Summary
The major lineages of nectar-feeding birds (hummingbirds, sunbirds, honeyeaters, flowerpiercers, and lorikeets) are considered examples of convergent evolution. We compared sucrose digestion capacity and sucrase enzymatic activity per unit intestinal surface area among 50 avian species from the New World, Africa, and Australia, including 20 nectarivores. With some exceptions, nectarivores had smaller intestinal surfaces, higher sucrose hydrolysis capacity, and greater sucrase activity per unit intestinal area. Convergence analysis showed high values for sucrose hydrolysis and sucrase activity per unit intestinal surface area in specialist nectarivores, matching the high proportion of sucrose in the nectar of the plants they pollinate. Plants pollinated by generalist nectar-feeding birds in the Old and New Worlds secrete nectar in which glucose and fructose are the dominant sugars. Matching intestinal enzyme activity in birds and nectar composition in flowers appears to be an example of convergent coevolution between plants and pollinators on an intercontinental scale.
Subject areas: Biological Sciences, Zoology, Evolutionary biology, Phylogenetics
Graphical abstract

Highlights
-
•
Nectarivory has evolved independently in birds in the New and Old Worlds
-
•
Nectarivorous birds have greater sucrose hydrolysis capacity than nonspecialists
-
•
Nectarivorous birds have a smaller intestinal surface area than nonspecialists
-
•
Capacity to digest sucrose and high nectar sucrose content coevolved independently
Biological sciences; Zoology; Evolutionary biology; Phylogenetics
Introduction
Three major lineages of extant birds have evolved independently as nectar-feeding specialists: hummingbirds (Trochilidae), sunbirds (Nectariniidae), and honeyeaters (Meliphagidae; Cronk and Ojeda 2008; Fleming and Kress 2013). In addition, there are specialized nectarivores scattered among other avian taxa, including lorikeets (Loriinae within the parrots, Psittacidae), white-eyes (Zosteropidae), Hawaiian honeycreepers (Fringillidae), the recently extinct Hawaiian family Mohoidae (Fleischer et al., 2008), and Neotropical flowerpiercers (Diglossa spp., Thraupidae; Fleming and Kress 2013).
Nectarivores must cope with a watery diet rich in sugars but poor in protein and electrolytes (Nicolson and Fleming 2003). After nectar is ingested, the sugars in it must be digested and absorbed and, if not used to directly fuel metabolism, synthesized into lipids (Suarez et al., 2011). The prodigious amounts of water ingested must also be processed (McWhorter et al., 2003). The challenges posed by a nectar diet raise the question of whether different lineages of nectarivores have converged in morphological and physiological traits (Nicolson and Fleming 2014).
Here, we focus on one of these potentially convergent traits: sucrose digestion rate at the brush-border membrane of intestinal cells. To be assimilated, the disaccharide sucrose must be hydrolyzed into its monosaccharide components glucose and fructose by a membrane-bound intestinal α-glucosidase enzyme called sucrase-isomaltase (henceforth “sucrase,” Brun et al., 2020a). The capacity to digest sucrose, defined as the rate at which the whole intestine hydrolyses sucrose, limits maximal food intake in hummingbirds (McWhorter and Martínez del Rio, 2000). Even so, the capacity of hummingbirds to digest sucrose is greater than that measured in other birds (Schondube and Martínez del Rio, 2004). Sucrase activity appears to be absent in a large monophyletic clade of birds that includes starlings (Sturnidae), thrushes (Turdidae), and mockingbirds (Mimidae; Cibois and Cracraft 2004), and these birds are unable to assimilate sucrose (Martínez del Rio 1990b).
Sucrose assimilation efficiency is high in specialized nectarivores: they assimilate all or almost all the sucrose that they ingest (Napier et al., 2013). However, to our knowledge, the sucrose digestive capacity of sunbirds and honeyeaters has not been compared with that of other birds. Cinnamon-bellied flowerpiercers (Diglossa baritula) specialize in robbing nectar from hummingbird flowers but have sucrose digestion capacities that are lower than those of hummingbirds and similar to those of omnivorous songbirds (Schondube and Martínez del Rio, 2004). Specialized nectarivory thus appears not to be necessarily associated with a high capacity to digest sucrose and the digestive abilities of hummingbirds may be unique. Alternatively, perhaps other specialized passerine avian nectarivores, such as honeyeaters and sunbirds, have converged with hummingbirds in the capacity to digest sucrose rapidly and efficiently.
Although sucrose, glucose, and fructose are all present in the nectar of ornithophilous flowers, sucrose is predominant in those visited by nectar-feeding specialists (Johnson and Nicolson 2008). This is a pattern that was first documented in the New World between hummingbirds and the plants that they pollinate (Baker and Baker 1982) but that was later extended to plants pollinated by sunbirds and honeyeaters in Africa and Australia (Johnson and Nicolson 2008; Nicolson and Fleming 2003). Different plant lineages on different continents, pollinated by independently evolved specialized nectar-feeding birds, appear to have converged in nectar sugar composition (Figure 1).
Figure 1.
Geographical distribution of the three major clades of nectar-feeding birds and sucrose content in nectar
(A) Nectarivory in extant birds evolved independently in the Old and New Worlds. Hummingbirds are found exclusively in the Americas, whereas sunbirds are found in Africa, Southern Asia, and Australasia. Honeyeaters are found exclusively in Australasia.
(B) The relative content of the disaccharide sucrose is higher in plants pollinated by bird nectarivores than in those pollinated by nonspecialized birds (data from Johnson and Nicolson 2008). Data are represented as mean ± SEM.
Because plants pollinated by nectar-feeding specialist birds secrete sucrose-rich nectar, we hypothesized that the capacity of these birds to digest sucrose (which we call sucrase activity) would be higher than that of non-nectarivores and birds that feed on nectar only facultatively. This hypothesis is a corollary of Diamond's (2002) quantitative design hypothesis that poses that physiological capacities such as the capacity to digest sucrose should match sucrose-ingested loads. Because nectar-feeding evolved independently in hummingbirds (Trochilidae), sunbirds (Nectarinidae), honeyeaters (Meliphagidae), flowerpiercers (genus Diglossa, Thaupidae), and lorikeets (Loriinae), we hypothesized and quantified convergence in this trait among these taxa.
We compared sucrase activity and intestinal surface area among species using new measurements on sunbirds, honeyeaters, and lorikeets and previously published data on hummingbirds and a variety of other birds, including insectivores, granivores, frugivores, and omnivores that include a variety of food types (Figure 2, Table S1; Del Hoyo et al., 2013). We included 20 species of putative nectarivores (11 hummingbirds, 3 sunbirds, 4 honeyeaters, 1 parrot, and 1 flowerpiercer), a single folivore (Phytotoma rara), and 29 species considered omnivores but including differing amounts of plant and animal sources in their diets (from the strictly insectivorous such as Empidonax difficilis to omnivorous Sturnus vulgaris).
