Abstract
Background and Objectives
Multiple variables play a role in spinal cord stimulation (SCS) treatment outcomes, including patient anatomy, pain pattern, lead location, stimulation parameters, and so on. A wide range of stimulation parameters are considered safe and on‐label, and as a result a growing number of new frequencies and frequency‐combinations are being incorporated into standard practice. A standardized approach to therapy delivery may provide more consistent outcomes for more patients. The Vectors study evaluated whether there is significant sustained improvement in pain and functional outcomes when therapy is delivered using a standardized approach.
Materials and Methods
Vectors, a post‐market, single‐arm study evaluated the safety and efficacy of SCS with an implantable neurostimulator starting with 1 kHz stimulation, targeting the T9–T10 disc space following paresthesia mapping. Subjects with chronic intractable low back and leg pain (visual analogue scale [VAS] ≥ 50 mm) were enrolled. The primary endpoint was change in overall pain (VAS) at the three‐month visit compared to baseline. Subjects were followed through 12 months. Secondary endpoints included changes in low back and leg pain, quality of life (European Quality of Life – Five Dimensions, EQ‐5D‐5L), disability (Oswestry Disability Index, ODI), individual subject goals, and subject satisfaction.
Results
There was a significant reduction in overall pain (VAS; 45.4 mm) through the three‐month visit, which was sustained through 12 months. At 12 months, 79% of subjects had ≥50% improvement in at least one pain domain (overall, lowback or leg) with 85% of subjects reporting therapy satisfaction. There was a decrease in disability and an improvement in quality of life with 70% of subjects achieving a personal activity goal by the three‐month visit.
Conclusions
Long‐term pain relief and improvement in quality of life and function were achieved when following a standardized workflow.
Clinical Trial Registration: The Clinicaltrials.gov registration number for the study is NCT03345472.
Keywords: Chronic neuropathic pain, functional outcomes, high‐frequency stimulation, pain relief, prospective
INTRODUCTION
The clinical use of spinal cord stimulation (SCS) can be complex for various reasons, such as variability in pain type and/or location, programming, hardware choices, or individual patient preferences and expectations. A standardized approach to SCS therapy, including lead location and stimulation parameters may help physicians achieve improved outcomes for their patients. In medical practice, workflows have been used to standardize the approach to patient care with clinical benefits (1, 2). When considering a standardized workflow for SCS, there is established evidence for specific stimulation location and programming parameters (3, 4, 5, 6).
Research and clinical experience indicate that targeting midline of the spinal cord at the T9–T10 disc space is a favorable location for SCS for the treatment of back pain while limiting undesired chest or abdominal wall stimulation. In 1993, Barolat reported that conventional SCS parameters provided fair to excellent pain relief reported by 68.8% of patients for back pain and 88.2% of patients for leg pain after one year with the majority of leads spanning the T9–T10 disc space in 106 subjects (3). The significance of the T9–10 disc space was reinforced by the publication of independent case series by Sharan and North in which optimal programming for back pain was achieved at this location (7, 8, 9). More recently, SCS in the kilohertz frequency range has focused on energy delivery around T9–T10, with evidence and clinical experience supporting its continued use across multiple parameters (4, 5, 6).
The amount of electrical energy delivered by SCS devices is calculated from the frequency, pulse width, and amplitude (10, 11). The “pulse density” or duty cycle and the “charge per second” are two ways to characterize energy delivery without relying on specific waveform shape. These terms allow stimulation to be categorized as low density (duty cycle ~1–5%) or high density (duty cycle >5%), or low dose (LD) and high dose (HD) when amplitude is considered. There is a widening range of HD stimulation parameters for kilohertz SCS from 10 kHz down to 1 kHz and below (4, 5, 6, 12, 13, 14) being used for SCS. Stimulation at 1 kHz has provided pain relief at trial (15, 16) and after implant in a number of published studies reproduced at multiple sites using an array of devices (6, 12, 15, 16).
The EvolveSM workflow provides a standardized approach to SCS therapy incorporating both HD and LD therapy options for patients (15, 17). With increasing evidence for new stimulation modalities, offering patients a workflow‐based approach with multiple options may allow optimization and individualized therapy within a structured methodology. This standardized workflow suggests delivering therapy starting with HD programming parameters (90–220 μsec and 1 kHz) targeting the T9–T10 disc space after paresthesia mapping. If HD therapy does not provide adequate pain relief and symptom benefits, LD programming remains a viable proven option for patients (4, 18, 19, 20, 21). This workflow‐based approach also includes regular follow‐up for the duration of the SCS trial with the goal of optimized pain relief and functional outcomes.
The Vectors study was designed to assess the long‐term effectiveness of SCS when following a standardized workflow at trial with a structured follow‐up through 12 months after implant. The purpose of the study was to evaluate changes in pain and functional outcomes. The study design was intended to provide clinicians with a replicable approach to the use of SCS aligned with routine clinical practice.
MATERIALS AND METHODS
This study was approved by Western Institutional Review Board, Lehigh Valley Health Network IRB, and St. Luke's University Health Network IRB. The Vectors study is a post‐market, single‐arm study evaluating the efficacy of SCS therapy for pain relief starting with HD stimulation parameters targeting a specific anatomical location following paresthesia mapping. The study was registered on clinicaltrials.gov (NCT03345472).
Subjects with chronic intractable low‐back and leg pain were enrolled at 20 sites in the United States from November 2017 through July 2018. Subjects who were candidates for SCS were screened for eligibility. Inclusion and exclusion criteria are detailed in Table 1. Consented subjects who were indicated for SCS and reported a pain score (as assessed by the visual analogue scale [VAS, 0–100]) of at least 50 mm for both low back and leg pain and had an Oswestry Disability Index (ODI) score in the range of moderate to crippled at their baseline visit were eligible to continue to the temporary screening trial.
Table 1.
Inclusion and Exclusion Criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
For the screening trial, leads were placed based on intraoperative paresthesia mapping and if the lead placement spanned the T9–T10 disc space after mapping, the subject could remain in the study. During the trial, subjects were programmed using a sequential clinical workflow. This began with an initial HD program (pulse width of 90–220 μsec; rate of 1 kHz). Guidance was to start with 90 μsec and then to widen the PW, if necessary (depending on high impedances, subject's comfort or lack of efficacy).
