ABSTRACT
Background
Population-based studies generally show neutral associations between dairy consumption and ischemic heart disease (IHD) mortality, whereas weak inverse associations were found for cardiovascular disease (CVD) and stroke mortality. Whether dairy consumption affects long-term survival after myocardial infarction (MI) is unknown.
Objectives
We studied types of dairy and long-term mortality risk in drug-treated post-MI patients.
Methods
We included 4365 Dutch patients from the Alpha Omega Cohort aged 60–80 y (21% women) with an MI ≤10 y before enrollment. Dietary data were collected at baseline (2002–2006) using a 203-item FFQ and patients were followed for cause-specific mortality through December 2018. HRs of CVD, IHD, stroke, and all-cause mortality for types of dairy were obtained from Cox models, adjusting for age, sex, energy intake, physical activity, smoking, alcohol intake, diabetes, obesity, and dietary factors.
Results
Most patients were Dutch, 24% were obese, 20% had diabetes, and 97% used cardiovascular medication. Median intakes were 39 g/d for plain yogurt, 88 g/d for total nonfermented milk, and 17 g/d for hard cheeses. Of the cohort, 10% consumed high-fat milk. During ∼12 y of follow-up (48,473 person-years) 2035 deaths occurred, including 903 from CVD, 558 from IHD, and 170 from stroke. Yogurt was linearly inversely associated with CVD mortality (HR: 0.96; 95% CI: 0.93, 0.99; per 25 g/d) and nonlinearly inversely associated with all-cause mortality. Milk was not associated with any of the outcomes (HRs: ∼1.0 per 100 g/d), except for a higher mortality risk in high-fat milk consumers (HR: 1.30; 95% CI: 1.13, 1.49). Other dairy groups were not associated with mortality risk.
Conclusions
In Dutch post-MI patients, yogurt consumption was inversely associated with CVD mortality and all-cause mortality. Associations for milk and other dairy products were neutral or inconsistent.
This trial was registered at clinicaltrials.gov as NCT03192410.
Keywords: cardiovascular mortality, ischemic heart disease, stroke, all-cause mortality, myocardial infarction patients, prospective cohort study, dietary intake, dairy, milk, yogurt
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide with ischemic heart disease (IHD), especially myocardial infarction (MI), and stroke being the main contributors (1). Mortality rates for CVD have strongly declined in Western societies because of improved detection and treatment, but the number of chronic patients with IHD and stroke is on the increase (1).
Advanced drug therapies are available to control risk factors for recurring CVD, such as high blood pressure, high LDL cholesterol, and thrombotic factors (2, 3). Changing unhealthy lifestyle habits such as smoking, physical inactivity, alcohol use, and a poor diet also form a cornerstone in the prevention of recurrent CVD (1). Adherence to dietary recommendations may lead to a substantial health gain in MI patients (4, 5). In the Alpha Omega Cohort (AOC) of stable Dutch post-MI patients, we found that healthy eating was associated with a 30% lower mortality risk, on top of advanced cardiovascular drug treatment (6).
The role of dairy consumption in CVD has been examined extensively in apparently healthy populations, largely showing neutral associations between dairy products and IHD (7). Some studies, however, found favorable associations with CVD or stroke (7) and hypertension (8). Micronutrients in dairy could beneficially influence blood pressure and the cardiovascular system (9), whereas SFAs and trans fatty acids are associated with elevated LDL cholesterol concentrations (10, 11). Yogurt, on the other hand, has been suggested to lower the risk of diabetes, a major risk factor for IHD, for which the mechanisms are yet unknown (12). Dairy is a heterogeneous food group of milk-based products differing in terms of consistency, fat content, and micronutrients (13, 14). Dietary guidelines tend to emphasize low-fat rather than high-fat dairy products to reduce the intake of SFAs and calories (15, 16). Nutrient interactions in the dairy matrix are increasingly recognized as being important in relation to health, which argues for studying whole dairy foods rather than their specific nutrients (14, 17).
A vast amount of research has been done on different dairy products in relation to CVD and all-cause mortality in the general population (7). Whether dairy intake could affect long-term mortality risk after MI, however, is largely unknown. Associations may be different for post-MI patients because of alterations in the cardiovascular system, medication use (e.g., statins), and common comorbidities such as diabetes. We examined well-defined types of dairy products in relation to CVD (both IHD and stroke) and all-cause mortality in drug-treated post-MI patients from the Netherlands, where dairy intake is relatively high.
Methods
Study design
This current study makes use of the AOC (NCT03192410) in the Netherlands. During the first 40 mo of follow-up, patients participated in an intervention study of low doses of omega-3 fatty acids, which did not affect major CVD events (18, 19). At baseline (2002–2006), patients filled in questionnaires and were physically examined by trained research nurses at home or in the hospital, which included blood sampling. After the trial period, the AOC continued as a prospective cohort study for risk prediction after MI, and patients are continuously followed for cause-specific mortality. Patients provided written consent and the study was approved by a central ethics committee (Haga Hospital) and by the ethics committees of participating hospitals.
Patients
The AOC consists of 4837 patients who were 60–80 y old at baseline, with a history of MI ≤10 y before the start of the study. Patients with incomplete dietary data (n = 453) or implausibly high or low energy intakes (<800 or >8000 kcal/d for men, <600 or >6000 kcal/d for women; n = 19) were excluded, leaving a total of 4365 patients. Supplemental Figure 1 provides a flow diagram describing the selection of the population for analysis.
Dietary assessment
Baseline dietary intake over the past month was assessed with a 203-item FFQ, which was adapted and extended from a validated questionnaire on fatty acids and cholesterol intake (20). The frequency of consumption was asked for 42 items on dairy products, grouped by fat content. The Pearson correlation coefficient for energy intake was 0.83 for the FFQ compared with a dietary history measured over the same period (20). Spearman correlation coefficients for the reproducibility of 2 measurements of the FFQ were 0.69 for cheese and 0.80 for milk, yogurt, and custard combined (21). Returned questionnaires were checked for missing items by trained dietitians who collected these data from patients by telephone.
Categories of dairy intake comprised plain yogurt regardless of fat content (classified as <25; ≥25–50; ≥50 g/d), hard cheeses (Dutch regular 20%, 30%, 40%, 48% fat cheese, Parmesan, Gruyère, Emmental, and cheddar cheese; <25, ≥25–50, and ≥50 g/d), total fermented dairy (hard cheeses, luxury soft cheeses, curds, mozzarella, cream cheese, yogurt, buttermilk, and yogurt drinks; <50, ≥50–100, and ≥100 g/d), total milk (including nonfermented full-fat, semi skimmed, and skimmed milk; predominantly cow milk; <50, ≥50–100, ≥100–150, and ≥150 g/d), and low-fat milk (≤2% fat; <50, ≥50–100, ≥100–150, and ≥150 g/d). Because of low consumption, only 2 categories (any intake compared with zero intake) were used for high-fat milk (≥3.5% fat), liquid fermented dairy (buttermilk and yogurt drinks), and butter. Total dairy (<200, ≥200–300, ≥300–400, and ≥400 g/d) comprised all the aforementioned dairy groups plus (ice)cream and (sweetened) dairy desserts. Intakes of energy, macronutrients, and micronutrients were obtained through linkage with the Dutch Food Composition Database (22). The 2015 Dutch Healthy Diet index (DHD15-index) score was calculated to reflect adherence to dietary guidelines [DHD15-index; scale from 0 to maximal adherence (0–150)] (23).
