ABSTRACT
Background
Folate may play a preventive role in the early stages of colorectal carcinogenesis, but long latencies may be needed to observe a reduction in colorectal cancer (CRC) incidence. In addition, concerns have been raised about the potential for cancer promotion with excessive folate intake, especially after the mandatory folic acid fortification in the United States in 1998.
Objective
We aimed to examine the association between folate intake in different chemical forms and CRC risk, especially in the postfortification era in the United States.
Design
We prospectively followed 86,320 women from the Nurses’ Health Study (1980–2016). Folate intake was collected by validated food frequency questionnaires. CRC was self reported and confirmed by review of medical records. The association between the folate intake and CRC risk was assessed using Cox proportional hazards regression.
Results
We documented 1988 incident CRC cases during follow-up. Analyzing folate intake as a continuous variable, greater total folate intake 12–24 y before diagnosis was associated with lower risk of CRC (per increment of 400 dietary folate equivalents (DFE)/d, HR: 0.93, 95% CI: 0.85, 1.01 for 12–16 y; HR: 0.83, 95% CI: 0.75, 0.92 for 16–20 y; and HR: 0.87, 95% CI: 0.77, 0.99 for 20–24 y); and greater synthetic folic acid intake 16–24 y before diagnosis was also associated with a lower CRC risk (per increment of 400 DFE/d, HR: 0.91, 95% CI: 0.84, 0.99 for 16–20 y and HR: 0.91, 95% CI: 0.83–1.01 for 20–24 y). In the postfortification period (1998–2016), intake of total or specific forms of folate was not associated with CRC risk, even among multivitamin users.
Conclusions
Folate intake, both total and from synthetic forms, was associated with a lower risk of overall CRC after long latency periods. There was no evidence that high folate intake in the postfortification period was related to increased CRC risk in this US female population.
Keywords: folate, folic acid fortification, latency, colorectal cancer, prospective cohort
See corresponding editorial on page 1.
Introduction
Although the incidence of colorectal cancer (CRC) has been declining for the past several decades, in part due to the increased use of screening, CRC is still the third most commonly diagnosed cancer for both women and men in the United States (1). Folate, an essential B vitamin for DNA methylation, synthesis, and repair, has been extensively studied with CRC, as these DNA-related processes are critical for cell growth and differentiation (2, 3). In a large pooled analysis of 13 prospective cohort studies, higher total folate intake was associated with a lower risk of colon cancer (4). Similarly, in a recent meta-analysis, total folate intake was significantly associated with a lower risk of CRC in cohort studies; however, no significant effect of folic acid supplementation on CRC risk was seen in randomized clinical trials, though most of which were not studying CRC prevention initially and the duration was limited to 2–7 y (5). The evidence on the folate–CRC relationship was graded as “limited—no conclusion” due to inconsistency in 2 recent reports by the World Cancer Research Fund and American Institute for Cancer Research (6, 7).
In a previous clinical trial among participants with a recent history of colorectal adenoma, an increased risk of having ≥3 colorectal adenomas (precursor lesion of CRC) was seen in those randomized to 1 mg/d of folic acid for 3–5 y (8). Although the increased risk of multiple adenomas did not persist in the reanalysis of this trial with extended follow-up, the updated results suggested that folic acid might increase the risk of sessile serrated adenomas/polyps (9). Data from animal studies also suggested high folate intake might increase the risk of CRC, especially after the neoplastic lesion had developed (10–12). These findings have raised the concerns that mandatory folic acid fortification in the United States, which was fully implemented in 1998, may increase the incidence of CRC (13, 14). Serum folate levels in the overall United States population increased 2.5-fold from 1988–1994 to 1999–2000, followed by minor fluctuations thereafter (2001–2016) (15, 16). In 2 large prospective United States cohort studies, total folate intake in the postfortification period was associated with a significant decrease in the risk of CRC (17, 18). However, folate intake was only collected once in these 2 studies and the postfortification follow-up time was only ∼8 y, which might be insufficient given that the CRC development may take >10 y (19). No study, including our previous one (20). has extended the follow-up >10 y in the postfortification era.
To address these research gaps, we studied the association between long-term folate intake and risk of CRC using data from a large, well-characterized cohort of women, with validated and updated data on dietary intake. The follow-up period we used was from 1980 to 2016 and included 18 y following mandatory fortification, allowing us to further examine the long-term association of folate intake with CRC risk in the postfortification era.
Methods
Study population
The Nurses’ Health Study (NHS) cohort was established in 1976 and included 121,700 female registered nurses who were 30–55 y of age at recruitment. Participants are followed up with detailed questionnaires ascertaining lifestyle factors and medical history every 2 y, with cumulative follow-up rates of ∼90%. In the present study, we excluded participants who did not return the baseline food frequency questionnaire (FFQ), left an extensive number of items blank on the baseline FFQ, had missing information on folate intake or reported implausible energy intake levels (<600 or >3500 kcal/d) at baseline, had missing information on birthday and height, and had been previously diagnosed with cancer (except nonmelanoma skin cancer) or ulcerative colitis. After these exclusions, a total of 86,320 women were included in the analysis (Supplemental Figure 1). The study was approved by the institutional review boards of the Brigham and Women's Hospital and Harvard TH Chan School of Public Health and those of participating registries as required.
Dietary assessment
Dietary information was collected using validated semiquantitative FFQs in 1980,1984, 1986, and every 4 y thereafter. In each FFQ, participants were asked how frequently, on average, they had consumed 1 standard serving of a specific food item during the past year. Nine response options were provided, ranging from “never or less than once per month” to “six or more times per day.” Frequency responses were then converted to average daily intakes for each food item. Total folate intake was calculated from foods (both folic acid fortified and unfortified) and supplements, synthetic folic acid intake from fortified foods and supplements, dietary folate intake from unfortified and fortified foods, and natural folate intake from the folate naturally present in all foods. To account for the difference in the bioavailability of naturally occurring folate and synthetic folic acid, folate intakes in all 4 forms were expressed as dietary folate equivalents (DFE) (21). The conversion factor to DFE was 1.0 for natural folate, 1.7 for folic acid in fortified foods, and 2.0 for folic acid in supplements (22). The average daily intake of folate and other nutrients was calculated by multiplying the reported frequency of consumption of each food by its nutrient content, primarily based on the USDA Nutrient Database that corresponded to each time when the FFQ was administered. The nutrient consumption was further adjusted for total energy intake using the nutrient residual method (23).
The validity of the FFQs in evaluating folate intake has been assessed in validation studies conducted before and after folic acid fortification. The correlation coefficient between folate intake determined by FFQ and by diet record was 0.71 before fortification and 0.77 after fortification (24, 25). Folate intake from the FFQs has also been shown to predict red blood cell folate concentrations, with correlation coefficients of 0.55 for both pre- and postfortification intakes (24, 26).
Ascertainment of CRC cases
Self-reported information on the diagnosis of CRC was collected with biennial questionnaires. Participants who reported a diagnosis of CRC or next-of-kin who reported for participants who died were contacted for permission to review their medical records. Study physicians blinded to the exposure data reviewed records to confirm CRC diagnosis and extract information on anatomical location. In the present analysis, 1988 incident CRC cases were included. We identified deaths using information from next-of-kin, death certificates, and the National Death Index (27).