Therapy was delivered starting with a bi‐pole electrode targeting the T9–T10 disc space. Amplitude was increased to perception threshold and then adjusted to a comfort based on subject feedback. Subjects were instructed to increase or decrease their therapy amplitude to maintain pain relief and comfort. If necessary, a second HD program (adjusting electrode location) and a subsequent LD program (based on best medical judgment of the investigator) were evaluated. If subjects reported at least a 50% improvement in their overall pain to either of the HD programs, their leads were explanted, without exposure to the other available program(s), and they were eligible to receive a permanent SCS implant. Guidance suggested a minimum of 48 hours of exposure to each program before assessing pain relief to allow subjects time to respond to therapy. For all subjects, trials were limited to no longer than 10 days, per labeling.
Subjects who had a successful HD screening trial were then scheduled for a permanent implant. Implanted neurostimulators (Intellis with AdaptiveStim technology) were activated 9–16 days after implant, to allow time for healing, with stimulation parameters starting at 90–220 μsec and 1 kHz with amplitude set to comfort. After evaluation of initial programming, subjects could be programmed to other stimulation settings if needed as determined by the physician and the subject's response to therapy. Subjects were assessed for pain relief (VAS) in‐clinic at 2, 4, and 6 weeks and 3, 6, and 12 months postactivation. If subjects had ≤50% improvement in overall pain at a study visit, they were offered reprogramming.
The primary objective of the study was to demonstrate a significant improvement in overall pain intensity, as measured by the VAS, from baseline to the three‐month visit. Secondary objectives were to characterize the overall, low back, and leg pain responder rates, where a responder was defined as achieving at least a 50% improvement in pain compared to baseline scores, as measured by the VAS.
Following intention‐to‐treat principles, all implanted and activated subjects (treated analysis set) were included in the analysis of the changes in pain and the responder rates. Missing 3‐, 6‐, or 12‐month pain scores were imputed using multiple imputation methods, where missing data were imputed multiple times, analyzed within each imputation, and then combined into one analysis result at each follow‐up time point. The statistical models estimating changes in pain and responder rates were fit using the pain scores collected at the 2‐, 4‐, and 6‐week and 3‐, 6‐, and 12‐month visits. Sensitivity analyses were conducted for subjects with complete 3‐, 6‐, and/or 12‐month pain scores (completers analysis set) to summarize the observed data and explore the consistency of these results as compared to those from the treated analysis set.
Additional study endpoints were evaluated using the data available at the associated follow‐up visits. Using the European Quality of Life – Five Dimension, Five Level (EQ‐5D‐5L) (22), changes in health‐related outcomes (utility score (23) and health state [EQ VAS]) were measured at the 3‐, 6‐, and 12‐month visits. In addition to changes in the EQ‐5D, the percentage of subjects with a better health state as assessed by the EQ‐5D was characterized, where a better health state is defined as the EQ‐5D assessment had improved on at least one of the five dimensions (mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression) and had not worsened in any other dimension from the baseline visit. Changes in functional disability were assessed at the 3‐, 6‐, and 12‐month visits using the ODI (24, 25, 26). Self‐defined activity goals were created at the baseline visit. Achievement of the activity goal was assessed beginning at the two‐weeks visit through the three‐month visit. Subjects who achieved their activity goal set a new one. Patient Global Impression of Change (PGIC) (27) was measured at the 3‐, 6‐, and 12‐month visits. Subject satisfaction with the therapy was measured at the 3‐, 6‐, and 12‐month visits using a Likert scale from very unsatisfied to very satisfied. The safety assessment characterized adverse events and device deficiencies that occurred from device activation through the study exit.
Prespecified hypothesis testing of the primary objective was conducted using a one‐sample t‐test, which tested whether there was a significant improvement in overall pain from baseline to the three‐month visit, based on the estimated change in overall pain from the multiple imputations model. Post hoc statistical testing of the change from baseline through the 12‐month visit was conducted using a repeated measures analysis of variance (ANOVA) for each of the following patient‐reported outcome measures: overall pain, low back pain, leg pain, EQ‐5D utility score, EQ VAS, and ODI. The t‐value (df = 102) and corresponding p‐value associated with the evaluation of change from baseline to 12 months are shown for each outcome measure.
All analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The study enrolled 175 subjects from 20 sites in the United States. Of these subjects, 143 met all inclusion/exclusion criteria and proceeded to screening trial: 122 subjects reported at least a 50% improvement in their pain using HD settings at the trial day‐2 or day‐4 visit (85% HD trial success rate); an additional seven subjects, when evaluated at the trial day‐6 visit, reported at least a 50% improvement in their pain after using parameters that were all below a pulse density of 5% and based on physician preference. There was an overall trial success rate of 90%. Of the 122 subjects who reported a 50% or greater improvement in their pain with HD settings, 103 proceeded to permanent SCS system implant and device activation; the 19 subjects who had successful trials but did not go onto implant were discontinued for various reasons, such as physician decision, insurance denial, lost to follow‐up, or withdrawal by subject (Fig. 1). Ninety‐eight subjects completed the three‐month visit, 96 subjects completed the six‐month visit, and 91 subjects completed the 12‐month visit (Fig. 1).
Figure 1.
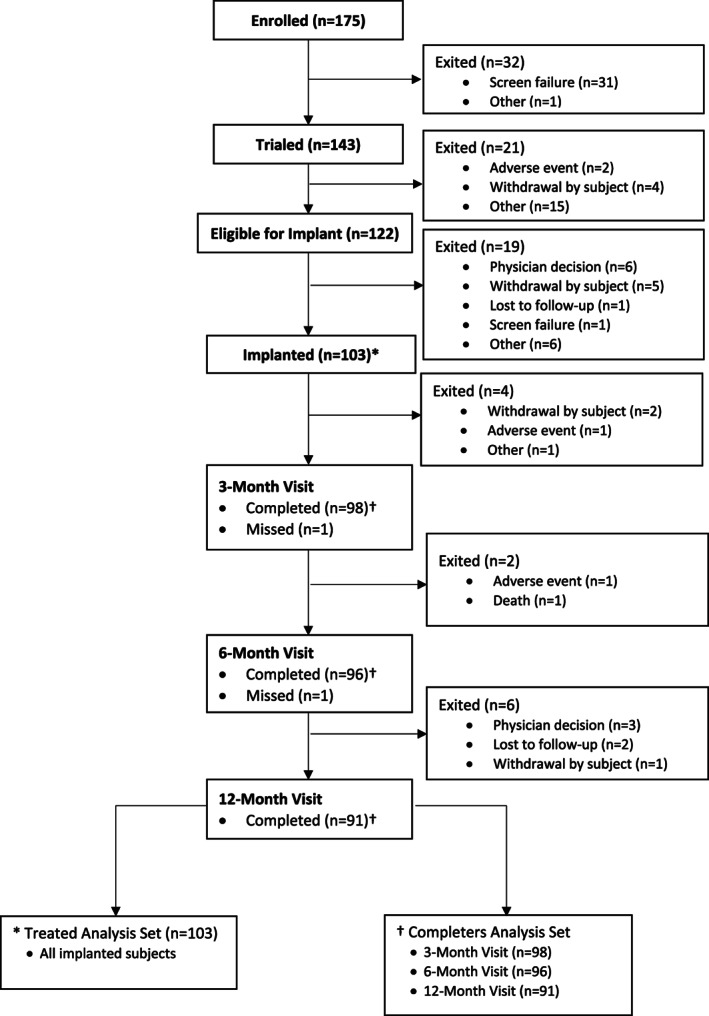
Subject disposition in study from enrollment through 12‐month visit or study exit.
