Abstract
Aims
Under‐ and, especially, overdosing of replacement therapy in haemophilia A patients may be prevented by application of other morphometric variables than body weight (BW) to dose factor VIII (FVIII) concentrates. Therefore, we aimed to investigate which morphometric variables best describe interindividual variability (IIV) of FVIII concentrate pharmacokinetic (PK) parameters.
Methods
PK profiling was performed by measuring 3 FVIII levels after a standardized dose of 50 IU kg−1 FVIII concentrate. A population PK model was constructed, in which IIV for clearance (CL) and central volume of distribution (V1) was quantified. Relationships between CL, V1 and 5 morphometric variables (BW, ideal BW [IBW], lean BW, adjusted BW, and body mass index [BMI]) were evaluated in normal weight (BMI < 25 kg m−2), overweight (BMI 25–30 kg m−2) and obese haemophilia A patients (BMI > 30 kg m−2).
Results
In total, 57 haemophilia A patients (FVIII≤0.05 IU mL−1) were included with median BW of 83 kg (range: 53–133) and median age of 48 years (range: 18–77). IBW best explained observed variability between patients, as IIV for CL and V1 was reduced from 45.1 to 37.6 and 26.% to 14.1%, respectively. CL, V1 and half‐life were similar for all BMI categories. The national recommended dosing schedule did not result in adequate trough levels, both in case of dosing based on BW and IBW. However, dosing based on IBW prevented unnecessary high FVIII peaks.
Conclusion
IBW is the most suitable morphometric variable to explain interindividual FVIII PK variability and is more appropriate to dose overweight and obese patients.
Keywords: haemostasis, modelling and simulation, obesity, pharmacokinetics
What is already known about this subject
Dosing of factor VIII (FVIII) concentrates in haemophilia A patients based on body weight leads to both under‐and overdosing.
Population pharmacokinetics (PK) enables investigation of the relationship between various morphometric variables and FVIII concentrate PK.
What this study adds
Ideal body weight (IBW) best explains PK variability between patients with varying body mass index.
Application of IBW avoids overdosing of factor VIIII concentrate in overweight and obese patients.
The national recommended dosing schedule did not result in adequate trough levels, both in case of dosing based on body weight and IBW. However, dosing based on IBW prevented unnecessary high FVIII peaks.
1. INTRODUCTION
Haemophilia A is an X‐linked inherited bleeding disorder caused by a deficiency in coagulation factor VIII (FVIII). 1 To prevent and treat bleeding in muscles and joints, haemophilia A patients are infused either prophylactically or on demand with intravenous replacement therapy consisting of recombinant or plasma‐derived FVIII concentrates. 2 , 3 Current FVIII concentrate dosing schedules are based on body weight (BW). It is, however, well‐known that a large interindividual variability (IIV) exists in observed FVIII levels after FVIII concentrate infusion, which results in both under‐ and overdosing when specific FVIII ranges are targeted. 4 , 5
Dosing based on pharmacokinetics (PK) is able to reduce under‐and overdosing. However, when applying this method, an individual's characteristics should be comparable to those individuals which have contributed data to the population PK model. In haemophilia A, this is especially important for age and BW, as these characteristics have been shown to be closely associated with FVIII PK. 5 Recently, a meta‐analysis in haemophilia patients demonstrated that the prevalence of overweight and obesity is steadily increasing with a pooled prevalence that has risen from 17% (95% CI: 15.0–19.3%) to 31% (95% CI: 26.8–36.2%) in the last decade. 6 This underlines the urgency to include representative numbers of overweight and obese patients in population PK dosing models for factor concentrates in haemophilia treatment.
More specifically, several authors report that obese patients defined as individuals with a body mass index (BMI) >30 kg m−2 have a higher in vivo recovery (IVR) of intravenously administered factor concentrates than patients with a normal BMI, defined as a BMI of 20–25 kg m−2. 7 , 8 This is explained by the fact that obese patients receive higher weight‐based doses that distribute over similar volumes of distribution to those measured in normal weight individuals, as the intravascular compartment does not increase with weight gain. This is, however, especially relevant for FVIII peak levels, as FVIII trough levels and steady state FVIII levels are more dependent on FVIII clearance. 9 As parameters from a population PK model are generally scaled using BW of the patient, 4 , 5 , 10 other morphometric variables that also take body composition into account may have a higher predictive performance when describing FVIII PK parameters in overweight and obese haemophilia A patients. 11 , 12 Various population models describe PK variability observed after FVIII concentrate dosing in haemophilia A patients, incorporating different morphometric variables such as BW, lean body mass (LBM) or ideal BW (IBW). 4 , 5 , 13 However, no study has yet investigated how different morphometric variables correlate with FVIII PK parameters using population PK modelling with real‐world data from haemophilia A patients treated with various FVIII concentrates. In addition, risk analyses for such an approach have not yet been reported.
Therefore, this study explores the extent to which morphometric variables other than BW explain IIV of FVIII PK parameters in normal, overweight and obese haemophilia A patients. Our study is the first to apply population PK analysis using real‐world data from patients of whom a representative number is overweight or obese. In addition, the risks of this real‐world population PK model are also simulated to analyse its results in critical circumstances when under‐dosing must be avoided.
2. METHODS
2.1. Patients
In this cross‐sectional study, severe and moderate haemophilia A patients were included with endogenous baseline FVIII activity levels ≤0.05 IU mL−1, aged ≥18 years, and without inhibitory FVIII antibodies (Bethesda Units [BU] <0.2 IU). Patients were enrolled from 6 Academic Hemophilia Treatment Centers in the Netherlands (Erasmus University Medical Center Rotterdam, University Medical Center Groningen, University Medical Center Utrecht, Radboud University Medical Center Nijmegen/Maxima Medical Center, Veldhoven, Leiden University Medical Center/Haga Hospital, The Hague, Amsterdam University Medical Centers). This study was approved by the Medical Ethics Committee of the Erasmus University Medical Center and all patients gave written informed consent before enrollment according to the declaration of Helsinki. Although data mostly originates from patients enrolled in the perioperative OPTI‐CLOT trial 14 (n = 48) and consists of preoperative individual PK profiling data, some patients (n = 9) were included from a separate study investigating PK tools for FVIII dosing. 15 This study was not subject to the Medical Research Involving Human Subjects Act (WMO) and was approved by the Medical Ethics Committee of the Erasmus University Medical Center.