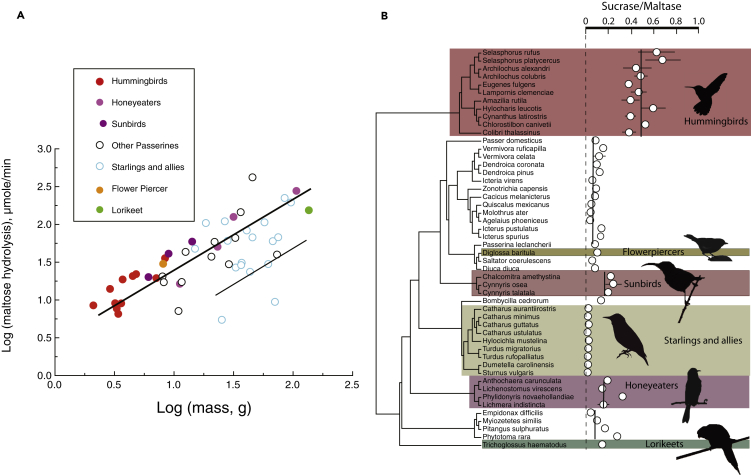
Figure 2.
Species included in this study and their sucrose activity per unit area
Hummingbirds, honeyeaters, and sunbirds have greater sucrase activity per unit intestinal surface area than other birds. Cinnamon-bellied flowerpiercers (Diglossa baritula) and rainbow lorikeets (Trichoglossus haematodus) have values similar to those of birds not specialized on nectar as a food source. Rufous-tailed plantcutters (Phytotoma rara) had extraordinarily high sucrase activity per unit intestinal area. The phylogenetic hypothesis was trimmed from Jetz et al. (2012). Data are represented as mean ± SEM.
See also Table S1.
Results and discussion
Intestinal surface area and capacity to digest sucrose
We found that intestinal surface area increased as an allometric function of body mass and was lower in nectar-feeding birds than in other birds (Figure 3A). As per the best supported model (Model 6′, F2,42 = 261.6, p < 0.0001), nectar-feeding birds had intestinal areas that were on average 28% smaller. A second model that included hummingbirds as a separate category from the nectarivorous passerines was also strongly supported (ΔAIC = 1.19), with hummingbirds having higher surface areas than honeyeaters and sunbirds. A comparison of phylogenetic generalized linear models supported these results with Model 6′ being best supported and Model 3′ receiving similar support (ΔAICc = 1.47).
Figure 3.
Allometric relationships between intestinal surface area and sucrose hydrolysis capacity among nectar-feeding birds and other bird groups
(A) Intestinal surface area (SA) increased allometrically with body mass with an exponent equal to 0.74 ± 0.03 (r2 = 0.96). Specialized nectarivores (heavy line, log(SA) = 0.01 + 0.73·log(mb)) had lower intestinal surface area than other birds (thin line, log(SA) = 0.42 + 0.73·log(mb)). Lines represent best-fit regression lines to those two groups.
(B) The capacity to hydrolyze sucrose (SH) increased allometrically with body mass (exponent = 0.78 ± 0.12) but differed among bird groups. Hummingbirds (dashed line, log(SH)hummingbirds = 0.35 + 0.78·log(mb)) had higher capacities than honeyeaters and sunbirds (heavy line, SHhoneyeaters and sunbirds = 0.08 + 0.78 log(mb)). Both nectarivore groups had higher capacities than other birds (thin line, SHother = −0.34 + 0.78 log(mb)). In the “other bird” group, there were three notable outliers: rufous-tailed plantcutters (Phytotoma rara) and orchard orioles (Icterus spurius) which had exceptionally high sucrose hydrolysis capacity and the insectivorous pacific-slope flycatcher (Empidonax difficilis) with low capacity.
The capacity to hydrolyze sucrose also scaled positively with body mass (Figure 3B). The best supported model distinguished hummingbirds from honeyeaters and sunbirds and these taxa again from other birds (Model 7′, F3,36 = 15.20, p < 0.001; r2 = 0.57). It described the capacity to digest sucrose as being ∼1.8 times greater in hummingbirds than in honeyeaters and sunbirds and ∼5 times greater in hummingbirds than in other birds. Sucrose hydrolysis capacity was 2.7 times greater in honeyeaters and sunbirds than in “other” taxa. However, because of overlap in sucrose digestion capacity between hummingbirds and honeyeaters and sunbirds, the model that grouped hummingbirds with sunbirds and honeyeaters was also well supported (Model 8, ΔAICc = 1.18). The best-supported phylogenetically explicit model included nectarivores (hummingbirds, honeyeaters, and sunbirds) in a single category with high sucrose hydrolysis capacity, and all other taxa in another with lower capacity (Model 8′). The phylogenetically explicit model distinguishing hummingbirds separately had similar support (Model 7′, ΔAICc = 0.35). Together, these results suggest that sucrose digestion capacity is higher in nectar-feeding birds than in other birds.
The best model describing sucrase activity per unit intestinal area (Model 7, F2, 43 = 38.03, p < 0.0001) indicated that hummingbirds had greater sucrase activity (3.2 ± 0.24 μmole/[min·cm2]) than other specialized nectarivores (2.4 ± 0.30 μmole/[min·cm2]), which had greater sucrase activities than all other birds (0.62 ± 0.24 μmole/[min·cm2], Figure 2). A second model that grouped all the “nectarivorous lineages” (i.e., hummingbirds, honeyeaters, and sunbirds) was also substantially supported (Model 8, ΔAICc = 1.6). The results of phylogenetic analyses indicated that nectar-feeding birds had higher sucrase activity per unit intestinal surface area (Model 8), but did not support a distinction between hummingbirds and other specialized nectarivores (ΔAICc >2). Sucrase activity per unit intestinal surface area appears to be higher in nectarivores than in other birds.
The high capacity of nectarivores to digest sucrose was owing, at least in part, to high expression of sucrase activity per unit intestinal surface area. High sucrase activities per unit intestinal surface area more than compensate for the reduced intestinal surface area and length that has been repeatedly documented in nectar-feeding birds (Richardson and Wooller, 1986; Wooller and Richardson 1988). Sucrase activity is owing to the action of a membrane-bound alpha-glucosidase enzyme called sucrase-isomaltase (coded by the SI gene) and expressed in the apical membrane of intestinal cells (called the brush-border membrane, Brun et al., 2020a). Recently developed methods in proteomics allow quantifying the abundance of different digestive enzymes in the brush-border membrane (Brun et al., 2020b). These proteomics methods have revealed that in the muscicapoid lineage (predominantly insectivorous) that includes starlings, thrushes, and mockingbirds, sucrase is present (Brun et al., 2020a) but has lost the ability to hydrolyze sucrose (Martínez del Rio, 1990b). In this light, we hypothesized that the abundance of the sucrose-isomaltase protein in the intestinal brush-border membrane is higher in nectarivorous birds than in other birds.