Demographics and Baseline Characteristics
See Table 2 for the demographics of implanted subjects.
Table 2.
Demographics and Baseline Characteristics of Implanted Subjects.
| Baseline characteristics (N = 103) | |
|---|---|
| Age in years | |
| Mean (SD) | 60.8 (13.4) |
| Minimum to maximum | 29–93 |
| Sex (n, %) | |
| Female | 56 (54.4%) |
| Race (n, %) | |
| Black or African American | 10 (9.7%) |
| White | 92 (89.3%) |
| Other | 1 (1.0%) |
| Ethnicity (n, %) | |
| Hispanic or Latino | 4 (3.9%) |
| Not Hispanic or Latino | 93 (90.3%) |
| Not reported | 5 (4.9%) |
| Unknown | 1 (1.0%) |
| Years from pain onset (n = 102) | |
| Mean (SD) | 12.9 (11.1) |
| Minimum to maximum | 1–53 |
| Primary diagnosis (n, %) | |
| Postlaminectomy pain/FBSS* | 46 (44.7%) |
| Radicular pain syndrome | 28 (27.2%) |
| Degenerative disc disease | 14 (13.6%) |
| Complex regional pain syndrome | 1 (1.0%) |
| Other | 14 (13.6%) |
| Number of prior surgical procedures | |
| Mean (SD) | 1.4 (1.4) |
| Median | 1 |
| Minimum to maximum | 0–7 |
| Overall pain (0–100) | |
| Mean (SD) | 77.2 (12.7) |
| Minimum to maximum | 45 to 100 |
| Low back pain | |
| Mean (SD) | 75.1 (12.8) |
| Minimum to maximum | 50–100 |
| Leg pain | |
| Mean (SD) | 74.4 (13.2) |
| Minimum to maximum | 52–100 |
| Top 3 classes of medications (n, %) | |
| Opioids | 66 (64.1%) |
| Anticonvulsants – GABAergic | 51 (49.5%) |
| NSAID | 28 (27.2%) |
Physicians had a choice to use diagnosis of either postlaminectomy pain or FBSS.
Primary Objective
Within the treated analysis set, the average (SE) overall pain score reduced from 77.2 (1.2) at baseline to 31.8 (2.5) at three months (Fig. 2a). The mean (SE) decrease in overall pain of 45.4 (2.6) was statistically significant (t = 17.7, p < 0.0001). Subjects demonstrated a significant improvement in overall pain intensity from baseline to three months; therefore, the study met its primary objective. This result was consistent with the sensitivity analysis of the completers analysis set (n = 98), which had an observed average (SE) decrease in overall pain of 45.8 (2.6) from baseline to three months (Fig. 2b). This reduction in pain was observed with the majority of subjects having three or fewer programming changes and one or no unscheduled visits (74.5% and 72.4%, respectively).
Figure 2.
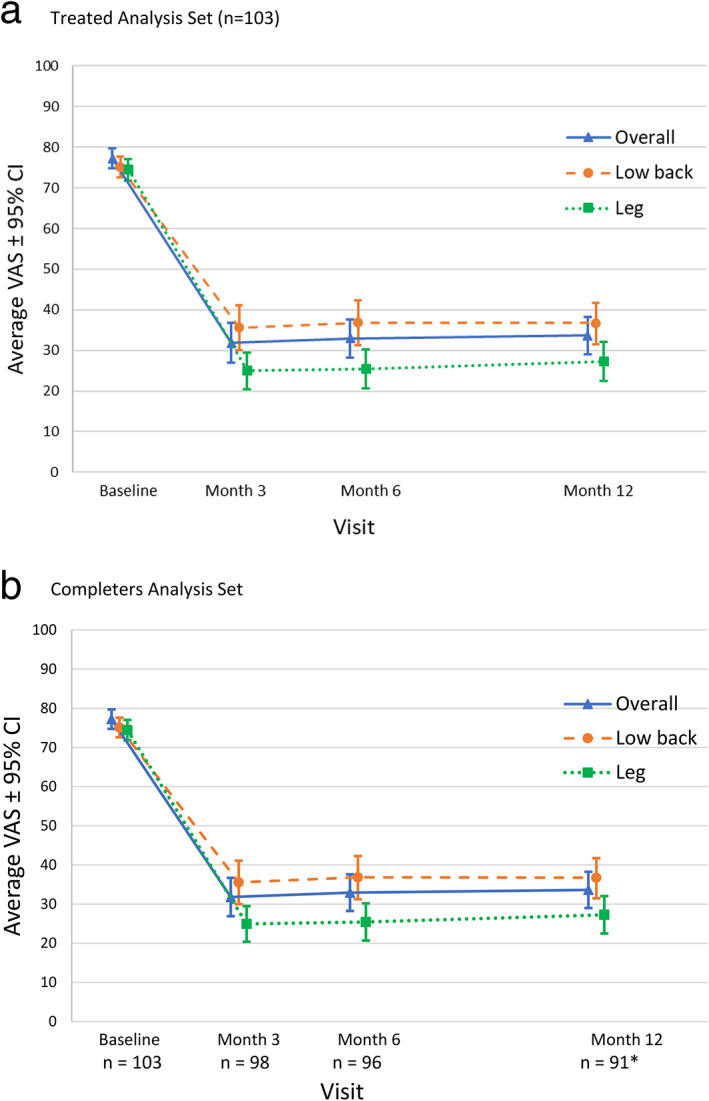
Longitudinal average overall, low‐back and leg VAS by visit. a. There is a decrease in pain in all three pain domains from baseline through the 12‐month visit (treated analysis set [n = 103]). b. There is a decrease in pain in all three pain domains from baseline through the 12‐month visit (completers analysis set). *Overall pain n=90. [Color figure can be viewed at wileyonlinelibrary.com]
Changes in Overall, Low Back, and Leg Pain Through 12 Months
Average pain scores at baseline and the 3‐, 6‐, and 12‐month visits are shown for the treated analysis set (Fig. 2a) and the completers analysis set (Fig. 2b). Within the treated analysis set, the estimated average (SE) reduction in pain from baseline was also significant and sustained through 12 months in overall (43.6 [2.3], t = 18.3, p < 0.0001), low back (38.4 [2.4], t = 16.0, p < 0.0001), and leg (47.5 [2.4], t = 19.7, p < 0.0001) pain. The average VAS scores and percent decrease in pain from baseline to the 3‐, 6‐, and 12‐month visits are shown in Table 3.