Assessment of covariates
Except for height being assessed at baseline, updated information was collected every 2 y for other lifestyle-, medical-, and health-related factors, such as body weight, cigarette smoking, physical activity, menopausal status, and menopausal hormone use; duration of use of regular aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs), duration of multivitamin use, history of lower gastrointestinal endoscopic examination, and family history of CRC. Physical activity was calculated by summing the product of reported hours spent on a variety of recreational activities, with the typical intensity expressed in metabolic equivalent of task (MET) for that activity. The duration of use of regular aspirin and other NSAIDs or multivitamins was assessed based on reported duration at baseline and updated using the responses to current aspirin/NSAIDs and multivitamin use during follow-up. Participants were considered to have a family history of CRC if ≥1 of their parents or siblings had been diagnosed with CRC.
Statistical analysis
Person-time of follow-up (in months) for each participant was calculated from the age at which the baseline questionnaire was returned to the age of CRC diagnosis, date of death, loss to follow-up, or end of follow-up (30 June 2016), whichever came first. Time-varying Cox proportional hazards regression models were used to examine the associations of folate intake with the risk of CRC. We stratified the analysis by age in months and calendar year of the current questionnaire cycle to control for potential confounding by these 2 time scales. We further adjusted for several risk factors for CRC in multivariable models, including height, BMI (kg/m2), pack-y of smoking before age 30 y, alcohol consumption, physical activity levels, menopausal status and menopausal hormone use, duration of regular aspirin and other NSAID use, duration of multivitamin use, history of lower gastrointestinal endoscopy, and family history of CRC. We also adjusted for other dietary factors, including total calorie intake and consumption of dietary fiber, total calcium (for total folate and synthetic folic acid analyses), dietary calcium (for dietary folate and natural folate analyses), total vitamin D (for total folate and synthetic folic acid analyses), dietary vitamin D (for dietary folate and natural folate analyses), red meat, and processed meat. We used cumulative averages up to the time of risk for BMI, physical activity, and intakes of dietary covariates during follow-up to represent long-term dietary and lifestyle patterns. The Cox proportional hazards assumption was evaluated by adding interaction terms between folate intake and age and calendar year to the multivariable model, and no significant deviations were observed.
To evaluate the latency between folate intake and CRC, we performed latency analyses based on dietary data collected at different time points (20, 28). In the cumulative average model, mean folate intake since 1980 through the follow-up period up to the time of risk was calculated; in the baseline-only model, folate intake was derived from the 1980 FFQ; in the simple update model, folate intake reported on the most recent FFQ before each follow-up interval was used; in the latency models, we used folate intake reported at different latencies (i.e.,, 4–8, 8–12, 12–16, 16–20, 20–24, and 24–28 y) before CRC diagnosis. For example, in the 12–16-y lag analysis, we examined the association of folate intake in 1980 with the risk of CRC between 1992 and 1996. Since the FFQs were collected every 4 y, the simple update model could be considered as a latency analysis of 0–4 y. Participants with missing information on folate intake for a particular FFQ cycle were excluded from the analysis.
Sensitivity analyses were conducted to test the influence of multivitamin use on the results for total folate and synthetic folic acid as follows: 1) we removed total vitamin D from the multivariable-adjusted model and 2) in the multivariable-adjusted model, we additionally adjusted for the duration of multivitamin use. We also corrected measurement errors in the assessment of folate intake by using a regression calibration approach (29) based on data from Women's Lifestyle Validation Study conducted by the NHS in 2010 (25). The mean intake of total or specific forms of folate assessed by two 7-d dietary records collected over 1 y showed good correlations with folate intake assessed by FFQ in the validation study (Supplemental Figure 2).
To investigate the association between folate intake and CRC risk before and after folic acid fortification, we conducted separate analyses for the prefortification (1980 –1998) and postfortification (1998–2016) periods, respectively. In the analysis for the postfortification period, we further adjusted for the cumulative average prefortification folate intake. We also examined the association between cumulative average prefortification folate intake and the risk of CRC diagnosed in the postfortification period. In subgroup analysis, we assessed whether the association for total and dietary folate intake varied by age, alcohol intake, family history of CRC, history of lower gastrointestinal endoscopy, aspirin or other NSAID use, and multivitamins use. A likelihood ratio test was used to compare the model with multiplicative interaction terms for total folate intake and the factors mentioned above to the model without such terms. All statistical tests were 2-sided, and p < 0.05 was considered statistically significant. We used SAS 9.4 (SAS Institute) for analyses.
Results
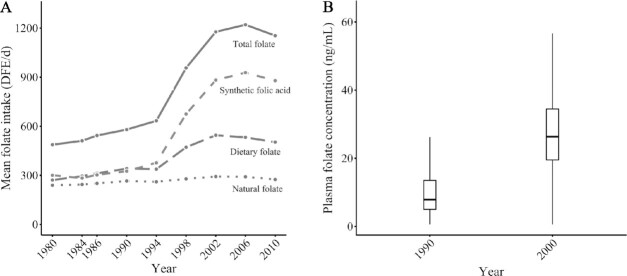
Due to the timing in which the implementation of folic acid fortification occurred, synthetic folic acid intake dramatically increased starting from 1998 and leveled off in 2002 (Figure 1A). The mean intake of energy-adjusted synthetic folic acid increased by 134% from 1994 to 2002. Intake of total folate and dietary folate (natural plus fortified) exhibited a similar increasing trend as synthetic folic acid. In contrast, natural folate intake did not change appreciably over the past 3 decades. Consistent with the change in folate intake, mean plasma folate levels substantially increased 2.5-fold from before folic acid fortification (1990) to after folic acid fortification (2000) (Figure 1B).
FIGURE 1.
Folate intake and plasma folate levels over 1980–2010. Mean folate intake over 1980–2010 (A). All folate intakes were energy adjusted. Total folate intake was calculated from foods (both unfortified and folic acid fortified) and supplements, synthetic folic acid intake from fortified foods and supplements, dietary folate intake from unfortified and fortified foods, and natural folate intake from the folate naturally present in both unfortified and fortified foods. Mean plasma folate levels before (1990) and after (2000) folic acid fortification (B). Within the Nurses’ Health Study, plasma folate was measured for 3880 participants in 1989–1990, and 696 participants in 2000–2001.
Characteristics of the participants in pre- and postfortification periods by quintiles of cumulative average total folate intake are shown in Table 1. At both periods, participants who consumed higher amounts of total folate tended to be more physically active and more likely to take aspirin/NSAIDs and multivitamins, and less likely to smoke before age 30. These participants were also somewhat more likely to have had endoscopy screening, use menopausal hormones, and have a greater dietary fiber intake.
TABLE 1.
