Abstract
Objective
Long‐term respiratory consequences of bronchopulmonary dysplasia (BPD) in preterm infants born in the post‐surfactant era (“new” BPD) remain partially unknown. The present study aimed to evaluate the respiratory outcomes of “new” BPD in adolescents who were born preterm.
Methods
This multicenter, cross‐sectional study included 286 adolescents born between 2003 and 2005 (mean age: 14.2 years); among them, 184 and 102 were born extremely preterm (EP; <28 weeks' gestation) and moderate–late preterm (32 to <37 weeks' gestation), respectively. Among EP adolescents, 92 had BPD, and 92 did not. All participants underwent lung function tests, skin prick testing, and questionnaires on asthma symptoms and quality of life.
Results
EP adolescents with BPD had significantly lower forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and forced expiratory flow between 25% and 75% of FVC than other included adolescents. FEV1/FVC ratios were below the lower limit of normal (z‐score <−1.645) in 30.4% of EP adolescents with BPD, 13.0% of EP adolescents without BPD, and 11.8% of adolescents who were born moderate–late preterm. Bronchodilator response and air‐trapping were significantly higher in BPD adolescents than in other adolescents. Diffusion capacity was significantly lower in EP adolescents than in moderate–late preterm adolescents. Asthma symptoms and quality‐of‐life scores were similar among groups.
Conclusion
EP adolescents with “new” BPD had poorer pulmonary function than EP adolescents without BPD or moderate–late preterm adolescents. Further studies are needed to determine whether “new” BPD is associated with early‐onset chronic obstructive pulmonary disease in adulthood.
Keywords: asthma, chronic lung disease, prematurity, pulmonary function
1. INTRODUCTION
Bronchopulmonary dysplasia (BPD), a chronic pulmonary disease that mainly affects extremely preterm (EP) infants (<28 weeks gestational age [GA]), is one of the most common and serious complications of prematurity 1 and is associated with increased respiratory morbidity and reduced pulmonary function throughout childhood and adolescence. 2 , 3 The condition is a consequence of an arrest of lung development during late fetal and early postnatal life and can predispose individuals with BPD to chronic obstructive pulmonary disease in adulthood. 4
Notably, the prevalence of asthma/wheezing disorders is reported to be higher in children and adolescents who were born preterm, and the risk of these conditions increases in proportion to the degree of prematurity. 5 Given that preterm infants with BPD have also experienced early lung injury, they would be expected to have a higher prevalence of long‐term asthma symptoms than preterm infants without BPD. However, this association has not been thoroughly assessed, and it remains unclear whether BPD is a risk factor for asthma/wheezing disorders in adolescence, irrespective of prematurity. 6
Most follow‐up studies on pulmonary function and respiratory morbidity in adolescents have focused on BPD occurring in the pre‐surfactant era or “classic” BPD, which is characterized by lung damage from oxygen toxicity and mechanical ventilation. To date, improvements in neonatal care have led to a less‐severe form of BPD that occurs in more premature infants. Nevertheless, most previous studies on this “new” BPD focused on patients between 6 and 12 years old, but data is sparse on patients >12 years old. 7
This study aimed to evaluate the pulmonary function, asthma symptom prevalence, and quality of life in a group of adolescents who were born EP in the post‐surfactant era and developed “new” BPD. The results were compared with those obtained from two other groups of former preterm adolescents, who did not develop BPD.
2. MATERIALS AND METHODS
We conducted a cross‐sectional, multicenter study at 11 hospitals from 5 Spanish regions. The study was sponsored by the Working Group of Perinatal Respiratory Diseases of the Spanish Society of Pediatric Pulmonology and was approved by the ethics committees at each participating institution. Written consent was obtained from the adolescents and their parents/caregivers after providing a full explanation of the study protocol. The inclusion period was from May 2017 to June 2019.
2.1. Study population and data source
This study included adolescents who were born between 2003 and 2005. They were classified into the following three groups: EP adolescents (up to 28 weeks' GA) with associated BPD (EP‐BPD); EP adolescents without BPD (EP‐noBPD); moderate–late preterm adolescents (MLP; 32 to <37 weeks' GA). The accepted definitions of BPD used by each center at the time of diagnosis were as follows: (1) Supplementary oxygen requirements for ≥28 days, regardless of the situation at 36 weeks post‐menstrual age. (2) National Heart, Lung and Blood Institution Workshop definition 8 : 2a, Mild BPD; 2b, Moderate BPD; 2c, Severe BPD. (3) Requirement for supplemental oxygen at 36 weeks post‐menstrual age. We regrouped EP‐BPD patients into the following two subgroups according to disease severity: “low severity BPD” (definitions 1 and 2a) and “high severity BPD” (definitions 2b, 2c, and 3). The following were considered exclusion criteria: (1) mental retardation; (2) disabling cerebral palsy; (3) severe gastroesophageal reflux; and (4) history of lung resection, airway surgery, or cardiac surgery.
In each hospital, data provided by the medical records department were used to create a list of patients for each group. Telephone calls were conducted in chronological order, beginning with participants born in January 2003, and appointments were made for those who were interested. For every EP‐BPD adolescent who agreed to participate, one EP‐noBPD adolescent and one MLP adolescent were also selected. They were matched in sex and date of birth as closely as possible.
