ABSTRACT
Background
Dietary protein may slow the decline in muscle mass and function with aging, making it a sensible candidate to prevent or modulate disability progression. At present, studies providing reliable estimates of the association between protein intake and physical function, and its interaction with physical activity (PA), in community-dwelling older adults are lacking.
Objectives
We investigated the longitudinal relation between protein intake and physical function, and the interaction with PA.
Methods
We undertook a pooled analysis of individual participant data from cohorts in the PROMISS (PRevention Of Malnutrition In Senior Subjects in the European Union) consortium (the Health Aging and Body Composition Study, Quebec Longitudinal Study on Nutrition and Successful Aging, Longitudinal Aging Study Amsterdam, and Newcastle 85+) in which 5725 community-dwelling older adults were followed up to 8.5 y. The relation between protein intake and walking speed was determined using joint models (linear mixed-effects and Cox proportional hazards models) and the relation with mobility limitation was investigated using multistate models.
Results
Higher protein intake was modestly protective of decline in walking speed in a dose-dependent manner [e.g., protein intake ≥1.2 compared with 0.8 g/kg adjusted body weight (aBW)/d: β = 0.024, 95% CI: 0.009, 0.032 SD/y], with no clear indication of interaction with PA. Participants with protein intake ≥0.8 g/kg aBW/d had also a lower likelihood of incident mobility limitation, which was observed for each level of PA. This association seemed to be dose-dependent for difficulty walking but not for difficulty climbing stairs. No associations between protein intake and other mobility limitations transitions were observed.
Conclusions
Higher daily protein intake can reduce physical function decline not only in older adults with protein intake below the current RDA of 0.8 g/kg BW/d, but also in those with a protein intake that is already considered sufficient. This dose-dependent association was observed for each level of PA, suggesting no clear synergistic association between protein intake and PA in relation to physical function.
Keywords: protein, walking speed, gait speed, physical activity, joint models, PROMISS, older adults, one-stage meta-analysis
Introduction
Life expectancy has reached 81.0, 82.1, and 78.7 y in the European Union, Canada, and United States, respectively (1–3). However, the increase in healthy life years (HLYs)—the number of years an individual can expect to live disability-free—has not kept pace (4). Bridging the gap between life expectancy and HLYs by compressing morbidity into the later years of life is of special interest, not only to increase quality of life, but also to relieve the immense strain on the health care systems of developed countries.
Diet is a major modifiable risk factor for the development and management of a range of age-related diseases that comprise the leading causes of morbidity, disability, and death (5, 6). Specifically, dietary protein can slow the decline of muscle mass and function with aging, making it a sensible candidate to prevent or modulate disability progression (7–9). For example, community-dwelling older adults (70–79 y) with lower protein intake [<1 g/kg body weight (BW)/d] from the Health Aging and Body Composition Study (Health ABC) study had an increased risk of mobility limitation over 6 y compared with those with higher protein intake (≥1 g/kg BW/d) (10). In the Newcastle 85+ Study, community-dwelling very old adults (≥85 y) with protein intake <1 compared with ≥1 g/kg adjusted body weight (aBW)/d had lower muscle strength over 5 y (9). However, at present, evidence for an association between protein intake and (performance-based) loss of physical function in older adults is limited. One study in older females showed that higher protein intake was associated with a slower rate of physical function decline (11), but other studies did not find protein intake to be associated with less decline in physical function (9, 12–15).
Inadequate protein intake is a cause for concern in older adults because protein intake is lower in older males (87 g/d) and females (69 g/d) than in their younger counterparts (97 g/d and 73 g/d, respectively) (16, 17) due to multimorbidity, tooth loss, changes in deglutition, appetite loss, and loss of functional independence (18). Additionally, a higher prevalence of disease-related tissue catabolism and inflammation, and anabolic resistance can offset protein requirements (19, 20). Expert groups, such as PROT-AGE, have suggested a possible synergistic protective effect of protein and physical activity (PA) on age-related loss of muscle strength and muscle function (18). This would be anticipated if higher PA and higher protein intake together were necessary to optimally stimulate the rate of muscle synthesis, which might consequently decrease the rate of loss of muscle mass and muscle function and slow the rate of functional decline in older adults. However, only a few cohort studies have addressed this issue (9, 12) and evidence from randomized controlled trials (RCTs) is inconclusive (21–23). This could be due to a lack of statistical power in individual studies, which can be overcome by pooling individual participant data from multiple studies. We hypothesized that higher protein intake (especially ≥1 g/kg aBW/d), alone or in combination with PA, is protective against physical function loss. We therefore aimed to: 1) investigate the prospective relation between protein intake and physical function (using objective and subjective measures); and 2) explore the interaction between protein intake and PA in relation to physical function in 4 longitudinal aging cohorts in the PROMISS (PRevention Of Malnutrition In Senior Subjects in the European Union) consortium.
Methods
Cohorts included
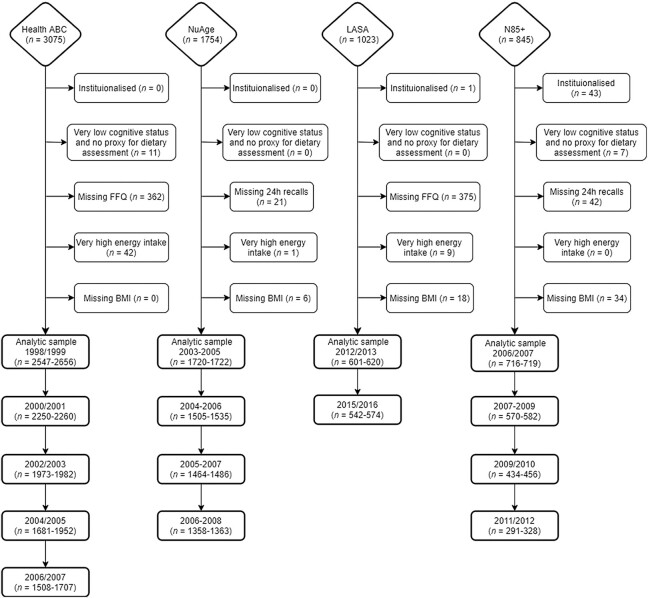
As part of the PROMISS consortium, 4 longitudinal prospective observational studies were included: 1) the Health, Aging and Body Composition Study (Health ABC); 2) the Quebec Longitudinal Study on Nutrition and Successful Aging (NuAge); 3) the Longitudinal Aging Study Amsterdam (LASA); and 4) the Newcastle 85+ Study (Figure 1). These studies are described in detail elsewhere (24–27). Briefly, Health ABC is a longitudinal cohort study that included 3075 well-functioning community-dwelling black and white males and females aged 70–79 y living in the United States at baseline. Participants were recruited from Medicare-eligible residents in the metropolitan areas of Memphis, Tennessee, and Pittsburgh, Pennsylvania, between April 1997 and June 1998 and followed annually (clinic visit) or every 6 mo (telephone interview) for 16 y (24). We used year 2 (baseline) and follow-up data until year 10. NuAge is a longitudinal cohort that recruited 1793 generally healthy community-dwelling males and females aged 67–84 y living in Montreal and Sherbrooke areas (Quebec, Canada) in 2003–2005 and followed them annually (clinic visit) or every 6 mo (telephone interview) for 3 y (27). We used data from baseline and all 3 follow-up waves for all participants who agreed to be part of the NuAge Database and Biobank (n = 1754 at baseline). LASA is an ongoing nationally representative longitudinal study of older males and females aged ≥55 y residing in The Netherlands. The study started in 1992/93 (n = 3107) and participants were followed every 3 y until 2018/2019 (most recent wave: wave J). Two additional cohorts were recruited from the same sampling frames at 10 y (2002/2003, n = 1002) and 20 y (2012/2013, n = 1023) after the baseline (25). We used data from wave 3B (2012/2013), the Nutrition and Food-related Behaviour substudy (2014/2015) and wave I (2015/2016). The Newcastle 85+ Study is a longitudinal population-based study that approached all people turning 85 y in 2006/2007 (born in 1921) in Newcastle and North Tyneside, United Kingdom. At baseline, there were 845 very old males and females who agreed to a health assessment and a review of their GP records (26), and who were re-examined after 18, 36, and 60 mo. We used data from baseline and all 3 follow-up waves.
FIGURE 1.
Flowchart of the 4 longitudinal aging cohorts included and the exclusion criteria for the analytic sample. Very low cognitive status was defined by a Mini-Mental State Examination score <18 or having dementia. Very high energy intakes were defined as >3500 kcal/d for females or >4000 kcal/d for males. The final sample depends on the availability of walking speed, self-reported ability to walk >200 m, or ability to climb stairs (hence a range of n is given). Health ABC, Health, Aging and Body Composition Study; LASA, Longitudinal Aging Study Amsterdam; N85+, Newcastle 85+ Study; NuAge, Quebec Longitudinal Study on Nutrition and Successful Aging.