Age-standardized characteristics of the study population in pre- and postfortification periods, according to quintiles of cumulative average total folate intake1
| Prefortification (1980–1998) | Postfortification (1998–2016) | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | |
| Person-y | 301,168 | 299,454 | 299,524 | 216,011 | 214,542 | 214,346 |
| Age at baseline, y | 45 | 47 | 47 | 63 | 63 | 64 |
| BMI | 25.1 | 25.1 | 24.6 | 26.2 | 25.8 | 25.5 |
| Height, cm | 164 | 164 | 164 | 163 | 164 | 164 |
| Physical activity, MET h/wk | 11.3 | 15.9 | 17.4 | 15.3 | 18.3 | 20.0 |
| Smoker before age 30 y, % | 57.4 | 50.6 | 51.7 | 52.0 | 50.6 | 50.6 |
| Pack-y of smoking before age 30 y, among smokers | 7.2 | 6.9 | 7.1 | 7.0 | 6.9 | 7.0 |
| History of previous endoscopy, % | 9.2 | 13.3 | 13.5 | 27.5 | 33.6 | 35.0 |
| Family history of CRC, % | 18.0 | 19.1 | 18.2 | 20.1 | 19.5 | 19.7 |
| Multivitamin use, % | 13.0 | 27.5 | 86.3 | 22.1 | 84.4 | 92.3 |
| Folic acid supplement, % | 2.2 | 3.4 | 7.9 | 5.8 | 12.3 | 33.9 |
| Regular aspirin or other NSAID use, % | 48.4 | 57.7 | 58.6 | 62.1 | 71.0 | 71.8 |
| Postmenopausal, % | 69.5 | 70.4 | 70.3 | 99.2 | 99.3 | 99.3 |
| Current menopausal hormone use, % | 22.9 | 32.8 | 36.5 | 23.7 | 23.3 | 24.7 |
| Never drinker, % | 28.9 | 24.1 | 25.0 | 21.2 | 17.2 | 18.8 |
| Dietary intake | ||||||
| Total folate, DFE/d | 189 | 377 | 1126 | 432 | 1073 | 1745 |
| Dietary folate, DFE/d | 190 | 332 | 329 | 422 | 481 | 593 |
| Synthetic folic acid, DFE/d | 13 | 101 | 1022 | 181 | 789 | 1430 |
| Natural folate, DFE/d | 179 | 273 | 269 | 254 | 278 | 316 |
| Alcohol among drinkers, g/d | 9.7 | 7.7 | 8.2 | 7.3 | 7.4 | 6.2 |
| Total vitamin D, IU/d | 184 | 257 | 629 | 188 | 202 | 219 |
| Total calcium, mg/d | 666 | 860 | 986 | 703 | 762 | 810 |
| Dietary fiber, g/d | 11 | 17 | 16 | 17 | 18 | 20 |
| Unprocessed red meat, svg/d | 0.8 | 0.7 | 0.6 | 0.6 | 0.6 | 0.4 |
| Processed red meat, svg/d | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 |
1All variables are standardized to the age distribution of the study population, except for age. Mean value is presented for continuous variables and percentage of participants for categorical variables. CRC, colorectal cancer; DFE: dietary folate equivalent; MET, metabolic equivalent of task; NSAID, nonsteroidal anti-inflammatory drug; svg: serving.
Over the whole follow-up period (1980–2016), we observed a significant inverse association between baseline intake of total folate (HR per 400 DFE/d greater: 0.90; 95% CI: 0.83, 0.97) and CRC risk (Table 2). The risk of CRC was not associated with baseline dietary folate, synthetic folic acid, or natural folate intake. We did not find statistically significant associations for the cumulative average intake of total folate, dietary folate, synthetic folic acid, or natural folate with CRC risk.
TABLE 2.
Risk of CRC according to folate intake with different latency periods, Nurses’ Health Study, 1980–20161
| Baseline | Cumulative | Simple update (0–4 y lag) | 4–8 y lag | 8–12 y lag | 12–16 y lag | 16–20 y lag | 20–24 y lag | 24–28 y lag | |
|---|---|---|---|---|---|---|---|---|---|
| Cases, n | 1988 | 1988 | 1687 | 1563 | 1385 | 1180 | 961 | 708 | 459 |
| Total folate | |||||||||
| Quintile 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Quintile 2 | 0.95 (0.82, 1.09) | 1.06 (0.91, 1.24) | 0.91 (0.77, 1.07) | 1.13 (0.96, 1.33) | 0.96 (0.81, 1.14) | 0.86 (0.72, 1.03) | 0.85 (0.70, 1.04) | 0.99 (0.79, 1.25) | 1.12 (0.84, 1.49) |
| Quintile 3 | 0.92 (0.79, 1.07) | 1.11 (0.94, 1.30) | 0.94 (0.78, 1.12) | 0.92 (0.77, 1.11) | 0.84 (0.69, 1.01) | 0.85 (0.70, 1.03) | 0.88 (0.71, 1.08) | 1.00 (0.79, 1.28) | 1.02 (0.74, 1.39) |
| Quintile 4 | 0.85 (0.73, 1.00) | 1.02 (0.85, 1.23) | 0.91 (0.75, 1.11) | 0.89 (0.73, 1.09) | 0.92 (0.74, 1.13) | 0.76 (0.61, 0.94) | 0.75 (0.59, 0.94) | 0.92 (0.71, 1.21) | 1.13 (0.81, 1.58) |
| Quintile 5 | 0.81 (0.66, 0.98) | 0.92 (0.73, 1.15) | 0.85 (0.68, 1.06) | 0.76 (0.60, 0.97) | 0.82 (0.63, 1.07) | 0.77 (0.58, 1.02) | 0.69 (0.52, 0.92) | 0.63 (0.45, 0.88) | 0.90 (0.59, 1.38) |
| P-trend | 0.007 | 0.50 | 0.25 | 0.25 | 0.46 | 0.09 | <0.001 | 0.03 | 0.24 |
| Per 400 DFE/d | 0.90 (0.83, 0.97) | 0.97 (0.88, 1.07) | 0.97 (0.93, 1.02) | 0.97 (0.91, 1.02) | 0.97 (0.91, 1.05) | 0.93 (0.85, 1.01) | 0.83 (0.75, 0.92) | 0.87 (0.77, 0.99) | 0.91 (0.78, 1.07) |
| Per 400 DFE/d2 | 0.86 (0.76, 0.97) | —3 | 0.96 (0.90, 1.03) | 0.95 (0.88, 1.04) | 0.96 (0.87, 1.07) | 0.90 (0.79, 1.02) | 0.77 (0.66, 0.91) | 0.82 (0.69, 0.99) | 0.87 (0.70, 1.10) |
| Dietary folate | |||||||||
| Quintile 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Quintile 2 | 0.98 (0.85, 1.13) | 0.98 (0.84, 1.15) | 0.93 (0.78, 1.11) | 1.00 (0.84, 1.19) | 1.08 (0.91, 1.29) | 0.89 (0.74, 1.06) | 0.88 (0.72, 1.08) | 1.00 (0.79, 1.26) | 0.97 (0.72, 1.32) |