2.2. Asthma symptoms/quality of life
Supervised by their parents/caregivers, the adolescents completed Spanish versions of validated questionnaires on asthma symptoms (Global Asthma Network [GAN] written questionnaire) 9 and quality of life (Kiddo‐KINDL®). 10 The Kiddo‐KINDL® questionnaire consists of 24 items, which are grouped into subsections based on physical well‐being, emotional well‐being, self‐esteem, family relationships, friends, and school. Each item is scored between 1 (never) and 5 (always). The questions refer to the week before the appointment, and the scores obtained in each subsection are transformed into a 0–100 scale that allows for comparison. A higher score represents a better quality of life.
Based on the methodology of the International Study of Asthma and Allergies in Childhood (ISAAC), 11 current asthma was defined as a positive answer to the question: “Have you had wheezing or whistling in the chest in the last 12 months?” Medical records from neonatal intensive care and pediatric pulmonology units were reviewed, and parents/caregivers were interviewed to obtain additional demographic and clinical information.
2.3. Pulmonary function
All patients underwent spirometry with bronchodilator testing. Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and forced expiratory flow at 25%–75% of FVC (FEF25–75) were calculated. A positive bronchodilator response was defined as an increase in the FEV1 of ≥12% from baseline (15 min after inhalation of 400 mcg of salbutamol by metered‐dose inhaler plus spacer). Where available, total body plethysmography and lung diffusion by the single breath diffusion method were performed, and the following parameters were collected: total lung capacity (TLC), functional residual capacity, residual volume (RV), RV/TLC ratio, diffusing capacity of the lung for carbon monoxide (DLCO), and DLCO adjusted for alveolar volume (KCO). All parameters were expressed as a percentage of the predicted value (%pred) and as a z‐score, except for the RV/TLC ratio, which was only expressed as a percentage. The FEV1/FVC and RV/TLC ratios were also expressed as actual values (percentages). The Global Lung Function Initiative equations 12 , 13 were used as references for spirometry and diffusion, while equations from Rosenthal et al. 14 were used for plethysmography. All measurements were performed according to American Thoracic Society/European Respiratory Society guidelines. 15 , 16 , 17
2.4. Allergy testing
A skin prick test using extracts of pollen, fungus, mites, and cat and dog epithelium was adopted to assess allergies. Saline and histamine (10 mg/ml) were used as negative and positive controls, respectively. The appearance of a papule >3 mm was considered a positive result.
2.5. Statistical analysis
The sample size was calculated with FEV1 %pred as the primary outcome. Accepting an α risk of .05 and a β risk of .2, we estimated a requirement of at least 90 participants in each group to recognize an FEV1 %pred difference of ≥5% between any pair of groups as statistically significant. The standard deviation was assumed to be 10%. 18
Descriptive data are expressed as mean, standard deviation (SD), median, and the first and third quartiles (Q) for continuous variables and counts and percentages for categorical variables. Continuous variables that followed a normal distribution were compared using a one‐way analysis of variance with post hoc Bonferroni correction or t tests. When the data distribution was not normal, we used the Mann–Whitney U test or Kruskal–Wallis test with Dunn's multiple comparison test. Categorical variables were compared using the χ 2 test or Fisher's exact test. To control for potentially confounding variables (allergic sensitization, GA, parental asthma/atopy, parental smoking, or sex), we examined differences between groups using multiple linear and logistic regression models. Adjusted differences and odds ratios with 95% confidence intervals (95% CIs) were reported. In addition, we performed a subanalysis for EP‐BPD adolescents and compared participants with “low” and “high severity” BPD.
p < .05 were considered statistically significant. Analyses were performed with SPSS 20 (SPSS Inc.).
3. RESULTS
This study included a total of 286 adolescents with technically acceptable spirometry results (Figure 1). Pulmonary volumes and lung diffusion measurements were obtained from 121 and 101 participants, respectively. Descriptive data during the neonatal period and at early follow‐up (first 6 years of life), findings of skin prick tests, and parental history are presented in Table 1. Body mass index z‐scores at the time of the study were significantly lower in the EP‐BPD group than in the MLP group. In addition, we found a significantly higher rate of allergen sensitization in the MLP group than in the other groups. E‐Table 1 shows the BPD definitions used in each center, the BPD subclassifications based on severity, and the incidence and duration of home oxygen therapy. Approximately 28% of infants with BPD required oxygen supplementation at home, with a median duration of 3 months (first and third Q: 2 and 6, respectively). Mean (SD) duration of home oxygen therapy was significantly longer in the “high‐severity” BPD subgroup than in the “low severity” BPD subgroup (7.42 [8.27] vs. 1.90 [0.22] months; p = .001).
Figure 1.