2.2. Blood sampling and analyses
A single intravenous dose of 50 IU kg−1 FVIII concentrate was administered to each patient. Patients received the following recombinant FVIII concentrates (Kogenate FS: Bayer, Berkeley, Ca, USA; Advate: Baxter Bioscience, Thousand Oaks, CA, USA; Refacto AF: Pfizer, New York, NY USA; NovoEight: Novo Nordisk, Bagsværd, Denmark) or plasma‐derived FVIII concentrates (Aafact: Blood Transfusion council of the Netherlands Red Cross, Amsterdam, the Netherlands). In general, 3 FVIII level measurements were obtained at 4, 24 and 48 hours after FVIII bolus administration. In a minority of patients, a preinfusion FVIII level was also measured. The need for a washout period and baseline measurement was avoided by collecting time of dosing and doses of 3 previous FVIII concentrate infusions. FVIII plasma levels were measured locally in each treatment centre, using a 1‐stage clotting assay.
2.3. Morphometric variables
For each patient, the following patient characteristics were collected: endogenous baseline FVIII level (IU mL–1); inhibitor status; age (mo); BW (kg) and height (cm). LBM was determined using a bioelectrical impedance analyser (Maltron BF‐906, Maltron International, Rayleigh, UK). Using the BW and height of the patient, the following morphometric variables were calculated: BMI (kg m−2) 16 ; IBW (kg) 17 ; adjusted BW (kg) 17 ; and calculated LBM (kg). 18 The equations used to calculate morphometric variables are presented in Supplementary Table S1. Patients were categorized into 3 BMI categories: normal weight patients (BMI < 25 kg m−2), overweight patients (25 kg m−2 ≤ BMI ≤ 30 kg m−2), and obese patients (BMI > 30 kg m−2).
2.4. PK modelling
A structural population PK model was constructed describing IIV of PK parameters. Models were compared using the objective function value (OFV). If the difference of the OFV between 2 models was larger than 3.84 it was considered to be statistically significant with a P < .05. Subsequently, the extent to which the various morphometric variables explained this variability was evaluated (applied equations are described in Supplementary S1)
The structural PK model was developed using nonlinear mixed‐effects modelling software NONMEM v7.4 (ICON Development Solutions, Ellicott City, MD, USA) with the FVIII level data of all patients simultaneously. The endogenous baseline FVIII activity level for each patient, especially important in the moderate haemophilia patients (FVIII 0.01–0.05 IU mL–1), at time of individual PK profiling was calculated by subtraction from predicted FVIII levels. Moreover, residual FVIII levels due to previous prophylactically administered FVIII concentrate doses were also taken into account.
The following PK parameters were estimated: clearance (CL), intercompartmental clearance (Q), and volume of distribution of the central (V1) and peripheral (V2) compartment. R software v3.4.1 (R Core Team; 2017) and Xpose v4.5.3 were used for data exploration and model diagnostics. 19
2.5. Evaluation of the morphometric variables
After establishing the structural population PK model, the ability of the morphometric variables to explain IIV of the obtained population PK parameters was evaluated. The difference between 2 values for OFV (dOFV) from 2 different models can be described by χ2 distribution in the case of nested models and, hence, a statistical significance can be calculated in terms of a P‐value. However, the OFV does not consider the difference in number of parameters between 2 evaluated models. The Akaike information criterion (AIC) is based on the OFV and adds a penalty for the number of parameters from the corresponding model. For calculating AIC, the evaluated models do not necessarily have to be nested. As the model with the least number of parameters and the highest ability to describe measured FVIII levels is most favourable, AIC was used instead of OFV in this study evaluation. Besides the predictive ability of the model, reduction in IIV of CL and V1 was considered for model selection as well.
Allometric scaling was used to describe the correlation between morphometric variables and PK parameters according to the following equation:
| (Eq 1) |
in which θTV is the typical value for the population PK parameter (i.e. the median value), θPop is the estimated population value for the population PK parameter, MVi and MVmed are the individual value and median for the morphometric variable, respectively, and EXPallo is the allometric exponent.
Allometric scaling was evaluated in 2 ways. Firstly, relationships between morphometric variables and PK parameters were evaluated with EXPallo fixed to the values of 0.75 and 1 for the clearance parameters (CL and Q) and for the volume of distribution parameters (V1 and V2), respectively. 20 Secondly, EXPallo values were estimated for clearance and volume of distribution parameters.
2.6. PK parameter evaluation
Individual (posthoc) PK parameters were estimated by maximum a posteriori Bayesian analysis using the final model with inclusion of the morphometric variable. For each patient, terminal elimination half‐life and IVR were calculated. The IVR was obtained by dividing BW of the patients by the individual PK parameter estimate for V1. A Kruskall–Wallis test was used to compare the individual PK parameter estimates obtained for the patients from the 3 BMI categories, as the data were not normally distributed. P‐values <.01 were considered statistically significant.
2.7. Simulations of single dose and dosing of FVIII concentrate in case of a life‐threatening bleed
For all patients in this cohort, peak and trough FVIII levels were simulated after a single dose of 50 IU kg−1, and after treating a life‐threatening bleed by infusing a loading dose of 50 IU kg−1 followed by a twice daily dose of 25 IU kg−1. The trough FVIII levels were obtained immediately before the sixth dose, which corresponded with 72 hours after administration of the loading‐dose. A Kruskall–Wallis test was used to compare the FVIII levels and P‐values <.01 were considered statistically significant.
Monte Carlo simulations were additionally performed to simulate a total of 1000 haemophilia A patients, receiving dosages based on BW and IBW for a life‐threatening bleed at time point of 72 hours, after 5 consecutive FVIII concentrate doses of 50 IU kg−1; 2 days of 25 IU kg−1 every 12 hours.