| Quintile 3 | 1.00 (0.87, 1.16) | 1.07 (0.91, 1.26) | 0.93 (0.77, 1.11) | 1.07 (0.90, 1.28) | 1.02 (0.85, 1.23) | 0.79 (0.65, 0.96) | 0.85 (0.68, 1.05) | 1.02 (0.80, 1.31) | 1.16 (0.85, 1.57) |
| Quintile 4 | 0.99 (0.84, 1.15) | 1.03 (0.86, 1.23) | 0.91 (0.75, 1.11) | 0.95 (0.78, 1.17) | 0.90 (0.73, 1.10) | 0.77 (0.62, 0.95) | 0.93 (0.75, 1.17) | 0.87 (0.66, 1.13) | 1.10 (0.79, 1.53) |
| Quintile 5 | 0.96 (0.80, 1.14) | 0.97 (0.79, 1.18) | 0.82 (0.66, 1.02) | 0.97 (0.77, 1.21) | 1.01 (0.80, 1.28) | 0.76 (0.60, 0.98) | 0.83 (0.64, 1.08) | 0.98 (0.73, 1.31) | 1.15 (0.80, 1.67) |
| P-trend | 0.54 | 0.78 | 0.36 | 0.44 | 0.73 | 0.03 | 0.15 | 0.49 | 0.22 |
| Per 400 DFE/d | 0.94 (0.79, 1.13) | 0.97 (0.77, 1.22) | 0.94 (0.83, 1.07) | 0.94 (0.81, 1.09) | 1.03 (0.87, 1.21) | 0.78 (0.62, 0.98) | 0.83 (0.64, 1.07) | 0.90 (0.67, 1.21) | 1.24 (0.88, 1.73) |
| Per 400 DFE/d2 | 0.84 (0.49, 1.46) | —3 | 0.83 (0.56, 1.23) | 0.84 (0.54, 1.31) | 1.09 (0.67, 1.78) | 0.48 (0.24, 0.97) | 0.57 (0.26, 1.24) | 0.73 (0.30, 1.79) | 1.88 (0.68, 5.21) |
| Synthetic folic acid | |||||||||
| Quintile 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Quintile 2 | 1.04 (0.90, 1.20) | 1.13 (0.97, 1.32) | 0.93 (0.79, 1.10) | 0.94 (0.80, 1.10) | 0.89 (0.76, 1.05) | 1.07 (0.90, 1.26) | 0.86 (0.72, 1.04) | 0.96 (0.77, 1.20) | 0.81 (0.61, 1.09) |
| Quintile 3 | 1.03 (0.90, 1.17) | 1.15 (0.98, 1.36) | 0.88 (0.73, 1.05) | 0.89 (0.74, 1.05) | 0.92 (0.77, 1.09) | 0.96 (0.80, 1.16) | 0.93 (0.77, 1.13) | 0.97 (0.77, 1.21) | 1.03 (0.78, 1.35) |
| Quintile 4 | 0.83 (0.72, 0.96) | 1.12 (0.94, 1.35) | 0.88 (0.72, 1.07) | 0.87 (0.72, 1.06) | 0.95 (0.78, 1.16) | 0.90 (0.73, 1.11) | 0.68 (0.54, 0.86) | 0.84 (0.65, 1.08) | 0.89 (0.65, 1.21) |
| Quintile 5 | 0.86 (0.72, 1.03) | 1.04 (0.83, 1.30) | 0.87 (0.70, 1.08) | 0.81 (0.64, 1.02) | 0.82 (0.64, 1.06) | 0.82 (0.62, 1.07) | 0.78 (0.59, 1.02) | 0.78 (0.57, 1.07) | 0.83 (0.56, 1.23) |
| P-trend | 0.32 | 0.60 | 0.32 | 0.97 | 0.67 | 0.37 | 0.02 | 0.07 | 0.21 |
| Per 400 DFE/d | 0.97 (0.92, 1.03) | 1.02 (0.95, 1.10) | 0.98 (0.93, 1.02) | 1.00 (0.95, 1.05) | 0.99 (0.93, 1.05) | 0.97 (0.90, 1.04) | 0.91 (0.84, 0.99) | 0.91 (0.83, 1.01) | 0.93 (0.82, 1.05) |
| Per 400 DFE/d1 | 0.96 (0.89, 1.04) | —3 | 0.97 (0.91, 1.03) | 1.00 (0.93, 1.07) | 0.98 (0.90, 1.07) | 0.96 (0.87, 1.06) | 0.88 (0.78,0.99) | 0.89 (0.77, 1.01) | 0.90 (0.76, 1.06) |
| Natural folate | |||||||||
| Quintile 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Quintile 2 | 0.93 (0.80, 1.07) | 1.05 (0.90, 1.21) | 1.02 (0.87, 1.19) | 0.89 (0.76, 1.05) | 1.01 (0.85, 1.19) | 0.86 (0.72, 1.03) | 0.88 (0.72, 1.08) | 1.07 (0.84, 1.34) | 1.15 (0.86, 1.55) |
| Quintile 3 | 0.94 (0.81, 1.09) | 0.93 (0.80, 1.09) | 1.04 (0.88, 1.23) | 1.00 (0.85, 1.17) | 1.03 (0.86, 1.23) | 0.88 (0.73, 1.06) | 0.91 (0.73, 1.12) | 0.92 (0.71, 1.18) | 1.01 (0.74, 1.39) |
| Quintile 4 | 0.94 (0.81, 1.10) | 0.93 (0.79, 1.10) | 1.10 (0.93, 1.30) | 0.87 (0.73, 1.04) | 0.92 (0.77, 1.11) | 0.71 (0.58, 0.87) | 0.93 (0.75, 1.16) | 0.99 (0.77, 1.28) | 1.05 (0.76, 1.46) |
| Quintile 5 | 0.99 (0.83, 1.18) | 1.04 (0.87, 1.24) | 1.05 (0.87, 1.26) | 0.95 (0.79, 1.14) | 0.97 (0.79, 1.18) | 0.87 (0.70, 1.08) | 0.95 (0.75, 1.20) | 0.93 (0.70, 1.23) | 1.15 (0.81, 1.64) |
| p trend | 0.65 | 0.84 | 0.42 | 0.89 | 0.74 | 0.31 | 0.66 | 0.70 | 0.10 |
| per 400 DFE/d | 1.06 (0.82, 1.37) | 0.96 (0.66, 1.40) | 1.13 (0.84, 1.51) | 1.02 (0.76, 1.38) | 0.95 (0.68, 1.31) | 0.83 (0.57, 1.19) | 0.91 (0.61, 1.36) | 0.91 (0.57, 1.45) | 1.58 (0.91, 2.72) |
| per 400 DFE/d2 | 1.24 (0.50, 3.07) | —3 | 1.53 (0.54, 4.31) | 1.08 (0.37, 3.15) | 0.82 (0.26, 2.62) | 0.51 (0.14, 1.90) | 0.73 (0.18, 2.99) | 0.72 (0.14, 3.73) | 5.04 (0.68, 37.1) |
All values are HRs (95% CIs), stratified by age and calendar year and adjusted for family history of CRC, endoscopy, height, BMI, pack-y of smoking before age 30 y, physical activity, duration of regular aspirin or other NSAIDs use, duration of multivitamin use (for dietary folate and natural folate analysis), menopausal status and menopausal hormone use, alcohol intake, total energy, dietary fiber, total vitamin D (for total folate and synthetic folic acid analysis), dietary vitamin D (for dietary folate and natural folate analysis), total calcium (for total folate and synthetic folic acid analysis), dietary calcium (for dietary folate and natural folate analysis), red meat, and processed meat. CRC, colorectal cancer; DFE, dietary folate equivalent; NSAID, nonsteroidal anti-inflammatory drug.
Corrected for measurement error using regression calibration approach.
The regression calibration approach was not appropriate for cumulative average models.