Cardiovascular endpoints
The study focuses on CVD mortality and all-cause mortality as primary endpoints, and IHD mortality and stroke mortality as secondary endpoints. The vital status of patients was monitored through linkage with municipal registries, from baseline through 31 December, 2018. Follow-up for cause-specific mortality occurred in 3 phases. From 2002–2009 (Alpha Omega Trial) (18, 19) information was obtained from the national mortality registry [Statistics Netherlands (CBS)], treating physicians, and close family members. Primary and contributing causes of death were coded by an independent Endpoint Adjudication Committee, as described previously (18, 19). After the trial through 2012, data on the primary and contributing causes of death were obtained from CBS. From 2013 onwards, CBS provided data on the primary cause of death only, and treating physicians were asked to fill out an additional cause-of-death questionnaire (response rate: 67%), which was coded by study physicians who were not involved in the current analysis. The endpoint CVD, IHD, or stroke was allocated to all patients for whom it was a primary or contributing cause of death, based on any of the data sources.
Mortality coding was performed according to the International Classification of Diseases, tenth revision (ICD-10) (24). IHD mortality comprised ICD-10 codes I20–I25 (ischemic heart disease), I46 (cardiac arrest), and R96 (sudden death, undefined). CVD mortality comprised I20–I25, I46, R96, I50 (heart failure), and I60–I69 (stroke).
Covariates
Data on ethnicity, smoking, physical activity, and dietary intake were collected using a self-administered lifestyle and health questionnaire and an FFQ at baseline. Smoking status was assessed in 3 categories (never, former, or current). Physical activity was assessed by the validated Physical Activity Scale for the Elderly (25) and categorized in 2 categories: no activity or only light activity [<3 metabolic equivalent tasks (METs)] and 0–7 d/wk of moderate or vigorous activity (>3 METs). Alcohol consumption (g/d) was derived from the FFQ and categorized into 4 categories of drinkers: abstainers, light (>0 to ≤10 g/d for men and >0 to ≤5 g/d for women), moderate (>10 to ≤30 g/d for men and >5 to ≤15 g/d for women), and heavy (>30 g/d for men and >15 g/d for women). Body weight and height were measured and BMI was calculated as kg/m2. Blood pressure was measured twice using an automatic device (HEM-711; Omron) and averaged. Serum lipids and plasma glucose were determined using standard assays and an automated analyzer (Hitachi 921; Roche Diagnostics). Medication use was self-reported and coded according to the Anatomical Therapeutic Chemical Classification system (26). Prevalent diabetes mellitus was defined as a self-reported physician diagnosis, use of antidiabetic medication, or elevated plasma glucose (≥7.0 mmol/L if fasted >4 h or ≥11.1 mmol/L if nonfasted). Dietary covariates (g/d) were derived from the FFQ and the following food groups were composed: fruits, vegetables, whole grains, refined grains, red meat, processed meat, sugar-sweetened beverages, coffee (caffeinated and decaffeinated), tea, fish, and sodium. Sodium intake was only estimated from foods, because discretionary salt use could not be assessed by means of the FFQ.
Statistical analysis
Missing data (all at random) were imputed using the age- and sex-specific mode for physical activity (n = 25) and smoking status (n = 1) and the age- and sex-specific median for BMI (n = 6). Patient characteristics across energy-adjusted categories of total milk consumption are presented as mean ± SD for normally distributed data, median [IQR] for skewed data, and n (%) for categorical data. The intakes of total dairy and dairy subgroups (except for high-fat milk, liquid fermented dairy, and butter, which were dichotomous) were adjusted for total energy intake using the residual method by Willett et al. (27).
Cox proportional hazards models were used to estimate HRs and 95% CIs for CVD, IHD, stroke, and all-cause mortality across categories of dairy products with the lowest intake group as the reference. Continuous (per increment) HRs were calculated for total milk (per 100 g/d), hard cheeses (per 25 g/d), and plain yogurt (per 25 g/d). The proportionality of the hazards assumption was checked visually using a log-minus-log plot of survival and time and was met. Person-years were calculated from date of study enrollment to date of death or end of the study (31 December, 2018), whichever came first. One patient was lost to follow-up and censored after 2.9 y.
HRs in the first model were adjusted for sex, age (y), and total energy intake (kcal). The second model was in addition adjusted for physical activity (2 categories), smoking (3 categories), alcohol intake (4 categories), diabetes (yes/no prevalent diabetes at baseline), and obesity (yes/no BMI ≥ 30 at baseline). The third model was in addition adjusted for fruits, vegetables, whole grains, refined grains, red meat, processed meat, sugar-sweetened beverages, coffee, tea, fish (yes/no), and sodium intake (only from foods). The analysis of cheese was adjusted for sodium from foods other than cheese. In an additional analysis, HRs for milk and yogurt were in addition adjusted for the DHD15-index.
Subgroup analyses were performed for gender, prevalent diabetes (yes/no), and prevalent obesity (yes/no). The Wald chi-square statistic was used for testing interaction. Sensitivity analyses were performed for CVD as the primary cause of death and excluding the first 2 y of follow-up. Restricted cubic splines were used to investigate the continuous associations of total milk, yogurt, hard cheeses, and total fermented dairy with CVD and all-cause mortality, using the fully adjusted model. The median intake was set as the reference and knots were placed at the 5th, 50th, and 95th percentiles. The Wald chi-square test was carried out for testing nonlinearity of the relations (28). Two-sided P values < 0.05 were considered statistically significant. All statistical analyses were performed using the statistical software SAS version 9.4 (SAS Institute Inc.). Forest plots were created using R version 3.6.1 (R Foundation for Statistical Computing).
Results
Table 1 presents baseline characteristics for the overall cohort and according to categories of energy-adjusted total milk consumption. Patients had a mean age of 69.0 ± 5.6 y, 21% were women, 24% were obese, and 20% had diabetes. Most patients were Dutch. The majority of patients used antihypertensive medication (89.8%) and/or statins (85.6%). Most patients (73.2%) consumed milk. Median daily intakes, adjusted for energy, were 39 g for total yogurt (33 g for low-fat yogurt), 17 g for hard cheeses, 55 g for total fermented dairy, 88 g for total milk (52 g for low-fat milk), and 273 g for total dairy (Supplemental Table 1). High-fat milk was consumed by 9.9% (median intake: 54 g/d in consumers), liquid fermented dairy products by 38.5% (75 g/d in consumers), and butter by 14% (4.4 g/d in consumers). During a median follow-up period of ∼12 y (48,473 person-years) we observed 2035 deaths including 903 from CVD, 558 from IHD, and 170 from stroke.
TABLE 1.