3. RESULTS
3.1. Patient characteristics
In total, 57 severe and moderate haemophilia A patients were included with a median BW of 83 kg (range: 53–133 kg) and a median age of 48 years (range: 18.4–76.9 years). In the 3 BMI categories, 26 patients (46%) had a normal BW, 21 patients (37%) were overweight, and 10 patients (18%) were obese. General patient characteristics of the study population and medians and ranges of the morphometric variables are presented in Table 1. In this study, LBM was not measured for 2 patients (4%), due to logistical reasons. In these patients, median measured LBM of the population was used instead.
TABLE 1.
General characteristics of the study population
| Total population | BMI | BMI | BMI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <25 kg m–2 | 25 kg m–2 ≤ and <30 kg m–2 | ≥30 kg m–2 | |||||||
| Patient characteristics | |||||||||
| Total no. of patients | 57 | 26 | 21 | 10 | |||||
| Age (y) | 48.0 | [18.4–76.9] | 36.0 | [18.4–69.1] | 53.4 | [26.9–76.9] | 48.7 | [32.3–70.9] | |
| Severe haemophilia A (<0.01 IU mL−1) | 37 | (65) | 18 | (69) | 12 | (57) | 7 | (70) | |
| Blood group O† | 31 | (54) | 14 | (54) | 12 | (57) | 5 | (50) | |
| Height (m) | 1.78 | [1.48–1.95] | 1.77 | [1.58–1.95] | 1.81 | [1.48–1.95] | 1.76 | [1.68–1.92] | |
| Body weight (kg) | 83.0 | [53.0–133] | 73.0 | [53.0–92.0] | 88.5 | [60.8–113] | 99.6 | [93.0–133] | |
| Adjusted body weight (kg) | 77.5 | [51.9–104] | 73.0 | [54.2–89.9] | 81.9 | [51.9–98.5] | 83.5 | [76.7–104] | |
| BMI (kg m–2) | 26.3 | [19.0–42.6] | 23.3 | [19.0–24.9] | 27.9 | [25.5–29.9] | 32.4 | [30.7–42.6] | |
| Ideal body weight (kg) | 73.1 | [46.0–88.5] | 72.2 | [55.0–88.5] | 75.9 | [46.0–88.5] | 71.3 | [64.1–85.8] | |
| Lean body mass measured (kg) | 61.4 | [39.4–83.5] | 57.9 | [39.4–76.5] | 62.5 | [40.6–80.8] | 64.9 | [57.7–83.5] | |
| Lean body mass calculated (kg) | 62.8 | [43.6–85.2] | 57.4 | [43.6–71.6] | 66.9 | [44.4–80.1] | 68.3 | [63.2–85.2] | |
| Type of clotting factor concentrate | |||||||||
| Advate | 34 | (59.6) | 15 | (57.7) | 13 | (61.9) | 6 | (60.0) | |
| Aafact | 1 | (1.8) | 0 | (0.0) | 1 | (4.8) | 0 | (0.0) | |
| Kogenate FS | 13 | (22.8) | 6 | (23.1) | 4 | (19.0) | 3 | (30.0) | |
| NovoEight | 5 | (8.8) | 3 | (11.5) | 1 | (4.8) | 1 | (10.0) | |
| Refacto AF | 4 | (7.0) | 2 | (7.7) | 2 | (9.5) | 0 | 0 | |
| Pharmacokinetic data | |||||||||
| Total number of observations | 173 | 80 | 63 | 30 | |||||
| No. of observations per individual | 3 | [2–4] | 3 | [3–4] | 3 | [2–4] | 3 | [3–3] | |
| No. of prior doses per individual | 3 | [1–6] | 3.5 | [1–6] | 2 | [1–5] | 4 | [1–4] | |
| No. of observations day 1 (>0–12 h) | 63 | (36) | 30 | (38) | 23 | (37) | 10 | (33) | |
| No. of observations day 2 (>12– 36 h) | 56 | (32) | 25 | (31) | 21 | (33) | 10 | (33) | |
| No. of observations day 3 (>36–72 h) | 54 | (31) | 25 | (31) | 19 | (30) | 10 | (33) | |
BMI = body mass index. † Blood group available in 56 patients. Data are n (%) or median [range]
3.2. PK modelling
A 2‐compartment model performed best as structural model (Table 2). IIV could be estimated for both CL and V1. Inclusion of a correlation between the IIV of both parameters allowed a significantly better fit of the model with measured FVIII levels. In Figure S2, the goodness‐of‐fit of the structural model is presented. Although a small deviation of population predictions of highest FVIII levels was observed, the main part of population predictions was distributed symmetrically around the line y = x demonstrating adequacy of the model to describe measured FVIII levels. When accounting for IIV by Bayesian analysis, the individual profiles were also well described, as practically all predictions were present on the line y = x (Figure S1‐B). Furthermore, the visual predictive check (VPC) showed a good description of the data, and that the model was able to accurately predict the FVIII levels of each patient (Figure S3).
TABLE 2.
Estimated population pharmacokinetic parameters for structural model and final model
| Structural model | Final model | |||||
|---|---|---|---|---|---|---|
| Estimate | RSE (%) | Shr. [%] | Estimate | RSE (%) | Shr. [%] | |
| Structural model | ||||||
| Clearance (CL; mL h−1) | 242 | (6) | 236 | (5) | ||
| Volume of central compartment (V1; mL) | 2620 | (19) | 2840 | (8) | ||
| Distribution CL to compartment 2 (Q; mL h−1) | 192 | (62) | 122 | (24) | ||
| Volume of compartment 2 (V2; mL) | 1070 | (37) | 821 | (47) | ||
| IIV | ||||||
| IIV on CL (%) | 45.1 | (20) | [1] | 37.6 | (19) | [2] |
| IIV on V1 (%) | 26.8 | (62) | [20] | 14.1 | (95) | [37] |
| Correlation between CL and V1 | 66.4 | (46) | 45.6 | (64) | ||
| Residual variability | ||||||
| Additive residual variability (IU dL−1) | 0.66 | (47.1) | 0.65 | (35) | ||
| Proportional residual variability (%) | 13.8 | (23) | 13.7 | (22) | ||
| Covariate relations | ||||||
| CL: allometric exponent | ‐ | 1.65 | (21) | |||
| V1: allometric exponent | ‐ | 1.34 | (19) | |||
| Model characteristics | ||||||
| Objective function value | −186.1 | −217 | ||||
| Condition number | 332 | 99 | ||||
in which IBW is the value for the ideal body weight and IBWmed is the median of for the ideal body weight from the studied population.