Table 3.
VAS Scores and Percent Decrease in Overall, Low Back, and Leg Pain From Baseline Through the 12‐Month Visit.
| Overall pain | Low back pain | Leg pain | |||||
|---|---|---|---|---|---|---|---|
| N | Mean VAS (SE) | Mean % Change | Mean VAS (SE) | Mean % Change | Mean VAS (SE) | Mean % Change | |
| Treated analysis set | |||||||
| Baseline | 103 | 77.2 (1.2) | ‐ | 75.1 (1.3) | ‐ | 74.4 (1.3) | ‐ |
| Three‐month visit | 103 | 31.6 (2.4) | 58.6% | 35.5 (2.8) | 52.9% | 24.9 (2.3) | 66.2% |
| Six‐month visit | 103 | 32.9 (2.4) | 57.9% | 36.8 (2.7) | 51.5% | 25.4 (2.4) | 65.8% |
| Twelve‐month visit | 103 | 33.6 (2.3) | 56.7% | 36.7 (2.5) | 51.9% | 27.3 (2.4) | 63.7% |
| Completers analysis set | |||||||
| Baseline | 103 | 77.2 (1.2) | ‐ | 75.1 (1.3) | ‐ | 74.4 (1.3) | ‐ |
| Three‐month visit | 98 | 31.4 (2.5) | 59.1% | 35.0 (2.8) | 53.6% | 24.4 (2.3) | 66.9% |
| Six‐month visit | 96 | 32.2 (2.4) | 58.8% | 36.5 (2.8) | 52.0% | 24.4 (2.4) | 67.1% |
| Twelve‐month visit | 91 | 32.6 (2.4)* | 57.9%* | 35.8 (2.6) | 53.2% | 26.0 (2.5) | 65.3% |
N = 90; one subject with a 12‐month visit did not report an overall pain score.
Secondary Objectives of Overall, Low Back, and Leg Pain Responder Rates Through 12 Months
Within the completers analysis set at three months, 69.4%, 61.2%, and 78.6%, of subjects achieved at least a 50% reduction in their overall, low back, and leg pain, respectively (Table 4). These results were sustained through 12 months and were consistent with the analyses of the treated analysis set. At 3, 6, and 12 months, 87%, 83%, and 79% of subjects were responders in at least one pain domain (overall, low back, or leg pain), respectively.
Table 4.
Responder Rates in Pain From Baseline Through the 12‐Month Visit.
| Responder rate in VAS % (95% CI) | N | Overall pain | Low back pain | Leg pain |
|---|---|---|---|---|
| Treated analysis set | ||||
| Three‐month visit | 103 | 68.3% (59.0–77.5%) | 59.8% (49.9–69.7%) | 77.4% (69.1–85.7%) |
| Six‐month visit | 103 | 66.2% (56.9–75.5%) | 58.4% (48.8–68.1%) | 72.2% (63.1–81.3%) |
| Twelve‐month visit | 103 | 59.1% (49.0–69.2%) | 57.1% (47.1–67.1%) | 67.9% (58.5–77.2%) |
| Completers analysis set | ||||
| Three‐month visit | 98 | 69.4% (59.7–77.6%) | 61.2% (51.3–70.3%) | 78.6% (69.5–85.5%) |
| Six‐month visit | 96 | 67.7% (57.8–76.2%) | 59.4% (49.4–68.7%) | 74.0% (64.4–81.7%) |
| Twelve‐month visit | 91 | 61.1% (50.8–70.5%)* | 59.3% (49.1–68.9%) | 69.2% (59.1–77.8%) |
N = 90; one subject with a 12‐month visit did not report an overall pain score.
Changes in Disability and Functional Outcomes and Subject Satisfaction Through 12 Months
The majority of subjects (75.5%, 75.0%, and 76.9%) had a better health state as assessed by the EQ‐5D at 3, 6, and 12 months, respectively, compared with baseline. This improvement in health state was also reflected in the EQ‐5D‐5L utility score, which significantly increased from an average (SD) baseline score of 0.36 (0.22) to 0.65 (0.22), 0.68 (0.20), and 0.73 (0.17) at 3, 6, and 12 months, respectively (t = 16.7, p < 0.0001). The EQ VAS health score significantly improved from an average (SD) of 56.4 (22.0) at baseline to 70.3 (16.9), 73.0 (16.4), and 73.8 (20.3) at 3, 6, and, 12 months, respectively (t = 5.9, p < 0.0001). The average (SD) ODI score was significantly reduced (i.e., improved) from a baseline score of 53.8 (12.1) to scores of 36.5 (16.9), 34.4 (16.3), and 32.2 (15.9) at 3, 6, and 12 months, respectively (t = 13.4, p < 0.0001); 65.3%, 66.7%, and 75.8% of subjects improved by at least one disability category at 3, 6, and 12 months. At baseline, 82.5% of subjects were classified as severe or crippled based on the ODI, which reduced to 28.6% at the 12‐month visit (Fig. 3).
Figure 3.
