lawrence JE, Yan M, et al. The learning curves of a validated virtual reality hip arthroscopy simulator. Arch Orthop Trauma Surg. 2020;140(6):761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernstein J, Bernstein JJ, Mehta S, et al. Defining the key parts of a procedure: implications for overlapping surgery. J Am Acad Orthop Surg. 2018;26(4):142–147. [DOI] [PubMed] [Google Scholar]
- 6. Bjorgul K, Novicoff WM, Saleh KJ. Learning curves in hip fracture surgery. Int Orthop. 2011;35(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Broadwell Martin M. Teaching for learning (XVI). Published February 20, 1969. Accessed May 25, 2020. http://www.wordsfitlyspoken.org/gospel_guardian/v20/v20n41p1-3a.html
- 8. Byrd JWT. Editorial commentary: great expectations or “we’ll see,” said the Zen master---hip arthroscopy patient selection. Arthroscopy. 2019;35(6):1817–1818. [DOI] [PubMed] [Google Scholar]
- 9. Curtiss PR, Warren PW. The Dynamics of Life Skills Coaching. NewStart Inc. for the Training Research and Development Station; 1973. [Google Scholar]
- 10. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286–1292. [DOI] [PubMed] [Google Scholar]
- 11. Cvetanovich GL, Harris JD, Erickson BJ, Bach BR, Jr, Bush-Joseph CA, Nho SJ. Revision hip arthroscopy: a systematic review of diagnoses, operative findings, and outcomes. Arthroscopy. 2015;31(7):1382–1390. [DOI] [PubMed] [Google Scholar]
- 12. Daniels AH, Digiovanni CW. Is subspecialty fellowship training emerging as a necessary component of contemporary orthopaedic surgery education? J Grad Med Educ. 2014;6(2):218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Degen RM, Pan TJ, Chang B, et al. Risk of failure of primary hip arthroscopy—a population-based study. J Hip Preserv Surg. 2017;4(3):214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409. [DOI] [PubMed] [Google Scholar]
- 15. Dreyfus S, Dreyfus H. A five-stage model of the mental activities involved in directed skill acquisition. California University Berkeley Operations Research Center. Published May 21, 1980. Accessed May 25, 2020. https://apps.dtic.mil/dtic/tr/fulltext/u2/a084551.pdf [Google Scholar]
- 16. Erturan G, Alvand A, Judge A, Pollard TCB, Glyn-Jones S, Rees JL. Prior generic arthroscopic volume correlates with hip arthroscopic proficiency: a simulator study. J Bone Joint Surg Am. 2018;100(1):e3. [DOI] [PubMed] [Google Scholar]
- 17. Frank RM, Wang KC, Davey A, et al. Utility of modern arthroscopic simulator training models: a meta-analysis and updated systematic review. Arthroscopy. 2018;34(5):1650–1677. [DOI] [PubMed] [Google Scholar]
- 18. Go CC, Kyin C, Maldonado DR, Domb BG. Surgeon experience in hip arthroscopy affects surgical time, complication rate, and reoperation rate: a systematic review on the learning curve. Arthroscopy. 2020;36(12):3092–3105. [DOI] [PubMed] [Google Scholar]
- 19. Griffin DR, Dickenson EJ, O’Donnell J, et al. The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med. 2016;50(19):1169–1176. [DOI] [PubMed] [Google Scholar]
- 20. Griffin DR, Dickenson EJ, Wall PDH, et al. Hip arthroscopy versus best conservative care for the treatment of femoroacetabular impingement syndrome (UK FASHIoN): a multicentre randomised controlled trial. Lancet. 2018;391(10136):2225–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589–595. [DOI] [PubMed] [Google Scholar]
- 22. Harris JD, Staheli G, LeClere L, Andersone D, McCormick F. What effects have resident work-hour changes had on education, quality of life, and safety? A systematic review. Clin Orthop Relat Res. 2015;473(5):1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoppe DJ, de Sa D, Simunovic N, et al. The learning curve for hip arthroscopy: a systematic review. Arthroscopy. 2014;30(3):389–397. [DOI] [PubMed] [Google Scholar]
- 24. Hsu CC, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval. 2007;12(10):1–8. [Google Scholar]