Baseline characteristics of 4365 patients in the Alpha Omega Cohort, overall and by categories of total milk consumption1
| Energy-adjusted total milk consumption, g/d | |||||
|---|---|---|---|---|---|
| Total population (n = 4365) | <50 (n = 1659) | ≥50 to 100 (n = 671) | ≥100 to 150 (n = 700) | ≥150 (n = 1335) | |
| Median total milk consumption, g/d | 88 | 12 | 72 | 125 | 249 |
| Women | 933 (21.4) | 303 (18.3) | 183 (27.3) | 127 (18.1) | 320 (24.0) |
| Age, y | 69.0 ± 5.6 | 68.7 ± 5.5 | 69.1 ± 5.5 | 69.1 ± 5.5 | 69.4 ± 5.6 |
| Dutch ethnicity | 4315 (98.9) | 1637 (98.7) | 663 (98.9) | 697 (99.6) | 1318 (98.7) |
| Smoking2 | |||||
| Never | 722 (16.5) | 262 (15.8) | 118 (17.6) | 106 (15.1) | 236 (17.7) |
| Former | 2929 (67.1) | 1116 (67.3) | 441 (65.8) | 491 (70.1) | 881 (66.0) |
| Current | 713 (16.3) | 281 (16.9) | 111 (16.6) | 103 (14.7) | 218 (16.3) |
| Physical activity3 | |||||
| No or only light activity | 1783 (41.1) | 673 (40.7) | 275 (41.3) | 292 (41.8) | 543 (41.0) |
| Moderate or vigorous activity | 2557 (58.9) | 979 (59.3) | 391 (58.7) | 406 (58.2) | 781 (59.0) |
| BMI,4 kg/m2 | 27.7 ± 3.8 | 27.6 ± 3.8 | 28.0 ± 3.8 | 27.8 ± 3.7 | 27.8 ± 3.9 |
| Obese | 1032 (23.6) | 364 (22.0) | 173 (25.8) | 162 (23.1) | 333 (25.0) |
| Alcohol consumption,5 g/d | |||||
| Abstainers | 231 (5.3) | 77 (4.6) | 41 (6.1) | 33 (4.7) | 80 (6.0) |
| Light | 2230 (51.1) | 780 (47.0) | 351 (52.3) | 350 (50.0) | 749 (56.1) |
| Moderate | 1212 (27.8) | 479 (28.9) | 189 (28.2) | 197 (28.1) | 347 (26.0) |
| Heavy | 692 (15.9) | 323 (19.5) | 90 (13.4) | 120 (17.1) | 159 (11.9) |
| Blood pressure,6 mm Hg | |||||
| Systolic | 141.9 ± 21.6 | 141.9 ± 21.9 | 142.9 ± 21.0 | 142.0 ± 21.8 | 141.4 ± 21.3 |
| Diastolic | 80.2 ± 11.1 | 80.2 ± 11.3 | 80.5 ± 11.0 | 80.6 ± 10.9 | 79.9 ± 11.0 |
| Antihypertensive drug use | 3919 (89.8) | 1497 (90.2) | 606 (90.3) | 625 (89.3) | 1191 (89.2) |
| Serum blood lipids, mmol/L | |||||
| Total cholesterol7 | 4.71 ± 0.95 | 4.69 ± 0.96 | 4.71 ± 0.95 | 4.71 ± 0.93 | 4.72 ± 0.97 |
| LDL cholesterol8 | 2.57 ± 0.82 | 2.55 ± 0.81 | 2.58 ± 0.82 | 2.55 ± 0.80 | 2.59 ± 0.84 |
| HDL cholesterol9 | 1.29 ± 0.34 | 1.29 ± 0.35 | 1.29 ± 0.33 | 1.27 ± 0.33 | 1.28 ± 0.34 |
| Triglycerides9 | 1.92 ± 1.05 | 1.91 ± 1.03 | 1.87 ± 1.02 | 2.03 ± 1.16 | 1.90 ± 1.01 |
| Statin use | 3747 (85.8) | 1426 (86.0) | 582 (86.7) | 605 (86.4) | 1134 (84.9) |
| Prevalent diabetes10 | 883 (20.2) | 313 (18.9) | 143 (21.3) | 131 (18.7) | 296 (22.2) |
| Plasma glucose,11 mmol/L | 6.17 ± 2.06 | 6.11 ± 2.03 | 6.19 ± 2.03 | 6.16 ± 1.96 | 6.25 ± 2.15 |
| Energy intake, kcal/d | 1914 ± 521 | 1979 ± 507 | 1808 ± 616 | 1939 ± 453 | 1875 ± 509 |
| DHD15-index score | 79 ± 14 | 77 ± 14 | 80 ± 13 | 81 ± 13 | 80 ± 14 |
Values are mean ± SD for normally distributed variables, or n (%) for categorical or discrete variables, unless otherwise indicated. DHD15-index, Dutch Healthy Diet 2015 index; MET, metabolic equivalent task.
Missing data for 1 patient.
Missing data for 25 patients. No or only light activity was defined as METs < 3. Moderate or vigorous activity was defined as METs > 3.
Missing data for 6 patients. Obesity was defined as BMI ≥ 30.
Light alcohol consumption was defined as >0 to ≤10 g/d for men or >0 to ≤5 g/d for women. Moderate alcohol consumption was defined as >10 to ≤30 g/d for men or >5 to ≤15 g/d for women. Heavy alcohol consumption was defined as >30 g/d for men or >15 g/d for women.
Missing data for 6 patients.
Nonfasted, missing data for 111 patients.
Nonfasted, missing data for 309 patients.
Nonfasted, missing data for 111 patients.
Defined as a self-reported physician diagnosis, use of antidiabetic medication, or elevated plasma glucose (≥7.0 mmol/L if fasted >4 h or ≥11.1 mmol/L if nonfasted).
Nonfasted, missing data for 86 patients.
CVD mortality
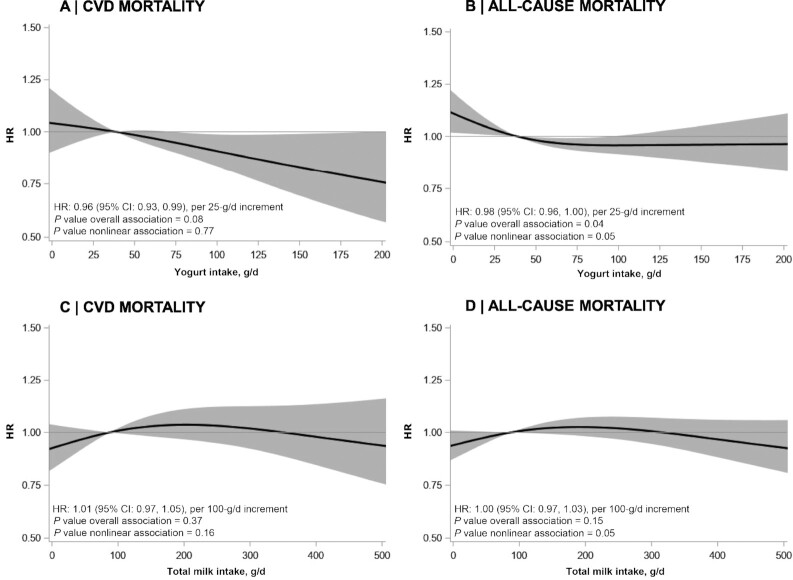
Tables 2 –4 present HRs for intake of specific dairy groups in relation to CVD mortality. Yogurt consumption of >50 g/d compared with <25 g/d was associated with a lower risk of CVD mortality using the fully adjusted model (HR: 0.86; 95% CI: 0.74, 0.99). When analyzed continuously, a 4% lower risk of CVD mortality was observed per 25-g/d increment in yogurt intake (HR: 0.96; 95% CI: 0.93, 0.99) (Figure 1A, Supplemental Figure 2). Yogurt was not associated with IHD mortality (HR: 1.01; 95% CI: 0.83, 1.22; upper compared with lower intake category) (Supplemental Tables 2–4). A trend toward a lower risk of stroke mortality was observed with yogurt consumption (HR: 0.83; 95% CI: 0.59, 1.17; upper compared with lower intake category) (Supplemental Table 2). Exclusion of high-fat yogurt from the analyses yielded similar HRs for CVD mortality (HR: 0.96; 95% CI: 0.93, 0.99) and other outcomes (data not shown).