In some digestive enzymes, such as salivary amylase, increased expression is a consequence of increased gene dosage (Pajic et al., 2019). In the two annotated hummingbird genomes available, one species, Calypte anna, has two copies of the sucrase-isomaltase gene, whereas the other species, Oreotrochilus melanogaster, has only one, as do the genomes of almost all other bird species that we examined (Table S2). The availability of more well-annotated genomes, as well as of transcriptomic data for the gastrointestinal tract of nectar-feeding birds, will allow quantifying sucrase-isomaltase gene copy number and expression.
Although the abundance of sucrase in the intestinal brush-border is likely an important contributor to the capacity to hydrolyze sucrose, it might not be the only factor that leads to higher sucrase capacity in nectar-feeding birds. Unlike mammals that have two discrete alpha-glucosidases with distinct, but partially overlapping, substrate specificities (Brun et al., 2020a), the birds that we studied have sucrose-isomaltase as the sole intestinal enzyme capable of hydrolyzing sucrose, maltose and isomaltose. We found that maltose hydrolysis capacity scaled positively with body mass (F2,38 = 53.46, p < 0.0001, Figure 4A). Birds in the muscicapoid lineage had maltase activities per unit area (1.98 ± 0.28 μmole/[min·cm2]) that were only about a third of those found in other birds (7.33 ± 0.76 μmole/[min·cm2], Model 9, F1,41 = 11.5, p < 0.001). The ratio of sucrase to maltase activity of the sucrose-isomaltase enzyme in hummingbirds (0.49 ± 0.018) is higher than that in other birds (0.22 ± 0.022, Model 7, F2, 43 = 128.24, p < 0.001, Figure 4B), but the molecular bases for this difference are unknown. In addition, the catalytic capacities of an enzyme are the result of the combined effect of enzyme “concentration” (which, in the case of sucrase, is related to its abundance in the membrane) and the enzyme's turnover number (quantified by kcat values, Davidi et al., 2016), neither of which has been compared among different bird groups. The biochemical and molecular bases that underlie convergence in sucrose digestion capacity among nectar-feeding birds remain to be explored.
Figure 4.
Maltose hydrolysis capacity as a function of body mass and ratio of sucrase to maltase activity for species included in this study
(A) The capacity to hydrolyze maltose (MH) increased allometrically with body mass (r2 = 0.74, exponent = 0.83 ± 0.09), but differed among bird groups with starlings and allies having lower capacity (thin line, MH = 0.009 + 0.83log(mb)) than other birds (heavy line, MH = 0.62 + 0.83log(mb)).
(B) Hummingbirds, honeyeaters, and sunbirds have greater sucrose activity per unit intestinal surface area and greater sucrase/maltase activities ratios than other birds. Hummingbirds had greater sucrase/maltase ratios than other nectar feeders, which had greater sucrase/maltase ratios than “other-starlings and their allies” taxa. Horizontal bars represent means for each group. Data are represented as mean ± SEM.
The high sucrase activity of nectar-feeding birds may have two nonexclusive explanations: 1) constitutively high expression of sucrase reflects a greater capacity to digest sucrose, because of high levels of sucrose intake in their natural diet and 2) high sucrase activity results from acclimation to a high-sucrose diet in captivity. However, disaccharidases are largely unresponsive to changes in diet in birds (Caviedes-Vidal et al., 2000). The adaptive modulation hypothesis, which poses that digestive traits should be increased by increased levels of their substrates (Karasov 1992), does not seem to apply for adult birds and sugar-digesting enzymes. In birds that modulate disaccharidase activities in response to diet changes (e.g., growing sparrows and chickens, Gatica-Sosa et al., 2015), the magnitude of this effect is smaller than the differences observed in this study. In addition, we included both species sampled immediately after trapping in the wild and species maintained in captivity on sucrose-rich diets. Our data do not suggest any differences in sucrase activity attributable to artificial diets. Although changes in enzyme expression or activity subsequent to a high-sucrose diet in captivity cannot be ruled out completely, this is an unlikely explanation for our results.
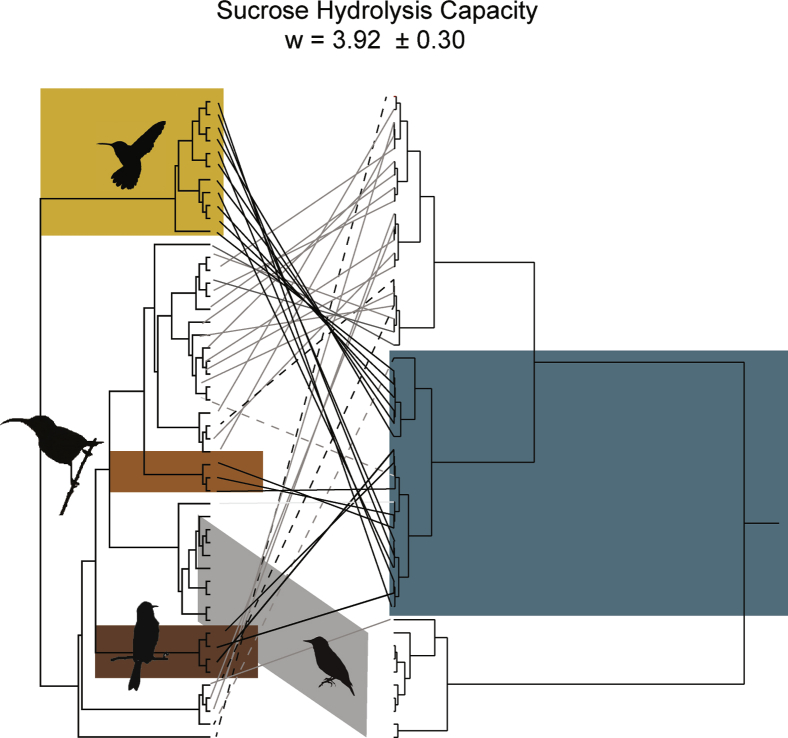
Our observations imply that hummingbirds, sunbirds, and honeyeaters have converged in traits that facilitate the digestion of sucrose. For this reason, we identified them as focal taxa in the estimation of strength of convergence Wheatsheaf indices (Arbuckle et al., 2014). Surprisingly, the flowerpiercer and rainbow lorikeet appear not to share these traits, so we excluded them as focal species. All hummingbirds, sunbirds, and three of the four honeyeaters (all except Lophocampa indistincta) coalesced in distinct clusters characterized by high sucrase capacity (Figure 5). These clusters also included the orchard oriole (Icterus spurius) and the folivorous rufous-tailed plantcutter (Phytotoma rara). Wheatsheaf indices indicated strong convergence in sucrose digestion capacity among hummingbirds, sunbirds, and most honeyeaters (w = 3.92 ± 0.30 95% CI).
