Abstract
Background:
Palbociclib, a CDK4/6 inhibitor, blocks proliferation and in a Rb and Cyclin D dependent manner in preclinical prostate cancer models. We hypothesized that co-targeting AR and cell-cycle with palbociclib would improve outcomes in patients with metastatic hormone sensitive prostate cancer (mHSPC).
Methods:
60 patients with Rb-intact mHSPC were randomized (1:2) to Arm 1: androgen deprivation (AD) or Arm 2: AD+ palbociclib. Primary endpoint was PSA response rate (RR) after 28-weeks of therapy. Secondary endpoints included safety, PSA and clinical progression-free survival (PFS), PSA and radiographic RR. Tumors underwent exome sequencing when available. Circulating tumor cells (CTC) were enumerated at various time points.
Results:
72 patients with mHSPC underwent metastatic disease biopsy and 64 had adequate tissue for RB assessment. 62/64 (97%) retained RB expression. 60 patients initiated therapy (Arm 1: 20; Arm 2: 40). Neutropenia was the most common G3/4 adverse event in Arm 2. 80% of pts (Arm 1: 16/20, Arm 2: 32/40; p = 0.87) met primary PSA endpoint ≤4ng/mL at 28 weeks. PSA undetectable rate at 28 weeks was 50% and 43% in Arms 1 and 2 respectively (p = 0.5). Radiographic RR was 89% in both arms. 12 month biochemical PFS was 69% and 74% in Arms 1 and 2, respectively (p=0.72). TP53, PIK3 pathway mutations, 8q gains and pretreatment CTCs were associated with reduced PSA PFS.
Conclusions:
Palbociclib did not impact outcome in RB intact mHSPC. Pretreatment CTC, TP53 and PIK3 pathway mutations, and 8q gain were associated with poor outcome.
Keywords: Prostate Cancer, AR signaling, Palbociclib, Cell cycle
INTRODUCTION
Metastatic prostate cancer (mPC) is an incurable disease with an estimated 33,330 deaths in the United States in 2020 (1). Androgen deprivation therapy (ADT) with or without androgen receptor (AR) blockade was the standard of care for men with newly diagnosed metastatic hormone sensitive prostate cancer (mHSPC) for many decades (2). While most men treated with ADT experience a clinical response, the majority will relapse and progress to castration resistance within 2-3 years (3,4). The overall survival for men with mHSPC treated with ADT as the sole therapy is around 4 years (3–6). Data from phase 3 trials, CHAARTED, STAMPEDE, LATITUDE, ENZAMET and TITAN have demonstrated significant survival benefits from early treatment intensification with docetaxel (6 cycles), abiraterone/prednisone, enzalutamide or apalutamide for patients with newly diagnosed mHSPC (3–8). These data illustrate that early intensive therapy for mHSPC results in significant clinical benefits. Despite these major advances, molecular methods to direct treatment intensification choice for mHSPC are lacking and targeting therapy to a specific tumor molecular profile is not yet validated.
Androgens drive PC proliferation by up-regulation of cyclin D which complexes with cyclin-dependent kinase (CDK) 4/6 resulting in phosphorylation of RB, a cell-cycle brake, which in turn allows the cell to progress through the cell-cycle. Loss of RB (RB1) by mutation, deletion or silencing has been shown in multiple preclinical models to promote the development of castrate-resistance and is associated with poor outcome (9,10). RB expression is lost in 1-20% of patients with localized PC (11) and up to 30-40% of patients with heavily treated metastatic castrate-resistant prostate cancer (mCRPC) (12). The frequency of RB loss in newly diagnosed M1 PC may be around 5% (13). In addition, CDK4/6 directly associates with AR increasing the AR-associated transcriptional activity (14,15). These results suggest that therapeutic targeting of the CDK 4/6 pathway may be important in previously untreated mHSPC which largely retain RB expression.
Palbociclib is a highly-selective reversible inhibitor of CDK 4 and 6 which has been shown to significantly improve progression-free survival when combined with letrazole in ER-positive, HER2-negative advanced breast cancer (16). It is administered orally on a 3 week on, 1 week off treatment schedule. Preclinical data demonstrated that treatment of PC cell lines and primary tumors which retained RB expression with palbociclib resulted in reduced proliferation in in vitro and in vivo models (17). Trials exploring the use of CDK 4/6 inhibitors in castrate resistant prostate cancer are ongoing (NCT02905318 and NCT02494921). However, we hypothesized that targeting this pathway in mHSPC with retained RB expression might be an ideal strategy because RB loss contributes to the development of castration resistance, is rare in mHSPC and because CDK4/6 participates with AR to promote PC growth.
To test this hypothesis, we conducted a multi-institutional randomized phase 2 trial in which patients with newly diagnosed mHSPC whose tumors retained RB expression were randomly assigned to ADT (LHRH analogue plus bicalutamide) vs ADT plus palbociclib. Primary endpoint was PSA response (≤ 4 ng/mL) after 28 weeks of therapy, an intermediate endpoint for efficacy (18,19). Secondary endpoints include safety/tolerability, biochemical and clinical PFS, PSA and radiographic RR. Circulating tumor cells (CTC) were enumerated from patients at various time points and mutations were examined in metastatic (and selected matched primary) tumor samples.
MATERIALS and METHODS
Trial Participants.
Eligible patients had pathologic diagnosis of PC, hormone-sensitive metastatic (M1) disease as evidenced by soft tissue and or bone metastases and were untreated or had started ADT for mHSPC less than 2 weeks prior to registration. Patients had to have a baseline PSA ≥ 5 ng/mL within 60 days of registration or prior to ADT initiation for patients starting prior to study registration; an ECOG performance status of 0-2; prior neoadjuvant/adjuvant hormonal therapy for non-metastatic disease was allowed if 12 months had elapsed since completion. Patients with known brain metastases and other uncontrolled illness were disqualified from the study.
Trial Design.
This phase 2 randomized biomarker-preselected multicenter trial involved registration of patients with newly diagnosed mHSPC (Fig 1). All eligible patients provided written informed consent and all studies were conducted in accordance with recognized ethical guidelines (e.g. Declaration of Helskinki, CIOMS, Belmont Report, U.S. Common Rule) and approved by institutional review boards. All registered patients underwent a metastatic disease biopsy unless metastatic archival samples were available. Metastatic biopsy tissue was evaluated for RB expression by IHC (Fig. 2). Patients with RB+ tumors were stratified by ADT start prior to registration and disease extent: limited (disease confined to spine, pelvic bones and/or lymph nodes) vs extensive disease (ribs, long bones and/or visceral organs) as previously described(20) and randomized 1:2 to ADT alone (LHRH agonist + bicalutamide 50 mg daily) or ADT (LHRH agonist + bicalutamide 50 mg daily) plus palbociclib (125 mg po daily, days 1-21 of a 28 day cycle).
Figure 1.