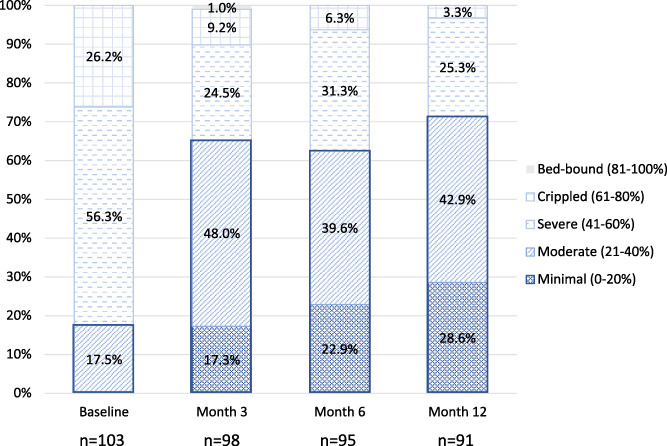
Longitudinal ODI categories by visit. The change in the percentage of subjects in each of the different ODI categories by visit is displayed. The number of subjects in the minimal and moderate categories increased from baseline through the 12‐month visit. [Color figure can be viewed at wileyonlinelibrary.com]
Secondary outcomes included achievement of personal activity goals, overall therapy satisfaction and subject recommendation of therapy to patients suffering from similar pain. At three‐months follow‐up, 70.4% of subjects had achieved at least one of their activity goals, which they had set at baseline. Fifty‐nine subjects achieved their baseline goal and set additional goals at a subsequent follow‐up visit through three months. On the Patient Global Impression of Change (PGIC) 56.1% of subjects rated their experience with SCS as “better” to “a great deal” better (Fig. 4a) at 12 months. In assessing overall therapy satisfaction, 81.6%, 89.6%, and 84.6% of subjects said they were very or somewhat satisfied with the therapy at 3, 6, and 12 months, respectively (Fig. 4b). Additionally, 82.4% of subjects said that they would recommend the therapy at 12 months.
Figure 4.
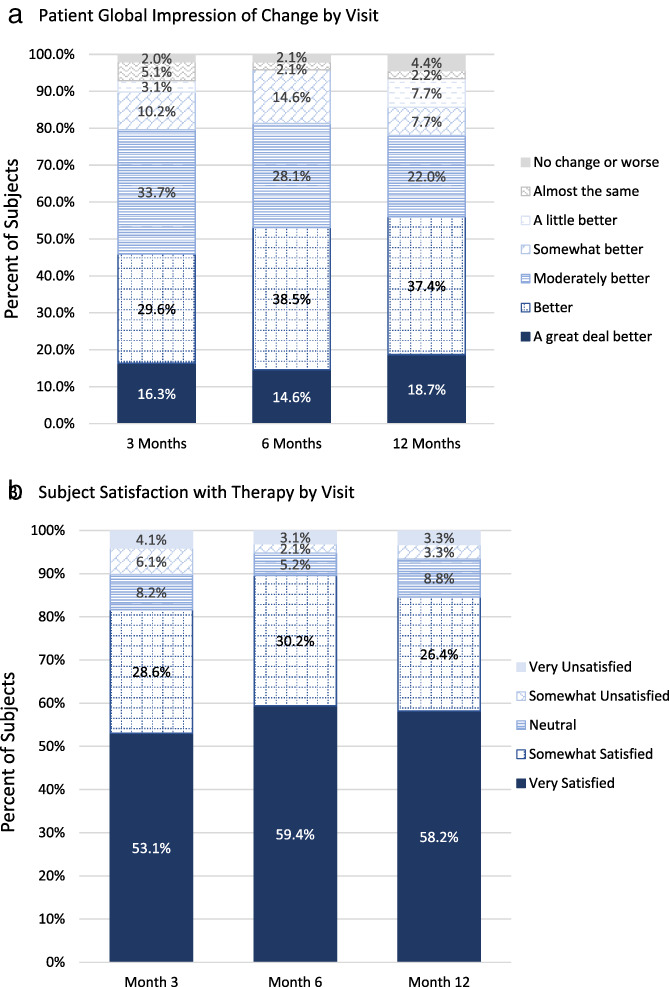
Subject impression of and satisfaction with the therapy. a. Subjects' impression of change through the 12‐month visit. b. Subjects' satisfaction with the therapy through the 12‐month visit. [Color figure can be viewed at wileyonlinelibrary.com]
Changes in Medications Through 12 Months
The mean (SD) number of prescribed pain medications at baseline and the 3‐months visit was 2.3 (1.4) and 2.2 (1.5), respectively. This was consistent through the 12‐month visit with a mean (SD) of 2.3 (1.5) and 2.4 (1.6) number of prescribed pain medications at 6 and 12 months, respectively. From baseline to the 12‐month visit, there was no change or a decrease in opioids in the majority of subjects (87.4%) and no opioid use in 34.1% of subjects at 12 months. The average (SD) prescribed morphine milligram equivalents (MME) for implanted subjects at baseline was 22.1 (36.5), and 35 subjects had 0 MMEs at baseline. There was no change through the 12‐month visit with 20.9 (34.1), 20.2 (33.2), and 20.6 (31.4) average MMEs prescribed at 3, 6, and 12 months. Nine subjects started with “high” MMEs at baseline (defined as ≥90); these subjects decreased from an average baseline MME of 120.5 (31.2) to 81.2 (56.1) at the 12‐month visit.
Programming Characterization
HD programming during the SCS screening trial consisted of 1 kHz and a mean (SD) pulse width of 139 (55) μsec (range, 90–220 μsec). Mean amplitude was 3.5 (1.6) mA, ranging from 1.0 to 9.5 mA. Based on these settings, the mean pulse density used at trial was 13.9% (range, 9%–20%) and the mean charge per second was 490 μC (range, 90–1900 μC). At three months, 97% of subjects remained on HD stimulation parameters. By 12 months, 95% were still using HD stimulation parameters; of these, 60% remained on 90–220 μsec and 1 kHz, and 40% continued with a different HD program above 5% pulse density. Mean amplitude for subjects using HD stimulation parameters was 3.9 (1.8) mA, ranging from 0.4 to 8.2 mA. Based on these settings, the mean pulse density used at 12 months was 17.2% (range, 5.4%–25.0%), and the mean charge per second was 683 μC (range, 80–1640 μC).
Safety
There was one device‐ or therapy‐related serious adverse event reported in the study, which was back pain exacerbation and it resolved with medication. There were 83 device‐, therapy‐, or procedure‐related adverse events that occurred in 41.7% (43/103) of subjects from device activation through study exit. The most common event was uncomfortable stimulation or paresthesia with 39 occurrences in 27.2% (28/103) of subjects. Of these 39 events, 38 resolved with reprogramming or without intervention, and one resolved with a repositioning of the neurostimulator. The second most common event was back pain with 11 occurrences in 9.7% (10/103) of subjects.