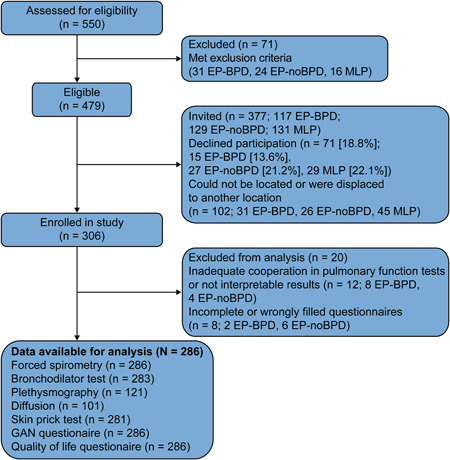
Flow diagram of the study. BPD, bronchopulmonary dysplasia; EP, extremely preterm; GAN, Global Asthma Network [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
General characteristics of the study population
| EP‐BPD, n = 92 | EP‐noBPD, n = 92 | MLP, n = 102 | EP‐BPD versus EP‐noBPD | EP‐BPD versus MLP | EP‐noBPD versus MLP | |
|---|---|---|---|---|---|---|
| p values | ||||||
| Perinatal period | ||||||
| GA (weeks) | 26.1 (1.36) | 27.0 (0.91) | 34.0 (1.13) | <.001 | ||
| Birth weight (g) | 839 (188) | 1004 (220) | 1983 (526) | <.001 | ||
| Female sex | 42 (45.7) | 41 (44.6) | 46 (45.1) | .88 | .94 | .94 |
| Smoking in pregnancy | 23 (25.0) | 16 (17.6) | 27 (26.5) | .22 | .82 | .14 |
| Prenatal steroids | 62 (67.4) | 57 (62.0) | 31 (30.4) | .44 | ||
| Surfactant | 86 (93.5) | 61 (67.0) | 4 (4.0) | <.001 | ||
| Invasive ventilationa | 15 (4, 30) | 1 (0, 3.75) | 0 (0, 0) | <.001 | ||
| Early follow‐upb | ||||||
| Inhaled corticosteroidsc | 51 (55.4) | 34 (37.8) | 29 (28.4) | .02 | <.001 | .17 |
| Any hospital admission | 49 (53.3) | 35 (38.0) | 28 (27.5) | .04 | <.001 | .12 |
| Any ICU admission | 13 (14.1) | 9 (9.78) | 2 (1.96) | .36 | .002 | .02 |
| At time of study | ||||||
| Age (years) | 14.1 (0.71) | 14.2 (0.61) | 14.5 (0.68) | .74 | <.001 | .001 |
| zBMI | −0.40 (0.99) | −0.04 (1.16) | 0.15 (1.23) | .10 | .002 | .70 |
| Positive skin prick testd | 22 (24.2) | 29 (32.2) | 55 (55.0) | .23 | <.001 | <.001 |
| Parental smoking | ||||||
| Mother | 21 (23.3) | 30 (33.3) | 28 (27.5) | .14 | .51 | .38 |
| Father | 22 (24.4) | 26 (28.6) | 30 (30.0) | .53 | .39 | .83 |
| Parental asthma/atopy | ||||||
| Mother | 21 (23.3) | 27 (29.3) | 30 (29.4) | .36 | .34 | .99 |
| Father | 14 (15.8) | 18 (19.6) | 19 (18.8) | .50 | .58 | .89 |
Abbreviations: BMI, body mass index; BPD, bronchopulmonary dysplasia; EP, extremely preterm; GA, gestational age; ICU, intensive care unit; MLP, moderate–late preterm; z, z‐score.
Note: Descriptive data are presented as the mean (standard deviation) or n (%) unless indicated otherwise.
Median number of days (first and third quartiles).
Refers to the first 6 years of life (data obtained from medical records and parents/caregivers interviews).
Periods >3 months.
Missing data = 5 (1 from EP‐BPD, 2 from EP‐noBPD, and 2 from MLP group).
3.1. Pulmonary function
Spirometry values were significantly lower in the EP‐BPD group than in the other groups (Table 2 and Figure 2). There were no statistically significant differences in spirometry between the EP‐noBPD and MLP groups. The percentage of patients with spirometry parameters below the lower limit of normal (LLN; 1.645 standard deviations below the predicted value) was significantly higher in the EP‐BPD group than in the other groups (Table 2).
Table 2.
Spirometry results and between‐group comparisons
| EP‐BPD, n = 92 | EP‐ noBPD, n = 92 | MLP, n = 102 | EP‐BPD versus EP‐noBPD | EP‐BPD versus MLP | EP‐noBPD versus MLP | |
|---|---|---|---|---|---|---|
| p values | ||||||
| FEV1 %pred | 85.4 (15.2) | 94.7 (12.5) | 97.0 (14.2) | <.001 | <.001 | .79 |
| zFEV1 | −1.22 (1.25) | −0.46 (1.05) | −0.29 (1.19) | <.001 | <.001 | .98 |
| FEV1 % Bdta , b | 5.57 (6.44) | 4.37 (5.06) | 3.54 (6.14) | .52 | .05 | 1.00 |
| FEV1 < LLN | 30 (32.6) | 13 (14.1) | 15 (14.7) | .005 | .002 | .54 |
| Positive Bdt | 15 (16.3) | 6 (6.52) | 8 (7.84) | .05 | .06 | .79 |
| FVC %pred | 91.7 (14.9) | 97.4 (11.9) | 97.7 (14.1) | .02 | .008 | 1.00 |
| zFVC | −0.71 (1.29) | −0.25 (1.00) | −0.22 (1.21) | .02 | .009 | 1.00 |
| FVC < LLN | 24 (26.1) | 8 (8.70) | 10 (9.80) | .003 | .004 | .81 |
| FEV1/FVCc | 81.8 (9.48) | 85.3 (7.95) | 86.7 (7.33) | .01 | <.001 | .72 |
| zFEV1/FVC | −0.82 (1.31) | −0.31 (1.18) | −0.11 (1.16) | .02 | <.001 | .72 |
| FEV1/FVC < LLN | 28 (30.4) | 12 (13.0) | 12 (11.8) | .007 | .001 | .83 |
| FEF25–75 %pred | 69.6 (23.4) | 82.7 (22.6) | 89.5 (24.5) | <.001 | <.001 | .14 |
| zFEF25–75 | −1.49 (1.31) | −0.89 (1.13) | −0.57 (1.15) | .002 | <.001 | .18 |
Note: Descriptive data are presented as the mean (standard deviation) or n (%).