3.3. Evaluation of the morphometric variables
The established structural model was used to evaluate the ability of morphometric variables to explain the IIV of CL and V1. In Table 3, a summary of all allometric scaling evaluations with the 5 morphometric variables is presented. Looking at the models in which the allometric exponents were fixed, scaling parameters with IBW produced the lowest AIC and the greatest reduction of IIV from V1. Interestingly, allometric scaling using the BW of the patient resulted in a worse fit as compared with the model without allometric scaling, which signifies the need for allometric scaling using an adequate predictor.
TABLE 3.
Summary of the covariate relationship selection process
| Model and parameter | Covariate | OFV | AIC | dAIC | IIV on CL | IIV on V1 | Correlation between CL & V1 |
|---|---|---|---|---|---|---|---|
| Comparator model | −186.1 | −168.1 | 45.1 | 26.8 | 66.4 | ||
| Allometric scaling with fixed exponents | |||||||
| WT | −176.2 | −158.2 | 9.9 | 43.7 | 26.7 | 60.1 | |
| LBM | −208.1 | −190.1 | −22.1 | 39.5 | 16.4 | 46.7 | |
| IBW | −212.1 | −194.1 | −26.0 | 40.2 | 15.6 | 52.9 | |
| BMI | −160.9 | −142.9 | 25.2 | 47.7 | 34.1 | 69.3 | |
| ABW | −200.7 | −182.7 | −14.6 | 41.2 | 18.9 | 55.7 | |
| LBMc | −197.3 | −179.3 | −11.3 | 42.1 | 20.4 | 58.1 | |
| Allometric exponents estimated for CL & V1 | |||||||
| WT | −190.6 | −168.6 | −0.5 | 43.9 | 23.8 | 64.2 | |
| LBM | −212.6 | −190.6 | −22.5 | 37.3 | 15.7 | 46.2 | |
| IBW | −217.2 | −195.2 | −27.2 | 37.6 | 14.1 | 45.6 | |
| BMI | −186.8 | −164.8 | 3.3 | 44.8 | 26.7 | 66.1 | |
| ABW | −202.3 | −180.3 | −12.2 | 40.7 | 18.7 | 57.0 | |
| LBMc | −198.8 | −176.8 | −8.7 | 41.9 | 20.2 | 60.1 | |
OFV: objective function value; AIC: Akaike information criterion; dAIC: change in the AIC as compared to the AIC from the comparator model; IIV: interindividual variability; CL: clearance of the central compartment; V1: volume of distribution of the central compartment; WT: body weight; LBM: measured lean body mass; IBW: ideal body weight; BMI: body mass index; ABW: adjusted body weight; LBMc: calculated lean body mass.
IBW best explained the interpatient variability in FVIII PK. Scaling PK parameters by IBW reduced the interpatient variability in CL and V1 from 45.1 to 37.6% and from 26.8 to 14.1%, respectively. However, the obtained IIV on CL using allometric scaling with LBM (37.3%) was similar to the IIV obtained using allometric scaling with IBW (37.6%), whereas not for the IIV obtained for V1 (15.7%). Nevertheless, a significant difference was obtained in the ability of the model to describe the measured FVIII data favouring allometric scaling using IBW.
3.4. PK parameter evaluation
In the final population model with parameters scaled for IBW, the allometric exponents for CL and V1 were estimated (Table 2). As both estimated allometric exponents were above 1, the relation with IBW allowed a more than proportional increase for the individual PK parameters CL and V1. The relationship between IBW and the individual PK parameter estimates is shown in Figure 1. However, the η‐shrinkage of the IIV on V1 is quite large (37%). Nevertheless, the conclusion still stands that with increasing IBW, the typical (median) values for CL and V1 also increase.
FIGURE 1.
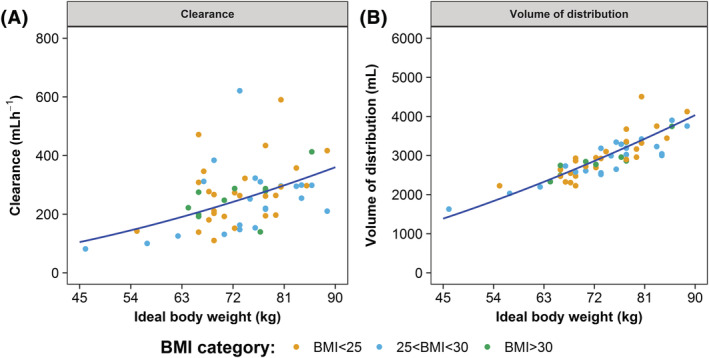
Clearance and volume of distribution increase when ideal body weight increases. (A) Clearance. (B) Volume of distribution of the central compartment. The individual PK parameter estimates were obtained by posthoc analysis using the final model. The blue line depicts the typical values, as calculated using the final model vs ideal body weight. In both figures, an increase is demonstrated for the typical value with increasing ideal body weight
In Figure 2, the individual posthoc PK parameter estimates for FVIII CL, V1, terminal half‐life and the calculated IVR are presented. For CL, V1 and terminal half‐life, no significant differences were obtained for the values obtained within the 3 BMI categories. This shows that CL, V1 and terminal half‐life were similar between normal weight, overweight and obese haemophilia A patients. However, the calculated IVR increased with increasing BMI and the values from the 3 BMI categories were significantly different (χ2 = 28.8, P < .001).
FIGURE 2.