DISCUSSION
The Vectors study demonstrated the long‐term effectiveness of SCS at 12‐months when following a standardized workflow at trial. The primary endpoint at three months showed a statistically significant decrease in overall pain as measured by the VAS of 45.4 mm from 77.2 to 31.8. This effect was maintained through 12 months with subjects experiencing an estimated average 43.6 mm decrease on the VAS. Nearly 70% of the subjects had at least a 50% improvement in overall pain at their three‐month visit, which was sustained through 12 months with 61.1% of subjects who completed the 12‐month visit being responders. Significant improvements were also seen in ODI and EQ‐5D. Additionally, subjects were satisfied with their therapy through 12 months, and 70% achieved an activity goal set at their baseline visit by the three‐month visit. The study supports the long‐term use of HD stimulation parameters as an effective option for pain management and the potential value of a standardized approach to therapy providing ongoing access to both HD and LD programming.
There are several limitations to this prospective, observational study. Only subjects with a successful HD trial were followed long‐term, so the potential for using HD after use of LD at trial is unknown. Only a small number of subjects (approximately 5%) switched to LD stimulation during the follow‐up, so no conclusions can be made regarding the cumulative benefit of providing access to multiple therapy options (HD and LD). Assessing the impact of SCS on the achievement of personalized subject activity goals was limited as goals were only captured and evaluated through the primary endpoint at three months, and a validated tool was not used to measure activity. Another limitation of the study is its single‐arm study design and the lack of a control group.
Subjects included in the study had back and leg pain scores of at least 50 mm and had a score between 21 and 80 on the ODI. The study showed successful reduction in both low‐back and leg pain scores. Within all implanted subjects, low back pain decreased by 39.5 mm at three months with a responder rate (≥ 50% reduction in pain) of 59.8% and was sustained through 12 months. Leg pain decreased by 49.5 mm at three months with a responder rate of 77.4% and was also sustained through 12 months. Although subjects were required to have at least 50 mm for both low back and leg pain at baseline, a subject could have had more predominant low back or leg pain. The improvements observed in low back pain using HD stimulation were better than what has been observed historically using conventional stimulation alone (4, 21). While not all subjects were responders in the low back pain domain, at three months 87% of subjects were responders in at least one pain domain (overall, low back, or leg pain). This trend was sustained through 12 months with 83% and 79% of subjects responding in at least one pain domain at 6 and 12 months, respectively. Although pain relief remains the primary objective of SCS, there might be more clinical utility in taking a more holistic view by assessing patients across multiple endpoints (pain, quality of life, function, and medications) in a combined analysis.
Improvements in quality of life and function as measured by ODI and EQ‐5D were achieved and sustained through 12‐months follow‐up. At three months, 65% of subjects improved by one or more ODI category and 76% of subjects were better in one or more dimensions of the EQ‐5D and no worse on any dimensions. These results were maintained through 12 months for both ODI (76% of subjects) and EQ‐5D (77% of subjects). The mean improvement in EQ‐5D utility score through 12 months (0.37) was greater than the average minimally important difference (MID) of 0.074 as determined in the study by Walters et al., which evaluated the EQ‐5D‐3L from 11 clinical studies across a range of conditions (28). The collection of individualized activity goal(s) provides insights into subject's expectations of SCS therapy beyond reduction in pain. Seventy percent of subjects achieved a self‐defined activity goal by three months. The achievement of activity goals, improvement in quality of life and meaningful pain relief can be inferred to relate to the high degree of satisfaction with the therapy reported by subjects (84.6%). There are multiple factors that impact a patient's response to therapy (SCS programming, pain profile, energy delivery at the spinal cord, duration of therapy, and patient expectations). It is important to consider these when assessing the benefits of SCS. Every patient can present a unique clinical profile extending beyond the direct management of their chronic pain symptoms (29).
Treating chronic pain with SCS may encourage the discontinuation of opioids (30, 31). This study was neither powered nor designed to examine changes in MME over time. Subjects were required to be on stable medication when entering the study and were instructed not to increase pain medications for back or leg pain through the three‐month visit. The protocol did not dictate medication management after three months; however, the majority of subjects remained on low opioids throughout the study and subjects entering the study with high opioid utilization ≥ 90 MMEs) were able to decrease from an average of 120 MMEs at baseline to 81 MMEs by the 12‐month visit. This suggests that SCS therapy may help patients in maintaining LD opioids or reducing HD opioid consumption.
Applicability of clinical study outcomes into clinical practice is often lacking. The Vectors study was intentionally designed so that its outcomes and methodology could be replicated in clinical practice. Subjects enrolled were representative of the general pain population. The study protocol prescribed the use of a standardized workflow, which provides clinical guidance starting with HD stimulation and also maintains patient access to LD stimulation or combination therapy as needed. The cadence of visits and subject interaction was also designed to mirror a standard of care as much as possible. The outcomes of the study demonstrate that using a workflow approach to SCS resulted in clinically meaningful outcomes and a predictable therapy experience for patients and physicians with minimal touchpoints and programming changes. At 12 months, 95% of subjects remained on HD while 5% of subjects switched to LD parameters. Results from Vectors are consistent with previous research on this standardized workflow and physician reported experience (15, 17, 32).
Published long‐term outcomes with the use of HD stimulation parameters as part of a clinical workflow is limited, and this study adds longitudinal outcomes with a large sample size to the literature. Recent prospective studies of HD therapies using 1–1.2 kHz have been short‐term (6, 10, 16, 33), have had small sample sizes (34), or have only assessed HD after conventional programming (35). In contrast, the Vectors study provides needed additional prospective 12‐months outcome evidence from a large cohort, with a majority of patients programmed to HD therapy. While the standardized workflow prescribed in this study provides a starting point for incorporation into clinical practice and Vectors demonstrated sustained clinically meaningful outcomes, there may be future learnings that enable further optimization and delivery of SCS therapy. The sustainability of outcomes and safety of new SCS modalities should continue to be evaluated through robust and transparent clinical studies.
CONCLUSIONS
This long‐term prospective study demonstrated that significant reduction in chronic pain is achieved when a workflow approach to SCS therapy is followed. Guidance on initial programming settings, electrode target, and patient follow‐up allowed for consistency in patient treatment across centers. Structured follow‐up and flexibility in programming provided long‐term HD and LD therapy options. The durable and sustained results at 12 months for overall pain, back pain, and leg pain and the improvement in quality of life demonstrated in the Vectors Study are expected to be repeatable in clinical practice.