Abbreviations: BPD, bronchopulmonary dysplasia; EP, extremely preterm; FEV1, forced expiratory volume in 1 s; FEF, forced expiratory flow; FVC, forced vital capacity; LLN, lower limit of normal; MLP, moderate–late preterm; z, z‐score.
% change after bronchodilator test (Bdt).
Missing data = 3 (all 3 from the EP‐noBPD group).
Actual value.
Figure 2.
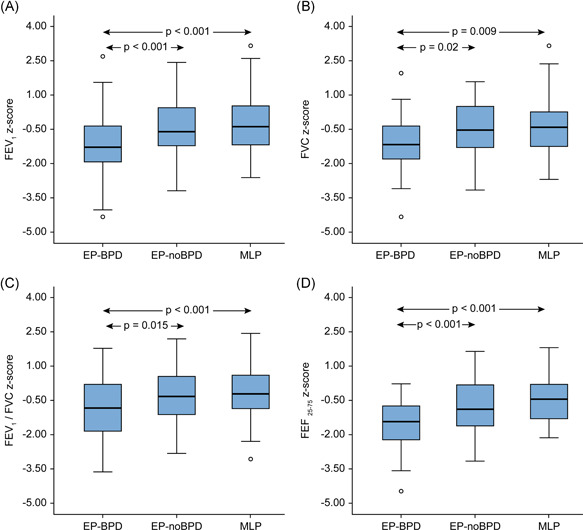
Box plots showing median and interquartile ranges of zFEV1 (a), zFVC (b), zFEV1/FVC (c), and zFEF25–75 (d) in each group. The circles show the outlier values. p values are provided for statistically significant mean differences between groups. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEF, forced expiratory flow; z, z‐score [Color figure can be viewed at wileyonlinelibrary.com]
The adolescents in the EP‐BPD group had significantly greater air‐trapping (represented by the ratio RV/TLC) than those in the MLP group. There were no differences in the remaining plethysmographic parameters between groups. DLCO values were significantly lower in the EP groups, regardless of whether participants had BPD, than in the MLP group. KCO values were also lower in the EP groups, but the differences only reached significance between the EP‐NoBPD and MLP groups (E‐Table 2).
In the adjusted regression models, the percentage of positive results in bronchodilator testing became significantly higher in the EP‐BPD group than in the other groups (E‐Table 3). In addition, RV/TLC was significantly higher in the BPD group than in the EP‐noBPD group (E‐Table 4). Moreover, pulmonary function differences between groups remained similar to those found in the unadjusted analysis.
The adolescents in the “high severity” BPD subgroup had significantly worse spirometry results than those in the “low severity” BPD subgroup, but there were no differences in lung volume or diffusion measurements. These two subgroups were comparable in terms of GA and birth weight (E‐Table 5).
3.2. Respiratory morbidity and GAN questionnaire
Throughout the early follow‐up, respiratory morbidity (inhaled corticosteroids ≥3 consecutive months and/or respiratory‐related hospitalizations) was higher in the EP‐BPD group than in other groups (Table 1). In addition, respiratory morbidity differed between the “high severity” and “low severity” BPD subgroups (E‐Table 5). The prevalence of ever wheezing was higher in the adolescents with BPD than in other adolescents, but differences only reached statistical significance between the EP‐BPD and MLP groups after adjusting for potential confounders. We found no significant differences between groups either in current asthma prevalence or in the remaining key questions of the GAN questionnaire (Table 3). There was no association between BPD severity and asthma symptom prevalence (E‐Table 5).
Table 3.
Prevalence of asthma symptoms based on the GAN questionnaire and between‐group comparisons by logistic regression (adjusted data)
| EP‐BPD, n = 92 | EP‐noBPD, n = 92 | MLP, n = 102 | EP‐BPD versus EP‐noBPD | EP‐BPD versus MLP | EP‐noBPD versus MLP | |
|---|---|---|---|---|---|---|
| Ever wheezing | 52 (56.5) | 43 (46.7) | 45 (44.1) | 1.46 (0.79, 2.71) | 2.02 (1.08, 3.77)* | 1.38 (0.75, 2.53) |
| Ever asthma | 30 (32.6) | 26 (28.3) | 28 (27.5) | 1.29 (0.64, 2.61) | 1.72 (0.85, 3.52) | 1.33 (0.67, 2.66) |
| Wheezing past 12 months (current asthma) | 9 (9.78) | 8 (8.70) | 12 (11.8) | 0.97 (0.31, 3.04) | 0.83 (0.28, 2.46) | 0.86 (0.32, 2.30) |
| Inhaled therapy past 12 months | 15 (16.3) | 13 (14.1) | 24 (23.5) | 1.45 (0.57, 3.72) | 0.92 (0.39, 2.16) | 0.63 (0.28, 1.45) |
| Nocturnal cough past 12 months | 27 (29.3) | 20 (21.7) | 26 (25.5) | 1.53 (0.75, 3.13) | 1.23 (0.61, 2.44) | 0.80 (0.39, 1.62) |
| Exercise‐induced wheeze past 12 months | 12 (13.0) | 9 (9.8) | 19 (18.6) | 1.47 (0.52, 4.18) | 0.84 (0.34, 2.13) | 0.57 (0.23, 1.43) |
| Awakening with wheezing past 12 months | 7 (7.61) | 9 (9.78) | 12 (11.8) | 0.81 (0.27, 2.48) | 0.72 (0.25, 2.14) | 0.89 (0.34, 2.32) |
Note: Data are presented as n of affirmative responses (%). Between‐group comparisons are presented as adjusted odds ratios (95% CI). The model was controlled for allergic sensitization, sex, parental history of asthma and atopy, and parental smoking.