Exclusion criteria
We excluded participants (Figure 1) who were institutionalized (n = 44), had very poor cognitive status (score <18 in the Mini-Mental State Examination or with diagnosed dementia) and no proxy for dietary assessment (n = 18), had missing dietary intake data (n = 800), had very high reported energy intake, that is, >3500 kcal/d for females or >4000 kcal/d for males (n = 52), or had no data on BMI (n = 58). The analytic sample comprised 5658–5725 (depending on the outcome, i.e., walking speed, difficulty walking >200 m, or difficulty climbing stairs) community-dwelling participants aged ≥55 y.
Missing and nonmissing data on walking speed and mobility limitation
Older adults who were smokers, had lower energy intake, low PA, and had more mobility limitation had more missing data on walking speed. Those with low education, poor cognitive status, non–alcohol drinkers, low PA, and slower walking speed had more missing data on mobility limitation (Supplemental Table 1).
Dietary assessment
Dietary intake was assessed by an FFQ in Health ABC and LASA, and by multiple 24-h recalls in NuAge and Newcastle 85+. In Health ABC, a 108-item interviewer-administered FFQ (28) that reflected the preceding 12 mo was used at the first follow-up (year 2; 1998/1999) and served as the baseline for the present study. In LASA, a validated (29) self-administered 238-item FFQ (30) that reflected the preceding 4 wk was used. In LASA, dietary intake was not assessed at a regular measurement cycle, but in the Nutrition and Food-related Behavior Study 2014–2015 substudy. In NuAge, 3 recalls per wave were collected by trained registered dietitians on 2 randomly chosen weekdays and 1 weekend day, 1 face-to face and 2 by telephone (27). Portion sizes were estimated with the aid of portion size models. In the Newcastle 85+ Study, two 24-h recalls were collected by trained research nurses on 2 nonconsecutive weekdays ≥1 wk apart (31, 32) and portion sizes were estimated with the aid of a photographic food atlas. In all studies, energy and protein intake were calculated by using country-specific food composition databases. In studies that assessed dietary intake using 24-h recall, individual intakes of protein and energy were averaged within the 2 (Newcastle 85+) or 1 or 3 (NuAge) measurements. Dietary intake was available at baseline for Health ABC, LASA, and Newcastle 85+, and at baseline and waves 2, 3, and 4 for NuAge.
For participants with an undesirable body weight (BW), BW was adjusted to be within the desired BMI for older adults of 22–27 kg/m2 and calculated as previously described (33, 34). By this, we attempted to control for the deficit and excess in BW in underweight and overweight people, respectively. All variables are described in Supplemental Table 2. Protein intake was expressed as a categorical variable using cut points of <0.8, 0.8–0.99, 1.0–1.19, and ≥1.2 g/kg BW/d and g/kg aBW/d. These cutoffs were based on expert recommendations for optimal protein intake (18, 20), on currently used RDAs for protein [e.g., 0.8 is recommended by the European Food Safety Authority (EFSA) (35) and the Institute of Medicine (IoM) (36), 1.0 by the European DACH (Germany, Austria, and Switzerland) countries (37), and 1.2 by the European Nordic countries (38)], and on previously published studies on protein intake in older adults (9, 24, 34, 39–43).
Physical activity
In Health ABC, total PA was measured (at baseline) by a specifically designed self-reported questionnaire as described previously (44). Participants indicated whether they had performed exercise (part of daily routine or leisure-time) in the past 7 d and how long they spent in each activity. These included gardening, heavy chores, light housework, grocery shopping, laundry, climbing stairs, walking, and moderate- and high-intensity exercise. A metabolic equivalent (MET) value in kilocalories per week per kilogram BW was determined for each activity. A total PA score was calculated as the sum of the MET values for each activity multiplied by the participant's BW in kilograms. In NuAge, PA was measured using the self-reported Physical Activity Scale for the Elderly (45). This validated questionnaire included work-related, household, and leisure time activities during a 1-wk period. The total PA score is derived from multiplying the amount of time spent on each activity (in hours per week) by the item weights and summing over all activities (46). PA in LASA was measured with a validated interviewer-administered questionnaire that estimates the frequency, duration, and intensity of specific activities in the previous 2 wk (47). These included walking, cycling, light and heavy household work, and first and second sport. MET scores were assigned to each activity based on published lists of MET scores (48). For each activity, the frequency, duration in minutes, and MET score were multiplied and then divided by 14 d. The minutes spent per activity per day were summed to a total PA score (minutes/day × MET). In Newcastle 85+, a validated purposely designed physical activity questionnaire included questions on how frequently [≥3 times/wk (score of 3), 1–2 times/wk (score of 2), 1–3 times/mo (score of 1), and hardly ever (score of 0)] the participants engaged in mildly energetic (e.g., light gardening, light housework), moderately energetic (e.g., moderate gardening, walking at moderate pace, heavy housework), and highly energetic (e.g., heavy gardening, swimming, cycling) activities. The resulting total PA score was calculated as 3 × highly energetic activities + 2 × moderately energetic activities + mildly energetic activities (49). PA was transformed into cohort-specific tertiles (categorized as low, medium, and high) at baseline, which were used to categorize the PA score for subsequent waves.
Walking speed
Walking speed was used as an objective (performance-based) measure of physical function. Walking speed (meters per second) was measured as the time (seconds) taken to walk a preset distance—20 m in Health ABC, 4 m in NuAge, and 3 m in LASA—at usual speed and calculated as distance (meters) divided by time (seconds). In Newcastle 85+, Timed-Up-and-Go (TUG) was measured instead of walking speed. To harmonize Newcastle 85+ with the other cohorts, the formula [6/TUG (s)]×1.62 was used to yield walking speed (m/s), as recommended (50).
Mobility limitation
Self-reported difficulty walking >200 m and climbing stairs were used as subjective measures of physical function and are here defined under the broad heading of mobility limitations. In Health ABC, difficulty walking was determined by asking the participants if they had any difficulty walking a quarter mile (400 m) because of a health or physical problem; difficulty in climbing stairs was determined by asking the participants if they were able to climb 1 flight of stairs (∼10 steps). In NuAge, participants were asked if their health status prevented them from walking >200 m and if they could go up and down a staircase (∼10 steps). In LASA, participants were asked if they had any difficulty walking for 5 min outside the house without stopping (equivalent to 240 m if walking speed was 0.8 m/s), and if they could walk up and down a staircase of 15 steps without resting. In Newcastle 85+, participants were asked if they had any difficulty walking ≥400 yards (366 m) and if they could go up and down stairs. Difficulty walking was harmonized by creating a binary variable: able to walk >200 m without difficulty, or not (with some difficulty, with help, or unable). Difficulty climbing stairs was harmonized as being able to climb stairs without difficulty or not (with some difficulty, with help, or unable).
Mortality
In Health ABC, deaths were confirmed by death certificate and review of hospital records, obituaries, and interviews with family members. Time to death was calculated as the time between age at baseline (1998/1999) and age of death or September 30, 2014, whichever came first. In NuAge, deaths were recorded during data collection. Age at baseline (2003–2005) and deaths (censored at May 3, 2010) were used to calculate the time to death. LASA was linked with mortality data of Statistics Netherlands (Centraal Bureau voor de Statistiek). Time to death was calculated as the time from baseline age (2012/2013) to death or July 22, 2018, whichever came first. In Newcastle 85+, information on date of death was provided by National Health Service Digital UK, and time to death was calculated as the time between baseline age (2006–2007) and age at death (censored at January 16, 2018).
The methods used to assess education, BMI, smoking, alcohol drinking, multimorbidity, and cognitive status in each cohort are described in the Supplemental Methods. All variables used and their operationalization are described in Supplemental Table 2.
Ethics approval and consent to participate
Health ABC was approved by the institutional review boards of the University of Tennessee, Memphis, Tennessee, and the University of Pittsburgh, Pittsburgh, Pennsylvania. LASA was approved by the medical ethics committee of the VU University Medical Center. The Newcastle 85+ Study was approved by the Newcastle & North Tyneside Local Research Ethics Committee 1. Signed consent was obtained from each participant, and a signed consultee approval was obtained whenever the patient lacked capacity. NuAge was approved by the ethics committees of both the University Institutes of Geriatrics of Sherbrooke and Montréal and the Research Ethics Board of the McGill University Health Centre. All studies were conducted in line with the Declaration of Helsinki, and all participants provided written informed consent and/or a signed consultee approval was obtained whenever the participant lacked capacity.