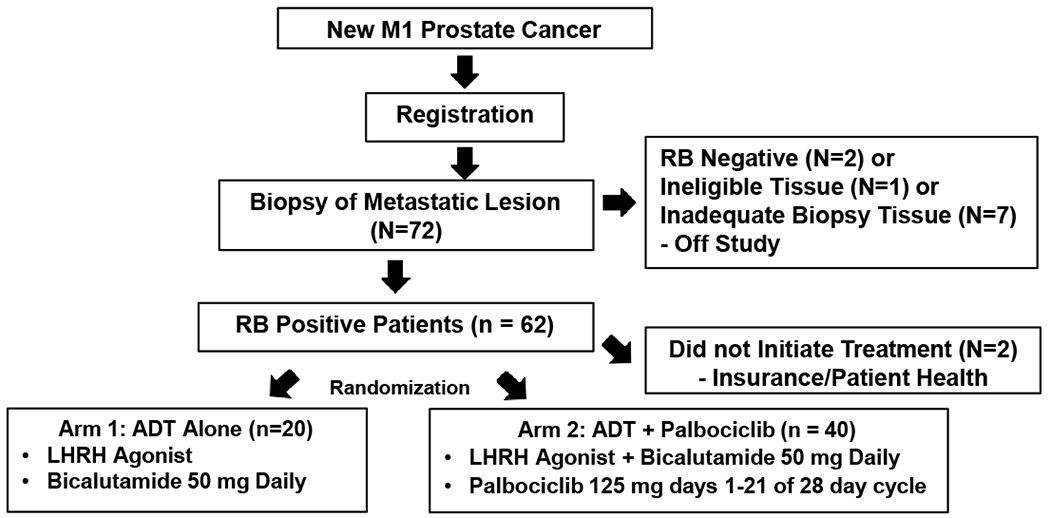
Study Schema.
Figure 2.
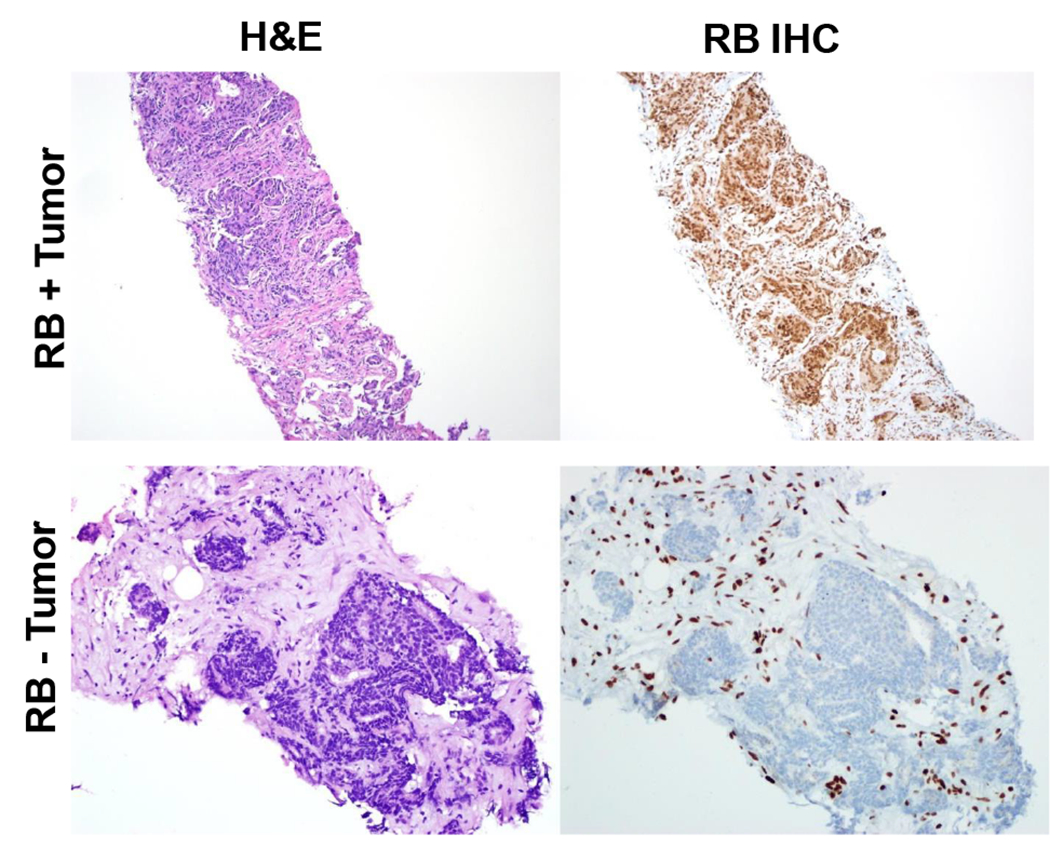
Representative RB staining in metastatic prostate cancer biopsies. Left panels shown H&E stained tissues. Right panels demonstrate typical RB staining in metastatic prostate cancers with (bottom panel) and without RB loss (top panel).
PSA and safety laboratory testing was monitored on a monthly basis. Radiologic assessments (including either CT or MRI abdomen/pelvis, either CXR, CT or MRI chest and a bone scan) occurred after 28 weeks of therapy, then every 24 weeks for 2 years and then annually until study therapy discontinuation. Patients came off study for clinical, radiographic or PSA progression, toxicity or per patient request. The full protocol is available on request. This multisite study was conducted at 7 institutions with coordination at the University of Michigan.
Assessment of RB Status.
Rb expression in tumor tissue was assessed by immunohistochemistry (IHC) for total Rb expression in a central CLIA-certified laboratory per previously published protocol (21). IHC was performed using a mouse anti-RB monoclonal antibody (BD Biosciences, San Jose, CA, G3-245) using automated IHC staining on the Ventana Medical Systems Benchmark Ultra autostainer. Nuclear RB staining intensity in tumor cells was assessed in 100 tumor cells and assigned a value of negative (0), weak (1+), moderate (2+) or strong (3+). Samples were considered positive for Rb expression and eligible for trial if >5% of cells had at least moderate staining or if > 20% cells had at least weak RB staining (representative results, Fig 2).
Trial Endpoints.
The primary endpoint was the rate of PSA ≤ 4 ng/mL after 28 weeks of treatment in patients treated with ADT vs those treated with ADT + palbociclib. This endpoint has been previously demonstrated to be a strong predictor of overall survival for patients with mHSPC treated with ADT. (18,19) Secondary endpoints included rate of undetectable PSA (< 0.2ng/mL) after 28 weeks, best PSA response (CR = <0.2ng/mL; PR = > 0.2ng/mL and <4ng/mL; SD (not CR, PR or PD); PD = 25% increase over baseline or nadir, whichever is lower, and a confirmed increase in the absolute value of PSA by 2ng/mL), biochemical and clinical progression-free survival, radiographic response rate and safety and tolerability of ADT plus palbociclib.