Abbreviations: BPD, bronchopulmonary dysplasia; EP, extremely preterm; GAN, Global Asthma Network; MLP, moderate–late preterm.
p < .05.
3.3. Quality of life
There were no significant differences either in the total scores or in the subscales of the Kiddo‐KINDL® questionnaire between groups (Table 4). The adjusted model (controlled for allergic sensitization, sex, parental history of asthma and atopy, and parental smoking) found similar findings. Quality‐of‐life total scores were also similar between the “high” and “low severity” BPD subgroups (E‐Table 5).
Table 4.
Quality of life scores (Kiddo‐KINDL® questionnaire) and between‐group comparisons
| EP‐BPD, n = 92 | EP‐noBPD, n = 92 | MLP, n = 102 | EP‐BPD versus EP‐noBPD | EP‐BPD versus MLP | EP‐noBPD versus MLP | |
|---|---|---|---|---|---|---|
| Subscales | ||||||
| Physical well‐being | 77.4 (15.3) | 79.1 (13.7) | 75.9 (17.7) | −1.63 (−7.22, 3.96) | 1.57 (−3.88, 7.02) | 3.20 (−2.25, 8.66) |
| Emotional well‐being | 79.6 (14.9) | 81.4 (11.4) | 79.3 (14.1) | −1.74 (−6.56, 3.08) | 0.37 (−4.33, 5.07) | 2.11 (−2.58, 6.82) |
| Self‐esteem | 74.8 (19.4) | 76.7 (19.3) | 70.0 (18.8) | −1.84 (−8.65, 4.98) | 4.89 (−1.77, 11.6) | 6.72 (0.08, 13.4) |
| Family | 82.4 (14.3) | 80.4 (15.4) | 79.8 (17.2) | 2.00 (−3.59, 7.59) | 2.62 (−2.85, 8.08) | 0.61 (−4.87, 6.09) |
| Friends | 84.0 (17.6) | 85.8 (13.9) | 83.3 (14.2) | −1.89 (−7.24, 3.64) | 0.68 (−4.65, 6.02) | 2.49 (−2.83, 7.80) |
| School | 59.2 (18.7) | 60.7 (18.6) | 62.0 (17.8) | −1.56 (−8.07, 4.95) | −2.83 (−9.20, 3.53) | −1.27 (−5.09, 7.63) |
| Total | 76.1 (10.9) | 77.4 (10.3) | 74.8 (10.3) | −1.26 (−4.96, 2.44) | 1.08 (−2.54, 4.69) | 2.34 (−1.28, 5.95) |
Note: Data, which were transformed to 0–100, are presented as the mean (standard deviation). Between‐group comparisons are presented as post hoc mean difference (95% CI). A higher score represents a better quality of life.
Abbreviations: BPD, bronchopulmonary dysplasia; EP, extremely preterm; MLP, moderate‐late preterm.
4. DISCUSSION
4.1. Pulmonary function
Pulmonary function has been investigated extensively in patients with BPD at various ages, and the findings have led to a better understanding of the nature of the disease. 2 , 3 , 19 BPD prognosis has improved considerably over the last decades owing to advances in neonatal care, and infants who develop “new” BPD are much more immature. 7 Therefore, long‐term pulmonary function is expected to be different between patients with “new” and “classic” BPD.
4.1.1. Spirometry/pulmonary volumes
Previous studies showed that adolescents who developed BPD in the pre‐surfactant era had greater airflow limitation and air‐trapping than EP adolescents without BPD and full‐term control individuals; these differences tended to persist over time. 2 , 3
With regard to adolescents born in the post‐surfactant era, there is less information available, and only a few have reached adulthood. 20 Available data show that differences between children with BPD aged 6–12 years and full‐term controls are similar to those observed previously. 2 However, studies comparing pulmonary function between EP children aged 6–12 years with and without BPD have shown somewhat contradictory findings. Some studies reported that those with BPD had greater airway obstruction and air‐trapping, 21 , 22 , 23 , 24 , 25 while other studies found no differences in either spirometry or pulmonary volumes between EP children with and without BPD. 18 , 23 , 24 This study focused on 14‐year‐old adolescents who developed BPD in the post‐surfactant era. We found that they had greater airflow limitation and air‐trapping than EP adolescents without BPD and MLP adolescents. The differences between the EP adolescents with and without BPD suggest that BPD is associated with deficits in pulmonary function in addition to the complications related to prematurity itself. Notably, most adolescents with BPD in our study had mild pulmonary function deficits, and even patients in the “high severity” BPD subgroup reached acceptable results. Despite this, further follow‐up is necessary to assess the long‐term consequences of early lung injury, particularly in patients with spirometry values below the LLN. Indeed, it is possible that BPD is linked to the development of chronic obstructive pulmonary disease in adulthood. 4
Interestingly, we found that spirometry values were within the normal range in most adolescents in the EP‐noBPD group, and the findings suggest that the lung may be able to resume its growth and alveolarization process after the disruption caused by premature birth, although the recovery might not be completely achieved. 26 These results differ from those reported by Fawke et al., 21 who showed a slightly higher deficit in lung function in EP‐noBPD adolescents. This discrepancy may be due to the differences in participants' age and clinical characteristics. The patients in Fawke et al.'s 21 study were 11 years old with a mean GA of 25 weeks, and the patients in our study were 14 years old with a mean GA of 27 weeks.