Figure 5.
Tanglegram joining species in the phylogenetic tree (left) with a phenetic tree (right) constructed using hierarchical cluster analysis on the residuals of log(sucrose hydrolysis capacity) and log(mb)
The analysis defined three distinct clusters: (1) a high hydrolytic capacity cluster (blue block on the phenetic tree on the right-hand side) that includes (1a) all hummingbirds (11 species; yellow block on the phylogenetic tree on the left-hand side), (1b) all sunbirds (3 species; orange), and (1c) honeyeaters (3 species, except Lichmera indistincta; brown). (2) A cluster for starlings and their allies, which have no sucrase activity, indicated by a gray block. (3) An intermediate capacity cluster that included all other species, as well as included the putative specialized nectarivores L. indistincta, Diglossa baritula, and Trichoglossus haematodus, joined by the strict insectivore Empidonax difficilis. Black lines identify putative specialized nectarivores, whereas dashed ones identify those that were outside of the high capacity cluster. Non-nectarivores are identified by gray lines. Note that two putative non-nectarivores (Phytotoma rara and Icterus spurius, dashed gray lines) were included in the high capacity cluster. Wheatsheaf (w ± 95% CI) indices estimated strong convergence among hummingbirds, sunbirds, and honeyeaters (except L. indistincta) in sucrose hydrolysis capacity.
In sum, several complementary analyses support our hypotheses that hummingbirds, sunbirds, and some, albeit not all, honeyeaters converged in having high sucrose hydrolysis capacity. This convergence appears to be a consequence of high sucrase activity per unit intestinal surface area. Greater sucrase activity per unit area appears to be shared by nectarivores, and this trait likely contributes to the ability to ingest and efficiently assimilate higher quantities of the sucrose-rich nectar secreted by the plants that they pollinate (Napier et al., 2013). It also supports the intriguing idea that the high nectar sucrose concentration of many flowers pollinated by hummingbirds, sunbirds, and honeyeaters, which independently evolved on different continents, evolved in parallel with the ability of these birds to assimilate sucrose efficiently and rapidly.
Although we found strong support for convergent evolution in nectarivores, we also found exceptions that reveal evolutionary lability in the expression of avian sucrase. Three species of putative nectarivores, brown honeyeaters (L. indistincta), rainbow lorikeets (Trichoglossus haematodus), and cinnamon-bellied flowerpiercers (D. baritula), had relatively low intestinal sucrase capacities and low sucrase per unit of intestinal surface area, whereas orchard orioles (I. spurius) and rufous-tailed plantcutters (P. rara) had high sucrase capacities and high sucrase activity per unit intestinal surface area. The low sucrose hydrolysis capacity of wild-caught L. indistincta is surprising because this species is considered highly reliant on nectar (Pyke 1980). The differences in this trait between L. indistincta and its close relative Phylidonyris novaehollandiae (Gardner et al., 2010) are difficult to explain. The morphology of nectar-feeding lorikeets suggests adaptations to a nectar-and-pollen diet (Schweizer et al., 2014). However, lorikeets appear to feed primarily on the open flowers of Eucalyptus spp. whose nectar contains little or no sucrose (Nicolson 1994). The low sucrose hydrolysis capacity found in D. baritula is perplexing because this species is a nectar-robber of hummingbird-pollinated flowers with sucrose-rich nectars (Arizmendi 2001). Other Diglossa species have been documented feeding on insects in addition to nectar (Montenegro et al., 2015).
I. spurius (Icteridae) and P. rara had both high sucrose hydrolysis capacities and high sucrase per intestinal surface area. I. spurius frequently visit and pollinate flowers, visit hummingbird feeders (commonly containing sucrose solutions), rob the nectar of hummingbird-pollinated flowers and have been hypothesized to have coevolved with a large-flowered plant species whose flowers secrete sucrose-rich nectar (Erythrina fusca, Morton 1979). It thus appears to be a facultative nectar-feeder with traits that have converged with those of more specialized species. P. rara expressed high sucrose hydrolysis capacity and high sucrase per unit surface area. The three species in the genus Phytotoma are among the very few almost completely leaf- and bud-eating passerines (López-Calleja and Bozinovic 2000). It is possible that small avian folivores such as Phytotoma, with very simple tubular gastrointestinal tracts, very short digesta retention time, and hence minimal fermentation capacity (traits shared by all species in this study), must rely on very high expression of sucrase to rapidly and efficiently assimilate sucrose in the cytoplasm of leaf and bud cells (Meynard et al., 1999).
In the New World, hummingbird-pollinated flowers secrete nectar predominantly containing sucrose, whereas nectars of passerine-pollinated plants by contain primarily the hexoses glucose and fructose (Martínez del Rio, 1990a). Using a larger sample that included more Old World flowering species and accounting for phylogenetic relationships, Johnson and Nicolson (2008) found that the differences in sugar composition were not between hummingbird- and passerine-pollinated plants but between plants pollinated by specialized nectarivores (i.e., hummingbirds and sunbirds), which produce low volumes of concentrated nectar with high sucrose content, and those pollinated by birds that feed on nectar occasionally, which secrete dilute hexose-rich nectars. Taking these analyses a step further, Abrahamczyk et al. (2017) analyzed an enormously large data set of asterid plants (∼2100 species in 660 genera and 55 families) for nectar sugar composition of plants pollinated by either specialized nectarivores or generalists including insects, bats, and birds. They found an association between sucrose content and degree of pollinator specialization. Consistent with the earlier analyses, asterid plants pollinated by more specialized nectarivores tend to have higher sucrose content. However, Abrahamczyk et al. (2017) concluded that the preferences or nutritional needs of pollinators only provides a partial explanation for the sugar composition in nectar, and associations between pollinator type and nectar sugar composition does not necessarily imply selection of sucrose-containing nectar by specialized pollinators.