Statistical analyses
Data cleaning, quality control, and harmonizing were performed separately for each cohort prior to merging. Because the methods to assess energy intake and walking speed were different between cohorts, these were transformed into cohort-specific z-scores [z = (x − µ)/σ)]. For walking speed, the baseline mean and SD was used to create z-scores for the other waves. Normality was assessed by Q-Q plots: normally distributed variables are presented as means and SDs, non-Gaussian distributed variables as medians and IQRs, and categorical data as percentages (with corresponding frequency). To determine the relation between protein intake and walking speed we performed hierarchical linear mixed effects models with the lme4 package (version 1.1-20) (51) and Cox proportional hazards for time-to-event data (mortality) with the survival package (version 2.43-3) (52). Data missing at random were accounted for in the linear mixed models. However, as in any longitudinal cohort of aging, attrition was high (53) and failure to account for mortality (nonrandom attrition) would likely result in biased estimates toward the null (54). These outcomes (walking speed and mortality) are typically analyzed separately, but joint models analyze the 2 outcomes together with shared parameters in a single likelihood function (maximum likelihood estimation). We therefore fitted joint models with the JoineRmeta package (version 0.1.2) (55) in R v3.6.3. To determine the association between protein intake and transitions in mobility limitation (i.e., difficulty walking >200 m and difficulty climbing stairs) we fitted multistate models with 3 states: no limitation, limitation, and death (absorbing state); the illness-death model with the allowed transitions is shown in Supplemental Figure 1. Multistate models describe the movement of an individual between a number of finite states in a continuous time stochastic process under the Markov assumption that the next state is only influenced by the current state (56, 57). Multistate models were fitted with the msm package in R v3.2.2 (58). For walking speed and mobility limitation (i.e., difficulty walking and difficulty climbing stairs), we fitted 3 models with increasing complexity with protein intake <0.8, 0.8–0.99, 1.0–1.19, and ≥1.2 g/kg BW/d (actual BW) and g/kg aBW/d (adjusted BW) as the primary exposure. Separate models for walking speed and mobility limitation were also stratified by PA level. Model l included protein intake, study (random effect in the joint models), age, sex, and education; Model 2 was adjusted for the previous variables plus energy intake, smoking, and alcohol drinking; Model 3 was further adjusted for multimorbidity, cognitive status, and PA (except if the model was stratified by PA level). Point estimates and CIs were used to assess statistical and clinical significance. Results are presented as βs and 95% CIs for the joint models, and as HRs and 95% CIs for the multistate models.
Results
Health and sociodemographic characteristics and functional status
The analytic sample consisted of 5725 participants with a median age of 75.0 y (IQR: 71.6–79.0 y) at baseline, 53% of which (n = 3035) were females (Table 1). Most participants were from the Health ABC study (46.5%), followed by NuAge (30.1%), Newcastle 85+ (12.6%), and LASA (10.8%) at baseline (Table 1, Figure 1). At baseline, 28%, 23%, 21%, and 28% of the participants had protein intake <0.8, 0.8–0.99, 1.0–1.19, and ≥1.2 g/kg aBW/d, respectively (Table 1). The protein intake groups were of similar age (age was “statistically” different but this difference was not meaningful), sex ratio, education, multimorbidity, cognition, and smoking status, but differed in alcohol consumption and energy intake (higher protein intake category included a higher proportion of alcohol drinkers and higher energy intake) and in PA (e.g., 29.6% of those with protein intake ≥1.2 g/kg aBW/d had a low level of PA, whereas this was 36.7% in those with protein intake <0.8 g/kg aBW/d). Participants with higher protein intake had slightly faster walking speed (z-score) and were less likely to have mobility limitation (Table 1). Maximum follow-up time was 8.5 y (mean: 2.5 ± 2.4 y). Walking speed decreased modestly with age (from 1.06 ± 0.28 m/s at baseline to 1.01 ± 0.25 m/s at wave 4, and 1.03 ± 0.23 m/s at wave 5), and the proportion of participants with mobility limitation increased with age [e.g., 18% (n = 1031) of participants had difficulty walking >200 m at baseline, and this increased to 30.2% (n = 1092) at wave 4 and 35.4% (n = 605) at wave 5] (Supplemental Figure 2, Table 2). Health and sociodemographic characteristics by protein intake categories and cohort or PA are shown in Supplemental Tables 3 and 4.
TABLE 1.
Baseline health and sociodemographic characteristics of participants by protein intake category1
| Protein intake, g/kg aBW/d | |||||||
|---|---|---|---|---|---|---|---|
| All (n = 5725) | Missing, % | <0.8 (n = 1579) | 0.8–0.99 (n = 1335) | 1.0–1.19 (n = 1218) | ≥1.2 (n = 1593) | P | |
| Age, y, median (IQR) | 75.0 (71.6–79.0) | 0.0 | 75.0 (72.0–79.0) | 75.0 (72.0–79.2) | 75.0 (71.0–79.0) | 74.0 (70.8–79.0) | <0.001 |
| Females, % (n) | 53.0 (3035) | 0.0 | 53.8 (849) | 53.7 (717) | 53.5 (652) | 51.3 (817) | 0.448 |
| Cohort, % (n) | 0.0 | <0.001 | |||||
| Health ABC | 46.5 (2660) | 66.1 (1044) | 44.8 (598) | 37.6 (458) | 35.2 (560) | ||
| NuAge | 30.1 (1726) | 16.2 (256) | 32.5 (434) | 36.9 (450) | 36.8 (586) | ||
| LASA | 10.8 (620) | 5.0 (79) | 8.1 (108) | 13.7 (167) | 16.7 (266) | ||
| N85+ | 12.6 (719) | 12.7 (200) | 14.6 (195) | 11.7 (143) | 11.4 (181) | ||
| Education, % (n) | 0.1 | 0.071 | |||||
| Low | 31.9 (1822) | 31.1 (490) | 33.2 (443) | 30.1 (366) | 32.8 (523) | ||
| Medium | 37.7 (2156) | 36.8 (580) | 36.4 (486) | 37.8 (460) | 39.5 (630) | ||
| High | 30.4 (1741) | 32.1 (505) | 30.4 (406) | 32.1 (390) | 27.6 (440) | ||
| Multimorbidity, % (n) | 51.0 (2784) | 4.6 | 49.6 (763) | 52.6 (663) | 53.0 (605) | 49.6 (753) | 0.144 |
| Cognition, % (n) | 2.9 | 0.025 | |||||
| Low | 29.9 (1660) | 31.3 (472) | 29.8 (388) | 29.9 (357) | 28.5 (443) | ||
| Medium | 41.7 (2319) | 39.4 (594) | 40.9 (532) | 40.6 (484) | 45.5 (709) | ||
| High | 28.4 (1579) | 29.4 (443) | 29.2 (380) | 29.4 (351) | 26.0 (405) | ||
| Smokers, % (n) | 8.5 (488) | 0.3 | 8.1 (127) | 8.4 (112) | 8.6 (105) | 9.1 (144) | 0.784 |
| Alcohol drinkers, % (n) | 44.5 (2545) | 0.0 | 37.7 (596) | 42.6 (569) | 48.4 (589) | 49.7 (791) | <0.001 |
| Energy intake, z-score (mean ± SD) | −0.0 ± 1.0 | 0.0 | −0.8 ± 0.7 | −0.2 ± 0.7 | 0.2 ± 0.8 | 0.8 ± 0.9 | <0.001 |
| Physical activity, % (n) | 0.1 | <0.001 | |||||
| Low | 32.3 (1849) | 36.7 (580) | 30.0 (400) | 32.6 (397) | 29.6 (472) | ||
| Medium | 33.8 (1935) | 34.1 (539) | 35.5 (473) | 34.7 (422) | 31.5 (501) | ||
| High | 33.9 (1937) | 29.1 (460) | 34.5 (460) | 32.7 (398) | 38.9 (619) | ||
| Walking speed, z-score (mean ± SD) | −0.0 ± 1.0 | 0.9 | −0.1 ± 1.0 | 0.0 ± 1.0 | 0.0 ± 1.0 | 0.1 ± 1.0 | 0.005 |
| Mobility limitations, % (n) | |||||||
| Difficulty walking >200 m | 18.0 (1031) | 0.0 | 22.2 (351) | 17.8 (238) | 16.3 (198) | 15.3 (244) | <0.001 |
| Difficulty climbing stairs | 21.3 (1208) | 0.9 | 25.0 (390) | 21.6 (286) | 19.1 (231) | 19.1 (301) | <0.001 |
Cognition was assessed with the Mini-Mental State Examination. Smokers and alcohol drinkers represent current consumers. z-scores and tertiles are cohort-specific. Nondifference between protein intake categories was assessed with χ2 test for categorical variables and ANOVA/Kruskal–Wallis for continuous variables along with the effect size and SD or 95% CI. aBW, adjusted body weight; Health ABC, Health, Aging and Body Composition Study; LASA, Longitudinal Aging Study Amsterdam; N85+, Newcastle 85 + Study; NuAge, Quebec Longitudinal Study on Nutrition and Successful Aging.
TABLE 2.