Authorship Statements
John Hatheway, Michael Verdolin, Matthew Kelly, Brian Acklin, Heather Gardner, Hector Cantua, Katherine Stromberg, and Chris Hilker designed the described study. The Vectors Investigators (see list below) collected data for the study. Katherine Stromberg performed the analysis of the data. Katherine Stromberg, Kelly Hendrickson, Nancy O'Connell, Matthew Kelly, Chris Hilker, and the Vectors Investigator authors (see bold names in Vectors Investigators list below) interpreted and synthesized the data. Matthew Kelly, Lisa Johanek, and Lachlan Davis wrote the manuscript. Kelly Hendrickson, Katherine Stromberg, and the Vectors Investigator authors (see bold names in Vectors Investigators list below) provided critical revisions to the manuscript.
Vectors Investigators
John A. Hatheway, MD, Vipul Mangal, MD, Michael A Fishman, MD, MBA, Philip Kim, MD
Binit Shah, MD, Rainer Vogel, MD, Vincent Galan, MD, Steven Severyn, MD, Tristan E. Weaver, MD, David Provenzano, MD, Eric Chang, MD, Michael H. Verdolin, MD, Gregory Howes, DO, Armando Villarreal, MD, Steven Falowski, MD, Daniel Mankoff, MD, Steven Rosen, MD, Bruce Nicholson, MD, Aaron Calodney, MD, Youssef Josephson, MD, Chris Merrell, MD, Kelby Hutcheson, MD.
Comment
This is an interesting study that attempts to answer an important question about subthreshold stimulation and suprathreshold SCS. Although the authors conclude that the results are applicable in clinical practice; the study's extensive inclusion‐exclusion list argues against that statement. For example, we remain unsure if this combination is helpful in CRPS or other forms of neuropathic pain where SCS may be utilized in clinical practice. The study is otherwise well designed, and very well presented.
Sam Eldabe, MBBS
Middlesbrough, United Kingdom
Acknowledgements
The authors would like to thank Hector Cantua, Shannon Collins, and Janelle Blum for their significant contributions to the execution of the study.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: This study was funded by Medtronic.
Conflicts of Interest: John A. Hatheway reports the following disclosures: Faculty Medtronic and Vertiflex, Consultant to Medtronic, Research Medtronic, Research Biotronik. Vipul Mangal serves as a consultant to Medtronic. Michael A. Fishman reports the following disclosures: Consultant to Abbott, Biotronik, Cornerloc, Medtronic, and Nevro. He has minority equity in Celeri Health and Thermaquil. His institution received research payments from Abbott, Biotronik, Medtronic, Stimgenics, and Thermaquil. Dr. Fishman is a Director‐at‐Large of the North American Neuromodulation Society. Phil Kim, MD reports the following disclosures: Consultant to Medtronic, Biotronik board, Research for Abbott and Medtronic, Tersera Speaking Bureau. Rainer Vogel reports the following disclosures: Consultant to Medtronic, Boston Scientific, Vertiflex, Nevro, Daichi‐Sankyo, Collegium, Salix, US Worldmed, Kaleo, Flexxion. Vincent Galan reports the following disclosures: Research for Medtronic, Nevro, Abbott, SPR Therapeutics, Neuros. Tristian E. Weaver reports the following disclosures: Consultant to Medtronic, research for Medtronic, Consultant to PainTeq. David Provenzano reports the following disclosures: Consultant to Avanos, Boston Scientific, Medtronic, Nevro, Esteve, and Salix, Research support from Medtronic, Nevro, Stimgenics, and Abbott. Michael. H. Verdolin reports the following disclosures: Consultant to Medtronic, Vertiflex, and Boston Scientific. Armando Villarreal reports the following disclosures: Board Fruit Street and MyRx365. Steven Falowski reports the following disclosures: Consultant to Medtronic, Abbott, Saluda, Vertiflex; Research for Medtronic, Abbott, Saluda, Biotronik, Nevro; Equity in Thermaquil, SPR Therapeutics, Saluda, CornerLoc, Stimgenics, Neural Integrative Solutions, Spine Thera, AGR. Kelly Hendrickson, Katherine Stromberg, Lachlan Davies, Lisa Johanek, and Matt Kelly are or were employees of Medtronic. Binit Shah, Steven Severyn, Eric Chang and Gregory Howes report no conflicts of interest.
REFERENCES
- 1. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth 2016;117:iii62–iii72. 10.1093/bja/aew362. [DOI] [PubMed] [Google Scholar]
- 2. Bolton L, McNees P, van Rijswijk L et al. Wound‐healing outcomes using standardized assessment and care in clinical practice. J Wound Ostomy Continence Nurs 2004;31:65–71. 10.1097/00152192-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 3. Barolat G, Massaro F, He J, Zeme S, Ketcik B. Mapping of sensory responses to epidural stimulation of the intraspinal neural structures in man. J Neurosurg 1993;78:233–239. 10.3171/jns.1993.78.2.0233. [DOI] [PubMed] [Google Scholar]
- 4. Kapural L, Yu C, Doust MW et al. Novel 10‐kHz high‐frequency therapy (HF10 therapy) is superior to traditional low‐frequency spinal cord stimulation for the treatment of chronic Back and leg pain. Anesthesiology 2015;123:851–860. 10.1097/ALN.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 5. Kapural L, Yu C, Doust MW et al. Comparison of 10‐kHz high‐frequency and traditional low‐frequency spinal cord stimulation for the treatment of chronic Back and leg pain: 24‐month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery 2016;79:667–677. 10.1227/NEU.0000000000001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomson SJ, Tavakkolizadeh M, Love‐Jones S et al. Effects of rate on analgesia in kilohertz frequency spinal cord stimulation: results of the PROCO randomized controlled trial. Neuromodulation. 2018;21:67–76. 10.1111/ner.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. North RB, Kidd DH, Olin J et al. Spinal cord stimulation for axial low back pain: a prospective, controlled trial comparing dual with single percutaneous electrodes. Spine (Phila Pa 1976) 2005;30:1412–1418. 10.1097/01.brs.0000166502.05449.a8. [DOI] [PubMed] [Google Scholar]
- 8. North RB, Kidd DH, Olin J, Sieracki JN, Petrucci L. Spinal cord stimulation for axial low back pain: a prospective controlled trial comparing 16‐contact insulated electrodes with 4‐contact percutaneous electrodes. Neuromodulation 2006;9:56–67. 10.1111/j.1525-1403.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- 9. Sharan A, Cameron T, Barolat G. Evolving patterns of spinal cord stimulation in patients implanted for intractable low back and leg pain. Neuromodulation. 2002;5:167–179. 10.1046/j.1525-1403.2002.02027.x. [DOI] [PubMed] [Google Scholar]