TABLE 2.
HRs for yogurt, hard cheeses, and total fermented dairy in relation to CVD and all-cause mortality in 4365 patients from the Alpha Omega Cohort1
| Categories of energy-adjusted dairy consumption | P value2 | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| CVD mortality | ||||
| Yogurt3 | ||||
| n | 1661 | 806 | 1898 | |
| Intake,4 g/d | <25 (8.1) | ≥25–50 (36.0) | ≥50 (87.5) | |
| Events | 360 | 178 | 365 | |
| Person-years | 18,141 | 8828 | 21,504 | |
| Model 15,6 | 1 | 1.00 (0.83, 1.19) | 0.80 (0.69, 0.93) | <0.01 |
| Model 26,7 | 1 | 1.05 (0.88, 1.26) | 0.84 (0.73, 0.98) | <0.01 |
| Model 36,8 | 1 | 1.05 (0.87, 1.26) | 0.86 (0.74, 0.99) | 0.01 |
| Hard cheeses9 | ||||
| n | 3043 | 916 | 406 | |
| Intake,4 g/d | <25 (12.6) | ≥25–50 (36.2) | ≥50 (61.3) | |
| Events | 631 | 183 | 89 | |
| Person-years | 33,615 | 10,337 | 4521 | |
| Model 15,6 | 1 | 0.92 (0.78, 1.08) | 1.07 (0.85, 1.33) | 0.98 |
| Model 26,7 | 1 | 0.94 (0.80, 1.11) | 1.07 (0.85, 1.33) | 0.93 |
| Model 36,8 | 1 | 0.93 (0.78, 1.10) | 1.02 (0.81, 1.29) | 0.72 |
| Total fermented dairy10 | ||||
| n | 2067 | 1020 | 1278 | |
| Intake,4 g/d | <50 (5.5) | ≥50–100 (73.6) | ≥100 (162.2) | |
| Events | 446 | 186 | 271 | |
| Person-years | 22,585 | 11,557 | 14,331 | |
| Model 15,6 | 1 | 0.81 (0.68, 0.96) | 0.93 (0.80, 1.08) | 0.28 |
| Model 26,7 | 1 | 0.84 (0.71, 1.00) | 0.95 (0.82, 1.10) | 0.40 |
| Model 36,8 | 1 | 0.85 (0.71, 1.01) | 0.95 (0.82, 1.12) | 0.45 |
| All-cause mortality | ||||
| Yogurt | ||||
| n | 1661 | 806 | 1898 | |
| Intake,4 g/d | <25 (8.1) | ≥25–50 (36.0) | ≥50 (87.5) | |
| Events | 828 | 379 | 828 | |
| Person-years | 18,141 | 8828 | 21,504 | |
| Model 15,6 | 1 | 0.92 (0.81, 1.04) | 0.80 (0.73, 0.88) | <0.0001 |
| Model 26,7 | 1 | 0.97 (0.86, 1.10) | 0.85 (0.77, 0.94) | <0.001 |
| Model 36,8 | 1 | 0.97 (0.86, 1.10) | 0.87 (0.78, 0.96) | <0.01 |
| Hard cheeses | ||||
| n | 3043 | 916 | 406 | |
| Intake,4 g/d | <25 (12.6) | ≥25–50 (36.2) | ≥50 (61.3) | |
| Events | 1429 | 422 | 184 | |
| Person-years | 33,615 | 10,337 | 4521 | |
| Model 15,6 | 1 | 0.94 (0.84, 1.05) | 0.99 (0.85, 1.14) | 0.48 |
| Model 26,7 | 1 | 0.95 (0.85, 1.06) | 1.00 (0.85, 1.16) | 0.53 |
| Model 36,8 | 1 | 0.96 (0.85, 1.07) | 0.99 (0.84, 1.16) | 0.57 |
| Total fermented dairy | ||||
| n | 2067 | 1020 | 1278 | |
| Intake,4 g/d | <50 (5.5) | ≥50–100 (73.6) | ≥100 (162.2) | |
| Events | 1021 | 433 | 581 | |
| Person-years | 22,585 | 11,557 | 14,331 | |
| Model 15,6 | 1 | 0.83 (0.74, 0.92) | 0.88 (0.79, 0.97) | <0.01 |
| Model 26,7 | 1 | 0.86 (0.77, 0.97) | 0.92 (0.83, 1.02) | 0.03 |
| Model 36,8 | 1 | 0.88 (0.78, 0.98) | 0.93 (0.84, 1.04) | 0.09 |
CVD, cardiovascular disease.
P-trend for categories was calculated by modeling the median values as a continuous variable.
Full-fat, semi skimmed, and skimmed plain yogurt.
Median in parentheses.
Adjusted for total energy intake, age, and sex.
Values are HRs (95% CIs) obtained from Cox proportional hazards models, using the lowest category as the reference.
Adjusted as model 1, plus for smoking status, physical activity, alcohol intake, diabetes, and obesity.
Adjusted as model 2, plus for intakes of whole grains, refined grains, fruits, vegetables, red and processed meat, sugar-sweetened beverages, coffee, tea, fish, and salt from foods.
Dutch regular 20%, 30%, 40%, 48% fat cheese, Parmesan, Gruyère, Emmental, and cheddar cheese.
Hard cheeses, luxury soft cheeses, curds, mozzarella, cream cheese, yogurt, buttermilk, and yogurt drinks.
TABLE 4.