4.1.2. Bronchodilator response
Consistent with the findings in other studies, 18 , 21 , 23 , 27 the EP adolescents with BPD in our study showed a greater bronchodilator response than those without BPD after adjusting for potential confounders. In addition, studies also found that EP children and adolescents had a greater bronchodilator response than full‐term controls. 18 , 21 However, we only found positive bronchodilator test results in 16% of the adolescents with history of BPD.
A lack of correlation between the degree of bronchial hyperresponsiveness and atopy and normal levels of exhaled nitric oxide has been documented in EP children and adolescents. 18 , 28 Therefore, it is thought that EP children and adolescents have no eosinophilic airway inflammation, as observed in typical asthma patients. Instead, airway obstruction might be related to structural changes due to immaturity and perinatal pulmonary damage induced by ventilation and prolonged use of oxygen. Over time, these changes could lead to fixed or irreversible airflow obstruction, which would explain the low rate of positive results in the bronchodilator test in our study, as reported by other studies. 18 , 21 , 28 Thus, BPD and asthma share some similarities in clinical manifestations and lung function, although they differ in the pathogenesis of bronchial obstruction.
4.1.3. Lung diffusion
Previous studies in the pre‐ 29 , 30 and post‐surfactant era 18 , 22 , 24 have reported significantly lower values of DLCO and KCO in EP children and adolescents than in full‐term controls. Similarly, we found that the above parameters were lower in the EP adolescents with or without BPD than in MLP adolescents. The reduction in diffusion capacity is probably a consequence of the arrest of lung development and reflects a decrease in the surface area for gaseous exchange. Lung parenchymal injury and vascular disease should lead to a more significant decline in diffusion capacity in patients with BPD. However, as observed in our study, diffusion capacity does not always differ between EP patients with and without BPD 18 , 24 ; in addition, changes in the diffusion capacity tend to persist over time. 31
4.2. Moderate–late preterm adolescents
Some studies showed no differences in spirometry between MLP adolescents and full‐term controls, 32 , 33 while others reported poorer results in MLP adolescents. 34 Regarding lung volumes, MLP children reportedly had higher RV and RV/TLC values than full‐term controls, although the values were within the normal range. 35 Our study found that pulmonary function tests were normal in most MLP adolescents, although around 12% showed FEV1/FVC values below the LLN. Notably, although impaired lung function is common in MLP infants early in life, 36 lung function tends to improve over time. 32 , 37
4.3. Respiratory morbidity /quality of life
Despite lung function impairment, the prevalence of current asthma symptoms in adolescents with BPD was not different from that in other adolescents, although our study was not powered to find differences in respiratory morbidity. This finding is consistent with that in a recent review, 6 and in general, it differs from that described in adolescents and adults who had BPD in the pre‐surfactant era. At that time, these individuals had greater respiratory morbidity than did controls without BPD. 2 The clinical improvement in adolescents with “new” BPD could be explained by “catch‐up” alveolar growth and airway repair after neonatal injury because they experienced less structural damage than did those with “classic” BPD. 7
Moreover, the prevalence of current asthma in each group in our study is comparable with that recorded in Spanish adolescents in the ISAAC Phase III study (10.6%) and is somewhat lower than that in the world population (14.1%). 38 We would like to emphasize that according to ISAAC methodology, the current asthma prevalence refers to the occurrence of wheezing episodes in the previous year. Therefore, the diagnostic approach of asthma‐like symptoms in BPD adolescents is not the same as that of typical asthma, although there may be overlap between the two conditions.
The scores of the quality‐of‐life questionnaire were similar across all groups, and they were within normal ranges when compared to the reference scores. 10 It has been documented that the quality of life is similar in adolescents who were born very preterm and those who were born full‐term. 39 In addition, a negative impact of BPD on quality of life in the early years has been demonstrated; however, the scores of EP infants with BPD do not seem to differ from those of healthy controls as they reach school age and adolescence. 40
4.4. Strengths and limitations
The participation rate was somewhat higher in the EP‐BPD group, probably because they are the most affected group and their families could be motivated to participate. We did not collect data from individuals who declined to participate. However, as the nonparticipation rate was low, and large differences between participants and nonparticipants are not expected, this seems unlikely to be a significant source of bias.
This study has several limitations. First, we excluded patients with severe neurological sequelae and other conditions that could cause further deterioration of pulmonary function and quality of life; therefore, our results cannot be extrapolated to all EP adolescents. Second, we did not include a control group of adolescents who were born full‐term. Therefore, interpretation of the degree of lung dysfunction and asthma symptoms is partially limited. Because studies on pulmonary function and respiratory morbidity in MLP adolescents are limited and the findings are discordant, we considered the inclusion of an MLP group to be appropriate. Further studies are needed to better understand the repercussions of moderate–late prematurity on long‐term pulmonary development. Third, there was a high percentage of participants with sensitization to allergens in the MLP group. We do not have a clear explanation for this finding. Although analyses of pulmonary function and respiratory morbidity were adjusted for this potential confounder, there might be an overestimation of the prevalence of symptoms such as night‐time coughing or ever wheezing. However, the prevalence of asthma symptoms in this group did not appear to be higher than that in the general population. Fourth, considering the variability of criteria used to define BPD at the time of diagnosis between the centers, the EP‐BPD adolescents were a heterogeneous group. We regrouped the EP‐BPD adolescents according to BPD severity into two subgroups, and the main differences between them have been reported. Although the “high severity” subgroup had higher respiratory morbidity during follow‐up and worse spirometry results than the “low severity” subgroup, the prevalence of current asthma symptoms and quality‐of‐life scores in the “high severity” subgroup were similar to those in the “low severity” subgroup and remaining groups. Fifth, this was not a prospective study that allowed for real follow‐up of patients over time.