Correlative endpoints.
Tumor biopsy specimens also underwent additional molecular and genomic analyses. Flash-frozen biopsies were processed for genomic DNA and total RNA isolation using Qiagen AllPrep Kit (Hilden, Germany) and then underwent targeted exon sequencing and capture transcriptome analysis at University of Michigan (Ann Arbor, MI), as previously detailed (22). The variant reporting protocol followed the guidelines set out for reporting germline variants by ACMG and the guidelines for reporting somatic variants as presented by CAP/AMP. Specifically, the current version of the ClinVar database was queried for all variants with a population frequency less than 1% and the ClinVar status for all germline alleles designated as pathogenic or likely pathogenic are reported as such. For truncating alleles with no ClinVar entry in genes with known pathogenic truncating alleles, the allele was reported as likely pathogenic if it is 5’ to the most 3’ known pathogenic allele of that gene. For splice site mutations, the splicing pattern in the matched tumor RNA-SEQ library was reviewed using IGV, to aid in assessing functional effects. For missense mutations, expression levels in the matched RNA-SEQ libraries were considered when assessing significance. For somatic mutations, recurrence in both COSMIC and the OncoSEQ databases were examined, along with entries in the online version of OncoKB, MyCancerGenome, the Jackson Laboratory clinical Knowledgebase, and the Atlas of Genetics and Cytogenetics in Oncology and Haematology. Literature searches for rare non-recurrent alleles were additionally performed using PubMed. Genes with absolute copy number of 4-6 were reported as a gain. Genes with absolute copy number of 7 or greater were reported as amplified.
Circulating tumor cell number were also measured using Epic Biosystems platform at baseline, 12 weeks, 28 weeks of therapy and after progression to determine if CTC corresponded to treatment response. CTCs were stained as previously described (23–25). Total CTC/mL were measured using immunofluorescence and microscopy.
Statistical Analyses.
The primary endpoint was to compare the proportion of patients who had a PSA ≤ 4ng/mL after 28 weeks of protocol treatment between patients stratified by preregistration ADT treatment and extent of disease and then randomized to combined ADT: LHRH agonist + bicalutamide and those randomized to combined ADT + palbociclib. For purposes of the primary endpoint, day 1 is the randomization date for patients with pre-registration ADT treatment and the treatment start date for patients’ naïve to ADT at registration. ADT alone (Arm 1) was expected to result in 70% of patients achieving PSA ≤ 4ng/mL after seven months of protocol treatment. It was hypothesized that the ADT + palbociclib arm (Arm 2) would have a 20% absolute increase in proportion to 90% (HA: p2 – p1 > 0). The null hypothesis was no difference in proportions (H0: p2 – p1 ≤ 0). All patients randomized were considered evaluable if they received 1 cycle of treatment or were removed during treatment due to toxicity. With 20 patients randomized to Arm 1 and 40 to Arm 2 there was a 64.2% power to detect a 20% difference in proportions with a one-sided type I error of 0.10 using the mid p-value method of the Fisher’s exact test. Although this power was lower than typical, we felt that the benefit of inclusion of randomization and negative control justified this design. The difference in proportions are reported with the mid p-value test of the 2X2 table with a one-sided type I error of 10%. The associated binomial proportions with the corresponding Wilson 80% binomial confidence intervals (CI) are reported by arm. Secondary endpoints of undetectable PSA, PSA response, and radiographic response are reported using counts and proportions by treatment arm with mid-p-values of the Fisher’s exact test or Jonckheere-Terpstra exact test. Biochemical PFS, clinical PFS, and time to castration resistant prostate cancer by treatment arm are described using product limit estimates using Kaplan-Meier methods and log-rank p-values. CTC correlatives are described at timepoints in relation to treatment. CTC counts per mL were dichotomized into <2 compared to 2 or greater for outcome associations based on published data from this platform(26) with the cutpoint decided apriori. Genomic biomarker prevalence is reported in the subset with non-prostate tissue containing tumor. Associations between CTC pre-treatment and genomic biomarkers with PSA response were explored with 2X2 tables and Fisher’s exact test and biochemical PFS with Cox proportional hazards models. Thirteen exploratory biomarkers were assessed; effect size, 95% confidence intervals and p-values were presented. The analysis was completed using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Trial Patients.
To identify 60 pts with RB positive tumors, between July 2014 and February 2017, a total of 72 eligible patients were enrolled from 7 centers (University of Utah: 27, University of Michigan: 14, City of Hope: 10, Vanderbilt: 9, Thomas Jefferson: 7, John Hopkins: 3, University of Washington: 2) and evaluated for their RB status (Schema: Figure 1). Baseline demographics are shown in Table 1. No statistically significant differences in patient characteristics were observed, although there was a nonsignificant trend towards increased measurable disease in the PD + ADT vs ADT arms (67.5% vs 45% respectively, p = 0.09). All patients underwent metastatic disease biopsy (n = 47) or had previously undergone metastatic biopsy (n = 25). Forty-one patients had a biopsy of soft tissue metastasis and 31 had a biopsy of bone lesion; 90% (64/72) had adequate tissue for RB assessment after a maximum of 2 biopsy attempts. Ninety-seven percent (62/64) retained RB expression and were stratified by early initiation of ADT and extent of disease and randomized. Sixty patients initiated treatment (Arm 1: 20, Arm 2: 40). Patients had median age of 66 (Arm 1) and 68 (Arm 2). Median baseline PSA was 55.7 (range 10.9 – 2882.5) for Arm 1 and 77.2 (range 6.1 – 2123) for Arm 2 (p = 0.63). The median number of cycles (cycle = 28 days) on study was 24.5 for Arm 1 and 24.5 for Arm 2. The most common reason for study discontinuation was disease progression (55% in arm 1; 50% in Arm 2). 1 patient on Arm 2 discontinued palbociclib and bicalutamide after 16 cycles due to an adverse event, development of interstitial lung disease. 4 patients on Arm 2 discontinued treatment for reasons other than progression or adverse events (after 6–32 cycles). All patients were evaluable for the primary endpoint.
Table 1.