Nectar-feeding birds perform equally well on sugar solutions containing sucrose, glucose and fructose mixtures, and even solutions containing only glucose or fructose (Martínez del Rio, 1990a; Fleming et al., 2004; Fleming et al., 2008; Chen and Welch 2014). In addition, it is unlikely that the relatively weak preferences of specialized nectar feeders for sucrose are the selective drive for the sugar composition of nectar (Martínez del Rio, 1990a). Higher sucrase activity confers nectar-feeding birds with increased capacity to assimilate sucrose and is correlated with diet preference (Napier et al., 2013), but does not hinder the assimilation of glucose and fructose. Nicolson and Fleming (2003) have summarized alternative hypotheses. Our currently favored hypothesis is that the preferences of facultative nectarivores including starlings and their allies, many of which avoid sucrose-containing nectars, drives this pattern. Nectar is secreted from sucrose-rich phloem. Plants that secrete hexose-rich nectars must hydrolyze the phloem's sucrose with the enzyme invertase, the plant analog of sucrase (Heil 2011). Pollination by nectar-feeding bird lineages that have higher sucrose hydrolysis capacity and which are therefore not sucrose-averse or have a weak preference for sucrose (Nicolson and Fleming 2014), might have led to reduced invertase expression in nectaries favored by reduced invertase synthesis costs (Martínez del Rio, 1990a).
Although we do not have a complete explanation yet for the association between the secretion of sucrose-rich nectars in plants and high sucrose digestion capacity in nectar feeding birds, the pattern is clear. This association is a striking example of convergent evolution in birds that appears to have taken place independently across continents. Just as flowers pollinated by specialized nectar-feeding birds converged in nectar sugar content, so have the birds that feed on them converged in their capacity to digest sucrose. This result appears to be an example of intercontinental convergent coevolution between flowering plants and the birds that pollinate them.
Limitations of the study
The inclusion of only one species from two of the putative specialized nectarivore lineages analyzed (flower piercers: D. baritula, Thraupidae; lorikeets: T. haematodus, Loriinae) limited the inferences made regarding these lineages. Inclusion of species from other specialist (e.g., Hawaiian honeycreepers, Fringillidae) and generalist lineages would strengthen the study. In addition, quantifying both enzyme abundance in the cell membrane and catalytic turnover rate (kcat value, Davidi et al., 2016) would allow a more sophisticated comparison of enzyme functional capacity among lineages. Finally, the availability of annotated genomes for more species of specialized nectar-feeding birds, as well as transcriptomic data for the gastrointestinal tract, will allow comparison of sucrase-isomaltase gene copy number and expression.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical Commercial Assays | ||
| Glucose (GO) Assay Kit | Sigma Aldrich | GAGO20 |
| Deposited Data | ||
| Calypte anna genome | NCBI | bCalAnn1_v1.p |
| Oreotrochilus melanogaster genome | NCBI | ASM1340099v1 |
| Software and Algorithms | ||
| Basic Local Alignment Search Tool (BLAST) | NCBI | https://blast.ncbi.nlm.nih.gov/Blast.cgi |
Resource availability
Lead contact
-
•
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Todd McWhorter (todd.mcwhorter@adelaide.edu.au).
Materials availability
-
•
This study did not generate new unique reagents.
Data and code availability
-
•
The published article and supplemental information include all data generated and analyzed during this study.
Experimental model and subject details
Birds and their maintenance
Rainbow lorikeets (Trichoglossus haematodus) of mixed sex were captured by canon-netting in July 2006 on the grounds of Perth Domestic Airport (Western Australia). Honeyeaters of mixed sex were captured by mist netting on the grounds of Murdoch University (Perth, Western Australia) in 2007. Singing honeyeaters (Lichenostomus virescens) and brown honeyeaters (Lichmera indistincta) were euthanized immediately upon capture. Rainbow lorikeets were held in captivity for >10 months, red wattlebirds (Anthochaera carunculata) for five months, and New Holland honeyeaters (Phylidonyris novaehollandiae) for 14 months before they were euthanized for analysis. During the period of captivity, the birds were fed a maintenance diet consisting of Wombaroo® powder (main sugar ingredient sucrose, Wombaroo Food Products, Adelaide, South Australia) supplemented with additional sucrose (∼25% m/m of total dry matter) supplied ad libitum from inverted, stoppered syringes and water ad libitum. Male Palestine sunbirds (Cinnyris osea) were captured with drop nets on the grounds of Midreshet Ben-Gurion, home of the Sede Boqer Campus of Ben-Gurion University of the Negev, Israel, in 2000–2001 and were held in captivity and fed an artificial nectar diet including 18% m/m sucrose, avian vitamin supplement, CaCO3, NaCl, KCl, and corn oil, periodically supplemented with fruit flies (Drosophila sp.) for up to three months before their tissues were analyzed. White-bellied sunbirds (Cinnyris talatala) and amethyst sunbirds (Chalcomitra amethystina) of mixed sex were mist-netted at Jan Celliers Park (Pretoria, South Africa) in May 2007 and were euthanized immediately upon capture. All birds used were adults.
Birds were euthanized by asphyxiation with CO2 (Palestine sunbirds) or halothane or isoflurane overdose (all other species) and their intestines immediately removed. Intestines were rinsed in chilled 1.02% saline and divided into three sections of approximately equal length. Each tissue section was slit longitudinally, unfolded flat, and its length and width were measured to obtain a nominal estimate of surface area. The tissue was then blotted dry, weighed and stored in liquid N2 or in a freezer at -80°C until analysis.
Animal use approval
Animal use in this study was approved by the animal ethics committees of Murdoch University (AEC protocol R1137/05), the University of Arizona (protocol 99-103) and the University of Pretoria (AUCC 060515-012) as applicable.
Method details
Intestinal enzymatic activity
Intestinal tissues were thawed at 5°C and homogenized (30 s at 24,000 rpm, OMNI 5000 homogenizer or similar) in approximately nine volumes of 350 mmol∙L-1 mannitol in 1 mmol∙L-1 Hepes/KOH at pH 7.5 (resulting in a concentration of 80–100 mg of intestinal tissue per mL of homogenate). Disaccharidase activities were measured following the methods reported in studies by Martínez del Rio (1990b) and Schondube and Martínez del Rio (2004) with slight modifications. In brief, tissue homogenate aliquots of 33 μl previously diluted with 350 mmol∙L-1 mannitol in 1 mmol∙L-1 Hepes/KOH were incubated at 40°C with 33 μl of 56 mmol∙L-1 sugar (sucrose or maltose) solutions in 0.1 mol∙L-1 maleate/NaOH buffer at pH 6.5. After 10–20 min of incubation, 400 μL of a stop-develop reagent (glucose assay kit GAGO20, Sigma-Aldrich, St. Louis, MO, USA; made up with equal parts of 1.0 mol∙L-1 TRIS/HCL at pH 7 and 0.5 mol∙L-1 NaH2PO4/Na2HPO4 at pH 7) was added to each tube which was then incubated at 40°C for a further 30 min. Finally, 400 μL of 12 N H2SO4 was added to each tube to stop the develop reaction, and the absorbance was read at 540 nm with a spectrophotometer (Spectronic 20 GENESYS, Spectronic Instruments, Rochester, NY, USA or similar). Disaccharidases in Palestine sunbirds were measured similarly, but after the initial 10- to 20-minute incubation, reactions were arrested by adding 1 mL of a stop/developing Glucose-Trinder reagent (one bottle of Glucose-Trinder 500 reagent, Sigma-Aldrich, St. Louis, MO, USA; made up with equal parts of 1.0 mol∙L-1 TRIS/HCL at pH 7 and 0.5 mol∙L-1 NaH2PO4/Na2HPO4 at pH 7). After exactly 15 min at 20°C, absorbance of the resulting solution was read at 505 nm with a spectrophotometer.