Sociodemographic characteristics and functional outcomes by wave of follow-up1
| Baseline (n = 5725) | Wave 2 (n = 5337) | Wave 3 (n = 4419) | Wave 4 (n = 3980) | Wave 5 (n = 1865) | |
|---|---|---|---|---|---|
| Age, y, median (IQR) | 75.0 (71.6–79.0) | 76.0 (73.0–80.0) | 78.4 (76.0–82.0) | 80.0 (77.2–83.3) | 82.0 (80.0–84.0) |
| Females, % (n) | 53.0 (3035) | 53.5 (2856) | 54.3 (2398) | 54.6 (2172) | 54.9 (1023) |
| Cohort, % (n) | |||||
| Health ABC | 46.5 (2660) | 46.6 (2487) | 53.2 (2353) | 52.4 (2085) | 100.0 (1865) |
| NuAge | 30.1 (1726) | 31.2 (1666) | 36.4 (1610) | 39.4 (1567) | 0.0 (0) |
| LASA | 10.8 (620) | 11.3 (602) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| N85+ | 12.6 (719) | 10.9 (582) | 10.3 (456) | 8.2 (328) | 0.0 (0) |
| Education, % (n) | |||||
| Low | 31.9 (1822) | 31.0 (1651) | 30.9 (1366) | 30.4 (1208) | 20.5 (382) |
| Medium | 37.7 (2156) | 38.0 (2025) | 35.1 (1551) | 35.2 (1400) | 32.4 (603) |
| High | 30.4 (1741) | 31.0 (1655) | 33.9 (1499) | 34.4 (1368) | 47.1 (878) |
| Multimorbidity, % (n) | 51.0 (2784) | 55.7 (2856) | 56.1 (2396) | 57.7 (2147) | 58.9 (1099) |
| Cognition, % (n) | |||||
| Low | 29.9 (1660) | 31.4 (1475) | 30.8 (1212) | 36.6 (1201) | 37.1 (538) |
| Medium | 41.7 (2319) | 38.7 (1817) | 38.0 (1498) | 39.5 (1298) | 31.9 (462) |
| High | 28.4 (1579) | 29.8 (1399) | 31.2 (1230) | 23.9 (786) | 31.0 (449) |
| Smokers, % (n) | 8.5 (488) | 7.7 (396) | 6.6 (282) | 6.0 (224) | 7.0 (130) |
| Alcohol drinkers, % (n) | 44.5 (2545) | 45.3 (2359) | 40.6 (1738) | 41.7 (1574) | 37.8 (705) |
| Energy intake, z-score (mean ± SD) | −0.0 ± 1.0 | −0.0 ± 1.0 | −0.0 ± 1.0 | −0.0 ± 1.0 | −0.0 ± 1.0 |
| Protein intake, g/kg BW/d, % (n) | |||||
| <0.8 | 33.6 (1924) | 34.0 (1752) | 36.8 (1566) | 36.7 (1373) | 45.5 (848) |
| 0.8–0.99 | 23.3 (1332) | 23.2 (1198) | 22.9 (976) | 22.4 (838) | 21.2 (395) |
| 1.0–1.19 | 19.3 (1107) | 19.0 (981) | 18.2 (773) | 18.5 (691) | 15.7 (292) |
| ≥1.2 | 23.8 (1362) | 23.8 (1227) | 22.1 (942) | 22.5 (842) | 17.7 (330) |
| Protein intake, g/kg aBW/d, % (n) | |||||
| <0.8 | 27.6 (1579) | 28.1 (1427) | 30.4 (1293) | 30.0 (1117) | 39.4 (734) |
| 0.8–0.99 | 23.3 (1335) | 23.1 (1173) | 24.5 (1043) | 23.7 (881) | 22.6 (421) |
| 1.0–1.19 | 21.3 (1218) | 20.8 (1056) | 19.7 (836) | 20.9 (777) | 17.4 (324) |
| ≥1.2 | 27.8 (1593) | 27.9 (1415) | 25.4 (1078) | 25.5 (948) | 20.7 (386) |
| Physical activity, % (n) | |||||
| Low | 32.3 (1849) | 37.9 (1933) | 44.8 (1848) | 43.7 (1541) | 51.0 (870) |
| Medium | 33.8 (1935) | 39.7 (2025) | 31.2 (1287) | 32.0 (1127) | 30.1 (514) |
| High | 33.9 (1937) | 22.5 (1146) | 24.1 (993) | 24.3 (856) | 18.9 (322) |
| Walking speed, m/s (mean ± SD) | 1.06 ± 0.28 | 1.06 ± 0.29 | 1.03 ± 0.27 | 1.01 ± 0.25 | 1.03 ± 0.23 |
| Walking speed, z-score (mean ± SD) | −0.00 ± 1.00 | −0.02 ± 1.00 | −0.14 ± 1.05 | −0.28 ± 1.06 | −0.53 ± 1.08 |
| Mobility limitation, % (n) | |||||
| Difficulty walking >200 m | 18.0 (1031) | 21.2 (1048) | 25.2 (981) | 30.2 (1092) | 35.4 (605) |
| Difficulty climbing stairs | 21.3 (1208) | 24.2 (1233) | 30.0 (1246) | 31.5 (1129) | 25.5 (414) |
Not all waves are at the same time of follow-up among cohorts. In Health ABC the necessary variables were available at year 2 (operationalized as baseline), 4 (wave 2), 6 (wave 3), 8 (wave 4), and 10 (wave 5). In NuAge data were available at year 1 (baseline), 2 (wave 2), 3 (wave 3), and 4 (wave 4). In LASA variables were available at wave 3B (baseline) and at wave I after 3 y (wave 2). The Newcastle 85+ has data at baseline, after 18 mo (wave 2), after 36 mo (wave 3), and after 60 mo (wave 4). Cognition was assessed with the Mini-Mental State Examination. Smokers and alcohol drinkers represent current consumers. z-scores and tertiles are cohort-specific. aBW, adjusted body weight; BW, body weight; Health ABC, Health, Aging and Body Composition Study; LASA, Longitudinal Aging Study Amsterdam; N85+, Newcastle 85+ Study; NuAge, Quebec Longitudinal Study on Nutrition and Successful Aging.
Protein intake and walking speed
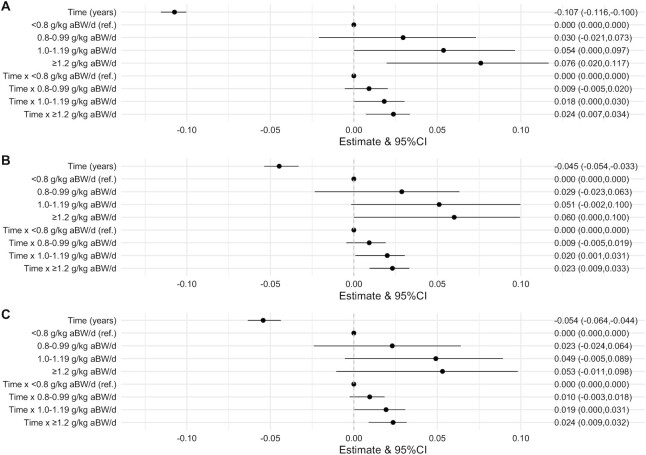
In the model adjusted for sex, age, and education, walking speed (z-score) decreased on average by 0.107 SD (95% CI: −0.116, −0.100) per year. In the same model, higher protein intake was modestly associated with faster walking speed and less decline in walking speed over time (Figure 2A). For example, a protein intake ≥1.2 g/kg aBW/d was associated with 0.076 SD (95% CI: 0.020, 0.117) faster walking speed and 0.024 SD (95% CI: 0.007, 0.034) per year slower decline in walking speed compared with a protein intake <0.8 g/kg aBW/d. There was also evidence of a dose-dependent association between protein intake category and decline in walking speed. The same trends remained with further adjustment for confounding factors (e.g., energy intake and multimorbidity) (Figure 2B, C). For example, in the fully adjusted model, a protein intake ≥1.2 compared with <0.8 g/kg aBW/d was associated with 0.053 SD (95% CI: −0.011, 0.098) faster walking speed and 0.024 SD (95% CI: 0.009, 0.032) per year slower decline in walking speed (Figure 2C). Results were similar for protein g/kg actual BW/d (Supplemental Table 5).
FIGURE 2.
Association between protein intake categories (g/kg aBW/d) and walking speed (z-score) over time (β coefficients and 95% CIs). The analytic sample consisted of 5725 participants. A joint model (hierarchical linear mixed effects and Cox proportional hazards models) was fitted to assess the association between protein intake and walking speed over time. Model 1 (A) is adjusted for categories of adjusted protein intake, sex, age, and education. Model 2 (B) is further adjusted for energy, smoking, and alcohol intake, and Model 3 (C) is further adjusted for cognition, multimorbidity, and physical activity. aBW, adjusted body weight.