Patient Characteristics.
| ADT Alone | PD + ADT | p-value | |
|---|---|---|---|
| Median Age (Min - Max) | 66 (44 - 81) | 68 (47 - 87) | 0.61 |
| Median Baseline PSA (Min - Max) | 55.7 (10.9 – 2883) | 77.2 (6.1 - 2123) | 0.63 |
| Race | |||
| White | 17 (85%) | 35 (87.5%) | 0.18 |
| Black | 1 (5%) | 5 (12.5%) | |
| Other/Not Reported | 2 (10%) | 0 (0%) | |
| ECOG | 0.77 | ||
| 0 | 15 (75%) | 27 (67.5%) | |
| 1 | 5 (25%) | 13 (32.5%) | |
| Primary Gleason Sum | 1.00 | ||
| 6 | 0 (0%) | 1 (2.5%) | |
| 7 | 5 (25%) | 9 (22.5%) | |
| 8-10 | 11 (55%) | 23 (57.5%) | |
| Unknown | 4 (20%) | 7 (17.5%) | |
| Prior Treatment | |||
| Radical Prostatectomy | 9 (45%) | 14 (35%) | |
| Primary Radiotherapy | 2 (10%) | 4 (10%) | |
| Neoadjuvant/Adjuvant Systemic Therapy | 5 (25%) | 10 (25%) | |
| Neoadjuvant/Adjuvant Radiotherapy | 3 (7.5%) | 2 (5%) | |
| Salvage Radiotherapy | 3 (15%) | 4 (10%) | |
| Other Radiotherapy | 0 (0%) | 5 (12.5%) | |
| Disease Sites at Baseline | |||
| Visceral Mets | 1 (5%) | 2 (5%) | 1.00 |
| Bone Mets | 14 (70%) | 30 (75%) | 0.76 |
| LN Only | 5 (25%) | 8 (20%) | 0.74 |
| Measurable Disease | 9 (45%) | 27 (67.5%) | 0.09 |
| Baseline Bone Pain | 5 (25%) | 6 (15%) | 0.48 |
| Strata | |||
| Limited Disease/ADT Initiation Prior to Bx | 4 (20%) | 8 (20%) | |
| Extensive Disease/ADT Initiation Prior to Bx | 8 (40%) | 16 (40%) | |
| Limited Disease/ADT Initiation after Randomization | 4 (20%) | 9 (22.5%) | |
| Extensive Disease/ADT Initiation after Randomization | 4 (20%) | 7 (17.5%) | |
| Median Number of Cycles of Treatment | 24.5 (4 - 47) | 24.5 (6 - 53) | 0.98 |
RB Loss in metastatic HSPC.
In total, 64 out of 72 biopsied patients had metastatic tissue available for RB assessment. Representative H&E and RB staining is shown in Figure 2 for RB + and RB − patients. Two out of 64 (3%) patients demonstrated loss of RB expression which was similar to previously published data (13). Ten primary prostate samples were obtained from Rb+ patients and also retained Rb. Histologically, the RB −ve tumors demonstrated neuroendocrine features. One of the Rb – ve patients also underwent genomic and transcriptomic analysis of the metastatic tumor tissue which confirmed presence of RB1 homozygous deletion as well as PTEN loss and presence of TMPRSS2-ERG fusion. These findings demonstrate that RB loss is rare in the mHSPC population and suggest that RB loss in this setting may be associated with neuroendocrine/small cell differentiation.
Safety.
Grade 3/4 adverse events by arm are shown in Table 2. No adverse events > grade 2 were observed in Arm 1. Fifty-eight percent of patients in Arm 2 experienced grade 3/4 adverse events and 48% experienced grade 3/4 hematologic events (19/40) with the most common being neutropenia (40%, 16/40). 12.5% (5/40) of patients in Arm 2 had a grade 3 decreased white blood cell count. Other grade 3/4 adverse events occurred in ≤ 5% of patients.
Table 2.
Adverse Events.
| Arm | NAME | Grade | Number of patients | Percent |
|---|---|---|---|---|
| Arm 1: ADT | Any Grade 3/4 AE | 0 | 0.0% | |
| Arm 2: Palbociclib + ADT | Any Grade 3/4 AE | 23 | 57.5% | |
| Arm 2: Palbociclib + ADT | Non-Hematologic AE | 3 | 6 | 15% |
| Arm 2: Palbociclib + ADT | Hematologic AE | 3/4 | 19 | 47.5% |
| Arm 2: Palbociclib + ADT | Abdominal pain | 3 | 2 | 5.0% |
| Arm 2: Palbociclib + ADT | Alanine aminotransferase increased | 3 | 1 | 2.5% |
| Arm 2: Palbociclib + ADT | Aspartate aminotransferase increased | 3 | 1 | 2.5% |
| Arm 2: Palbociclib + ADT | Hyperglycemia | 3 | 1 | 2.5% |
| Arm 2: Palbociclib + ADT | Hypertension | 3 | 1 | 2.5% |
| Arm 2: Palbociclib + ADT | Left ventricular systolic dysfunction | 3 | 1 | 2.5% |
| Arm 2: Palbociclib + ADT | Lymphocyte count decreased | 3 | 1 | 2.5% |
| Arm 2: Palbociclib + ADT | Neutrophil count decreased | 3 | 16 | 40.0% |
| Arm 2: Palbociclib + ADT | Neutrophil count decreased | 4 | 1 | 2.5% |
| Arm 2: Palbociclib + ADT | Platelet count decreased | 3 | 1 | 2.5% |
| Arm 2: Palbociclib + ADT | Vomiting | 3 | 1 | 2.5% |
| Arm 2: Palbociclib + ADT | White blood cell decreased | 3 | 5 | 12.5% |
Primary and Secondary Endpoints.
All patients who initiated treatment were evaluable for the primary and secondary endpoints (Table 3). In total, 80% of patients in both arms achieved PSA ≤ 4ng/mL (Arm 1: 16/20=80%, 80% CI (64% - 91%), Arm 2: 32/40=80%, 90% CI (70%-88%), p = 0.87). The rate of PSA ≤ 4 ng/mL in the control Arm 1 was higher than the anticipated rate of 70% based on historical data. Sixty-five percent (13/20) of patients in Arm 1 and 55% (22/40) of patients in Arm 2 achieved an undetectable PSA (< 0.2ng/mL) a difference which was not statistically significant (p = 0.50). To determine if palbociclib prolonged PSA response, we examined biochemical PFS (Fig. 3A). Rate of biochemical PFS at 12 months was 69% (95%CI: 44-85%) for Arm 1 and 74% (95%CI: 57-85%) for Arm 2 (p = 0.72) suggesting that palbociclib did not significantly delay PSA progression.
Table 3.