To determine pH optima, we used a 0.1 mol∙L-1 maleate/NaOH buffer system for sucrase and maltase with pH ranging from 5 to 8.5 in 0.5 pH increments with disaccharide concentrations held constant (56 mmol∙L-1). Measurements reported in the results were corrected to optimal pH if this differed from assay pH (6.5 for disaccharidases) by simply dividing activity at pH optima by relative activity at assay pH. We standardized enzyme activities by intestine nominal (smooth bore tube) surface area (i.e., μmol∙min-1∙cm-2). We used the log10-transformed total summed activity under standardized assay conditions (μmol∙min-1).
Determination of sucrase-isomaltase gene copy numbers
We used the whole genomic information of 44 avian species available in the NCBI database to determine the gene copy numbers for the sucrase-isomaltase (SI) intestinal gene. We examined the gene copy number of SI reported in the annotation files (gff) for each bird species. To determine the SI gene copy number in the available hummingbird species genomes for Calypte anna (GenBank: bCalAnn1_v1.p) (Korlach et al., 2017) and Oreotrochilus melanogaster (Genbank: ASM1340099v1) (Feng et al., 2020), we extracted the SI sequence from each genome and performed a blastn (NCBI-BLAST) (Altschul et al., 1990) search to find regions of local similarity between the SI gene and the whole genome assembly (using an e-value = 1∗10-8, identity percent ≥ 75% and coverage percent ≥ 90%).
Quantification and statistical analysis
Published values for gut nominal (smooth bore tube) surface area, body mass (mb), and summed enzymatic activities for 11 hummingbird and 31 passerine species measured using similar methods are available from various sources (Table S1). These were compared with new data collected for eight nectarivorous species: one Australian lorikeet (Loriidae), four Australian honeyeater (Meliphagidae), and three African/Asian sunbird (Nectariniidae) species (Table S1). We analyzed two kinds of dependent variables: those that scale allometrically with body mass (mb in grams), including nominal intestinal surface area (cm2) and sucrase hydrolytic capacity (μmole/min), and those that do not scale with body mass (sucrase activity/intestinal area [μmole/min.cm2]). We did both standard statistical analyses and analyses that accounted for phylogenetic relationships (phylogenetic ANOVA and phylogenetic generalized linear models; Revell 2011). The phylogenetic hypothesis in Figure 2 represents species in our analyses and was “pruned” from the global phylogeny of birds super tree (Jetz et al., 2012; Rubolini et al., 2015; BirdTree.org). Table 1 lists the discrete categories that we used in our models. We added log-transformed(mb) as a continuous independent variable to the discrete variables listed in Table 1 for response variables scaling with body mass. Briefly, in our most complete model, we categorized birds as belonging to the following groups: hummingbirds, honeyeaters, sunbirds, flowerpiercers, lorikeets, starlings and their allies, and other birds. We then constructed nested subsets of reduced models that pooled groups of birds (Table 1). For example, some models pooled honeyeaters and sunbirds as “specialized nectarivorous passerines,” whereas others pooled hummingbirds, honeyeaters, and sunbirds as “specialized lineages.” Because starlings and their allies lack significant sucrase activity, they were placed in their own category. Finally, because the categories “lorikeets” and “flowerpiercers’ were never included in the set of best-supported models, these species were pooled with other birds. We used AIC values corrected for small sample size (AICc) to compare among all the models in Table 1. In result details (supplemental information), we only describe inferences from models with ΔAICc < 2 relative to the best-supported model. We used the same model sets for standard and phylogenetic analyses and present the results of both sets of analyses to assess robustness of our inferences, that is, similarity of outcomes of models with different assumptions. The values of the statistics that accompany each model are given in result details. We predicted lower intestinal surface areas in specialized nectarivores and greater sucrose hydrolysis capacity than in other birds.
Table 1.
Nested set of driving variables used to compare among bird groups. Response variables were sucrase and maltase activity/intestinal area (μmole/[min.cm2]), and sucrase/maltase ratios. In analyses of nominal intestinal area and hydrolysis capacity, response variables were log(hydrolase capacity, μmole/min). In generalized linear models used to construct allometric relationships we included log(mb, g) as a covariate, and in results, we add an apostrophe to designate these models (e.g., Model x’). None of the models best supported by data included sunbirds and honeyeaters each as a single category or did they include flower-piercers or lorikeets. Hence, we grouped the former into “nectarivorous passerines” and the latter with other birds. Some models grouped hummingbirds, honeyeaters, and sunbirds into “nectarivorous lineages.”
| Model | Taxonomic classifications tested |
|---|---|
| 1 | Hummingbirds (H); Honeyeaters (HE); Sunbirds (S); Flowerpiercers (FP); Lorikeets (L); Starlings; Other birds (O). |
| 2 | Hummingbirds; nectarivorous passerines (HE&S); Starlings; Other (O + FP&L). |
| 3 | Hummingbirds; nectarivorous passerines (HE&S); Other (O + FP&L + Starlings). |
| 4 | Hummingbirds; All other. |
| 5 | Nectarivorous lineages (H, HE&S); Other (O + FP&L); Starlings. |
| 6 | Nectarivorous lineages (H, HE&S); All other (O + FP&L + including Starlings). |
| 7 | Hummingbirds; Nectarivorous passerines (HE&S); Other (O + FP&L; no Starlings). |
| 8 | Nectarivorous lineages (H, HE&S); All other (O + FP&L; no Starlings). |
| 9 | Starlings; All other (H + HE&S + FP&L + O). |
To assess convergence in sucrase/area and sucrase/maltase ratios among nectar-feeding groups, we constructed “tanglegrams” (Agrawal and Fishbein 2006; Speed and Arbuckle 2017). These match the position of species within a phylogenetic tree with their position in a phenetic tree that joins species based on a hierarchical cluster analysis of the magnitude of a phenotypic variable. Following Agrawal and Fishbein (2006), we used Ward's minimum variance method which minimizes the total within cluster variance. Species with convergent traits join independently evolved clades in the same clusters defined by phenotypic similarity (Agrawal and Fishbein 2006). We assessed the strength of convergence in sucrase capacity (estimated as the residuals of the allometric relationship between log(sucrose hydrolysis capacity) and log(mb)), sucrase/area, and sucrase/maltase ratios with Wheatsheaf indices (Arbuckle et al., 2014). Our analyses suggested convergence only among hummingbirds, sunbirds, and honeyeaters (excluding Lichmera indistincta), and we used these three groups as the foci of strength of convergence calculations. Wheatsheaf indices are calculated by first estimating phenetic distances corrected by phylogenetic relatedness, and then calculating the ratio of the average phenetic distances among all species pairs (and the average phenetic distance among all focal species pairs; Arbuckle et al., 2014). The value of w = 1 when these two averages are equal (i.e., there is no convergence) and increases as the phenetic similarity among focal species increases (and hence as the average phenetic distance among them decreases). We estimated 95% confidence intervals for w by jackknifing our data sets (Arbuckle et al., 2014). Because we did not use inferential statistics in our tanglegrams and Wheatsheaf index analyses, we consider them exploratory.