The latency analyses indicated a 12–24-y lag for the associations between total folate intake and CRC risk (Figure 2). Each 400 DFE/d greater total folate intake 12–24 y before diagnosis was associated with a 7–17% lower risk of CRC (HR: 0.93, 95% CI: 0.85, 1.01 for 12–16 y; HR: 0.83, 95% CI: 0.75, 0.92 for 16–20 y; and HR: 0.87, 95% CI: 0.77, 0.99 for 20–24 y). The association disappeared at the 24–28-y lag (HR per 400 DFE/d greater: 0.91; 95% CI: 0.78, 1.07). For the association between dietary folate intake and CRC risk, a 12–16-y lag was observed (HR per 400 DFE/d greater: 0.78; 95% CI: 0.62, 0.98), whereas the time lag for synthetic folic acid was 16–24 y and for each 400 DFE/d greater synthetic folic acid intake was 16–20 y (HR: 0.91; 95% CI: 0.84, 0.99) and 20–24 y (HR: 0.91; 95% CI: 0.83, 1.01) before diagnosis was associated with a 9% lower CRC risk. We did not observe significant associations for natural folate in any lags. Results were similar in analyses using quintiles or absolute intake cut points (Table 2 and Supplemental Table 1). Although not all statically significant, the associations of total folate, dietary folate, and synthetic folic acid with CRC risk comparing extreme quintiles/categories were the strongest at the above latencies.
FIGURE 2.
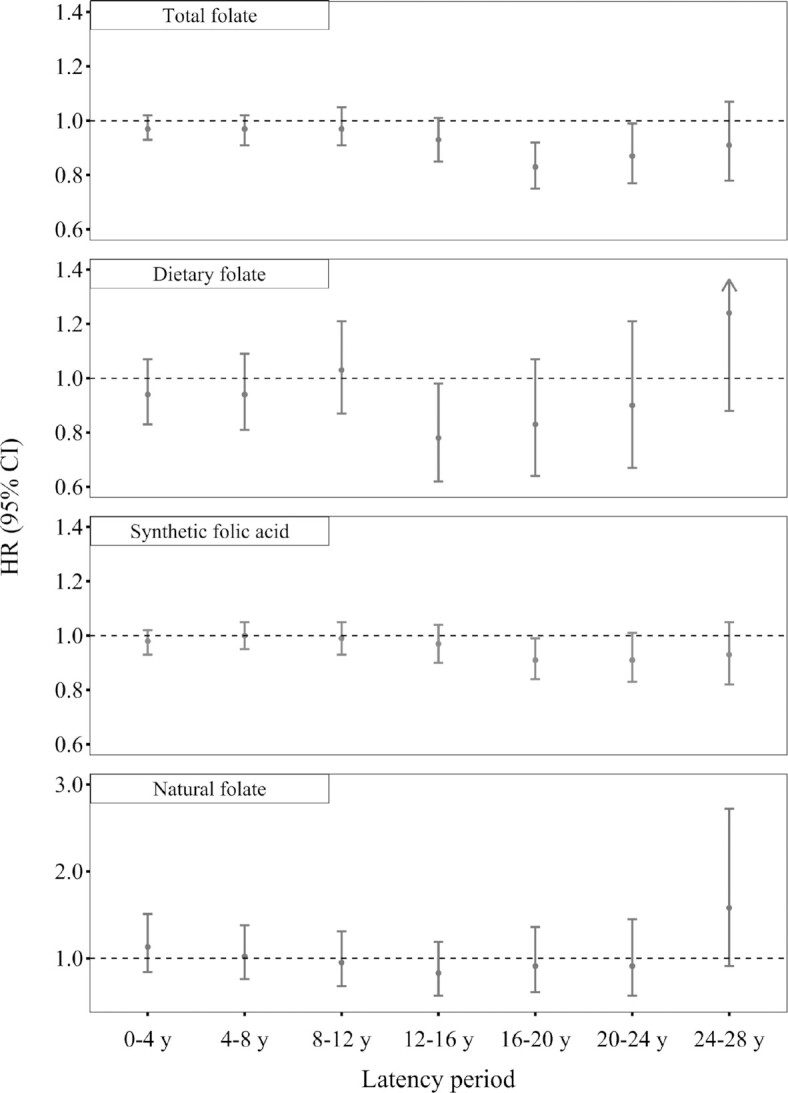
Risk of CRC per 400 DFE/d greater folate intake with different latency periods, Nurses’ Health Study, 1980–2016. Cox models were stratified by age and calendar year and adjusted for family history of CRC, endoscopy, height, BMI, pack-y of smoking before age 30 y, physical activity, duration of regular aspirin/NSAIDs use, duration of multivitamin use (for dietary folate and natural folate analysis), menopausal status and menopausal hormone use, alcohol intake, total energy, dietary fiber, total vitamin D (for total folate and synthetic folic acid analysis), dietary vitamin D (for dietary folate and natural folate analysis), total calcium (for total folate and synthetic folic acid analysis), dietary calcium (for dietary folate and natural folate analysis), red meat, and processed meat. For different latency periods, we used folate intake reported at various latencies (i.e., 4–8, 8–12, 12–16, 16–20, 20–24, and 24–28 y) before diagnosis. n = 86,320 for 0–4 y, 84,071 for 4–8 y, 81,029 for 8–12 y, 77,200 for 12–16 y, 73,080 for 16–20 y, 67,780 for 20–24 y, and 61,722 for 24–28 y. CRC, colorectal cancer; DFE: dietary folate equivalent; NSAIDs, nonsteroidal anti-inflammatory drugs.
The association between intake of total folate and synthetic folic acid and CRC became statistically significant for most latency periods after removing total vitamin D from the multivariable-adjusted model (Supplemental Table 2). However, when further adjusting for the duration of multivitamin use in the multivariable-adjusted model, the associations for different latency periods were all attenuated. In the sensitivity analysis accounting for measurement error in folate intake, we observed the same latencies for the inverse associations of total folate and synthetic folic acid with CRC, but the associations were strengthened (Table 2).
In the separate analysis for the prefortification period (1980–1998), we did not observe any significant associations between folate intake and CRC risk at various latencies (Supplemental Table 3). In the analysis for the postfortification period (1998–2016), simple updated postfortification total folate and synthetic folic acid intakes were significantly inversely associated with CRC risk. However, the associations were attenuated and were no longer statistically significant after adjusting for cumulative average prefortification folate intake (Table 3). On the contrary, we found significant inverse associations for the cumulative average of prefortification total folate and synthetic folic acid intakes with the risk of CRC diagnosed after 1998, even after adjusting for baseline, cumulative average, or simple updated postfortification folate intake. We did not observe significant associations between postfortification folate intake and CRC risk at other latencies (Supplemental Table 4). To examine any potential adverse effects of postfortification high folate intake, we analyzed deciles of cumulative average total folate and synthetic folic acid intake. No evidence of adverse impact for either total folate or synthetic folic acid was observed when we compared the highest deciles of postfortification total folate (median: 1881 DFE/d; HR: 0.95; 95% CI: 0.68, 1.32) and synthetic folic acid (median: 1573 DFE/d; HR: 0.76; 95% CI: 0.55, 1.05) to the lowest, even among multivitamin users (Supplemental Table 5).
TABLE 3.