- 10. Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of spinal cord stimulation and their role in electrical charge delivery: a review. Neuromodulation. 2016;19:373–384. 10.1111/ner.12438. [DOI] [PubMed] [Google Scholar]
- 11. De Ridder D, Vanneste S, Plazier M, van der Loo E, Menovsky T. Burst spinal cord stimulation: toward paresthesia‐free pain suppression. Neurosurgery 2010;66:986–990. 10.1227/01.NEU.0000368153.44883.B3. [DOI] [PubMed] [Google Scholar]
- 12. North JM, Hong K‐SJ, Cho PY. Clinical outcomes of 1 kHz subperception spinal cord stimulation in implanted patients with failed paresthesia‐based stimulation: results of a prospective randomized controlled trial. Neuromodulation 2016;19:731–737. 10.1111/ner.12441. [DOI] [PubMed] [Google Scholar]
- 13. Provenzano DA, Rebman J, Kuhel C, Trenz H, Kilgore J. The efficacy of high‐density spinal cord stimulation among trial, implant, and conversion patients: a retrospective case series. Neuromodulation. 2017;20:654–660. 10.1111/ner.12612. [DOI] [PubMed] [Google Scholar]
- 14. Wille F, Breel JS, Bakker EWP, Hollmann MW. Altering conventional to high density spinal cord stimulation: an energy dose‐response relationship in neuropathic pain therapy. Neuromodulation 2017;20:71–80. 10.1111/ner.12529. [DOI] [PubMed] [Google Scholar]
- 15. Verdolin M, Hatheway J, Roy L. A large retrospective, multi‐center cohort study evaluating a novel SCS workflow for failed back surgery syndrome (FBSS) back and leg pain: final analysis with 31 month outcomes. Pain Med (United States) 2018;19:1105–1106. 10.1093/pm/pny044. [DOI] [Google Scholar]
- 16. Benyamin R, Hatheway JA, Galan V et al. Evaluating high dose parameters with spinal cord stimulation in failed back surgery syndrome patients. Neuromodulation 2018;21:e1–e149. 10.1111/ner.12774. [DOI] [PubMed] [Google Scholar]
- 17. Preuss C, Arcioni R, Cooper R et al. HD‐SCS using the evolve® workflow: a multicentric European experience report for treatment of FBSS. Neuromodulation 2019;22:E40–E295. 10.1111/ner.12950. [DOI] [PubMed] [Google Scholar]
- 18. Kumar K, North R, Taylor R et al. Spinal cord stimulation vs. conventional medical management: a prospective, randomized, controlled, multicenter study of patients with failed Back surgery syndrome (PROCESS study). Neuromodulation. 2005;8:213–218. 10.1111/j.1525-1403.2005.00027.x. [DOI] [PubMed] [Google Scholar]
- 19. North RB, Kidd D, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery 2007;61:361–368; discussion 368‐9. 10.1227/01.NEU.0000255522.42579.EA. [DOI] [PubMed] [Google Scholar]
- 20. Schultz DM, Webster L, Kosek P, Dar U, Tan Y, Sun M. Sensor‐driven position‐adaptive spinal cord stimulation for chronic pain. Pain Physician 2012;15:1–12. [PubMed] [Google Scholar]
- 21. Deer T, Slavin KV, Amirdelfan K et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel Burst waveform. Neuromodulation. 2018;21:56–66. 10.1111/ner.12698. [DOI] [PubMed] [Google Scholar]
- 22. EuroQol . A new facility for the measurement of health‐related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 23. Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health‐related quality of life: An EQ‐5D‐5L value set for England. Health Econ 2018;27:7–22. 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–273. [PubMed] [Google Scholar]
- 25. Fairbank JC, Pynsent PB. The Oswestry disability index. Spine (Phila Pa 1976). 2000;25:2940–2952; discussion 2952. 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 26. Hakkinen A, Kautiainen H, Jarvenpaa S, Arkela‐Kautiainen M, Ylinen J. Changes in the total Oswestry index and its ten items in females and males pre‐ and post‐surgery for lumbar disc herniation: a 1‐year follow‐up. Eur Spine J 2007;16:347–352. 10.1007/s00586-006-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther 2004;27:26–35. 10.1016/j.jmpt.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 28. Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ‐5D and SF‐6D. Qual Life Res 2005;14:1523–1532. 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 29. Goudman L, Bruzzo A, van de Sande J, Moens M. Goal identification before spinal cord stimulation: a qualitative exploration in potential candidates. Pain Pract 2019;20:247–254. 10.1111/papr.12845. [DOI] [PubMed] [Google Scholar]
- 30. Gee L, Smith HC, Ghulam‐Jelani Z et al. Spinal cord stimulation for the treatment of chronic pain reduces opioid use and results in superior clinical outcomes when used without opioids. Neurosurgery 2019;84:217–226. 10.1093/neuros/nyy065. [DOI] [PubMed] [Google Scholar]
- 31. Sharan AD, Riley J, Falowski S et al. Association of opioid usage with spinal cord stimulation outcomes. Pain Med 2018;19:699–707. 10.1093/pm/pnx262. [DOI] [PubMed] [Google Scholar]
- 32. Benyamin R, Galan V, Hatheway J et al. Options: a prospective, open‐label study of high‐dose spinal cord stimulation in patients with chronic Back and leg pain. Pain Physician 2020;23:87–98. [PubMed] [Google Scholar]
- 33. Al‐Kaisy A, Palmisani S, Pang D et al. Prospective, randomized, sham‐control, double blind, crossover trial of subthreshold spinal cord stimulation at various kilohertz frequencies in subjects suffering from failed Back surgery syndrome (SCS frequency study). Neuromodulation. 2018;21:457–465. 10.1111/ner.12771. [DOI] [PubMed] [Google Scholar]
- 34. Hamm‐Faber TE, Gultuna I, van Gorp E‐J, Aukes H. High‐dose spinal cord stimulation for treatment of chronic low Back pain and leg pain in patients with FBSS, 12‐month results: A prospective pilot study. Neuromodulation 2019;23:118–125. 10.1111/ner.12940. [DOI] [PubMed] [Google Scholar]
- 35. North J, Loudermilk E, Lee A et al. Outcomes of a multicenter, prospective, crossover, randomized controlled trial evaluating subperception spinal cord stimulation at </=1.2 kHz in previously implanted subjects. Neuromodulation 2020;23:102–108. 10.1111/ner.13015. [DOI] [PMC free article] [PubMed] [Google Scholar]