Acknowledgments
This research was funded by a variety of sources including a U.S. National Science Foundation dissertation improvement grant to CMR (many years ago), a United States-Israel Binational Science Foundation grant to BP and CMR (98-178), and an Australian Research Council Discovery Program grant (DP0665730) to PAF, SWN, and TJM.
Author contributions
Gathering of new data and compilation of existing data – TJM; data analyses and figures – CMR and JAR; gene duplication analysis - YGG; writing –CMR, TJM (who wrote the first draft), with contributions by all co-authors. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors declare no conflict of interest. Funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Published: July 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102717.
Supplemental information
References
- Abrahamczyk S., Kessler M., Hanley D., Karger D.,N., Müller M.P.J., Knauer A.C., Keller F., Schwertfeger M., Humphreys A.M. Pollinator adaptation and the evolution of floral nectar composition. J. Evol. Biol. 2017;30:112–127. doi: 10.1111/jeb.12991. [DOI] [PubMed] [Google Scholar]
- Agrawal A.A., Fishbein M. Plant defense syndromes. Ecology. 2006;87:S132–S148. doi: 10.1890/0012-9658(2006)87[132:pds]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arbuckle K., Bennett C.M., Speed M.P. A simple measure of the strength of convergent evolution. Meth. Ecol. Evol. 2014;5:685–693. [Google Scholar]
- Arizmendi M.C. Multiple ecological interactions: nectar robbers and hummingbirds in a highland forest in Mexico. Can. J. Zool. 2001;79:997–1006. [Google Scholar]
- Baker H.G., Baker I. Chemical constituents in nectar in relation to pollination mechanisms and phylogeny. In: Nitecki M.H., editor. Biochemical Aspects of Evolutionary Biology. Univ. Chicago Press; 1982. pp. 117–171. [Google Scholar]
- Brun A., Mendez-Aranda D., Magallanes M.E., Karasov W.H., Martínez del Rio C., Baldwin M.W., Caviedes-Vidal E. Duplication and functional convergence of intestinal carbohydrate-digesting enzymes. Mol. Biol. Evol. 2020;37:1657–1666. doi: 10.1093/molbev/msaa034. [DOI] [PubMed] [Google Scholar]
- Brun A., Magallanes M.E., Martinez del Rio C., Barrett-Witt G.A., Karasov W.H., Caviedes-Vidal E. A fast and accurate method to identify and quantify enzymes in brush-border membranes: in-situ hydrolysis followed by nano LC-MS/MS. Methods Protoc. 2020;3:15–23. doi: 10.3390/mps3010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviedes-Vidal E., Afik D., Martínez del Rio C., Karasov W.H. Dietary modulation of intestinal enzymes of the house sparrow (Passer domesticus): testing an adaptive hypothesis. Comp. Biochem. Physiol. A. 2000;125:11–24. doi: 10.1016/s1095-6433(99)00163-4. [DOI] [PubMed] [Google Scholar]
- Chen C.C.W., Welch K.C. Hummingbirds can fuel expensive hummingbird flight with either exogenous glucose or fructose. Funct. Ecol. 2014;28:589–600. [Google Scholar]
- Cibois A., Cracraft J. Assessing the passerine ‘Tapestry’: phylogenetic relationships of the Muscicapoidea inferred from nuclear DNA sequences. Mol. Phyl. Evol. 2004;32:264–273. doi: 10.1016/j.ympev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Cronk Q., Ojeda I. Bird-pollinated flowers in an evolutionary and molecular context. J. Exp. Bot. 2008;59:715–727. doi: 10.1093/jxb/ern009. [DOI] [PubMed] [Google Scholar]
- Davidi D., Noor E., Liebermeister W., Bar-even A., Flamholz A., Tummler K., Barenholz U., Goldenfeld M., Shlomi T., Milo R. Global characterization of in vivo catalytic rates and their correspondence to in vitro kcat measurements. Proc. Nat. Acad. Sci. 2016;113:3401–3406. doi: 10.1073/pnas.1514240113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Hoyo J., Elliott A., Sargatal J. Vol 1–16. Lynx Editions; 2013. Handbook of the Birds of the World. [Google Scholar]
- Diamond J. Quantitative evolutionary design. J. Physiol. 2002;542:337–345. doi: 10.1113/jphysiol.2002.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Stiller J., Deng Y., Armstrong J., Fang Q., Reeve A.H., Xie D., Chen G., Guo C., Faircloth B.C. Dense sampling of bird diversity increases power of comparative genomics. Nature. 2020;587:252–257. doi: 10.1038/s41586-020-2873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer R.C., James H.F., Storrs L. Convergent evolution of Hawaiian and Australo-Pacific Honeyeaters from distant songbird ancestors. Curr. Biol. 2008;19:1927–1931. doi: 10.1016/j.cub.2008.10.051. [DOI] [PubMed] [Google Scholar]
- Fleming P.A., Hartman Bakken B., Lotz C.N., Nicolson S.W. Concentration and temperature effects on sugar intake and preferences in a sunbird and a hummingbird. Funct. Ecol. 2004;18:223–232. [Google Scholar]
- Fleming P.A., Xie S., Napier K.R., McWhorter T.J., Nicolson S.W. Nectar concentration affects sugar preferences in two Australian honeyeaters and a lorikeet. Funct. Ecol. 2008;22:599–605. [Google Scholar]
- Fleming T.H., Kress J. Univ. of Chicago Press; 2013. The Ornaments of Life. [Google Scholar]
- Gardner J.L., Trueman J.W.H., Elbert D., Joseph L., Magrath R.D. Phylogeny and evolution of the Meliphagoidea: the largest radiation of Australasian songbirds. Mol. Phyl. Evol. 2010;55:1087–1102. doi: 10.1016/j.ympev.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Gatica-Sosa C., Brzec P., Chediack J.G., Cid F.D., Karasov W.H., Caviedes-Vidal E. Differential transcriptional responses underlie dietary induction of intestinal carbohydrase activities in house sparrow nestlings. Anim. Physiol. Anim. Nutr. 2015;100:236–242. doi: 10.1111/jpn.12354. [DOI] [PubMed] [Google Scholar]