Protein intake and mobility limitation
There were 1478 incident cases (i.e., transition from no limitation to mobility limitation) of difficulty walking >200 m and 742 who recovered (Table 3). There were also 1612 incident cases of difficulty climbing stairs and 932 who recovered (Table 4) from one wave to another (mean: 1.70 ± 0.55 y between each wave). In the fully adjusted models, higher protein intake was associated with a lower incidence of mobility limitation (for both difficulty walking >200 m and difficulty climbing stairs). Participants with a protein intake of 0.8–0.99 g/kg aBW/d were 16% less likely to develop difficulty walking (HR: 0.84; 95% CI: 0.72, 0.99) (Table 3) and 22% less likely to develop difficulty climbing stairs (HR: 0.78; 95% CI: 0.67, 0.92) (Table 4) compared with participants with protein intake <0.8 g/kg aBW/d. This protective association was also observed for a protein intake of 1.0–1.19 g/kg aBW/d (difficulty walking, HR: 0.71; 95% CI: 0.59, 0.86; difficulty climbing stairs, HR: 0.76; 95% CI: 0.63, 0.91), and of ≥1.2 g/kg aBW/d (difficulty walking, HR: 0.69; 95% CI: 0.56, 0.84; difficulty climbing stairs, HR: 0.76; 95% CI: 0.62, 0.92) compared with <0.8 g/kg aBW/d. Higher protein intake, even in participants with a protein intake ≥0.8 g/kg aBW/d, seemed to be beneficial for lower incidence of difficulty walking (Table 3), but not for difficulty climbing stairs, where all protein intake categories >0.8 cutoff had similar HRs (Table 4). No associations between protein intake and other transitions of mobility limitation were observed. Results were similar for protein in g/kg actual BW/d (Supplemental Tables 6 and 7).
TABLE 3.
HRs and 95% CIs for the contribution of protein intake categories to transitions in self-reported difficulty walking1
| Protein intake, g/kg aBW/d | |||||||
|---|---|---|---|---|---|---|---|
| <0.8 (ref.) | 0.8–0.99 | 1.0–1.19 | ≥1.2 | ||||
| HR | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Incident mobility limitation (n = 1478) | |||||||
| Model 1 | 1.0 | 0.90 | 0.79, 1.04 | 0.82 | 0.71, 0.96 | 0.83 | 0.72, 0.95 |
| Model 2 | 1.0 | 0.83 | 0.72, 0.97 | 0.72 | 0.60, 0.85 | 0.68 | 0.56, 0.82 |
| Model 3 | 1.0 | 0.84 | 0.72, 0.99 | 0.71 | 0.59, 0.86 | 0.69 | 0.56, 0.84 |
| No mobility limitation to death (n = 542) | |||||||
| Model 1 | 1.0 | 1.07 | 0.71, 1.59 | 1.24 | 0.84, 1.84 | 1.20 | 0.82, 1.76 |
| Model 2 | 1.0 | 1.02 | 0.68, 1.52 | 1.13 | 0.74, 1.73 | 1.00 | 0.61, 1.64 |
| Model 3 | 1.0 | 1.04 | 0.69, 1.56 | 1.20 | 0.78, 1.85 | 1.08 | 0.66, 1.78 |
| Recovery from mobility limitation (n = 742) | |||||||
| Model 1 | 1.0 | 0.98 | 0.79, 1.20 | 0.98 | 0.79, 1.23 | 1.05 | 0.85, 1.29 |
| Model 2 | 1.0 | 1.03 | 0.82, 1.30 | 1.04 | 0.81, 1.34 | 1.10 | 0.82, 1.48 |
| Model 3 | 1.0 | 1.03 | 0.81, 1.32 | 1.06 | 0.81, 1.39 | 1.09 | 0.80, 1.48 |
| Mobility limitation to death (n = 557) | |||||||
| Model 1 | 1.0 | 0.98 | 0.82, 1.16 | 0.98 | 0.81, 1.19 | 0.98 | 0.81, 1.17 |
| Model 2 | 1.0 | 1.06 | 0.88, 1.27 | 1.10 | 0.89, 1.36 | 1.16 | 0.92, 1.46 |
| Model 3 | 1.0 | 1.10 | 0.91, 1.33 | 1.13 | 0.91, 1.41 | 1.20 | 0.95, 1.52 |
Multistate models were used to determine the association between protein intake and transitions in difficulty walking. Model 1 is adjusted for categories of adjusted protein intake, sex, age, and education. Model 2 is further adjusted for energy intake, smoking, and alcohol intake, and Model 3 is further adjusted for cognition, multimorbidity, and physical activity. aBW, adjusted body weight; ref., referent.
TABLE 4.
HRs and 95% CIs for the contribution of protein intake categories to transitions in self-reported difficulty climbing stairs1
| Protein intake, g/kg aBW/d | |||||||
|---|---|---|---|---|---|---|---|
| <0.8 (ref.) | 0.8–0.99 | 1.0–1.19 | ≥1.2 | ||||
| HR | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Incident mobility limitation (n = 1612) | |||||||
| Model 1 | 1.0 | 0.83 | 0.72, 0.96 | 0.83 | 0.72, 0.97 | 0.82 | 0.71, 0.95 |
| Model 2 | 1.0 | 0.79 | 0.68, 0.92 | 0.74 | 0.62, 0.88 | 0.70 | 0.58, 0.85 |
| Model 3 | 1.0 | 0.78 | 0.67, 0.92 | 0.76 | 0.63, 0.91 | 0.76 | 0.62, 0.92 |
| No mobility limitation to death (n = 608) | |||||||
| Model 1 | 1.0 | 1.18 | 0.84, 1.66 | 1.27 | 0.89, 1.81 | 1.13 | 0.80, 1.61 |
| Model 2 | 1.0 | 1.16 | 0.81, 1.66 | 1.28 | 0.86, 1.91 | 1.07 | 0.67, 1.71 |
| Model 3 | 1.0 | 1.16 | 0.83, 1.61 | 1.23 | 0.85, 1.79 | 1.12 | 0.73, 1.72 |
| Recovery from mobility limitation (n = 932) | |||||||
| Model 1 | 1.0 | 0.88 | 0.73, 1.07 | 0.84 | 0.68, 1.03 | 0.91 | 0.75, 1.10 |
| Model 2 | 1.0 | 0.89 | 0.73, 1.09 | 0.86 | 0.68, 1.08 | 0.93 | 0.71, 1.20 |
| Model 3 | 1.0 | 0.91 | 0.73, 1.13 | 0.92 | 0.72, 1.18 | 1.05 | 0.80, 1.38 |
| Mobility limitation to death (n = 598) | |||||||
| Model 1 | 1.0 | 0.89 | 0.75, 1.06 | 0.96 | 0.79, 1.17 | 0.92 | 0.77, 1.10 |
| Model 2 | 1.0 | 0.98 | 0.81, 1.18 | 1.03 | 0.83, 1.28 | 1.07 | 0.85, 1.34 |
| Model 3 | 1.0 | 1.01 | 0.83, 1.23 | 1.06 | 0.84, 1.33 | 1.12 | 0.88, 1.43 |
Multistate models were used to determine the association between protein intake and transitions in difficulty climbing stairs. Model 1 is adjusted for categories of adjusted protein intake, sex, age, and education. Model 2 is further adjusted for energy intake, smoking, and alcohol intake, and Model 3 is further adjusted for cognition, multimorbidity, and physical activity. aBW, adjusted body weight; ref., referent.
Interaction of protein intake with PA
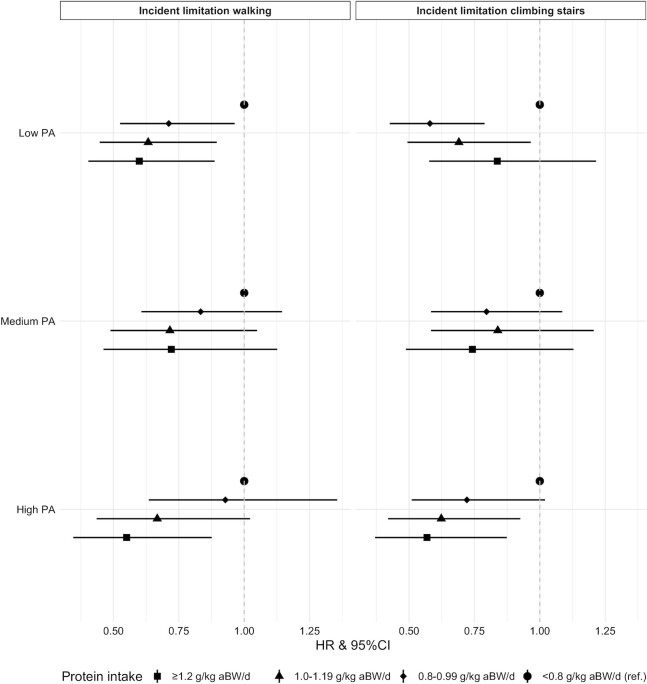
The fully adjusted model for protein intake and walking speed was stratified by low, medium, and high PA (Supplemental Figure 3). Although there was a trend within the same PA category for a slightly slower decline in walking speed for higher protein intake [e.g., participants with ≥1.2 compared with <0.8 g protein/kg aBW/d and medium PA had a slower decline in walking speed of 0.03 SD (95% CI: 0, 0.05)/y], there was no evidence of other associations (Supplemental Figure 3). No interactions between protein intake and PA were observed for any of the transitions between mobility limitation (i.e., difficulty walking and climbing stairs), except for incident mobility limitation. For the latter, we ran the same fully adjusted models by PA category (Figure 3) and observed that higher protein intake was associated with a decreased likelihood of transitioning from no mobility limitation to mobility limitation for both difficulty walking and difficulty climbing stairs, and within low PA and high PA but not within medium PA. These associations seemed to be largely dose-dependent, where participants with higher protein intake had a lower likelihood of incident mobility limitation, even within the same PA category. For example, participants with a protein intake ≥1.2 g/kg aBW/d and high PA were 43% less likely (HR: 0.57; 95% CI: 0.37, 0.87) than those with a protein intake <0.8 g/kg aBW/d and high PA to transition to difficulty climbing stairs, whereas participants with a protein intake of 1.0–1.19 g/kg aBW/d were 38% less likely (HR: 0.62; 95% CI: 0.42, 0.93) and those with a protein intake of 0.8–0.99 g/kg aBW/d were 28% less likely (HR: 0.72; 95% CI: 0.51, 1.02) to transition to difficulty climbing stairs within the high PA category (Figure 3).