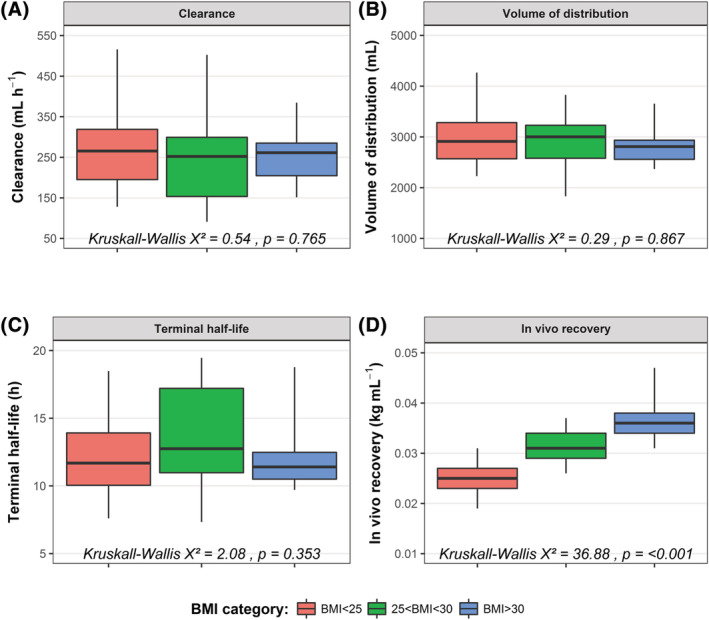
No differences in individual pharmacokinetic (PK) parameters (clearance, volume of distribution and terminal half‐life) between BMI categories. (A) Clearance. (B) volume of distribution of the central compartment. (C) Terminal elimination half‐life. (D) Calculated in vivo recovery. The in vivo recovery was calculated using the body weight of the patient divided by the individual PK parameters estimates for V1. For each boxplot, the whiskers depict the 2.5th and 97.5th percentile of the data, whereas the box depicts the interquartile range. The median of the data is depicted by the black horizontal line inside the boxplot
3.5. Dosing in case of life‐threatening bleed
PK simulations were performed using posthoc PK parameters from each patient included in this study. BW and IBW were used to calculate the dose required in 2 clinical situations: a single dose of 50 IU kg−1, which may be a test dose for PK profiling, and a dose required to treat a life‐threatening bleed with a loading dose of 50 IU kg−1 followed by a twice daily dose of 25 IU kg−1. In Figure 3, simulations are presented for 1 typical normal weight, 1 typical overweight and 1 typical obese patient. For the patient with a BMI <25 kg m−2, no difference was observed between the FVIII levels calculated using BW or IBW. For the 2 other BMI categories, differences increased with increasing BMI for both achieved FVIII peak and trough levels. Importantly, dosing based on IBW resulted in similar peak and trough levels for each BMI category.
FIGURE 3.
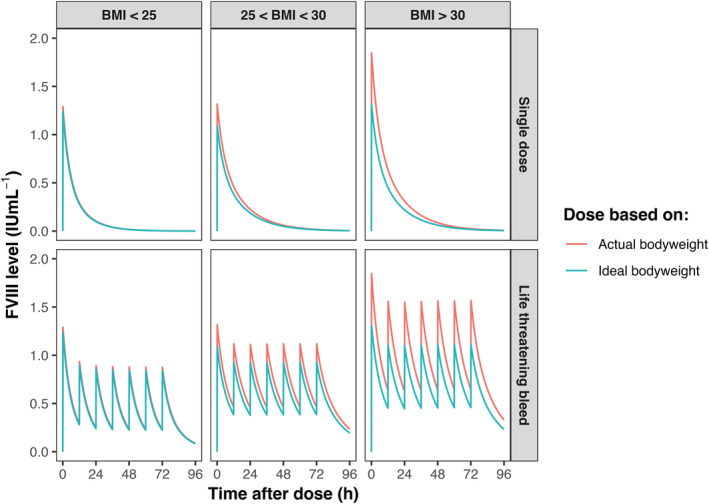
Coagulation factor VIII (FVIII) dosing based on body weight and ideal body weight in a normal weight, overweight and obese patient. The 3 examples were obtained by selecting typical patients from each body mass index (BMI) category; normal weight (BMI <25 kg m−2), overweight (BMI 25–30 kg m−2) and obese (BMI >30 kg m−2) patient. The lines from the plot depict the individual predicted FVIII levels after a simulated single‐dose of 50 IU kg−1 (upper panels). In the lower panels, a simulated loading‐dose was administered of 50 IU kg−1 followed by twice daily dosing of 25 IU kg−1 to treat a life‐threatening bleed. The individual FVIII levels were estimated using the individual pharmacokinetic (PK) parameters from the corresponding example patients
Figure 4 depicts the simulated FVIII trough and peak levels for all patients included in this cohort when treating a life‐threatening bleed by a loading dose of 50 IU kg−1 followed by doses of 25 IU kg−1 twice daily. In all patients with dosing based on IBW, no statistical differences were obtained for both FVIII peak (χ2 = 1.1, P = .57) and trough (χ2 = 2, P = .37) levels, whereas dosing based on BW resulted in significant differences for both FVIII peak (χ2 = 33.5, P < .001) and trough (χ2 = 9.43, P < .009) levels. This demonstrates that dosing based on BW results in unnecessary high peak levels in overweight/obese patients.
FIGURE 4.

Dosing based on ideal body weight results in adequate coagulation factor VIII (FVIII) peak and trough levels when treating a life‐threatening bleed. The FVIII peak levels (upper panels) were obtained 5 minutes after the simulated loading‐dose of 50 IU kg−1, which was followed by twice daily dosing of 25 IU kg−1 to treat a life‐threatening bleed. The trough FVIII levels (lower panels) were obtained immediately before the sixth dose, which corresponded with 72 hours after administration of the loading‐dose. The individual FVIII levels were simulated using the individual pharmacokinetic (PK) parameters from each included real‐life patient of the studied population. The blue bar depicts the median FVIII level. To enhance the visibility for the number of FVIII levels in each category, the FVIII levels were scattered horizontally
Figure 5 shows additional Monte Carlo simulations for a total of 1000 simulated patients to assess potential risks involved with the proposed model. Simulations show that dosing based on IBW for a life‐threatening bleed results in median trough levels of 0.40, 0.46 and 0.40 IU mL−1 for normal BMI, overweight and obese patients, respectively when dosed according to national guidelines. In contrast, dosing based on BW compensated the lower FVIII trough levels in the overweight and obese patients, although strikingly under dosing still occurred in patients with normal BMI when dosed according to BW. For the FVIII peak levels, dosing based on IBW prevent high FVIII peaks, while dosing based on BW resulted in unnecessary high peaks (supplementary table 3). In conclusion, dosing based on IBW prevents high FVIII peak levels, but is not superior when taking into account FVIII trough levels to treat life‐threatening bleeds. Critical analysis of current dosing strategies shows that dosing based on BW may lead to under dosing in cases of life‐threatening bleeds.