HRs for high-fat milk, liquid fermented dairy, and butter in relation to CVD and all-cause mortality in 4365 patients from the Alpha Omega Cohort1
| Categories of dairy consumption | |||
|---|---|---|---|
| Zero intake | Any intake | P value2 | |
| CVD mortality | |||
| High-fat milk (≥3.5% fat) | |||
| n | 3935 | 430 | |
| Intake, g/d | 0 | 54.0 | |
| Events | 810 | 93 | |
| Person-years | 44,055 | 4418 | |
| Model 13,4 | 1 | 1.18 (0.95, 1.46) | 0.13 |
| Model 24,5 | 1 | 1.10 (0.89, 1.37) | 0.39 |
| Model 34,6 | 1 | 1.06 (0.85, 1.32) | 0.60 |
| Liquid fermented dairy7 | |||
| n | 2686 | 1679 | |
| Intake, g/d | 0 | 75.0 | |
| Events | 570 | 333 | |
| Person-years | 29,596 | 18,878 | |
| Model 13,4 | 1 | 0.95 (0.83, 1.09) | 0.45 |
| Model 24,5 | 1 | 0.98 (0.85, 1.12) | 0.76 |
| Model 34,6 | 1 | 0.98 (0.86, 1.13) | 0.79 |
| Butter | |||
| n | 3693 | 672 | |
| Intake, g/d | 0 | 4.4 | |
| Events | 770 | 133 | |
| Person-years | 41,258 | 7215 | |
| Model 13,4 | 1 | 0.95 (0.79, 1.14) | 0.55 |
| Model 24,5 | 1 | 0.93 (0.77, 1.12) | 0.44 |
| Model 34,6 | 1 | 0.91 (0.76, 1.10) | 0.32 |
| All-cause mortality | |||
| High-fat milk (≥3.5% fat) | |||
| n | 3935 | 430 | |
| Intake, g/d | 0 | 54.0 | |
| Events | 1791 | 244 | |
| Person-years | 44,055 | 4418 | |
| Model 13,4 | 1 | 1.42 (1.24, 1.62) | <0.001 |
| Model 24,5 | 1 | 1.33 (1.16, 1.52) | <0.001 |
| Model 34,6 | 1 | 1.30 (1.13, 1.49) | <0.001 |
| Liquid fermented dairy | |||
| n | 2686 | 1679 | |
| Intake, g/d | 0 | 75.0 | |
| Events | 1292 | 743 | |
| Person-years | 29,596 | 18,878 | |
| Model 13,4 | 1 | 0.94 (0.86, 1.03) | 0.19 |
| Model 24,5 | 1 | 0.96 (0.88, 1.06) | 0.43 |
| Model 34,6 | 1 | 0.97 (0.89, 1.06) | 0.52 |
| Butter | |||
| n | 3693 | 672 | |
| Intake, g/d | 0 | 4.4 | |
| Events | 1706 | 329 | |
| Person-years | 41,258 | 7215 | |
| Model 13,4 | 1 | 1.07 (0.95, 1.20) | 0.28 |
| Model 24,5 | 1 | 1.04 (0.93, 1.18) | 0.47 |
| Model 34,6 | 1 | 1.04 (0.91, 1.16) | 0.63 |
CVD, cardiovascular disease.
P for HR.
Adjusted for total energy intake, age, and sex.
Values are HRs (95% CIs) obtained from Cox proportional hazards models, using the lowest category as the reference.
Adjusted as per model 1, plus for smoking status, physical activity, alcohol intake, diabetes, and obesity.
Adjusted as per model 2, plus for intakes of whole grains, refined grains, fruits, vegetables, red and processed meat, sugar-sweetened beverages, coffee, tea, fish, and salt from foods.
Buttermilk and yogurt drinks.
FIGURE 1.
Associations of yogurt (A, B) and total milk (C, D) consumption with mortality from CVD and all causes in 4365 patients from the Alpha Omega Cohort. Lines are restricted cubic splines, showing continuous associations, with 3 knots located at the 5th, 50th, and 95th percentiles. The y-axis shows the predicted HRs for mortality for any value of intake, compared with the reference value set at the median intake. HRs are adjusted for energy intake, age, sex, smoking, physical activity, alcohol intake, diabetes, and dietary factors (see text). Continuous covariates were modeled using restricted cubic splines (knots at the 5th, 50th, and 95th percentiles). CVD, cardiovascular disease.
Total milk consumption showed no association with CVD mortality, with HRs (95% CIs) of 0.93 (0.75, 1.14), 1.03 (0.85, 1.26), and 1.05 (0.90, 1.24) in consecutive intake categories when compared with the reference (<50 g/d), using the full model (Table 3). Also when analyzed continuously, total milk (per 100 g/d) was not associated with CVD mortality (HR: 1.01; 95% CI: 0.97, 1.05) (Figure 1C) and also not with IHD mortality (HR: 1.00; 95% CI: 0.95, 1.06) or stroke mortality (HR: 1.00; 95% CI: 0.92, 1.09) (Supplemental Figure 2). Likewise, no associations were observed for hard cheeses, total fermented dairy, low-fat milk, total dairy, high-fat milk, liquid fermented dairy, and butter intake. Additional adjustment for the DHD15-index did not change the HRs for yogurt and milk in relation to CVD mortality (Supplemental Table 5). Correlations of total dairy, milk, and yogurt with the DHD15-index were weak (all r < 0.1).
TABLE 3.
HRs for milk and total dairy in relation to CVD and all-cause mortality in 4365 patients from the Alpha Omega Cohort1
| Categories of energy-adjusted dairy consumption | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | P value2 | |
| CVD mortality | |||||
| Total milk3 | |||||
| n | 1659 | 671 | 700 | 1335 | |
| Intake,4 g/d | <50 (11.6) | ≥50–100 (71.9) | ≥100–150 (125.4) | ≥150 (248.5) | |
| Events | 334 | 126 | 146 | 297 | |
| Person-years | 18,649 | 7463 | 7744 | 14,616 | |
| Model 15 | 1 | 0.91 (0.74, 1.12) | 1.02 (0.84, 1.24) | 1.08 (0.92, 1.27) | 0.23 |
| Model 26 | 1 | 0.93 (0.75, 1.14) | 1.03 (0.84, 1.25) | 1.07 (0.91, 1.25) | 0.31 |
| Model 37 | 1 | 0.93 (0.75, 1.14) | 1.03 (0.85, 1.26) | 1.05 (0.90, 1.24) | 0.41 |
| Low-fat milk (<2% fat)8 | |||||
| n | 2118 | 554 | 703 | 990 | |
| Intake,4 g/d | <50 (0.0) | ≥50–100 (77.6) | ≥100–150 (127.3) | ≥150 (310.5) | |
| Events | 434 | 102 | 145 | 222 | |
| Person-years | 23,477 | 6249 | 7882 | 10,865 | |
| Model 15 | 1 | 0.88 (0.71, 1.09) | 0.95 (0.78, 1.14) | 1.08 (0.92, 1.27) | 0.35 |
| Model 26 | 1 | 0.89 (0.72, 1.10) | 0.97 (0.81, 1.17) | 1.09 (0.92, 1.28) | 0.29 |
| Model 37 | 1 | 0.91 (0.73, 1.13) | 0.99 (0.82, 1.19) | 1.08 (0.91, 1.27) | 0.35 |
| Total dairy9 | |||||
| n | 1321 | 1136 | 820 | 1088 | |