Risk of CRC during the postfortification period (1998–2016) according to postfortification and prefortification folate intakes1
| Cases, n | Baseline | Cumulative | Simple update (0–4 y lag) |
|---|---|---|---|
| 965 | 965 | 892 | |
| Postfortification folate intake | |||
| Total folate, per 400 DFE/d | 0.97 (0.91, 1.03) | 0.94 (0.88, 1.01) | 0.93 (0.88, 0.99) |
| Prefortification intake adjusted2 | 0.97 (0.92, 1.03) | 0.94 (0.88, 1.00) | 0.96 (0.90, 1.01) |
| Dietary folate per 400 DFE/d | 1.03 (0.87, 1.23) | 1.03 (0.86, 1.23) | 1.00 (0.86, 1.16) |
| Prefortification intake adjusted2 | 1.04 (0.87, 1.24) | 1.04 (0.86, 1.25) | 1.01 (0.87, 1.17) |
| Synthetic folic acid, per 400 DFE/d | 0.97 (0.91, 1.03) | 0.94 (0.88, 1.01) | 0.93 (0.88, 0.98) |
| Prefortification intake adjusted2 | 0.97 (0.91, 1.03) | 0.94 (0.87, 1.00) | 0.95 (0.90, 1.01) |
| Natural folate, per 400 DFE/d | 1.00 (0.70, 1.42) | 0.95 (0.64, 1.42) | 1.04 (0.70, 1.53) |
| Prefortification intake adjusted2 | 1.00 (0.70, 1.43) | 0.96 (0.64, 1.43) | 1.06 (0.71, 1.57) |
| Prefortification folate intake2 | |||
| Total folate, per 400 DFE/d | 0.82 (0.72, 0.93) | 0.82 (0.72, 0.93) | 0.81 (0.74, 0.90) |
| Post-fortification intake adjusted | 0.82 (0.72, 0.93)3 | 0.82 (0.72, 0.93)4 | 0.83 (0.75, 0.92)5 |
| Dietary folate, per 400 DFE/d | 0.96 (0.69, 1.34) | 0.96 (0.69, 1.34) | 0.90 (0.66, 1.23) |
| Postfortification intake adjusted | 0.94 (0.67, 1.33)3 | 0.95 (0.67, 1.33)4 | 0.90 (0.65, 1.24)5 |
| Synthetic folic acid per 400 DFE/d | 0.83 (0.77, 0.90) | 0.83 (0.77, 0.90) | 0.85 (0.78, 0.92) |
| Postfortification intake adjusted | 0.85 (0.78, 0.93)3 | 0.86 (0.79, 0.94)4 | 0.88 (0.81, 0.95)5 |
| Natural folate, per 400 DFE/d | 0.89 (0.52, 1.51) | 0.89 (0.52, 1.51) | 0.90 (0.55, 1.47) |
| Postfortification intake adjusted | 0.88 (0.52, 1.50)3 | 0.88 (0.52, 1.51)4 | 0.90 (0.55, 1.47)5 |
All values are HRs (95% CIs), stratified by age and calendar year and adjusted for family history of CRC, endoscopy, height, BMI, pack-y of smoking before age 30 y, physical activity, duration of regular aspirin or other NSAID use, duration of multivitamin use (for dietary folate and natural folate analysis), menopausal status and menopausal hormone use, alcohol intake, total energy, dietary fiber, total vitamin D (for total folate and synthetic folic acid analysis), dietary vitamin D (for dietary folate and natural folate analysis), total calcium (for total folate and synthetic folic acid analysis), dietary calcium (for dietary folate and natural folate analysis), red meat, and processed meat. DFE, dietary folate equivalent; NSAID, nonsteroidal anti-inflammatory drug.
Prefortification folate intake was calculated as the cumulative average of folate intake before 1998.
Baseline (1998) postfortification folate intake was adjusted.
Cumulative average postfortification folate intake was adjusted.
Simple updated postfortification folate intake was adjusted.
In subgroup analysis, we did not observe any significant interactions between total and dietary folate intake and age, alcohol intake, family history of CRC, lower gastrointestinal endoscopy history, aspirin/NSAIDs use, and multivitamin use (all P-interaction > 0.05) (Supplemental Table 6).
Discussion
This large prospective cohort study directly addressed the concern about excessive folate intake and higher CRC risk that has impeded folic acid fortification for the prevention of neural tube defects in the United Kingdom and some other countries (30). With 36 y of follow-up, we observed an inverse association between folate intake and CRC risk that was limited to latency periods of at least 12 y. Additionally, in the postfortification period, high postfortification folate intake in any chemical form was not associated with increased CRC risk, but high prefortification intake of both total folate and synthetic folic acid was associated with a reduced risk of CRC diagnosed in the postfortification period, consistent with lower risks with long latency.
The adenoma–carcinoma sequence is the classic pathway accounting for the majority of CRC (19, 31). In this model, CRC develops over decades, progressing from microscopic lesions to small adenomas, advanced adenomas, and finally to CRC. Even development from a small adenoma, the first observable macroscopic lesion, typically requires >10 y to develop to CRC. Laboratory evidence from animal studies generally supports a causal role of folate supplementation in CRC prevention (32). However, the timing of folate intervention is critical for its chemopreventive effects (33). In a mouse model, folate supplementation has been effective in preventing the formation of colonic aberrant crypt foci and adenomas only if it started before the establishment of neoplastic foci (34). Thus, we would anticipate a long latency period to observe any preventive effect of high folate intake on CRC if folate also selectively inhibits the early stages of colorectal carcinogenesis in humans. We observed these latency periods, specifically, approximately ≥12 y for total folate and dietary folate, and ≥16 y for synthetic folic acid, by following up participants for up to 36 y. These latency periods are consistent with previous findings that the use of folic acid–containing multivitamins was associated with reduced CRC risk only after long latency periods (>10 y) (35). Such a long duration is hard to achieve in randomized controlled trials (RCTs), and this might be one of the reasons why most RCTs have not shown a protective effect of folate. In several RCTs of folic acid plus B vitamins or multivitamins with relatively long durations such as >7 y, reduced risk of CRC was found, though the reduction was not statistically significant (36–38). Postintervention follow-up should receive more attention in future RCTs to better estimate the effect of folate in carcinogenesis.
In the present study, we derived the intake of total folate as well as folate in different chemical forms (synthetic folic acid and natural folate). The inverse association between folate and CRC risk was most clearly observed for total folate (especially among participants taking multivitamins) and synthetic folic acid. Further adjusting for multivitamin use attenuated the inverse association of synthetic folic acid with CRC risk, all suggesting folic acid may be essential for CRC prevention. These results are in line with participants in the previous 2 US cohorts, in whom a stronger inverse association with CRC risk of total folate or synthetic folic acid than of natural folate was observed (17, 18). Such a difference may be due to the higher bioavailability of folic acid than natural folate, which is entrapped in the food matrix and vulnerable to substantial losses during cooking and intestinal absorption (39, 40). Additionally, assessment of folic acid intake might have fewer measurement errors, especially when folic acid comes from supplements (41), which are different items with a standard dose of folic acid.