The main strength of the study is that it gathered information on lung function, asthma symptom prevalence, and quality of life in a broad sample of participants with different degrees of prematurity. Given that data on pulmonary function and respiratory morbidity in adolescents with “new” BPD are still limited, we believe that the present findings contribute to a better understanding of the underlying mechanisms of this disease. Since “new” BPD is a constantly moving target that changes over time, further periodical studies are needed because the number of EP infants who survive is increasing, and continuous advances in neonatal care will have an impact on long‐term outcomes.
4.5. Conclusions
The EP adolescents who developed “new” BPD had poorer pulmonary function than the EP adolescents without BPD and MLP adolescents; however, these adolescents did not have a higher prevalence of asthma symptoms or a poorer quality of life. Advances in neonatal care are leading to milder forms of BPD, and further research is needed to investigate the long‐term outcomes of these patients to better understand their risk of developing chronic obstructive pulmonary disease in adulthood.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Research conception/design, data acquisition, data analysis/interpretation, manuscript preparation, and final approval: Santiago Pérez‐Tarazona, Santiago Rueda Esteban, and Maria L. García‐García. Data acquisition, data analysis/interpretation, and final approval: Inés de Mir Messa and Tatiana Acevedo Valarezo. Research conception/design, data acquisition, manuscript preparation, and final approval: Salome Albi Rodriguez. Data acquisition and final approval: Orlando Mesa Medina, Alicia Callejón Callejón, Elisa M. Canino Calderín, Roser Ayats Vidal, Antonio Salcedo Posadas, Jordi Costa Colomer, Xavier Domingo Miró, Montserrat Berrocal Castañeda, and Ana Villares Porto‐Dominguez.
Supporting information
Supplementary information.
Supplementary information.
Supplementary information.
Supplementary information.
Supplementary information.
ACKNOWLEDGMENTS
The authors would like to express their sincere thanks to the adolescents and their families for their collaboration in this study and to all the medical and nursing staff from the different pediatric pulmonology units that contributed to recruiting the participants and carrying out the measurements. The authors would like to thank Wiley Editing Services for their English language editing assistance.
This project was funded by the Spanish Society of Pediatric Pulmonology in their 2016 round of Senior Research Grants.
Pérez‐Tarazona S, Rueda Esteban S, García‐García ML, et al. Respiratory outcomes of “new” bronchopulmonary dysplasia in adolescents: A multicenter study. Pediatric Pulmonology. 2021;56:1205–1214. 10.1002/ppul.25226
DATA AVAILABILITY STATEMENT
All available data can be obtained by contacting the corresponding author.
REFERENCES
- 1. Alvarez‐Fuente M, Arruza L, Muro M, et al. The economic impact of prematurity and bronchopulmonary dysplasia. Eur J Pediatr. 2017;176:1587‐1593. [DOI] [PubMed] [Google Scholar]
- 2. Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short‐ and long‐term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192:134‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malleske DT, Chorna O, Maitre NL. Pulmonary sequelae and functional limitations in children and adults with bronchopulmonary dysplasia. Paediatr Respir Rev. 2018;26:55‐59. [DOI] [PubMed] [Google Scholar]
- 4. McGrath‐Morrow SA, Collaco JM. Bronchopulmonary dysplasia: what are its links to COPD? Ther Adv Respir Dis. 2019;13:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Been JV, Lugtenberg MJ, Smets E, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta‐analysis. PLOS Med. 2014;11:e1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pérez‐Tarazona S, Solano P, Bartoll E, Alfonso J. Bronchopulmonary dysplasia as a risk factor for asthma in school children and adolescents: a systematic review. Allergol Immunopathol (Madr). 2018;46:87‐98. [DOI] [PubMed] [Google Scholar]
- 7. Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946‐1955. [DOI] [PubMed] [Google Scholar]
- 8. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723‐1729. [DOI] [PubMed] [Google Scholar]
- 9. Ellwood P, Asher MI, Billo NE, et al. The Global Asthma Network rationale and methods for Phase I global surveillance: prevalence, severity, management and risk factors. Eur Respir J. 2017;49:1601605. [DOI] [PubMed] [Google Scholar]
- 10. Erhart M, Ellert U, Kurth BM, Ravens‐Sieberer U, Bullinger M. Measuring adolescents' HRQoL via self‐reports and parent proxy reports: an evaluation of the psychometric properties of both versions of the KINDL‐R instrument. Health Qual Life Outcomes. 2009;7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. ISAAC Steering Committee . International Study of Asthma and Allergies in Childhood, 2nd ed. ISAAC Phase One Manual. Auckland/Münster; 1993. [Google Scholar]
- 12. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi‐ethnic reference values for spirometry for the 3–95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J. 2017;50:1700010. [DOI] [PubMed] [Google Scholar]