| Intake,4 g/d | <200 (135.8) | ≥200–300 (245.6) | ≥200–300 (343.8) | ≥400 (527.5) | |
| Events | 272 | 216 | 176 | 239 | |
| Person-years | 14,823 | 12,597 | 9177 | 11,876 | |
| Model 15 | 1 | 0.86 (0.72, 1.03) | 0.95 (0.78, 1.15) | 0.99 (0.83, 1.18) | 0.80 |
| Model 26 | 1 | 0.90 (0.75, 1.08) | 0.98 (0.80, 1.18) | 0.99 (0.83, 1.19) | 0.95 |
| Model 37 | 1 | 0.91 (0.75, 1.09) | 0.98 (0.80, 1.19) | 0.97 (0.81, 1.17) | 0.88 |
| All-cause mortality | |||||
| Total milk | |||||
| n | 1659 | 671 | 700 | 1335 | |
| Intake,4 g/d | <50 (11.6) | ≥50–100 (71.9) | ≥100–150 (125.4) | ≥150 (248.5) | |
| Events | 749 | 311 | 327 | 648 | |
| Person-years | 18,649 | 7463 | 7744 | 14,616 | |
| Model 15 | 1 | 1.00 (0.88, 1.14) | 1.02 (0.90, 1.16) | 1.05 (0.95, 1.17) | 0.32 |
| Model 26 | 1 | 1.02 (0.90, 1.17) | 1.03 (0.91, 1.18) | 1.06 (0.95, 1.17) | 0.34 |
| Model 37 | 1 | 1.03 (0.90, 1.17) | 1.04 (0.91, 1.19) | 1.05 (0.94, 1.17) | 0.38 |
| Low-fat milk (<2% fat) | |||||
| n | 2118 | 554 | 703 | 990 | |
| Intake,4 g/d | <50 (0.0) | ≥50–100 (77.6) | ≥100–150 (127.3) | ≥150 (310.5) | |
| Events | 998 | 250 | 318 | 469 | |
| Person-years | 23,477 | 6249 | 7882 | 10,865 | |
| Model 15 | 1 | 0.94 (0.82, 1.08) | 0.90 (0.79, 1.02) | 1.00 (0.89, 1.11) | 0.87 |
| Model 26 | 1 | 0.96 (0.84, 1.11) | 0.93 (0.82, 1.05) | 1.02 (0.92, 1.14) | 0.80 |
| Model 37 | 1 | 0.98 (0.85, 1.13) | 0.94 (0.83, 1.07) | 1.02 (0.92, 1.14) | 0.78 |
| Total dairy | |||||
| n | 1321 | 1136 | 820 | 1088 | |
| Intake,4 g/d | <200 (135.8) | ≥200–300 (245.6) | ≥300–400 (343.8) | ≥400 (527.5) | |
| Events | 604 | 523 | 383 | 525 | |
| Person-years | 14,823 | 12,597 | 9177 | 11,876 | |
| Model 15 | 1 | 0.93 (0.83, 1.05) | 0.93 (0.81, 1.05) | 0.99 (0.88, 1.11) | 0.99 |
| Model 26 | 1 | 0.98 (0.87, 1.10) | 0.97 (0.85, 1.10) | 1.01 (0.90, 1.14) | 0.98 |
| Model 37 | 1 | 0.99 (0.88, 1.11) | 0.98 (0.86, 1.12) | 1.01 (0.90, 1.15) | 0.91 |
CVD, cardiovascular disease.
P-trend for categories was calculated by modeling their median values as a continuous variable.
Nonfermented full-fat, semi skimmed, and skimmed milk.
Median in parentheses.
Adjusted for total energy intake, age, and sex.
Adjusted as model 1, plus for smoking status, physical activity, alcohol intake, diabetes, and obesity.
Adjusted as model 2, plus for intakes of whole grains, refined grains, fruits, vegetables, red and processed meat, sugar-sweetened beverages, coffee, tea, fish, and salt from foods.
Semi skimmed (<2% fat) and skimmed milk (<1% fat).
Total milk, total fermented dairy, butter, (ice)cream, and (sweetened) dairy desserts.
All-cause mortality
Tables 2–4 present HRs for dairy groups in relation to all-cause mortality. Yogurt intakes > 50 g/d compared with <25 g/d were associated with a lower all-cause mortality risk (HR: 0.87; 95% CI: 0.78, 0.96) (Table 2). A similar association was found when excluding high-fat yogurt from the analysis (HR: 0.85; 95% CI: 0.77, 0.94; upper compared with lower intake category; data not shown). Associations between yogurt intake and all-cause mortality appeared to be borderline nonlinear (P for nonlinearity = 0.05; P for overall association = 0.04) (Figure 1B).
Total milk consumption per 100 g/d was not associated with all-cause mortality (HR: 1.00; 95% CI: 0.97, 1.03) (Figure 1D). High-fat milk intake was associated with an increased risk of all-cause mortality in the fully adjusted model (HR: 1.30; 95% CI: 1.13, 1.49) (Table 4). Total fermented dairy intakes of ≥50–100 g/d compared with <50 g/d were associated with a lower all-cause mortality risk (HR: 0.88; 95% CI: 0.78, 0.98) (Table 2). When analyzed continuously, a trend toward a nonlinear association was found for total fermented dairy (P for nonlinearity = 0.09) (Supplemental Figure 3). No associations were observed for hard cheeses, low-fat milk, total dairy, liquid fermented dairy, and butter with all-cause mortality.
Sensitivity analyses
When analyzing CVD as the primary cause of death, HRs did not essentially differ from the analyses that also included CVD as a contributing cause of death (Supplemental Tables 6, 7). Excluding the first 2 y of follow-up did not change the results (Supplemental Tables 8, 9).
Subgroup analyses
Supplemental Figures 4 and 5 depict associations of total milk, yogurt, and hard cheeses as continuous variables with CVD and all-cause mortality in subgroups.
For milk, associations with CVD and all-cause mortality were not modified by sex, diabetes, or obesity (HR: ∼1.0 per 100 g/d; in all subgroups). For yogurt, associations with CVD mortality were stronger in women (HR: 0.93; 95% CI: 0.87, 0.99; per 25 g/d) than in men (HR: 0.98; 95% CI: 0.94, 1.01; per 25 g/d; P-interaction = 0.12). Associations for yogurt and CVD mortality were also stronger in patients without obesity (HR: 0.94; 95% CI: 0.91, 0.98; per 25 g/d) than in patients with obesity (HR: 1.00; 95% CI: 0.95, 1.05; per 25 g/d; P-interaction = 0.07), whereas no effect modification was found for diabetes (P-interaction = 0.67).
Discussion
This prospective analysis of 4365 Dutch post-MI patients who received state-of-the-art drug treatment showed inverse associations of yogurt with CVD and all-cause mortality, but not IHD mortality. Associations of milk and other dairy products with mortality were generally neutral, except for an elevated risk in the small group of high-fat milk consumers.