The concern regarding excessive folate intake in the postfortification period was raised mainly from 2 ecological studies and 1 RCT (2, 8, 13). The 2 ecological studies examined the temporal trend of CRC incidence in the United States and Canada following folic acid fortification starting in 1996 and 1997. Each found a small transient increase in CRC incidence (began to increase in 1996 in the United States and 1998 in Canada) with the implementation of fortification, putting forward a hypothesis that folic acid fortification might be, at least in part, responsible for this increase (2, 13). However, ecological studies are limited by the lack of consideration of confounders and cannot provide definitive evidence to support the etiologic role of folic acid fortification in the transient increase in CRC incidence (42). In addition, this increase corresponded in time to the recommendation of CRC screening tests (fecal occult blood testing or colonoscopy) in the United States and Canada (43, 44), which would cause an artificial increase in incidence. Further, the observed increase in each country occurred too soon (within 1–2 y) to be plausibly ascribed to the introduction of folic acid fortification and did not persist thereafter (30). The RCT that reported an unexpected increase in advanced colorectal adenoma after 7 y of treatment with folic acid (8) was conducted among participants with a recent history of adenomas. To obtain more precise clinical evidence, a combined analysis of this trial and 2 other large trials in patients with an adenoma history was conducted (45), and no apparent decrease or increase in the occurrence of new adenomas was found. In another small trial in patients with colorectal adenoma, a significant reduction in the occurrence of colonic adenomas was associated with folic acid supplementation (46). Nevertheless, none of these trials provided information on the primary prevention of adenomas by folic acid (the potential for folic acid to reduce the incidence of first adenomas) (14). A primary prevention trial did show that the initial occurrence of colorectal adenoma was lower in those receiving 1 mg/d treatment of folic acid compared to placebo for 3 y (47). Results from our analysis conducted in the first 18 y of the postfortification period provide reassurance that high total folate and synthetic folic acid intake in the postfortification period is not associated with CRC promotion.
The main strengths of this prospective study are its uniquely long follow-up and multiple repeated measurements of folate intake, encompassing both the pre- and postfortification periods. In the absence of evidence from large, long-term RCTs on hard endpoints like cancer, evidence from prospective cohort studies is of great importance, as prospective cohort studies are the strongest observational study design in terms of minimizing bias and inferring causality (48). A total of 36 y of follow-up and multiple dietary assessments every 2–4 y allowed us to investigate the timing of folate intake with CRC progression over periods of >20 y and to examine the folate–CRC relationship in the postfortification era. Other strengths include correction for measurement error using dietary record data collected in the validation studies, derivation of different folate types to study the associations of each form separately, and comprehensive assessment of confounders such as diet during follow-up and duration of aspirin/NSAIDs and multivitamin use instead of just never use or ever use.
Nevertheless, this study has several limitations that require further discussion. First, almost all included participants were younger than 80 y when their folate intake was assessed. We are not able to investigate the possible association of folate intake with CRC for this older age group. In 1 RCT conducted in the Netherlands, folic acid and vitamin B12 supplementation was associated with a higher risk of overall cancer, with 18 of the 28 excess cancers being CRC among participants aged >80 y (49, 50). Second, we only included female health professionals, most of whom were white. However, our findings are mostly consistent with other cohorts that include both sexes (17, 18). Third, as with all other observational studies, we cannot rule out the possibility of residual confounding, though we conducted a comprehensive assessment of confounders. Vitamin D or other constituents of multivitamins or fortified foods are plausible confounders. However, when we adjusted for vitamin D intake, we still found the inverse association of total folate and synthetic folic acid intakes and CRC risk. In addition, although our analysis included 36 y of follow-up, the CIs became wider with the longer latencies due to the decreasing number of cases. Additional follow-up will be needed to fully elucidate the relation between folate intake and the risk of CRC in the postfortification period. Finally, we only examined the association between folate intake and overall CRC risk. Further investigation is needed to determine whether the association differs across various CRC stages.
In conclusion, the results of this study support an inverse association between folate intake and overall CRC risk that did not emerge until approximately ≥12 y after the exposure. More importantly, the present study did not reveal an increased risk of CRC among participants with high folate intake in the postfortification period, providing reassurance that the US folic acid fortification policy should not lead to higher CRC incidence.
Supplementary Material
Acknowledgements
We thank Biling Hong and Molin Wang for their help with measurement error correction analysis. We also thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. The authors assume full responsibility for analyses and interpretation of these data.
YL reports receiving grants from the California Walnut Commission and the Swiss Re Foundation. All other authors report no conflicts of interest.
The authors’ responsibilities were as follows—FW, ELG, WCW: were involved in the study concept and design; FW, KW, RS: participated in acquisition of data; FW, YL, YW: were involved in statistical analysis; FW, XZ, MS, LL, SAW, ELG, WCW: participated in interpretation of data; FW, WCW: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This work was supported by grants from the National Institutes of Health (UM1 CA186107, P01 CA87969). The sponsors had no role in the study design; the collection, analysis, or interpretation of data; the writing of the report; or in the decision to submit the article for publication.
Supplementary Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CRC, colorectal cancer; DFE, dietary folate equivalent; FFQ, food frequency questionnaire; MET, metabolic equivalent of task; NHS, Nurses’ Health Study; NSAID, nonsteroidal anti-inflammatory drug; RCT, randomized controlled trial.
Contributor Information
Fenglei Wang, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Kana Wu, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Yanping Li, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Rui Song, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
You Wu, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Xuehong Zhang, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA.
Mingyang Song, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA; Clinical and Translational Epidemiology Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA; Division of Gastroenterology, Massachusetts General Hospital, Boston, MA.
Liming Liang, Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA; Department of Biostatistics, Harvard TH Chan School of Public Health, Boston, MA.
Stephanie A Smith-Warner, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA.
Edward L Giovannucci, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA.
Walter C Willett, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval by the corresponding author.