FIGURE 3.
HRs and 95% CIs for the contribution of protein intake in grams per kilogram adjusted body weight per day to incident mobility limitations (i.e., difficulty walking >200 m and climbing stairs) by physical activity category over time. The analytic sample for the incident limitation climbing stairs model consisted of 1612 transitions, and for the incident limitation walking model consisted of 1478 transitions. Multistate models were used to determine the association between protein intake and transitions to mobility limitation stratified by physical activity. The models are adjusted for categories of adjusted protein intake, sex, age, and education, energy intake, smoking, alcohol intake, cognition, and multimorbidity and stratified by PA category at baseline. aBW, adjusted body weight; PA, physical activity.
Discussion
To the best of our knowledge, we are the first to perform a pooled analysis of individual participant data from multiple longitudinal aging cohorts on the prospective association between protein intake and (objective and subjective) physical function, and its interaction with PA. Our study indicated that community-dwelling older adults with protein intake higher than the EFSA's and IoM's RDA of 0.8 g/kg BW/d were more likely to retain objective and perceived physical function over time. Those with protein intake ≥0.8 g/kg aBW/d had (moderate) slower decline in walking speed and were less likely to develop self-reported mobility limitation, with some evidence for dose-dependency. Our study also indicated that a protein intake ≥1.0 g/kg aBW/d was associated with less functional decline compared with an intake of 0.8–0.99 g/kg aBW/d. We observed no synergistic relation between protein intake and PA for maintaining physical function over time.
Our finding that higher protein intake can be associated with a modest decline in walking speed over a follow-up of maximum 8.5 y is in contrast to the individual studies pooled (9, 13, 15), and to other aging cohorts (12, 14), except one (11). This could be explained by the larger sample size in our study, providing more power to detect this modest association, especially because the decline in walking speed was small (prior to z-score transformation it declined by 0.05 m/s over the study period), which could be insufficient to see a more clinically relevant association with higher protein intake. Our study mostly comprised well-functioning older adults at baseline, among whom the rate of functional decline might be slower than those who are frailer and/or have more comorbidities (59). Two recent meta-analyses of RCTs of protein supplementation and physical performance, in nonfrail and frail/ill older adults (22, 60), support this. That in nonfrail older adults failed to show an effect of protein supplementation on walking speed (22), whereas that in frail and ill older adults showed that protein supplementation improved physical function (60).
Regarding protein intake and mobility limitations, our finding that older adults who consumed ≥0.8 g protein/kg aBW/d were less likely to develop mobility limitations, compared with those with a protein intake <0.8 g/kg aBW/d, was in line with previous studies in individual cohorts used in this study (10, 61) and in other aging cohorts (12, 62–64). We observed no associations between protein intake and other mobility limitation transitions, that is, from no mobility limitation to death, recovery from mobility limitation, or from mobility limitation to death. These findings suggest that higher protein intake is especially beneficial in the prevention of mobility limitation rather than reversing it, because it might be too late when mobility limitation develops, and emphasize the importance of early screening for lower protein intake and early (dietary) intervention.
The debate about whether the RDA for protein for older adults, currently 0.8 g/kg BW/d according to the EFSA (35) and IoM (36), should be increased, and to what extent, is still ongoing. Our results tend to show that the relation between protein intake and decline in walking speed is dose-dependent. There was also a small reduction in the likelihood of incident difficulty walking with higher protein intake categories (0.8–0.99, 1.0–1.19, and ≥1.2 g/kg aBW/d) suggesting, as for walking speed, a dose-dependent association, although this was not observed for difficulty climbing stairs. These findings suggest that higher protein intake might benefit walking function not only in older adults with a protein intake below the RDA, but also in those with a habitual protein intake of ∼1.0 g/kg BW/d [mean protein intake in community-dwelling European and North American older adults (17)]. Our findings are in line with results from the study by Beasley et al. (12), in which the rate of self-reported physical function decline was 52% lower in females with a mean protein intake of 1.19 ± 0.20 g/kg BW/d than in females with a protein intake of 0.97 ± 0.17 g/kg BW/d. Together, these results suggest that even a protein intake >1.2 g/kg BW/d might be better in terms of slowing the rate of physical function decline. However, as such a (high) protein recommendation could have many substantial implications (e.g., for >70% of community-dwelling older adults, health care, food industry, and environment), such recommendations should only be set when sufficient high-quality evidence is available. More observational studies and RCTs in well-characterized study samples with adequate follow-up are needed to define the optimal yet feasible protein intake in older adults as well as the optimal food sources for the additional protein.
Several experts have suggested that (higher) PA could have an additive or synergistic effect on muscle and physical function when combined with protein intake (18). We did not find a clear indication for effect modification by PA or a synergistic effect of protein and PA. We observed a trend of higher protein intake categories being more protective for walking speed and walking speed decline within both the low and high PA category, but these did not reach statistical significance. This is probably a consequence of the small decline in walking speed over time. Participants with higher protein intake (even ≥0.8 g/kg aBW/d) were less likely to transition to mobility limitation, within each PA level. This suggests that 1) higher protein intake can protect from the incidence of mobility limitation independently of PA level, and 2) participants with protein intake ≥0.8 g/kg aBW/d still benefit from higher protein intake within the same PA level (i.e., a protein intake ≥1.2 g/kg aBW/d has a greater protective association with incident mobility limitations than 0.8–0.99 g/kg aBW/d). Similar to our findings, 1 systematic review of 15 RCTs in older adults on the additive effect of protein supplementation and progressive resistance exercise for 2–5 times/wk over 7 wk to 1 y found that improvements in physical function were no different with protein supplementation alone or in combination with resistance exercise (23). A later systematic review of 36 RCTs in nonfrail older adults concluded that walking speed tended to improve more with protein supplementation in combination with resistance exercise after 26 ± 26 wk compared with resistance exercise only (22). Differences in the type, intensity, and duration of PA or the timing in relation to protein intake might account for different effects of PA (and its interaction with protein) on physical function.
A major strength of this study is that we harmonized data from 4 large aging cohorts and performed an individual participant pooled analysis, allowing us to significantly increase our sample size and test for interactions that we could not test otherwise. The use of objective (walking speed) and subjective (mobility limitations) measures of physical function, the large range of covariates adjusted for, and the use of joint modeling and multistate models to account for nonrandom attrition and study membership, are other major strengths of this study.
One possibly important limitation in our study is misreporting of dietary intakes. However, because protein-rich foods are less commonly underreported (unlike snacks and sweets) (65) it is unlikely that protein intakes were underestimated. Additionally, although protein intake was harmonized, dietary intake was assessed by FFQs in Health ABC and LASA, and with multiple 24-h recalls in NuAge and Newcastle 85+, 2 methods that might give slightly different estimates. Another limitation is that protein intake was measured at baseline only and, therefore, intakes were assumed to be stable or have declined proportionally over the follow-up period. A fourth limitation is that mobility limitation states were assigned at every data collection point (mean =1.70 ± 0.55 y) between each wave but unobserved incidence and recovery from these mobility limitation states could have occurred between waves. Finally, although attempts were made to reduce the possibility of reverse causality it is impossible to fully exclude the possibility of protein intake being affected by physical function.
In conclusion, increasing daily protein intake can reduce physical function decline over 8.5 y not only in community-dwelling older adults with a protein intake at or below the current RDA of 0.8 g/kg BW/d, but also in those with a protein intake that is already considered sufficient. This dose-dependent association was observed for each level of PA, suggesting no clear synergistic association between protein intake and PA in relation to physical function.
Supplementary Material
Acknowledgements
We thank Jolanda Boer and Hanneke Wijnhoven for commenting on the research proposal, and Valérie Turcot, Jan Poppelaars, and Liset Elstgeest for preparing the datasets. Thanks are also due to everyone in the EpiDoC Unit, especially Ana Rodrigues and Rute de Sousa for their support.
The authors’ responsibilities were as follows—NM: drafted the research proposal; LMH, MV, and CJ: critically reviewed the research proposal; NM: conducted the study, analyzed the data, performed statistical analyses, and wrote the paper alongside LMH. NM and LMH: had primary responsibility for the final content; and all authors: contributed to the interpretation of the findings, and critically reviewed, commented on, read, and approved the final manuscript.