Intake of total (nonfermented, mainly low-fat) milk showed no associations with all-cause, CVD, and IHD mortality in our post-MI patients, in line with population-based studies (29–31). A dose-response meta-analysis of prospective studies by Guo et al. (29) showed an RR of 1.01 (95% CI: 0.93, 1.10; per 244 g/d) for milk and CVD risk and also no association with IHD or all-cause mortality. When analyzing high-fat milk (>3.5% fat) separately, we observed a 30% elevated risk of all-cause mortality. Studies of high-fat milk and mortality are scanty. In healthy Swedish individuals with 15 y of follow-up, high-fat milk was also associated with mortality risk (HR: 1.08; 95% CI: 1.03, 1.14, users compared with nonusers) (32). However, residual confounding may be present because no adjustment was made for socioeconomic and dietary factors. In the present analysis of the AOC, high-fat milk was related to all-cause mortality but not to CVD mortality. Given the small number of high-fat milk consumers (only 430 patients), we think that the increased mortality risk that we observed may be due to chance.
In our post-MI patients, the intake of plain (unsweetened) yogurt irrespective of fat content was associated with a lower risk of CVD and all-cause mortality, although not with IHD mortality. In the meta-analysis of population-based studies by Guo et al. (29), yogurt was not associated with CVD (RR: 1.03; 95% CI: 0.97, 1.09; per 50 g/d), IHD, or all-cause mortality (RR: 0.97; 95% CI: 0.85, 1.11). A pooled analysis of the Nurses’ Health Study and Health Professionals Follow-Up Study (>3 million person-years), on the other hand, showed a protective association of yogurt with CVD mortality, particularly in women (HR: 0.78; 95% CI: 0.70, 0.87; for ∼250 g/wk compared with zero intake) (33). Also in our cohort, the observed reductions in CVD mortality risk were more pronounced in women than in men (HRs of 0.93 and 0.98 per 25 g/d, respectively). For yogurt, we also observed a trend toward a lower risk of stroke mortality (nonsignificant HR of 0.83), a major cause of death in women (1). However, we only had 170 cases of stroke and insufficient power for studying sex-specific associations with stroke mortality.
In the present study, risks of all-cause, CVD, and stroke mortality tended to be lower for higher intake of fermented dairy products. Probiotics in fermented dairy may beneficially affect the gut microbiota and intestinal health, possibly enhancing the body's immune system and reducing low-grade inflammation (17, 34). Fermented dairy is a heterogenous group of products, including solid foods (e.g., cheese), semisolid foods (e.g., yogurt), and drinks (e.g., buttermilk), which differ in calories, nutrient density, and level of processing. Cheese is an important contributor to salt intake in the Dutch diet. Yogurt may contain added sugar, although in the present study mainly plain yogurt was consumed. In light of dietary recommendations, we consider risk estimates for separate fermented dairy groups more relevant than those for total fermented dairy. Based on our findings, unsweetened yogurt may be preferable to cheese (containing added salt) in IHD patients.
A general concern in patient cohorts is the alteration of diet and lifestyle because of health complaints or prescribed diets, potentially leading to “reverse causation bias”. However, sensitivity analyses excluding the first 2 y of follow-up showed no evidence for biased risk estimates due to pre-existing disease. Another limitation of observational studies is the possibility of residual confounding. Dairy consumption may be related to other dietary and lifestyle habits, for which we carefully adjusted. However, our FFQ was not designed to accurately measure sodium intake, a major risk factor for CVD. Systematic errors may occur when assessing diet by means of an FFQ, including underreporting of energy-dense products or overreporting of healthy foods. In our cohort with a traditional Dutch diet, yogurt intake and dairy intake in general were not correlated with adherence to dietary guidelines (DHD15-index). We therefore consider “health consciousness bias” unlikely to play a role in the present study. Also, associations did not materially change after excluding patients with obesity who may have underreported dietary intakes. The present analysis is based on dietary assessment at baseline, not accounting for changes in food intake during follow-up. Previous studies in Dutch elderly have shown relatively stable dietary patterns over time (35). Nevertheless, we cannot rule out misclassification of patients due to lack of repeated dietary assessments. This could have led to attenuated risk estimates for dairy intake.
The AOC comprised older post-MI patients, mainly Dutch men, and one-fifth had diabetes. Most patients received state-of-the-art drug treatment, reflected in largely controlled risk factor levels. It is possible that medication use and the underlying disease process affected the patients’ sensitivity to lifestyle and dietary factors, including dairy. Results of the present study cannot merely be translated to healthy populations, and may also be less applicable to women and people of African, Asian, or Hispanic origin. Our observations are largely in line with results from population-based studies in various parts of the world, showing neutral relations between milk consumption and IHD (29).
Strengths of our study include the unique cohort of post-MI patients with detailed data on dietary and lifestyle factors and >10 y of follow-up for CVD mortality. We could adequately assess the intake of different types of dairy, by fat content, based on an FFQ that had initially been designed for estimating fatty acid intakes (20, 21). This study was performed in a patient cohort in the Netherlands. Cow milk and hard cheeses are basic components of the traditional Dutch diet. Dairy is consumed at breakfast, lunch, and dinner (dessert), and also in between meals. Individual nutrients in dairy, such as minerals, may affect cardiovascular health (36). Apart from that, however, the interaction of nutrients with fat and other aspects of the dairy matrix may be important (14, 17). Therefore, we used a whole-food rather than nutrient-based approach for studying dairy intake and mortality risk.
In conclusion, we found an inverse association between yogurt consumption and the long-term risk of CVD and all-cause mortality in post-MI patients. Milk and other dairy products were not associated with a lower mortality risk. These findings may have implications for dietary advice after MI, and warrant confirmation in other cohorts of IHD patients.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—EC: conducted the research, performed the statistical analysis, interpreted the data, wrote the manuscript, and had primary responsibility for the final content; MGJC, MCB, LKK, and JMG: critically reviewed the manuscript; LKK: provided the data set; JMG: conceived and designed the study and performed data acquisition; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
The Alpha Omega Trial (2002–2009), from which this cohort study emerged, was supported by Netherlands Heart Foundation grant 200T401; NIH, National Heart, Lung, and Blood Institute, and Office of Dietary Supplements grant R01HL076200; Unilever R&D Vlaardingen; and Jaap Schouten Foundation grant JSF_SU_10_2018 (to LKK).
MGJC was supported by a Mexican National Council for Science and Technology (CONACYT) scholarship during the conduct of the study.
The funding sources had no role in the study design and conduct, data analysis, or manuscript preparation.
Supplemental Tables 1–9 and Supplemental Figures 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AOC, Alpha Omega Cohort; CBS, Statistics Netherlands; CVD, cardiovascular disease; DHD15-index, Dutch Healthy Diet 2015-index; ICD, International Classification of Diseases; IHD, ischemic heart disease; MET, metabolic equivalent task; MI, myocardial infarction.
Contributor Information
Esther Cruijsen, Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands.
Maria G Jacobo Cejudo, Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands.
Leanne K Küpers, Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands.
Maria C Busstra, Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands.
Johanna M Geleijnse, Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands.