Primary and Secondary Endpoints.
| Arm 1 (ADT only) | Arm 2 (Palbociclib + ADT) | ||||
|---|---|---|---|---|---|
| PSA Response at 28 weeks | n = 20 | % | n = 40 | % | p value |
| PSA < or = 4ng/dL | 16 | 80 | 32 | 80 | 0.87 |
| PSA undetectable (PSA <0.2ng/mL) | 10 | 50 | 17 | 43 | 0.50 |
| PSA PFS % (95% CI) | 80 | (55-92) | 90 | (76-96) | 0.72* |
| Best PSA Response Rate | 0.25^ | ||||
| CR (PSA <0.2ng/mL) | 13 | 65 | 22 | 55 | |
| PR (PSA ≥0.2-4ng/mL) | 5 | 25 | 12 | 30 | |
| Stable Disease (not PR or PD) | 1 | 5 | 6 | 15 | |
| Progression (25% increase and an absolute increase of 2 ng/ml) | 1 | 5 | 0 | 0 | |
| Measurable Disease | 0.42^ | ||||
| # evaluable | 9 | 45 | 27 | 67.5 | |
| CR | 4 | 44.4 | 11 | 40.7 | |
| PR | 4 | 44.4 | 13 | 48.2 | |
| Stable Disease | 1 | 11.1 | 2 | 7.4 | |
| Progressive Disease | 0 | 0 | 1 | 2.7 | |
| Best Bone Response | 0.10 | ||||
| # evaluable | 14 | 70 | 30 | 75 | |
| Stable/Improved | 14 | 100 | 25 | 83.3 | |
| Progression | 0 | 0 | 5 | 16.7 | |
Log-Rank test
Mid p-value Jonckheere-Terpstra Exact Test
Figure 3.
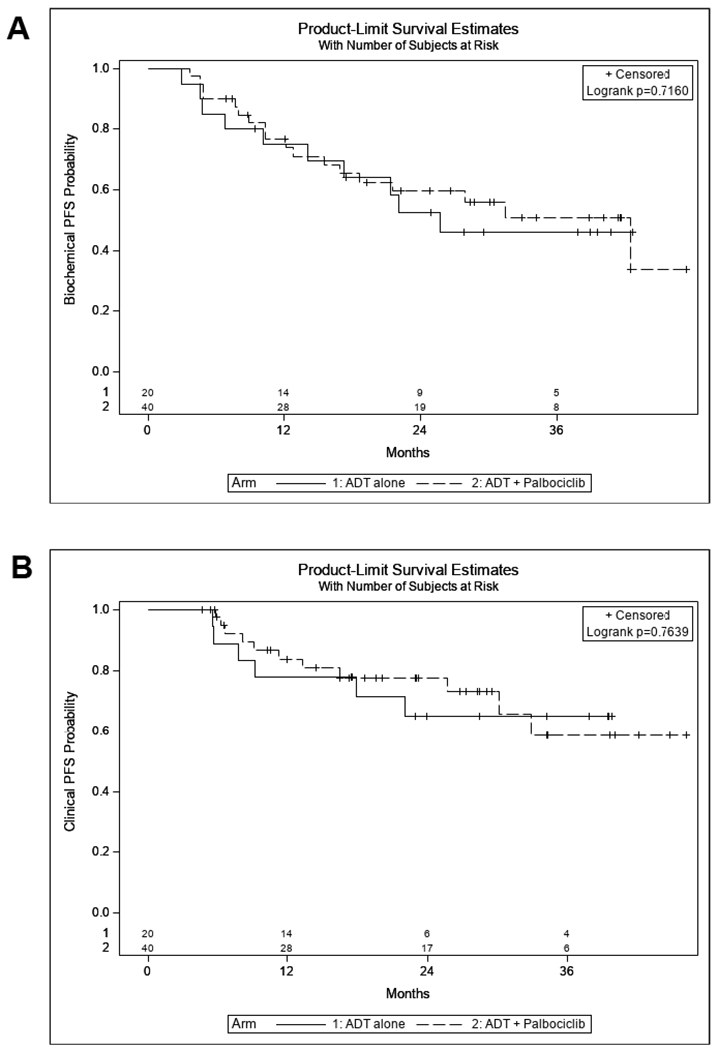
Progression free survival. There were no statistically significant differences in biochemical PFS (A) or clinical PFS (B) between ADT and ADT + palbociclib arms.
We examined the effect of palbociclib on measurable disease response and changes in bone metastasis burden. In total, 9/20 (45%) in Arm 1 had measurable disease as defined by RECIST criteria and 27/40 (67.5%) of patients in Arm 2 had measurable disease (p = 0.09). 30/40 (75%) patients in Arm 2 had bone disease compared to 14/20 (70%) in Arm 1 (p = 0.65). Figure 4 presents a waterfall plot of change in RECIST tumor measurements by treatment demonstrating high response rates in both arms. The measurable disease response rate was not different between arms (Arm 1: 89% vs Arm 2: 89%, p = 0.78, Table 3). Rates of bone scan improvement/stability (based on PCWG2 criteria) were also not significantly different between arms (Arm 1: 100%, 14/14 vs Arm 2: 83.3%, 25/30, p = 0.10). Figure 3B demonstrates the radiographic PFS data for both arms again demonstrating no significant difference between arms (p = 0.76). Lastly, median time to development of castrate resistant disease (biochemical/radiographic progression) was similar between arms [Arm 1: 25.8 months (95%CI: 9.2-NR) vs Arm 2: 27.9 months (95%CI:12.2 – NR); p=0.92]. Together, these data suggest that there was no significant clinical benefit from the addition of palbociclib to primary ADT in newly diagnosed M1 prostate cancer, although the higher rate of measurable disease in Arm 2 suggests that the two arms may not have been balanced.
Figure 4.
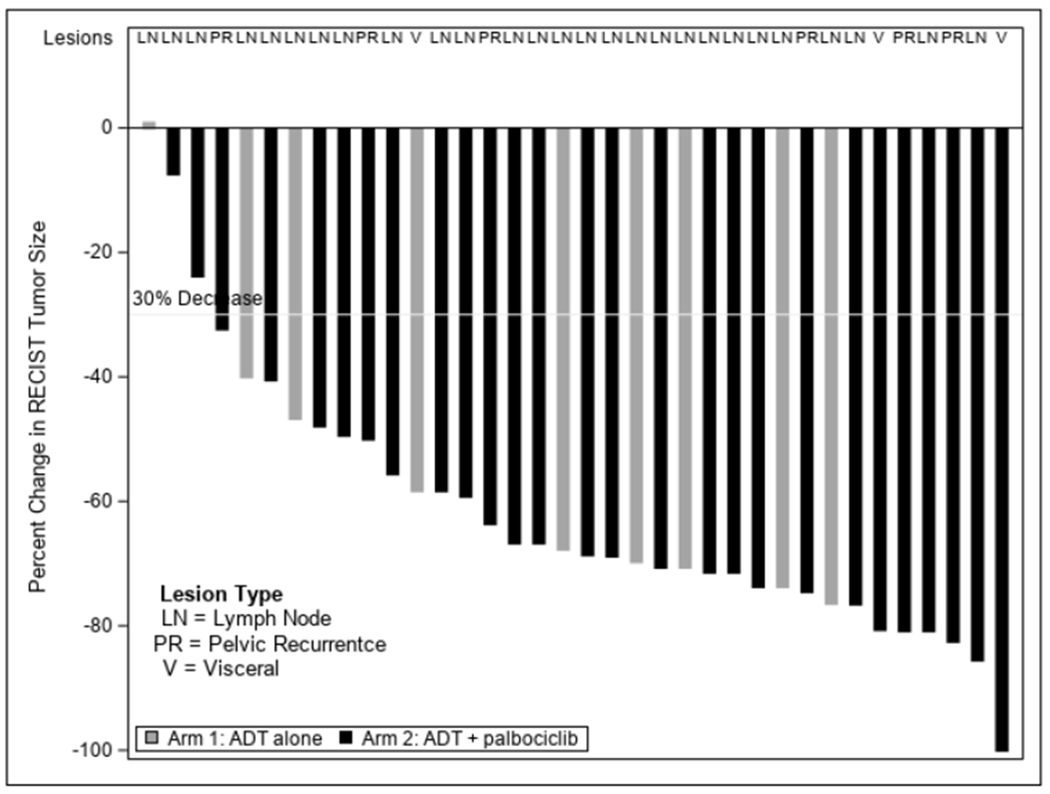
Waterfall plot for radiographic response. Black bars = ADT + palbociclib. Gray bars = ADT alone. Metastatic lesion type is indicated across top of graph.