- Heil M. Nectar: generation, regulation, and ecological functions. Trends Plant Sci. 2011;16:191–200. doi: 10.1016/j.tplants.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Jetz W., Thomas G.H., Joy J.B., Hartmann K., Mooers A.O. The global diversity of birds in space and time. Nature. 2012;491:444–448. doi: 10.1038/nature11631. [DOI] [PubMed] [Google Scholar]
- Johnson S.D., Nicolson S.W. Evolutionary associations between nectar properties and specificity in bird pollination systems. Biol. Lett. 2008;4:49–52. doi: 10.1098/rsbl.2007.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov W.H. Tests of the adaptive modulation hypothesis for dietary control of intestinal nutrient transport. Am. J. Physiol. 1992;263:R496–R502. doi: 10.1152/ajpregu.1992.263.3.R496. [DOI] [PubMed] [Google Scholar]
- Korlach J., Gedman G., Kingan S., Chin C., Howard J.T., Audet J., Cantin L. De novo PacBio long-read and phased avian genome assemblies correct and add to reference genes generated with intermediate and short reads. Gigascience. 2017;6:1–16. doi: 10.1093/gigascience/gix085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Calleja M.V., Bozinovic F. Energetics and nutritional ecology of small herbivorous birds. Rev. Chil. Hist. Nat. 2000;73:411–420. [Google Scholar]
- Martínez del Rio C. Sugar preferences in hummingbirds: the influence of subtle chemical difference on food choice. Condor. 1990;92:1022–1030. [Google Scholar]
- Martínez del Rio C. Dietary and phylogenetic correlates of intestinal sucrase and maltase activity in birds. Physiol. Zool. 1990;63:987–1011. [Google Scholar]
- McWhorter T., Martínez del Rio C. Does gut function limit hummingbird food intake? Physiol. Biochem. Zool. 2000;73:313–324. doi: 10.1086/316753. [DOI] [PubMed] [Google Scholar]
- McWhorter T., Martínez del Rio C., Pinshow B. Modulation of ingested water absorption by Palestine sunbirds: evidence for adaptive regulation. J. Exp. Biol. 2003;206:659–666. doi: 10.1242/jeb.00147. [DOI] [PubMed] [Google Scholar]
- Meynard C., López-Calleja M.V., Bozinovic F. Digestive enzymes of a small avian herbivore, the Rufous-tailed Plantcutter. Condor. 1999;101:904–907. [Google Scholar]
- Montenegro S., Álvarez S., Calderón J., Noguera E. Hábitos alimenticios y simpatría de tres robamieles (Diglossa) en un bosque andino de Nariño. Revista UNIMAR. 2015;33:215–227. [Google Scholar]
- Morton E.S. Effective pollination of Erythrina fusca by the orchard oriole (Icterus spurius): coevolved behavioral manipulation? Ann. Mo. Bot. Gard. 1979;66:482–489. [Google Scholar]
- Napier K.R., McWhorter T.J., Nicolson S.W., Fleming P.A. Sugar preferences of avian nectarivores are correlated with intestinal sucrase activity. Physiol. Biochem. Zool. 2013;86:499–514. doi: 10.1086/672013. [DOI] [PubMed] [Google Scholar]
- Nicolson S.W. Eucalyptus nectar: production, availability, composition and osmotic consequences for the larva of the eucalypt nectar fly, Drosophila Flavohirta. S. Afr. J. Sci. 1994;90:75–79. [Google Scholar]
- Nicolson A.W., Fleming P.A. Nectar as food for birds: the physiological consequence of drinking dilute sugar solutions. Plant Syst. Evol. 2003;238:139–153. [Google Scholar]
- Nicolson S.W., Fleming P.A. Drinking problems on a ‘simple’ diet: physiological convergence in nectar-feeding birds. J. Exp. Biol. 2014;217:1015–1023. doi: 10.1242/jeb.054387. [DOI] [PubMed] [Google Scholar]
- Pajic P., Pavlidis P., Lubov N., Romano R., Garneu D., Daugherty E., Globig A., Ruhl L. Independent amylase gene copy number bursts correlate with dietary preferences in humans. Elife. 2019;8:e44628. doi: 10.7554/eLife.44628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke G. The foraging behaviour of Australian honeyeaters: a review and some comparisons with hummingbirds. Aust. J. Ecol. 1980;5:343–369. [Google Scholar]
- Revell L.J. Phytools: an R package for phylogenetic comparative biology (and other things) Meth. Ecol. Evol. 2011;3:217–223. [Google Scholar]
- Richardson K.C., Wooller R.D. The structures of the gastrointestinal tracts of honeyeaters and other small birds in relation to their diets. Aust. J. Zool. 1986;34:119–124. [Google Scholar]
- Rubolini D., Liker A., Garamszegi L.Z., Møller A.P., Saino N. Using the BirdTree.org website to obtain robust phylogenies for avian comparative studies: a primer. Curr. Zool. 2015;61:959–965. doi: 10.1093/czoolo/61.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schondube J.E., Martínez del Rio C. Sugar and protein digestion in flowerpiercers and hummingbirds: a comparative test of adaptive convergence. J. Comp. Physiol. B. 2004;174:263–273. doi: 10.1007/s00360-003-0411-3. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Güntert M., Seehausen O., Leuenberger C., Hertwig S.T. Parallel adaptations to nectarivory in parrots, key innovations, and the diversification of the Loriinaee. Ecol. Evol. 2014;4:2867–2883. doi: 10.1002/ece3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed M.P., Arbuckle K. Quantification provides a conceptual basis for convergent evolution. Biol. Rev. 2017;92:815–829. doi: 10.1111/brv.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez R.K., Herrera-Montalvo L.G., Welch K.C. The sugar oxidation cascade: aerial refueling in hummingbirds and nectar bats. J. Exp. Biol. 2011;214:172–178. doi: 10.1242/jeb.047936. [DOI] [PubMed] [Google Scholar]
- Wooller R.D., Richardson K.C. Morphological relationships of passerine birds from Australia and New Guinea in relation to diets. Zool. J. Linn. Soc. 1988;94:193–210. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The published article and supplemental information include all data generated and analyzed during this study.