- 25. Konan S, Rhee SJ, Haddad FS. Hip arthroscopy: analysis of a single surgeon’s learning experience. J Bone Joint Surg Am. 2011;93(suppl 2):52–56. [DOI] [PubMed] [Google Scholar]
- 26. Kruger J, Dunning D. Unskilled and unaware of it: how difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J Pers Soc Psychol. 1999;77(6):1121–1134. [DOI] [PubMed] [Google Scholar]
- 27. Leopold SS. Editorial: are we all better-than-average drivers, and better-than-average kissers? Outwitting the Kruger-Dunning effect in clinical practice and research. Clin Orthop Relat Res. 2019;477(10):2183–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leopold SS. Editorial: transition from training to practice—is there a better way? Clin Orthop Relat Res. 2014;472(5):1351–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301(25):1364–1369. [DOI] [PubMed] [Google Scholar]
- 30. Lynch TS, Minkara A, Aoki S, et al. Best practice guidelines for hip arthroscopy in femoroacetabular impingement: results of a Delphi process. J Am Acad Orthop Surg. 2020;28(2):81–89. [DOI] [PubMed] [Google Scholar]
- 31. Mather RC, Nho SJ, Federer A, et al. Effects of arthroscopy for femoroacetabular impingement syndrome on quality of life and economic outcomes. Am J Sports Med. 2018;46(5):1205–1213. [DOI] [PubMed] [Google Scholar]
- 32. Mehta N, Chamberlin P, Marx RG, et al. Defining the learning curve for hip arthroscopy: a threshold analysis of the volume-outcomes relationship. Am J Sports Med. 2018;46(6):1284–1293. [DOI] [PubMed] [Google Scholar]
- 33. O’Neill DC, Mortensen AJ, Cannamela PC, Aoki SK. Clinical and radiographic presentation of capsular iatrogenic hip instability after previous hip arthroscopy. Am J Sports Med. 2020;48(12):2927–2932. [DOI] [PubMed] [Google Scholar]
- 34. Palmer AJR, Ayyar Gupta V, Fernquest S, et al. Arthroscopic hip surgery compared with physiotherapy and activity modification for the treatment of symptomatic femoroacetabular impingement: multicentre randomised controlled trial. BMJ. 2019;364:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pena A. The Dreyfus model of clinical problem solving skills acquisition: a critical perspective. Med Educ Online. 2010;15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Philibert I, Lieh-Lai M, Miller R, et al. Scholarly activity in the next accreditation system: moving from structure and process to outcomes. J Grad Med Educ. 2013;5(4):714–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pollard TC, Khan T, Price AJ, et al. Simulated hip arthroscopy skills: learning curves with the lateral and supine patient positions: a randomized trial. J Bone Joint Surg Am. 2012;94(10):e68. [DOI] [PubMed] [Google Scholar]
- 38. Ross J. Simulation and psychomotor skill acquisition: a review of the literature. Clin Simul Nurs. 2012;8:e429–e435. [Google Scholar]
- 39. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025–1033. [DOI] [PubMed] [Google Scholar]
- 40. Singh V, Gress DR, Higashida RT, et al. The learning curve for coil embolization of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2002;23(5):768–771. [PMC free article] [PubMed] [Google Scholar]
- 41. Sochacki KR, Jack RA, Safran MR, Nho SJ, Harris JD. There is a significant discrepancy between “big data” database and original research publications on hip arthroscopy outcomes: a systematic review. Arthroscopy. 2018;34(6):1998–2004. [DOI] [PubMed] [Google Scholar]
- 42. White SM. The ethics of anaesthesia learning curves. Anaesth Intensive Care. 2009;37(5):824–829. [DOI] [PubMed] [Google Scholar]
- 43. Wiese M, Krämer J, Bernsmann K, Ernst Willburger R. The related outcome and complication rate in primary lumbar microscopic disc surgery depending on the surgeon’s experience: comparative studies. Spine J. 2004;4(5):550–556. [DOI] [PubMed] [Google Scholar]