CTC.
CTC have previously shown promise as surrogate markers for overall survival in mCRPC as well in other malignancies (27). In SWOG S0925, lower baseline CTC also correlated with higher rates of PSA response in patients with mHSPC (28). To explore whether CTC enumeration provided additional information regarding treatment response in mHSPC patients during treatment with ADT +/− palbociclib, CTC counts were monitored at pretreatment, 12 and 28 weeks after starting treatment and at progression using the EPIC platform. Thirty-three out of 45 patients (73.3%) had detectable CTCs at pretreatment blood draw (Figure 5A). There was no significant difference in pretreatment CTC by treatment arm. Pretreatment CTC counts of ≥ 2 (n = 9) were associated with decreased PSA CR rates (p = 0.04, Table 4) and decreased clinical PFS times (p = 0.031, Figure 5B) in the entire cohort. Blood samples at progression also showed a statistically significant increase in CTC counts (Mean increase 1.9 CTCs (95%CI: 0.1-3.6) paired t-test p = 0.038, Figure 5C) as compared to week 12 values. There was no statistically significant difference in CTC counts between the treatment arms at any of the assessed time points.
Figure 5.
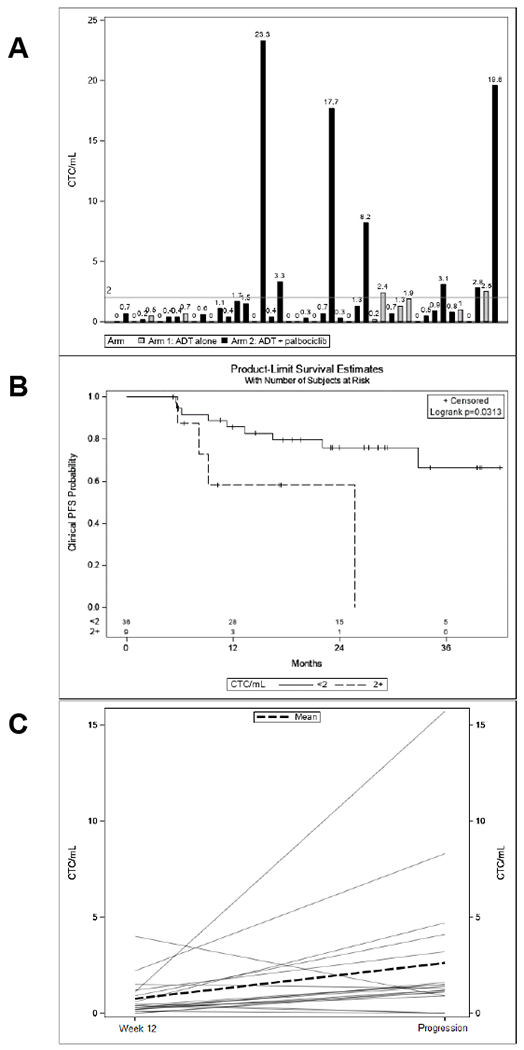
CTC correlative data. (A) Pretreatment CTC counts by patient and treatment arm. (B) Stratification of patients by pretreatment CTC (≥ 2 or < 2 CTC/mL) demonstrated that pts with higher CTC counts have significantly shorter cPFS. (C) CTC counts increase between week 12 and progression for most patients (N=19).
Table 4.
CTC Counts Correlated with PSA Response.
| Pretreatment CTC Count / mL | PSA ≤ 4 at 28 Weeks | PSA > 4 at 28 Weeks | p-value |
|---|---|---|---|
| <2 | 30 (83.3%) | 6 (16.7%) | 0.49 |
| 2+ | 7 (77.8%) | 2 (22.2%) | |
| Pretreatment CTC Count / mL | PSA CR | PSA PR/SD | p-value |
| <2 | 25 (69.4%) | 11 (30.6%) | 0.04 |
| 2+ | 3 (33.3%) | 6 (66.7%) |
Molecular Data.
41 metastatic biopsy samples were sequenced. 10 matched primary PC specimens were also sequenced. Table 5 summarizes the mutations and chromosomal aberrations identified in this study population. ETS Fusions 22/40 (55%), PTEN 17/40 (42.5%), TP53 11/40 (27.5%), WNT pathway (APC, CTNNB1, ZNRF3, RSPO) 9/40 (22.5%), KMT2C 8/40 (20%), SPOP 6/40 (15%), PI3K pathway (PIK3R1, PIK3CB, PIK3CA) 5/40 (12.5%), CHD1 5/40 (12.5%), FOXA1 4/40 (10%) and BRCA1/2 3/40 (7.5%) were the most commonly detected mutations. 8p loss (35/40, 87.5%), 8q gain (18/40, 45%), 13q (18/40, 45%) and 7p gain (6/40, 15%) were the most commonly detected chromosomal aberrations. 8q amplification, CCND1 amplification, KMT2C and PTEN mutations were observed in metastatic biopsies, but not matched primary prostate cancer samples. Clinically, TP53 (HR 2.95, C.I. 1.21-7.22, p=0.01), PI3K pathway (HR 3.24, C.I. 1.03-10.1, p=0.04) mutations and 8q gain (HR 4.68, C.I. 1.82-12.0, p=0.001) were associated with reduced time to biochemical progression regardless of treatment arm in this exploratory analysis (Figure 6). Of these molecular markers, only 8q gain, which is associated with MYC amplification, was also significantly associated with ≥2 CTCs (p = 0.01) and extensive stage disease (p = 0.02)(29).
Table 5.