References
- 1. American Cancer Society . Cancer facts & figures 2019. Atlanta, GA: American Cancer Society, 2019. [Google Scholar]
- 2. Ulrich CM. Folate and cancer prevention–where to next? Counterpoint. Cancer Epidemiol Biomarkers Prev. 2008;17:2226–30. [DOI] [PubMed] [Google Scholar]
- 3. Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer?. Cancer Epidemiol Biomarkers Prev. 2004;13:511–9. [PubMed] [Google Scholar]
- 4. Kim DH, Smith-Warner SA, Spiegelman D, Yaun SS, Colditz GA, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Harnack Let al. . Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes Control. 2010;21:1919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moazzen S, Dolatkhah R, Tabrizi JS, Shaarbafi J, Alizadeh BZ, de Bock GH, Dastgiri S. Folic acid intake and folate status and colorectal cancer risk: a systematic review and meta-analysis. Clin Nutr. 2018;37:1926–34. [DOI] [PubMed] [Google Scholar]
- 6. World Cancer Research Fund/American Institute for Cancer Research . Continuous Update Project Report. Food, nutrition, physical activity, and the prevention of colorectal cancer. 2011. [Google Scholar]
- 7. World Cancer Research Fund/American Institute for Cancer Research . Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Available from: https://dietandcancerreport.org. [Google Scholar]
- 8. Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CAet al. . Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–9. [DOI] [PubMed] [Google Scholar]
- 9. Passarelli MN, Barry EL, Rees JR, Mott LA, Zhang D, Ahnen DJ, Bresalier RS, Haile RW, McKeown-Eyssen G, Snover DCet al. . Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song J, Medline A, Mason JB, Gallinger S, Kim YI. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000;60:5434–40. [PubMed] [Google Scholar]
- 11. Le Leu RK, Young GP, McIntosh GH. Folate deficiency reduces the development of colorectal cancer in rats. Carcinogenesis. 2000;21:2261–5. [DOI] [PubMed] [Google Scholar]
- 12. Lindzon GM, Medline A, Sohn KJ, Depeint F, Croxford R, Kim YI. Effect of folic acid supplementation on the progression of colorectal aberrant crypt foci. Carcinogenesis. 2009;30:1536–43. [DOI] [PubMed] [Google Scholar]
- 13. Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, Rosenberg IH. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16:1325–9. [DOI] [PubMed] [Google Scholar]
- 14. Ulrich CM, Potter JD. Folate and cancer–timing is everything. JAMA. 2007;297:2408–9. [DOI] [PubMed] [Google Scholar]
- 15. Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82:442–50. [DOI] [PubMed] [Google Scholar]
- 16. Pfeiffer CM, Sternberg MR, Zhang M, Fazili Z, Storandt RJ, Crider KS, Yamini S, Gahche JJ, Juan W, Wang CYet al. . Folate status in the US population 20 y after the introduction of folic acid fortification. Am J Clin Nutr. 2019;110:1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gibson TM, Weinstein SJ, Pfeiffer RM, Hollenbeck AR, Subar AF, Schatzkin A, Mayne ST, Stolzenberg-Solomon R. Pre- and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. Am J Clin Nutr. 2011;94:1053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stevens VL, McCullough ML, Sun J, Jacobs EJ, Campbell PT, Gapstur SM. High levels of folate from supplements and fortification are not associated with increased risk of colorectal cancer. Gastroenterology. 2011;141:98–105, 101-105. [DOI] [PubMed] [Google Scholar]
- 19. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713–32. [DOI] [PubMed] [Google Scholar]
- 20. Lee JE, Willett WC, Fuchs CS, Smith-Warner SA, Wu K, Ma J, Giovannucci E. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am J Clin Nutr. 2011;93:817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saldanha LG, Dwyer JT, Haggans CJ, Mills JL, Potischman N. Perspective: time to resolve confusion on folate amounts, units, and forms in prenatal supplements. Adv Nutr. 2020;11:753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline.Dietary reference intakes for thiamin, riboflavin, niacin, vitamin b₆, folate, vitamin b₁₂, pantothenic acid, biotin, and choline. Washington, DC: National Academies Press, 1998: 564. [PubMed] [Google Scholar]
- 23. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S, 1229S-31S. [DOI] [PubMed] [Google Scholar]
- 24. Giovannucci E, Stampfer MJ, Colditz GA, Rimm EB, Trichopoulos D, Rosner BA, Speizer FE, Willett WC. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst. 1993;85:875–84. [DOI] [PubMed] [Google Scholar]
- 25. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185:570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Rood JC, Harnack LJ, Sampson LKet al. . Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187:1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH. Test of the National Death Index. Am J Epidemiol. 1984;119:837–9. [DOI] [PubMed] [Google Scholar]
- 28. Willett W. Nutritional Epidemiology. 3rd ed. New York, NY: Oxford University Press, 2012: 40. [Google Scholar]
- 29. Rosner B, Willett WC, Spiegelman D. Correction of logistic regression relative risk estimates and confidence intervals for systematic within-person measurement error. Stat Med. 1989;8:1051–69. [DOI] [PubMed] [Google Scholar]
- 30. Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, Armitage J, Manson JE, Hankey GJ, Spence JDet al. . Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502. [DOI] [PubMed] [Google Scholar]
- 32. Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem. 1999;10:66–88. [DOI] [PubMed] [Google Scholar]
- 33. Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention?. Gut. 2006;55:1387–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song J, Sohn KJ, Medline A, Ash C, Gallinger S, Kim YI. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/-Msh2-/- mice. Cancer Res. 2000;60:3191–9. [PubMed] [Google Scholar]
- 35. Jacobs EJ, Connell CJ, Chao A, McCullough ML, Rodriguez C, Thun MJ, Calle EE. Multivitamin use and colorectal cancer incidence in a US cohort: does timing matter?. Am J Epidemiol. 2003;158:621–8. [DOI] [PubMed] [Google Scholar]
- 36. Zhang SM, Cook NR, Albert CM, Gaziano JM, Buring JE, Manson JE. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA. 2008;300:2012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Sleight P, Peto Ret al. . Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303:2486–94. [DOI] [PubMed] [Google Scholar]
- 38. Gaziano JM, Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, Schvartz M, Manson JE, Glynn RJ, Buring JE. Multivitamins in the prevention of cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2012;308:1871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McNulty H, Pentieva K. Folate bioavailability. Proc Nutr Soc. 2004;63:529–36. [DOI] [PubMed] [Google Scholar]
- 40. Herbert V. Making sense of laboratory tests of folate status: folate requirements to sustain normality. Am J Hematol. 1987;26:199–207. [DOI] [PubMed] [Google Scholar]
- 41. Satia-Abouta J, Patterson RE, King IB, Stratton KL, Shattuck AL, Kristal AR, Potter JD, Thornquist MD, White E. Reliability and validity of self-report of vitamin and mineral supplement use in the vitamins and lifestyle study. Am J Epidemiol. 2003;157:944–54. [DOI] [PubMed] [Google Scholar]
- 42. Kim Y. Current status of folic acid supplementation on colorectal cancer prevention. Curr Pharmacol Rep. 2016;2:21–33. [Google Scholar]
- 43.US Preventive Services Task Force. Guide to Clinical Preventive Services. Baltimore, MD: Williams & Wilkins, 1996. [Google Scholar]
- 44. National Committee on Colorectal Cancer Screening . Recommendations for population-based colorectal cancer screening. Version 20 December 2002. [Internet]. Available from: https://www.canada.ca/en/public-health/services/reports-publications/technical-report-national-committee-colorectal-cancer-screening/national-committee-colorectal-cancer-screening.html(accessed 10 August 2020). [Google Scholar]
- 45. Figueiredo JC, Mott LA, Giovannucci E, Wu K, Cole B, Grainge MJ, Logan RF, Baron JA. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials. Int J Cancer. 2011;129:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jaszewski R, Misra S, Tobi M, Ullah N, Naumoff JA, Kucuk O, Levi E, Axelrod BN, Patel BB, Majumdar AP. Folic acid supplementation inhibits recurrence of colorectal adenomas: a randomized chemoprevention trial. WJG. 2008;14:4492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gao QY, Chen HM, Chen YX, Wang YC, Wang ZH, Tang JT, Ge ZZ, Chen XY, Sheng JQ, Fang DCet al. . Folic acid prevents the initial occurrence of sporadic colorectal adenoma in Chinese older than 50 years of age: a randomized clinical trial. Cancer Prev Res. 2013;6:744–52. [DOI] [PubMed] [Google Scholar]
- 48. Satija A, Yu E, Willett WC, Hu FB. Understanding nutritional epidemiology and its role in policy. Adv Nutr. 2015;6:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Wijngaarden JP, Swart KM, Enneman AW, Dhonukshe-Rutten RA, van Dijk SC, Ham AC, Brouwer-Brolsma EM, van der Zwaluw NL, Sohl E, van Meurs JBet al. . Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578–86. [DOI] [PubMed] [Google Scholar]
- 50. Oliai AS, Kiefte-de JJ, van Dijk SC, Swart K, van Laarhoven HW, van Schoor NM, de Groot L, Lemmens V, Stricker BH, Uitterlinden AGet al. . Folic acid and vitamin b12 supplementation and the risk of cancer: long-term follow-up of the B Vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval by the corresponding author.