The authors report no conflicts of interest.
Notes
Funding for this research is provided by the European Horizon 2020 PROMISS Project “PRevention Of Malnutrition In Senior Subjects in the EU,” grant agreement no. 678732. NM has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement no. 835577. Health ABC has been supported by the Intramural Research Program of the NIH, National Institute on Aging (NIA) (NIA contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050; National Institute of Nursing Research grant R01-NR012459). The NuAge study was funded by the Canadian Institutes of Health Research (CIHR; MOP-62842). The NuAge Database and Biobank are supported by the Fonds de recherche du Québec (FRQ; 2020-VICO-279753), the Quebec Network for Research on Aging, a thematic network funded by the Fonds de Recherche du Québec—Santé (FRQS), and by the Merck-Frosst Chair funded by La Fondation de l'Université de Sherbrooke. NP is a Junior 1 Research Scholar of the FRQS. The Longitudinal Aging Study Amsterdam is supported by a grant from the Netherlands Ministry of Health, Welfare and Sports, Directorate of Long-Term Care. The Newcastle 85+ Study has been funded by a joint grant from the Medical Research Council and Biotechnology and Biological Sciences Research Council (G0500997), and the Dunhill Medical Trust (R124/0509).
The content reflects the authors’ views only and the European Commission is not responsible for any use made of the information the article contains.
The data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval from individual cohorts, and payment for management costs.
Supplemental Methods, Supplemental Tables 1–7, and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: aBW, adjusted body weight; BW, body weight; Health ABC, the Health Aging and Body Composition Study; EFSA, European Food Safety Authority; HLY, healthy life year; IoM, Institute of Medicine; LASA, Longitudinal Aging Study Amsterdam; MET, metabolic equivalent; Miss, missing; NuAge, Quebec Longitudinal Study on Nutrition and Successful Aging; PA, physical activity; PROMISS, PRevention Of Malnutrition In Senior Subjects in the EU; RCT, randomized controlled trial; TUG, Timed-Up-and-Go.
Contributor Information
Nuno Mendonça, EpiDoC Unit, CEDOC, NOVA Medical School, Universidade Nova de Lisboa (UNL), Lisbon, Portugal; Comprehensive Health Research Centre (CHRC), NOVA Medical School, Lisbon, Portugal; Population Health Sciences Institute, Newcastle University, Newcastle-upon-Tyne, United Kingdom.
Linda M Hengeveld, Department of Health Sciences, Faculty of Science, Amsterdam Public Health Research Institute, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Marjolein Visser, Department of Health Sciences, Faculty of Science, Amsterdam Public Health Research Institute, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Nancy Presse, Research Centre on Aging, CIUSSS de l'Estrie-CHUS, Sherbrooke, Quebec, Canada; Faculty of Medicine and Health Sciences, University of Sherbrooke, Sherbrooke, Quebec, Canada; Centre de recherche de l'Institut universitaire de gériatrie de Montréal, Montréal, Quebec, Canada.
Helena Canhão, EpiDoC Unit, CEDOC, NOVA Medical School, Universidade Nova de Lisboa (UNL), Lisbon, Portugal; Comprehensive Health Research Centre (CHRC), NOVA Medical School, Lisbon, Portugal.
Eleanor M Simonsick, National Institute on Aging Intramural Research Program, Baltimore, MD, USA.
Stephen B Kritchevsky, Sticht Center on Aging, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Anne B Newman, Center for Aging and Population Health, Department of Epidemiology, University of Pittsburgh, PA, USA.
Pierrette Gaudreau, Department of Medicine, University of Montréal, H3T 1J4, Montréal, Quebec, Canada; Research Centre of the Centre hospitalier de l'Université de Montréal (CHUM), H2X 0A9, Montréal, Quebec, Canada.
Carol Jagger, Population Health Sciences Institute, Newcastle University, Newcastle-upon-Tyne, United Kingdom.
References
- 1. European Commission – Eurostat.. Mortality and life expectancy statistics. 2020. [Google Scholar]
- 2. Statistics Canada.. Table 13-10-0114-01 Life expectancy and other elements of the life table, Canada, all provinces except Prince Edward Island. 2020. [Google Scholar]
- 3. CDC – National Center for Health Statistics.. National vital statistics reports. 2020. [Google Scholar]
- 4. Kingston A, Davies K, Collerton J, Robinson L, Duncan R, Bond J, Kirkwood TB, Jagger C. The contribution of diseases to the male-female disability-survival paradox in the very old: results from the Newcastle 85+ study. PLoS One. 2014;9(2):e88016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leenders M, Sluijs I, Ros MM, Boshuizen HC, Siersema PD, Ferrari P, Weikert C, Tjonneland A, Olsen A, Boutron-Ruault MCet al. Fruit and vegetable consumption and mortality: European prospective investigation into cancer and nutrition. Am J Epidemiol. 2013;178(4):590–602. [DOI] [PubMed] [Google Scholar]
- 6. O'Flaherty M, Bandosz P, Critchley J, Capewell S, Guzman-Castillo M, Aspelund T, Bennett K, Kabir K, Björck L, Bruthans Jet al. Exploring potential mortality reductions in 9 European countries by improving diet and lifestyle: a modelling approach. Int J Cardiol. 2016;207:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150–5. [DOI] [PubMed] [Google Scholar]
- 8. McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham offspring cohort. J Gerontol A Biol Sci Med Sci. 2016;71(3):356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Granic A, Mendonça N, Sayer AA, Hill TR, Davies K, Adamson A, Siervo M, Mathers JC, Jagger C. Low protein intake, muscle strength and physical performance in the very old: the Newcastle 85+ study. Clin Nutr. 2018;37(6 Pt A):2260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Houston DK, Tooze JA, Garcia K, Visser M, Rubin S, Harris TB, Newman AB, Kritchevsky SB, Health A. Protein intake and mobility limitation in community-dwelling older adults: the Health ABC Study. J Am Geriatr Soc. 2017;65(8):1705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, Erkkila AT. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br J Nutr. 2016;115(7):1281–91. [DOI] [PubMed] [Google Scholar]
- 12. Beasley JM, Wertheim BC, LaCroix AZ, Prentice RL, Neuhouser ML, Tinker LF, Kritchevsky S, Shikany JM, Eaton C, Chen Zet al. Biomarker-calibrated protein intake and physical function in the Women's Health Initiative. J Am Geriatr Soc. 2013;61(11):1863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farsijani S, Payette H, Morais JA, Shatenstein B, Gaudreau P, Chevalier S. Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3-y physical function decline, in free-living older adults: the Quebec longitudinal study on Nutrition as a Determinant of Successful Aging (NuAge study). Am J Clin Nutr. 2017;106(1):113–24. [DOI] [PubMed] [Google Scholar]
- 14. Chan R, Leung J, Woo J, Kwok T. Associations of dietary protein intake on subsequent decline in muscle mass and physical functions over four years in ambulant older Chinese people. J Nutr Health Aging. 2014;18(2):171–7. [DOI] [PubMed] [Google Scholar]
- 15. Elstgeest LE, Schaap LA, Heymans MW, Hengeveld LM, Naumann E, Houston DK, Kritchevsky SB, Simonsick EM, Newman AB, Farsijani Set al. Sex- and race-specific associations of protein intake with change in muscle mass and physical function in older adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2020;112:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Commission , EU Science Hub, Dietary protein, [Internet]. [cited 2019 Jan 10]. Available from: https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/nutrition/protein. [Google Scholar]
- 17. Hengeveld LM, Boer JMA, Gaudreau P, Harris TB, Heymans MW, Jagger C, Mendonça N, Ocké MC, Presse N, Sette Set al. Prevalence of protein intake below recommended in community-dwelling older adults: a meta-analysis across cohorts from the PROMISS consortium. J Cachexia Sarcopenia Muscle. 2020;11(5):1212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta Det al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–59. [DOI] [PubMed] [Google Scholar]
- 19. Deer RR, Volpi E. Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care. 2015;18(3):248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznaric Z, Nair KSet al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, Reginster JY, Chapurlat R, Chan DC, Bruyere Oet al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28(6):1817–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ten Haaf DSM, Nuijten MAH, Maessen MFH, Horstman AMH, Eijsvogels TMH, Hopman MTE. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;108(5):1043–59. [DOI] [PubMed] [Google Scholar]