- 14. Rosenthal M, Cramer D, Bain SH, Denison D, Bush A, Warner JO. Lung function in white children aged 4 to 19 years: II—single breath analysis and plethysmography. Thorax. 1993;48:803‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Graham BL, Steenbruggen I, Miller MR, et al. Standardisation of spirometry 2019 update. An official American Respiratory Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200:e70‐e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graham BL, Brusasco V, Burgos F, et al. ERS/ATS standards for single‐breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49:1600016. [DOI] [PubMed] [Google Scholar]
- 17. Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511‐522. [DOI] [PubMed] [Google Scholar]
- 18. Kaplan E, Bar‐Yishay E, Prais D, et al. Encouraging pulmonary outcome for surviving, neurologically intact, extremely premature infants in the postsurfactant era. Chest. 2012;142:725‐733. [DOI] [PubMed] [Google Scholar]
- 19. Gibson AM, Doyle LW. Respiratory outcomes for the tiniest or most immature infants. Semin Fetal Neonatal Med. 2014;19:105‐111. [DOI] [PubMed] [Google Scholar]
- 20. Doyle LW, Irving L, Haikerwal A, Lee K, Ranganathan S, Cheong J. Airway obstruction in young adults born extremely preterm or extremely low birth weight in the postsurfactant era. Thorax. 2019;74:1147‐1153. [DOI] [PubMed] [Google Scholar]
- 21. Fawke J, Lum S, Kirkby J, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182:237‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ronkainen E, Dunder T, Peltoniemi O, et al. predicts lung function at school age: follow‐up study and meta‐analysis. Pediatr Pulmonol. 2015;50:1090‐1098. [DOI] [PubMed] [Google Scholar]
- 23. Joshi S, Powell T, Watkins WJ, Drayton M, Williams EM, Kotecha S. Exercise‐induced bronchoconstriction in school‐aged children who had chronic lung disease in infancy. J Pediatr. 2013;162:813‐818. [DOI] [PubMed] [Google Scholar]
- 24. Cazzato S, Ridolfi L, Bernardi F, Faldella G, Bertelli L. Lung function outcome at school age in very low birth weight children. Pediatr Pulmonol. 2013;48:830‐837. [DOI] [PubMed] [Google Scholar]
- 25. Fortuna M, Carraro S, Temporin E, et al. Mid‐childhood lung function in a cohort of children with “new bronchopulmonary dysplasia”. Pediatr Pulmonol. 2016;51:1057‐1064. [DOI] [PubMed] [Google Scholar]
- 26. Narayanan M, Beardsmore CS, Owers‐Bradley J, et al. Catch‐up alveolarization in ex‐preterm children: evidence from (3)He magnetic resonance. Am J Respir Crit Care Med. 2013;187:1104‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pelkonen AS, Hakulinen AL, Turpeinen M. Bronchial lability and responsiveness in school children born very preterm. Am J Respir Crit Care Med. 1997;156:1178‐1184. [DOI] [PubMed] [Google Scholar]
- 28. Baraldi E, Bonetto G, Zacchello F, Filippone M. Low exhaled nitric oxide in school‐age children with bronchopulmonary dysplasia and airflow limitation. Am J Respir Crit Care Med. 2005;171:68‐72. [DOI] [PubMed] [Google Scholar]
- 29. Hakulinen AL, Järvenpää AL, Turpeinen M, Sovijärvi A. Diffusing capacity of the lung in school‐aged children born very preterm, with and without bronchopulmonary dysplasia. Pediatr Pulmonol. 1996;21:353‐360. [DOI] [PubMed] [Google Scholar]
- 30. Satrell E, Røksund O, Thorsen E, Halvorsen T. Pulmonary gas transfer in children and adolescents born extremely preterm. Eur Respir J. 2013;42:1536‐1544. [DOI] [PubMed] [Google Scholar]
- 31. Um‐Bergström P, Hallberg J, Pourbazargan M, et al. Pulmonary outcomes in adults with a history of bronchopulmonary dysplasia differ from patients with asthma. Respir Res. 2019;20:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kotecha SJ, Dunstan FD, Kotecha S. Long term respiratory outcomes of late preterm‐born infants. Semin Fetal Neonatal Med. 2012;17:77‐81. [DOI] [PubMed] [Google Scholar]
- 33. EJLE Vrijlandt, Reijneveld SA, Aris‐Meijer JL, Bos AF. Respiratory health in adolescents born moderately‐late preterm in a community‐based cohort. J Pediatr. 2018;203:429‐436. [DOI] [PubMed] [Google Scholar]
- 34. Thunqvist P, Gustafsson PM, Schultz ES, et al. Lung function at 8 and 16 years after moderate‐to late preterm birth: a prospective cohort study. Pediatrics. 2016;137: e20152056. [DOI] [PubMed] [Google Scholar]
- 35. Todisco T, de Benedictis FM, Iannacci L, et al. Mild prematurity and respiratory functions. Eur J Pediatr. 1993;152:55‐58. [DOI] [PubMed] [Google Scholar]
- 36. Colin AA, McEvoy C, Castile RG. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks' gestational age. Pediatrics. 2010;126:115‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Näsänen‐Gilmore P, Sipola‐Leppänen M, Tikanmäki M, et al. Lung function in adults born preterm. PLOS One. 2018;13:e0205979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S, International Study of Asthma and Allergies in Childhood Phase Three Study Group . Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2009;64:476‐483. [DOI] [PubMed] [Google Scholar]
- 39. Vieira ME, Linhares MB. Quality of life of individuals born preterm: a systematic review of assessment approaches. Qual Life Res. 2016;25:2123‐2139. [DOI] [PubMed] [Google Scholar]
- 40. Bozzetto S, Carraro S, Tomasi L, Berardi M, Zanconato S, Baraldi E. Health‐related quality of life in adolescent survivors of bronchopulmonary dysplasia. Respirology. 2016;21:1113‐1117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Supplementary information.
Supplementary information.
Supplementary information.
Supplementary information.
Data Availability Statement
All available data can be obtained by contacting the corresponding author.