Data Availability
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FNet al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–596. [DOI] [PubMed] [Google Scholar]
- 2. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade Tet al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mills E, Wu P, Chong G, Ghement I, Singh S, Akl E, Eyawo O, Guyatt G, Berwanger O, Briel M. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170 255 patients from 76 randomized trials. QJM. 2011;104(2):109–24. [DOI] [PubMed] [Google Scholar]
- 4. Cole JA, Smith SM, Hart N, Cupples ME. Systematic review of the effect of diet and exercise lifestyle interventions in the secondary prevention of coronary heart disease. Cardiol Res Pract. 2011:232351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li S, Chiuve SE, Flint A, Pai JK, Forman JP, Hu FB, Willett WC, Mukamal KJ, Rimm EB. Better diet quality and decreased mortality among myocardial infarction survivors. JAMA Intern Med. 2013;173(19):1808–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sijtsma FP, Soedamah-Muthu SS, De Goede J, Oude Griep LM, Geleijnse JM, Giltay EJ, De Boer MJ, Jacobs DR Jr, Kromhout D. Healthy eating and lower mortality risk in a large cohort of cardiac patients who received state-of-the-art drug treatment. Am J Clin Nutr. 2015;102(6):1527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fontecha J, Calvo MV, Juarez M, Gil A, Martínez-Vizcaino V. Milk and dairy product consumption and cardiovascular diseases: an overview of systematic reviews and meta-analyses. Adv Nutr. 2019;10(suppl_2):S164–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soedamah-Muthu SS, Verberne LD, Ding EL, Engberink MF, Geleijnse JM. Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension. 2012;60(5):1131–7. [DOI] [PubMed] [Google Scholar]
- 9. McGrane MM, Essery E, Obbagy J, Lyon J, MacNeil P, Spahn J, Van Horn L. Dairy consumption, blood pressure, and risk of hypertension: an evidence-based review of recent literature. Curr Cardiovasc Risk Rep. 2011;5(4):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mölenberg FJ, de Goede J, Wanders AJ, Zock PL, Kromhout D, Geleijnse JM. Dietary fatty acid intake after myocardial infarction: a theoretical substitution analysis of the Alpha Omega Cohort. Am J Clin Nutr. 2017;106(3):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mensink RP, World Health Organization . Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. Geneva, Switzerland: WHO; 2016. [Google Scholar]
- 12. Guo J, Givens DI, Astrup A, Bakker SJ, Goossens GH, Kratz M, Marette A, Pijl H, Soedamah-Muthu SS. The impact of dairy products in the development of type 2 diabetes: where does the evidence stand in 2019?. Adv Nutr. 2019;10(6):1066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soedamah-Muthu SS, De Goede J. Dairy consumption and cardiometabolic diseases: systematic review and updated meta-analyses of prospective cohort studies. Curr Nutr Rep. 2018;7(4):171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thorning TK, Bertram HC, Bonjour J-P, de Groot L, Dupont D, Feeney E, Ipsen R, Lecerf JM, Mackie A, McKinley MCet al. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr. 2017;105(5):1033–45. [DOI] [PubMed] [Google Scholar]
- 15. Van Horn L, Carson JAS, Appel LJ, Burke LE, Economos C, Karmally W, Lancaster K, Lichtenstein AH, Johnson RK, Thomas RJet al. Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) guidelines: a scientific statement from the American Heart Association. Circulation. 2016;134(22):e505–e29. [DOI] [PubMed] [Google Scholar]
- 16. Kromhout D, Spaaij C, de Goede J, Weggemans R. The 2015 Dutch food-based dietary guidelines. Eur J Clin Nutr. 2016;70(8):869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Astrup A, Geiker NRW, Magkos F. Effects of full-fat and fermented dairy products on cardiometabolic disease: food is more than the sum of its parts. Adv Nutr. 2019;10(5):924S–30S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kromhout D, Giltay EJ, Geleijnse JM. n–3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015–26. [DOI] [PubMed] [Google Scholar]
- 19. Geleijnse JM, Giltay EJ, Schouten EG, de Goede J, Griep LMO, Teitsma-Jansen AM, Katan MB, Kromhout D; Alpha Omega Trial Group . Effect of low doses of n-3 fatty acids on cardiovascular diseases in 4,837 post-myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. Am Heart J. 2010;159(4):539–46.e2. [DOI] [PubMed] [Google Scholar]
- 20. Feunekes GI, Van Staveren WA, De Vries JH, Burema J, Hautvast JG. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr. 1993;58(4):489–96. [DOI] [PubMed] [Google Scholar]
- 21. Feunekes IJ, Van Staveren WA, Graveland F, De Vos J, Burema J. Reproducibility of a semiquantitative food frequency questionnaire to assess the intake of fats and cholesterol in The Netherlands. Int J Food Sci Nutr. 1995;46(2):117–23. [DOI] [PubMed] [Google Scholar]
- 22. Dutch Nutrition Center . Dutch Food Composition Table 2006 NEVO-tabel. The Hague, Netherlands: Nederlands Voedingsstoffenbestand/NEVO Foundation; 2006. [Google Scholar]
- 23. Looman M, Feskens EJ, de Rijk M, Meijboom S, Biesbroek S, Temme EH, de Vries J, Geelen A. Development and evaluation of the Dutch Healthy Diet index 2015. Public Health Nutr. 2017;20(13):2289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization . International statistical classification of diseases and related health problems: instruction manual. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 25. Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol. 1997;50(5):541–6. [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization . Anatomical Therapeutic Chemical classification system (ATC). Oslo, Norway: WHO; 2009. [Google Scholar]
- 27. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4):1220S–8S. [DOI] [PubMed] [Google Scholar]
- 28. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57. [DOI] [PubMed] [Google Scholar]
- 29. Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose–response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2017;32(4):269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alexander DD, Bylsma LC, Vargas AJ, Cohen SS, Doucette A, Mohamed M, Irvin SR, Miller PE, Watson H, Fryzek JP. Dairy consumption and CVD: a systematic review and meta-analysis. Br J Nutr. 2016;115(4):737–50. [DOI] [PubMed] [Google Scholar]
- 31. Bergholdt HK, Nordestgaard BG, Varbo A, Ellervik C. Milk intake is not associated with ischaemic heart disease in observational or Mendelian randomization analyses in 98 529 Danish adults. Int J Epidemiol. 2015;44(2):587–603. [DOI] [PubMed] [Google Scholar]
- 32. Tognon G, Nilsson LM, Shungin D, Lissner L, Jansson J-H, Renström F, Wennberg M, Winkvist A, Johansson I. Nonfermented milk and other dairy products: associations with all-cause mortality. Am J Clin Nutr. 2017;105(6):1502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmid D, Song M, Zhang X, Willett WC, Vaidya R, Giovannucci EL, Michels KB. Yogurt consumption in relation to mortality from cardiovascular disease, cancer, and all causes: a prospective investigation in 2 cohorts of US women and men. Am J Clin Nutr. 2020;111(3):689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wen L, Duffy A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J Nutr. 2017;147(7):1468S–75S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jankovic N, Steppel MT, Kampman E, de Groot LC, Boshuizen HC, Soedamah-Muthu SS, Kromhout D, Feskens EJ. Stability of dietary patterns assessed with reduced rank regression; the Zutphen Elderly Study. Nutr J. 2014;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88:987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.