FIGURE 5.
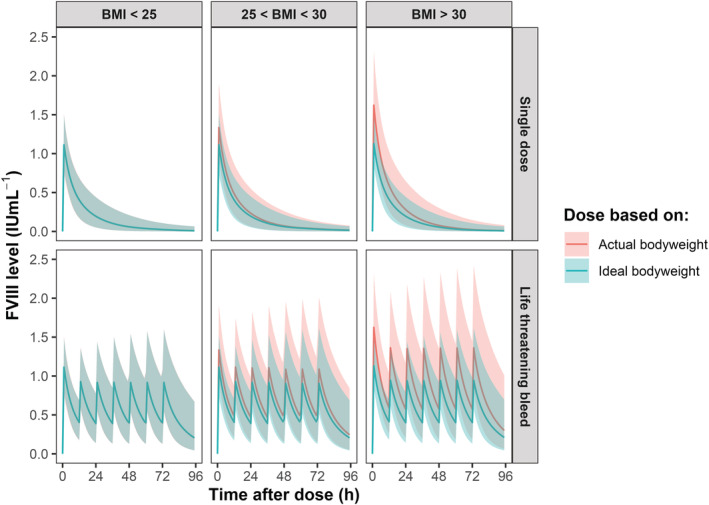
Monte Carlo simulations show adequate coagulation factor VIII (FVIII) levels when dosing is based on ideal body weight. Simulations were performed (n = 1000) using Monte Carlo simulations. The green bold line depicts the median FVIII level when dosing is based on ideal bodyweight and the green shade represents the corresponding 95% confidence interval. The bold red line represents the median FVIII level when dosing is based on actual bodyweight, whereas the red shades represent the 95% confidence interval. Both the upper and lower left panel do not show red lines (dosing based on bodyweight) or shades as they completely overlap with the green lines (dosing based on ideal bodyweight). The lines from the upper plots depict the predicted FVIII levels after a simulated single‐dose of 50 IU kg−1. In the lower panels, a simulated loading‐dose was administered of 50 IU kg−1 followed by twice daily dosing of 25 IU kg−1 to treat a life‐threatening bleed
4. DISCUSSION
In this study, the relationship between 5 morphometric variables (e.g. IBW, BMI, LBM, adjusted BW and calculated LBM) and the PK of FVIII concentrates was investigated in normal weight, overweight and obese haemophilia A patients. IBW best explained the interpatient variability in FVIII PK. Scaling PK parameters by IBW reduced the interpatient variability in CL and V1 from 45.1 to 37.6% and from 26.8 to 14.1%, respectively. CL, V1 and FVIII half‐life were similar between normal, overweight and obese patients while IVR increased with increasing BMI. This was also demonstrated in additional simulations of included study patients, in which it was observed that FVIII peak levels increased to unnecessary high values when dosing was based on BW in the overweight/obese patients. Dosing based on IBW was shown to prevent excessively high FVIII peak levels, but was not able to maintain FVIII trough levels >0.50 IU mL−1, when dosing according to national guidelines of The Netherlands.
Allometric scaling can be applied to partly explain IIV for a population PK parameter. By inclusion of allometric scaling, the fit of the model to collected data may improve, resulting in more accurate estimation of individual PK parameters required to calculate individualized doses. Although BW is generally used for allometric scaling, other morphometric variables can also be applied. Therefore, which morphometric variable correlated best with FVIII PK parameters was investigated. It was demonstrated that a more adequate fit was obtained using allometric scaling with IBW, reducing IIV of CL by 7.5% and V1 by 12.7%. If BW was used, IIV of CL and V1 was only reduced with 1.2 and 3%, respectively. Therefore, we conclude that IBW should be used for allometric scaling instead of BW in overweight and obese haemophilia A patients.
No differences were found in the estimated individual PK parameters (CL, V1 and half‐life) between normal weight, overweight and obese patients. Previous studies have demonstrated that increasing BMI results in higher von Willebrand factor (VWF) levels in both healthy individuals and haemophilia A patients. 21 , 22 , 23 This may be caused by increased shear stress upon the vessel wall caused by hypertension and atherosclerosis and subsequent secretion of VWF. 24 Furthermore, it has been shown that adipose tissue expresses VWF. 25 As VWF protects FVIII from proteolytic cleavage, it could be expected that FVIII CL may decrease with increasing BMI. However, the estimated individual PK parameters showed no differences in FVIII CL, V1 and FVIII half‐life between normal weight, overweight and obese haemophilia A patients.
Applying maximum a posteriori Bayesian analysis using the established population PK model to calculate FVIII doses is only possible when the source data are generalizable to the population which you wish to dose. Due to rising prevalence of obesity, overweight and obese patients should be adequately represented in the population used to construct population PK models. This fact also emphasizes that other morphometric variables instead of BW may be more adequate to calculate doses. The applied population PK model in this study was able to adequately fit FVIII levels obtained in all patients, as in the study population 36.8% of the patients were overweight and 17.5% were obese. Moreover, the population PK model was able to adequately fit FVIII levels obtained in all patients. Therefore, the established population PK model can be applied to the current haemophilia A patient population.
Simulations of the included real‐life patients on the basis of this population PK model using real‐world data, showed that dosing based on IBW resulted in adequate FVIII peak to treat or prevent a life‐threatening bleed, a circumstance during which it is required to achieve FVIII peak of 0.80–1.0 IU mL−1. However, the recommended dosing schedule of a loading dose of 50 IU kg−1 followed by 25 IU kg−1 twice a day does not result in adequate trough levels, both in case of dosing based on BW and IBW. This suggests that higher dosages may be needed to maintain adequate trough levels. These dosages should be based on IBW to prevent unnecessary high FVIII peak levels. When dosing FVIII based on IBW, interindividual differences in FVIII PK however do still exist. The variation in FVIII peak and trough levels in obese patients were comparable to levels obtained for the nonobese patients. It is always important to realize that IIV in bleeding tendency e.g. bleeding phenotype is notably large and not always explained by the FVIII levels measured after FVIII concentrate infusion. Nevertheless, clinical guidelines advise specific FVIII levels to prevent or treat bleeding in certain settings, and in clinical practice these dosing strategies reduce this variability in response. It must also be realized and anticipated that clearance and volume of distribution may alter in a situation of a life‐threatening bleeding risk, which we have not been able to simulate in this study.