Molecular Alterations in Study Cohort.
| N | % | |
|---|---|---|
| ETS Fusion | 22 | 55.0% |
| PTEN | 17 | 42.5% |
| TP53 | 11 | 27.5% |
| WNT | 9 | 22.5% |
| KMT2C | 8 | 20.0% |
| SPOP | 6 | 15.0% |
| PIK3 pathway | 5 | 12.5% |
| CHD1 | 5 | 12.5% |
| FoxA1 | 4 | 10.0% |
| BRCA1/2 | 3 | 7.5% |
| AR | 2 | 5.0% |
| CDK12 | 2 | 5.0% |
| ZFHX3 | 2 | 5.0% |
| KRAS | 2 | 5.0% |
| CCND1 | 1 | 2.5% |
| ATM | 1 | 2.5% |
| 8p loss | 35 | 87.5% |
| 8q gain | 18 | 45.0% |
| 13q loss | 18 | 45.0% |
| 7p gain | 6 | 15.0% |
Figure 6.
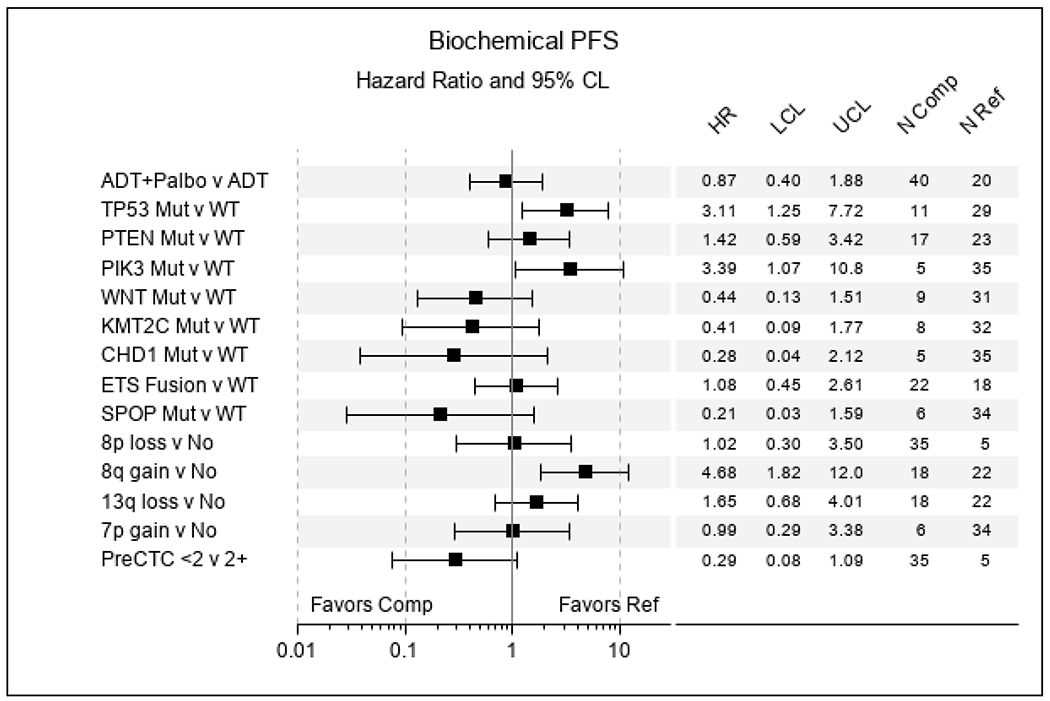
Forrest Plot of Biochemical PFS and association with mutation and CTC counts.
DISCUSSION
Despite decades of progress, mHSPC remains incurable. Preclinical data utilizing PC models supported the hypothesis that co-targeting CDK4/6 in conjunction with AR could provide benefit to patients with mHSPC (17). Of note women with ER+ advanced breast cancer demonstrated increased PFS when the hormonal and CDK4/6 axes are targeted in parallel (16).
The addition of the CDK4/6 selective inhibitor, palbociclib, to ADT did not improve PSA or radiographic response in men with new mHSPC with intact RB expression in this randomized phase 2 trial. The addition of palbociclib treatment resulted in expected toxicities including neutropenia. While the results of this study do not support the use of CDK4/6 targeted therapies in mHSPC patients, other ongoing studies are examining whether there is a role for these agents in mCRPC alone or in combination with other therapies (NCT02555189, NCT02494921, NCT02905318). It remains possible that CDK4/6 inhibition may offer clinical benefit to patients with more advanced disease or may work synergistically with other agents such as chemotherapy and newer androgen targeted therapies. Moreover, it will be important in future studies to address the efficacy of CDK4/6 targeted agents to effectively suppress kinase activity and engage Rb function in prostate cancer.
Various factors may have contributed to the negative results observed here. First, shortly after enrollment started on this study, the CHAARTED data showing survival benefit to those with extensive metastatic disease was presented. After these results were presented, patients who were candidates for docetaxel were encouraged to receive docetaxel. We hypothesize that patients with more extensive or higher risk disease which were underrepresented in this study may have been the population most likely to realize benefit from the treatment intensification strategy presented here. In SWOG 9346, which compared intermittent versus continuous ADT in men with mHSPC, 71% (965/1345) and 45% (604/1345) of patients had PSA ≤4ng/mL and ≤0.2ng/mL, respectively (18,19), while in this study, the rates were 80% and 50% respectively. The comparatively greater PSA responses in our study population with ADT alone may have limited our ability to detect significant improvements in the designated endpoints with intensified therapy. Additionally, progression to castration resistance may not depend on CDK4/6 activation, or treatment with palbociclib may drive earlier loss of RB and thus escape from CDK4/6 inhibition.
Correlative studies from this study suggest potentially informative CTC and molecular markers. Similar to S0925, a randomized phase 2 study in a similar mHSPC population, where higher baseline CTC was associated with decreased PSA response (28), we found that base line CTCs ≥ 2 were associated with lower PSA response and PFS. Similar to other studies, we found that RB and TP53 loss correlated with neuroendocrine differentiation (30). Molecular analysis of tumor samples from our patient population also revealed that ETS fusions, PTEN and TP53 mutations were the most common molecular events identified in this cohort of mHSPC similar to other studies (13). We observed that TP53 mutations are associated with reduced time to PSA progression but also identified 8q gain and PIK3 mutations as having potential prognostic significance. These results are comparable to other published data for this patient population (31). Taken together, this data further suggests that CTCs and mutations have potential prognostic importance in patients with mHSPC which will require further validation.
This study illustrates the feasibility of biopsy- and biomarker-driven studies in the treatment naïve mHSPC population. Since most studies of new therapies have focused on the castrate-resistant patient population, few metastatic disease biopsy-driven biomarker-based studies have been attempted in the treatment naïve mHSPC population. The survival benefit of treatment intensification with abiraterone, enzalutamide, apalutamide or docetaxel in newly diagnosed mHSPC patients further raises the bar for development of biomarker targeted therapies in this clinical space. Our data suggests that molecular profiling of mHSPC and testing of appropriate molecularly targeted therapies in this patient population is feasible, but that targeting Rb was not associated with benefit. This does not rule out potential benefit from other appropriately selected targeted therapies. The goal is to better personalize care for patients as much as possible. Efforts towards this goal are in progress specifically in relation to DNA repair defective tumors (NCT03413995, NCT04332744). Thus, while biopsy- and biomarker driven strategies may not be appropriate for all patients with mHSPC, precision-based therapy is a feasible and attractive approach for those with high-risk disease or DRD tumors.