- 23. Thomas DK, Quinn MA, Saunders DH, Greig CA. Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: a systematic review. J Am Med Dir Assoc. 2016;17(10):959.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hengeveld LM, Wijnhoven HAH, Olthof MR, Brouwer IA, Harris TB, Kritchevsky SB, Newman AB, Visser M, Health A. Prospective associations of poor diet quality with long-term incidence of protein-energy malnutrition in community-dwelling older adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2018;107(2):155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoogendijk EO, Deeg DJ, Poppelaars J, van der Horst M, Broese van Groenou MI, Comijs HC, Pasman HR, van Schoor NM, Suanet B, Thomese Fet al. The Longitudinal Aging Study Amsterdam: cohort update 2016 and major findings. Eur J Epidemiol. 2016;31(9):927–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collerton J, Davies K, Jagger C, Kingston A, Bond J, Eccles MP, Robinson LA, Martin-Ruiz C, von Zglinicki T, James OFet al. Health and disease in 85 year olds: baseline findings from the Newcastle 85+ cohort study. BMJ. 2009;339:b4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaudreau P, Morais JA, Shatenstein B, Gray-Donald K, Khalil A, Dionne I, Ferland G, Fulop T, Jacques D, Kergoat MJet al. Nutrition as a determinant of successful aging: description of the Quebec longitudinal study Nuage and results from cross-sectional pilot studies. Rejuvenation Res. 2007;10(3):377–86. [DOI] [PubMed] [Google Scholar]
- 28. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–69. [DOI] [PubMed] [Google Scholar]
- 29. Visser M, Elstgeest LEM, Winkens LHH, Brouwer IA, Nicolaou M. Relative validity of the HELIUS food frequency questionnaire for measuring dietary intake in older adult participants of the Longitudinal Aging Study Amsterdam. Nutrients. 2020;12(7):1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elstgeest LEM, Visser M. Nutrition and food-related behaviour study 2014–2015 (side study)[Internet]. Longitudinal Aging Study Amsterdam [cited 2018 Oct 18]. Available from: https://www.lasa-vu.nl/themes/physical/nutrition.html [Google Scholar]
- 31. Mendonça N, Hill TR, Granic A, Mathers JC, Wrieden W, Siervo M, Seal C, Jagger C, Adamson AJ. Micronutrient intake and food sources in the very old: analysis of the Newcastle 85+ Study. Br J Nutr. 2016;116(4):751–61. [DOI] [PubMed] [Google Scholar]
- 32. Mendonca N, Hill TR, Granic A, Davies K, Collerton J, Mathers JC, Siervo M, Wrieden WL, Seal CJ, Kirkwood TBet al. Macronutrient intake and food sources in the very old: analysis of the Newcastle 85+ Study. Br J Nutr. 2016;115(12):2170–80. [DOI] [PubMed] [Google Scholar]
- 33. Berner LA, Becker G, Wise M, Doi J. Characterization of dietary protein among older adults in the United States: amount, animal sources, and meal patterns. J Acad Nutr Diet. 2013;113(6):809–15. [DOI] [PubMed] [Google Scholar]
- 34. Mendonça N, Granic A, Mathers JC, Hill TR, Siervo M, Adamson AJ, Jagger C. Prevalence and determinants of low protein intake in very old adults: insights from the Newcastle 85+ Study. Eur J Nutr. 2018;57(8):2713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. European Food Safety Authority (EFSA).. Dietary reference values for nutrients: summary report. 2017. [Google Scholar]
- 36. Institute of Medicine.. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): The National Academies Press; 2005. [Google Scholar]
- 37. Richter M, Baerlocher K, Bauer JM, Elmadfa I, Heseker H, Leschik-Bonnet E, Stangl G, Volkert D, Stehle P. Revised reference values for the intake of protein. Ann Nutr Metab. 2019;74(3):242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nordic Council of Ministers.. Nordic nutrition recommendations 2012: integrating nutrition and physical activity. Copenhagen (Denmark): Nordisk Ministerråd; 2014. [Google Scholar]
- 39. Mendonca N, Granic A, Hill TR, Siervo M, Mathers JC, Kingston A, Jagger C. Protein intake and disability trajectories in very old adults: the Newcastle 85+ Study. J Am Geriatr Soc. 2019;67(1):50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Granic A, Mendonca N, Sayer AA, Hill TR, Davies K, Siervo M, Mathers JC, Jagger C. Effects of dietary patterns and low protein intake on sarcopenia risk in the very old: the Newcastle 85+ study. Clin Nutr. 2020;39(1):166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hung Y, Wijnhoven HAH, Visser M, Verbeke W. Appetite and protein intake strata of older adults in the European Union: socio-demographic and health characteristics, diet-related and physical activity behaviours. Nutrients. 2019;11(4):777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wijnhoven HAH, Elstgeest LEM, de Vet HCW, Nicolaou M, Snijder MB, Visser M. Development and validation of a short food questionnaire to screen for low protein intake in community-dwelling older adults: the Protein Screener 55+ (Pro55+). PLoS One. 2018;13(5):e0196406–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mendonça N, Kingston A, Granic A, Jagger C. Protein intake and transitions between frailty states and to death in very old adults: the Newcastle 85+ Study. Age Ageing. 2020;49(1):32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Visser M, Simonsick EM, Colbert LH, Brach J, Rubin SM, Kritchevsky SB, Newman AB, Harris TB. Type and intensity of activity and risk of mobility limitation: the mediating role of muscle parameters. J Am Geriatr Soc. 2005;53(5):762–70. [DOI] [PubMed] [Google Scholar]
- 45. Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52(7):643–51. [DOI] [PubMed] [Google Scholar]
- 46. Fiocco AJ, Shatenstein B, Ferland G, Payette H, Belleville S, Kergoat MJ, Morais JA, Greenwood CE. Sodium intake and physical activity impact cognitive maintenance in older adults: the NuAge Study. Neurobiol Aging. 2012;33(4):829.e21–8. [DOI] [PubMed] [Google Scholar]
- 47. Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. Comparison of the LASA physical activity questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57(3):252–8. [DOI] [PubMed] [Google Scholar]
- 48. Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, Sallis JF, Paffenbarger RS Jr. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exercise. 1993;25(1):71–80. [DOI] [PubMed] [Google Scholar]
- 49. Innerd P, Catt M, Collerton J, Davies K. A comparison of physical activity measures from self-report and raw accelerometry in the very old: results from the Newcastle 85+ study. Age Ageing. 2015;44(4):691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cooper R, Bann D, Wloch EG, Adams JE, Kuh D. "Skeletal muscle function deficit" in a nationally representative British birth cohort in early old age. J Gerontol A Biol Sci Med Sci. 2015;70(5):604–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Soft. 2015;67(1):48. [Google Scholar]
- 52. Terry M., Therneau PMG. Modeling survival data: extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 53. Davies K, Kingston A, Robinson L, Hughes J, Hunt JM, Barker SAH, Edwards J, Collerton J, Jagger C, Kirkwood TBL. Improving retention of very old participants in longitudinal research: experiences from the Newcastle 85+ Study. PLoS One. 2014;9(10):e108370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Amelia MH, Bobby LJ, Daniel SN. Group-based trajectory modeling extended to account for nonrandom participant attrition. Sociol Methods Res. 2011;40(2):367–90. [Google Scholar]
- 55. Sudell M, Kolamunnage-Dona R, Tudur SC. joineRmeta: joint modelling for meta-analytic (multi-study) data. R package version 012. R Project; 2020. [Google Scholar]
- 56. Cox DR, Miller HD. The theory of stochastic processes. London (UK): Chapman and Hall; 1965. [Google Scholar]
- 57. Meira-Machado L, de Uña-Álvarez J, Cadarso-Suárez C, Andersen PK. Multi-state models for the analysis of time-to-event data. Stat Methods Med Res. 2009;18(2):195–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jackson C. Multi-state models for panel data: the msm package for R. J Stat Softw. 2011;38(8):28. [Google Scholar]
- 59. Auyeung TW, Lee SWJ, Leung J, Kwok T, Woo J. Age-associated decline of muscle mass, grip strength and gait speed: a 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr Gerontol Int. 2014;14(Suppl 1):76–84. [DOI] [PubMed] [Google Scholar]
- 60. Cheng H, Kong J, Underwood C, Petocz P, Hirani V, Dawson B, O'Leary F. Systematic review and meta-analysis of the effect of protein and amino acid supplements in older adults with acute or chronic conditions. Br J Nutr. 2018;119(5):527–42. [DOI] [PubMed] [Google Scholar]
- 61. Mendonça N, Kingston A, Granic A, Hill TR, Mathers JC, Jagger C. Contribution of protein intake and its interaction with physical activity to transitions between disability states and to death in very old adults: the Newcastle 85+ Study. Eur J Nutr. 2020;59(5):1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hruby A, Sahni S, Bolster D, Jacques PF. Protein intake and functional integrity in aging: the Framingham heart study offspring. J Gerontol A Biol Sci Med Sci. 2020;75(1):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bradlee ML, Mustafa J, Singer MR, Moore LL. High-protein foods and physical activity protect against age-related muscle loss and functional decline. J Gerontol A Biol Sci Med Sci. 2018;73(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Imai E, Tsubota-Utsugi M, Kikuya M, Satoh M, Inoue R, Hosaka M, Metoki H, Fukushima N, Kurimoto A, Hirose Tet al. Animal protein intake is associated with higher-level functional capacity in elderly adults: the Ohasama study. J Am Geriatr Soc. 2014;62(3):426–34. [DOI] [PubMed] [Google Scholar]
- 65. Macdiarmid J, Blundell J. Assessing dietary intake: who, what and why of under-reporting. Nutr Res Rev. 1998;11(2):231–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.