The potential use of alternative morphometric variables for allometric scaling of model parameters has been addressed in previous studies. Garmann et al. constructed a population PK model for recombinant FVIII (rFVIII) concentrate, in which scaling was investigated using only BW and LBM. 26 This model was based on information from both children and adults with a median age of 22 years (range: 1–61 year). It is known that LBM should preferably be corrected when used in children. 27 However, the authors did not report whether they used different formulas to calculate LBM, while they did include LBM for allometric scaling of parameters in their final model. 26 In McEneny‐King et al., this model was subsequently applied to perform a simulation study, in which alternative dosing strategies of 1 brand of rFVIII concentrate based on various morphometric variables were simulated in 1000 normal weight (BMI <29.6 kg m−2) and 1000 overweight/obese (BMI 29.6–40.0 kg m−2) patients. 13 Although a different cut‐off point (BMI: 29.6 kg m−2) was applied to discriminate between normal and overweight/obese patients than used in the present study, it was also concluded that IBW demonstrates best predictive performance across all of the investigated dosing regimens. The present study using real‐world and simulated data, substantiates the conclusion that IBW is the best morphometric variable to explain the variability in PK in haemophilia A patients with varying FVIII concentrates.
In conclusion, this study investigated the relationship between 5 morphometric variables and FVIII PK in normal weight, overweight and obese haemophilia A patients. Scaling of the model parameters using IBW best explained the IIV of PK parameters and provided the most optimal description of the measured FVIII levels. Additional research is needed to specifically develop adequate dosing schedules based on IBW to treat life‐threatening bleeds. If future dosing strategies are indeed based on IBW to prevent excessive dosing in daily practice, higher dosing may be needed to adequately treat bleeding in critical circumstances.
COMPETING INTERESTS
R.E.G. Schutgens has received research support from CSL Behring and Sanquin. B. Laros‐van Gorkom has received unrestricted educational grants from Baxter and CSL Behring. F.J.M. van der Meer received grants from Bayer, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Sanquin and Sobi for the development of a registry of haemophilia patients in the Netherlands (HemoNED). K. Fijnvandraat is a member of the European Hemophilia Treatment and Standardization Board sponsored by Baxalta, has received unrestricted research grants from CSL Behring, Pfizer and Bayer, and has given lectures at educational symposiums organized by Pfizer, Baxalta, Novonordisk and Bayer. F.W.G. Leebeek received research support from CSL Behring and Shire, and is consultant for uniQure, Novo Nordisk and Shire, of which the fees go to the institution. He is a member of the DSMB for a of Roche study. K. Meijer received research support from Bayer, Sanquin and Pfizer; speaker fees from Bayer, Sanquin, Boehringer Ingelheim, BMS and Aspen; consulting fees from Uniqure (all fees go to the institution). R.A.A. Mathôt has served as advisor for Bayer, CSL Behring, Merck Sharp & Dohme, Shire and Zeria (all honoraria/fees paid to the department); He has received unrestricted research grants from Bayer, CSL Behring and Shire. M.H. Cnossen has received unrestricted research/educational and travel funding from the following companies: Pfizer, Baxter, Bayer Schering Pharma, CSL Behring, Novo Nordisk, Novartis and Nordic Pharma over the years, and has served as a member on steering boards of Roche and Bayer. All received fees go to the institution. The remaining authors declare no competing financial interests.
CONTRIBUTORS
I.M., H.H. and M.C. were responsible for protocol development and implementation of the study. I.M. enrolled patients, performed blood sampling for PK analysis and collected patient data. T.P. performed population PK modelling and Monte Carlo simulations. I.M. and T.P. analysed the data and are the main authors of the article with M.C. R.S., B.L., L.H., F.M., K.F., F.L. and K.M. monitored patient inclusion. M.C., R.M., F.L., K.F. and K.M. gave critical guidance during the project. M.H. and R.M. supervised the study. All authors substantially contributed to the writing and critically revised the manuscript, with approval of the final draft.
Supporting information
Table S1. Equations for the morphometric variables
Table S2. Model building‐steps for constructing the structural model for covariate analysis
Figure S1. Observed concentrations vs time plot
Figure S2. Goodness‐of‐fit plots for the final model
Figure S3. Prediction‐corrected visual predictive check plot of the final model
Figure S4. Correlation plot of all tested morphometric variables
Figure S5. Difference in OFV between the models using allometric scaling with body weight and ideal body weight.
ACKNOWLEDGEMENTS
This study is part of the international multicentre OPTI‐CLOT consortium research program (Patient tailOred PharmacokineTIc‐guided dosing of CLOTting factor concentrate and desmopressin in bleeding disorders), which aims to implement PK‐guided dosing of clotting factor replacement therapy and desmopressin by initiating studies that emphasize the impact of PK‐guided dosing, by constructing prophylactic and on‐demand population PK models, and by evaluating the cost‐effectiveness of a PK‐guided approach. A complete list of the members of the OPTI‐CLOT research program is available in the appendix. This study is part of the OPTI‐CLOT randomized controlled trial (Netherlands Trial Registry: NL3955) which was kindly funded by a grant from NWO‐ZonMw (grant number 836011011), a governmental research institution and by an unrestricted investigator‐initiated research grant provided by Baxter/Shire/Baxalta/Takeda.
van Moort I, Preijers T, Hazendonk HCAM, et al. Dosing of factor VIII concentrate by ideal body weight is more accurate in overweight and obese haemophilia A patients. Br J Clin Pharmacol. 2021;87:2602–2613. 10.1111/bcp.14670
The authors confirm that the principal investigator for this paper is Dr M.H. Cnossen, MD, PhD, and that she was clinically responsible for patients.
Dr. Iris van Moort and Dr. Tim Preijers are shared first authors, and both Prof.dr. Ron Mathôt and Dr. Marjon Cnossen are shared last authors.