TRANSLATION RELEVANCE.
Co-targeting of hormonal and cell cycle pathways by the CDK4/6 inhibitor, palbociclib, has demonstrated clinical activity in hormone receptor-positive breast cancer patients leading to its FDA approval. Metastatic hormone sensitive prostate cancer (mHSPC) also retains dependence on hormonal signaling and retinoblastoma (RB) protein related cell cycle checkpoints suggesting that co-targeting CDK4/6 and androgen signaling might offer benefit to prostate cancer patients. In this randomized phase 2 clinical study, we assessed the utility of palbociclib in patients with mHSPC undergoing androgen deprivation therapy. The study did not identify a clinical benefit for palbociclib but did establish that metastatic tissue-based biomarker preselected trials are feasible in mHSPC.
Acknowledgements:
This work was funded by grants from Pfizer, Movember-PCF Challenge, (M.Hussain), NIH K08 CA201335 (P.Palmbos), EDRN U01CA214170 and SPORE P50 CA186786 (J.Siddiqui). The authors would like to thank the patients and their families for participation in this study. Clinical Trial Registration: NCT02059213.
Funding provided by Pfizer for the clinical trial
Footnotes
Conflict of Interest: Authors report no additional conflict of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70(1):7–30 doi 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Palmbos PL, Hussain M. Non-castrate metastatic prostate cancer: have the treatment options changed? Semin Oncol 2013;40(3):337–46 doi 10.1053/j.seminoncol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 3.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 2017;377(4):338–51 doi 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol 2018;36(11):1080–7 doi 10.1200/JCO.2017.75.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017;377(4):352–60 doi 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 6.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387(10024):1163–77 doi 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019;381(2):121–31 doi 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 8.Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2019;381(1):13–24 doi 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 9.Sharma A, Yeow WS, Ertel A, Coleman I, Clegg N, Thangavel C, et al. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. J Clin Invest 2010;120(12):4478–92 doi 10.1172/JCI44239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNair C, Xu K, Mandigo AC, Benelli M, Leiby B, Rodrigues D, et al. Differential impact of RB status on E2F1 reprogramming in human cancer. J Clin Invest 2018;128(1):341–58 doi 10.1172/JCI93566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet 2012;44(6):685–9 doi 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012;487(7406):239–43 doi 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stopsack KH, Nandakumar S, Wibmer AG, Haywood S, Weg ES, Barnett ES, et al. Oncogenic genomic alterations, clinical phenotypes, and outcomes in metastatic castration-sensitive prostate cancer. Clin Cancer Res 2020. doi 10.1158/1078-0432.CCR-20-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim JT, Mansukhani M, Weinstein IB. Cyclin-dependent kinase 6 associates with the androgen receptor and enhances its transcriptional activity in prostate cancer cells. Proc Natl Acad Sci U S A 2005;102(14):5156–61 doi 10.1073/pnas.0501203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comstock CE, Augello MA, Schiewer MJ, Karch J, Burd CJ, Ertel A, et al. Cyclin D1 is a selective modifier of androgen-dependent signaling and androgen receptor function. J Biol Chem 2011;286(10):8117–27 doi 10.1074/jbc.M110.170720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375(20):1925–36 doi 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 17.Comstock CE, Augello MA, Goodwin JF, de Leeuw R, Schiewer MJ, Ostrander WF, et al. Targeting cell cycle and hormone receptor pathways in cancer. Oncogene 2013. doi 10.1038/onc.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol 2006;24(24):3984–90 doi 24/24/3984 [pii] 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 19.Hussain M, Goldman B, Tangen C, Higano CS, Petrylak DP, Wilding G, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol 2009;27(15):2450–6 doi JCO.2008.19.9810 [pii] 10.1200/JCO.2008.19.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, Liu G, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med 2013;368(14):1314–25 doi 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldhoff P, Clarke J, Smirnov I, Berger MS, Prados MD, James CD, et al. Clinical stratification of glioblastoma based on alterations in retinoblastoma tumor suppressor protein (RB1) and association with the proneural subtype. J Neuropathol Exp Neurol 2012;71(1):83–9 doi 10.1097/NEN.0b013e31823fe8f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015;162(2):454 doi 10.1016/j.cell.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 23.Cho EH, Wendel M, Luttgen M, Yoshioka C, Marrinucci D, Lazar D, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys Biol 2012;9(1):016001 doi 10.1088/1478-3975/9/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol 2012;9(1):016003 doi 10.1088/1478-3975/9/1/016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieva J, Wendel M, Luttgen MS, Marrinucci D, Bazhenova L, Kolatkar A, et al. High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: a longitudinal analysis. Phys Biol 2012;9(1):016004 doi 10.1088/1478-3975/9/1/016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDaniel AS, Ferraldeschi R, Krupa R, Landers M, Graf R, Louw J, et al. Phenotypic diversity of circulating tumour cells in patients with metastatic castration-resistant prostate cancer. BJU Int 2017;120(5B):E30–E44 doi 10.1111/bju.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantel K, Hille C, Scher HI. Circulating Tumor Cells in Prostate Cancer: From Discovery to Clinical Utility. Clin Chem 2019;65(1):87–99 doi 10.1373/clinchem.2018.287102. [DOI] [PubMed] [Google Scholar]
- 28.Yu EY, Li H, Higano CS, Agarwal N, Pal SK, Alva A, et al. SWOG S0925: A Randomized Phase II Study of Androgen Deprivation Combined With Cixutumumab Versus Androgen Deprivation Alone in Patients With New Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol 2015;33(14):1601–8 doi 10.1200/JCO.2014.59.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hovelson DH, Udager AM, McDaniel AS, Grivas P, Palmbos P, Tamura S, et al. Targeted DNA and RNA Sequencing of Paired Urothelial and Squamous Bladder Cancers Reveals Discordant Genomic and Transcriptomic Events and Unique Therapeutic Implications. Eur Urol 2018;74(6):741–53 doi 10.1016/j.eururo.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 30.Aparicio AM, Shen L, Tapia EL, Lu JF, Chen HC, Zhang J, et al. Combined Tumor Suppressor Defects Characterize Clinically Defined Aggressive Variant Prostate Cancers. Clin Cancer Res 2016;22(6):1520–30 doi 10.1158/1078-0432.CCR-15-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mateo J, Seed G, Bertan C, Rescigno P, Dolling D, Figueiredo I, et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J Clin Invest 2020;130(4):1743–51 doi 10.1172/JCI132031. [DOI] [PMC free article] [PubMed] [Google Scholar]