[Correction added on 31 January 2021, after first online publication: Author information has been added in this current version.]
Funding information Baxalta, Grant/Award Number: GHOL 6238; NWO‐ZonMw, Grant/Award Number: 836011011
DATA AVAILABILITY STATEMENT
The data that support the findings of this study will be made available by the corresponding author after a reasonable request.
REFERENCES
- 1. Alexander SPH, Kelly E, Mathie A, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: introduction and other protein targets. Br J Pharmacol. 2019;176:S1‐S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. Treatment guidelines working group on behalf of the world Federation of H. guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1‐e47. [DOI] [PubMed] [Google Scholar]
- 3. Fijnvandraat K, Cnossen MH, Leebeek FW, Peters M. Diagnosis and management of haemophilia. BMJ. 2012;344:e2707. [DOI] [PubMed] [Google Scholar]
- 4. Bjorkman S, Folkesson A, Jonsson S. Pharmacokinetics and dose requirements of factor VIII over the age range 3‐74 years: a population analysis based on 50 patients with long‐term prophylactic treatment for haemophilia a. Eur J Clin Pharmacol. 2009;65(10):989‐998. [DOI] [PubMed] [Google Scholar]
- 5. Bjorkman S, Oh M, Spotts G, et al. Population pharmacokinetics of recombinant factor VIII: the relationships of pharmacokinetics to age and body weight. Blood. 2012;119(2):612‐618. [DOI] [PubMed] [Google Scholar]
- 6. Wilding J, Zourikian N, Di Minno M, et al. Obesity in the global haemophilia population: prevalence, implications and expert opinions for weight management. Obes Rev. 2018;19(11):1569‐1584. [DOI] [PubMed] [Google Scholar]
- 7. Henrard S, Speybroeck N, Hermans C. Body weight and fat mass index as strong predictors of factor VIII in vivo recovery in adults with hemophilia a. J Thromb Haemost. 2011;9(9):1784‐1790. [DOI] [PubMed] [Google Scholar]
- 8. Henrard S, Hermans C. Impact of being overweight on factor VIII dosing in children with haemophilia a. Haemophilia. 2016;22(3):361‐367. [DOI] [PubMed] [Google Scholar]
- 9. Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation. 1977;56(4):605‐612. [DOI] [PubMed] [Google Scholar]
- 10. Mahmood I. Allometric extrapolation of factors VII, VIII and IX clearance in children from adults. J Thromb Haemost. 2012;10(8):1609‐1613. [DOI] [PubMed] [Google Scholar]
- 11. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71‐87. [DOI] [PubMed] [Google Scholar]
- 12. Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58(2):119‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McEneny‐King A, Chelle P, Henrard S, Hermans C, Iorio A, Edginton AN. Modeling of body weight metrics for effective and cost‐efficient conventional factor VIII dosing in hemophilia a prophylaxis. Pharmaceutics. 2017;9(4):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hazendonk HC, van Moort I, Fijnvandraat K, et al. The "OPTI‐CLOT" trial. A randomised controlled trial on periOperative PharmacokineTIc‐guided dosing of CLOTting factor concentrate in haemophilia a. Thromb Haemost. 2015;114(09):639‐644. [DOI] [PubMed] [Google Scholar]
- 15. Preijers T, van Moort I, Fijnvandraat K, et al. Cross‐evaluation of pharmacokinetic‐guided dosing tools for factor VIII. Thromb Haemost. 2018;118(3):514‐525. [DOI] [PubMed] [Google Scholar]
- 16. Garrow JS, Webster J. Quetelet's index (W/H2) as a measure of fatness. Int J Obes (Lond). 1985;9(2):147‐153. [PubMed] [Google Scholar]
- 17. Devine BJ. Gentamicin therapy. Drug Intell Clin Pharm. 1974;8:650‐655. [Google Scholar]
- 18. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051‐1065. [DOI] [PubMed] [Google Scholar]
- 19. Jonsson EN, Karlsson MO. Xpose‐‐an S‐PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58(1):51‐64. [DOI] [PubMed] [Google Scholar]
- 20. Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25‐36. [DOI] [PubMed] [Google Scholar]
- 21. Mertens I, Van Gaal LF. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3(2):85‐101. [DOI] [PubMed] [Google Scholar]
- 22. Blann AD, Bushell D, Davies A, Faragher EB, Miller JP, von McCollum CN. Willebrand factor, the endothelium and obesity. Int J Obes Relat Metab Disord. 1993;17(12):723‐725. [PubMed] [Google Scholar]
- 23. Tuinenburg A, Biere‐Rafi S, Peters M, et al. Obesity in haemophilia patients: effect on bleeding frequency, clotting factor concentrate usage, and haemostatic and fibrinolytic parameters. Haemophilia. 2013;19(5):744‐752. [DOI] [PubMed] [Google Scholar]
- 24. Galbusera M, Zoja C, Donadelli R, et al. Fluid shear stress modulates von Willebrand factor release from human vascular endothelium. Blood. 1997;90(4):1558‐1564. [PubMed] [Google Scholar]
- 25. Consortium GT. The genotype‐tissue expression (GTEx) project. Nat Genet. 2013;45:580‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garmann D, McLeay S, Shah A, Vis P, Maas Enriquez M, Ploeger BA. Population pharmacokinetic characterization of BAY 81‐8973, a full‐length recombinant factor VIII: lessons learned ‐ importance of including samples with factor VIII levels below the quantitation limit. Haemophilia. 2017;23(4):528‐537. [DOI] [PubMed] [Google Scholar]
- 27. Peters AM, Snelling HL, Glass DM, Bird NJ. Estimation of lean body mass in children. Br J Anaesth. 2011;106(5):719‐723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Equations for the morphometric variables
Table S2. Model building‐steps for constructing the structural model for covariate analysis
Figure S1. Observed concentrations vs time plot
Figure S2. Goodness‐of‐fit plots for the final model
Figure S3. Prediction‐corrected visual predictive check plot of the final model
Figure S4. Correlation plot of all tested morphometric variables
Figure S5. Difference in OFV between the models using allometric scaling with body weight and ideal body weight.
Data Availability Statement
The data that support the findings of this study will be made available by the corresponding author after a reasonable request.


