ABSTRACT
Background
Evidence points to diverse risk factors associated with small- (SGA) and large-for-gestational-age (LGA) births. A more comprehensive understanding of these factors is imperative, especially in vulnerable populations.
Objectives
To estimate the occurrence of and sociodemographic factors associated with SGA and LGA births in poor and extremely poor populations of Brazil.
Methods
The study population consisted of women of reproductive age (14–49 y), whose last child was born between 2012 and 2015. INTERGROWTH 21st consortium criteria were used to classify weight for gestational age according to sex. Multinomial logistic regression modeling was performed to investigate associations of interest.
Results
Of 5,521,517 live births analyzed, SGA and LGA corresponded to 7.8% and 17.1%, respectively. Multivariate analysis revealed greater odds of SGA in children born to women who self-reported as black (OR: 1.21; 95% CI: 1.19, 1.22), mixed-race (parda) (OR: 1.08; 95% CI: 1.07, 1.09), or indigenous (OR: 1.11; 95% CI: 1.06, 1.15), were unmarried (OR: 1.08; 95% CI: 1.07, 1.08), illiterate (OR: 1.47; 95% CI: 1.42, 1.52), did not receive prenatal care (OR: 1.57; 95% CI: 1.53, 1.60), or were aged 14–20 y (OR: 1.21; 95% CI: 1.20, 1.22) or 35–49 y (OR: 1.12; 95% CI: 1.10, 1.13). Considering LGA children, higher odds were found in infants born to women living in households with ≥3 inadequate housing conditions (OR: 1.11; 95% CI: 1.10, 1.12), in indigenous women (OR: 1.22; 95% CI: 1.19, 1.25), those who had 1–3 y of schooling (OR: 1.18; 95% CI: 1.17, 1.19), 1–3 prenatal visits (OR: 1.16; CI 95%: 1.14, 1.17), or were older (OR: 1.26; 95% CI: 1.25, 1.27).
Conclusions
In poorer Brazilian populations, socioeconomic, racial, and maternal characteristics are consistently associated with the occurrence of SGA births, but remain less clearly linked to the occurrence of LGA births.
Keywords: small-for-gestational age, large-for-gestational age, cohort, linkage, poor population
Introduction
The newborn size is a product of the duration of pregnancy and rate of fetal growth. It is an important indicator of prenatal health and it has been associated with infant mortality, as well as short- and long-term morbidity (1). According to the Gaussian distribution of birth weight specific to sex, 3 main groups of live births have been conventionally defined: 1) small-for-gestational-age (SGA: weight at gestational age <10th percentile); 2) appropriate-for-gestational-age (weight at gestational age between the 10th and 90th percentiles); and 3) large-for-gestational-age (LGA: weight at gestational age >90th percentile) (2–5).
In high-income countries, the prevalence of SGA and LGA is 4.6–15.3% and 5–20%, respectively (6, 7). Higher SGA burdens are evidenced in low- and middle-income countries (LMIC), as the prevalence of SGA varies from 7.0% in East Asia to 44.5% in South Asia (2). In Latin America and the Caribbean, 12.5% of all live births are considered SGA (8). The prevalence of macrosomia also varies among LMIC, ranging from 0.5% in India to 14.9% in Algeria (9). Variability in these estimates of SGA and LGA can be explained both by socioenvironmental factors and differences among populations, as well as by differences in the methodological approaches used to build these indicators (9–11).
There is evidence of a diversity of risk factors associated with SGA, such as: smoking, maternal short stature, underweight and low weight gain during pregnancy, chronic and infectious disease, nulliparity, extremes in maternal age, and placental pathology (12–15). The best-known risk factors for LGA are high pregestational BMI, pre-existing and gestational diabetes mellitus, the prior occurrence of LGA in pregnancy, and significant weight gain during pregnancy (12, 16–20). Some studies have shown SGA to be associated with social status, especially family income and schooling (21, 22); however, the role that socioeconomic factors play in LGA births is not well understood.
Despite significant improvements in maternal and child health indicators over recent decades in Brazil, neonatal and infant mortality rates remain unacceptably high, and regions with limited resources and specialized care (obstetric emergencies and high-quality prenatal care services) remain disproportionately affected (23, 24). Poverty and social inequality have been increasingly identified as main social causes underlying negative health outcomes in different populations (25, 26). Moreover, abnormal birth weight can introduce additional risks for both mothers and newborns living in poverty (27, 28).
A comprehensive understanding of the importance of socio-economic factors is imperative to develop strategies designed to improve maternal and infant health, especially in vulnerable populations in countries with high inequalities. In light of these considerations, our study aimed to estimate the frequencies of and identify socioeconomic factors associated with SGA and LGA in poor and extremely poor mothers in Brazil.
Methods
Population, study design, and data collection procedures
The present study considered baseline population-based data from the 100 Million (100M) Brazilian Cohort (29) linked with the National System of Information on Live Births (SINASC) from January 1, 2012 to December 31, 2015. The 100M Brazilian Cohort contains information on low-income families with a monthly per capita income <BRL200 (US$50). The cohort database consists of records containing socioeconomic data from 114,008,179 low-income individuals who applied for social assistance programs via the Unified Registry for Social Programs, and represents ∼55% of the entire Brazilian population (29).
Baseline data in the 100M Brazilian Cohort pertaining to women who gave birth between 2012 and 2015 were linked to the live birth registry from SINASC according 2 stages using Centre for Data and Knowledge Integration for Health-Record Linkage (30). The first was a deterministic linkage, and the second based on the similarity index. The novel record linkage tool considers the mother's name, mother's municipality of residence at time of registry/delivery, and mother's date of birth in the matching process. For current linkage, the estimated accuracy was >90% by year (0.94, 0.92, 0.91, and 0.93 for the years 2012, 2013, 2014, and 2015, respectively).
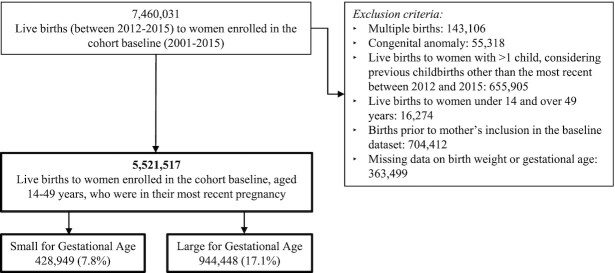
The study population included the most recent live birth to women aged 14–49 y who were registered in the 100M Cohort at any time between 2001 and 2015 prior to giving birth (Figure 1). Only the most recent live birth to each woman was considered, because the inclusion of gestational age (measured in complete weeks) could influence the outcome of analysis. In an effort to avoid bias, multiple births, which accounted for <1.9% of the total live births in the presently studied population, as well as live births with congenital anomalies, were excluded, because these conditions are known to be strongly associated with low birth weight (31, 32) (Figure 1).
FIGURE 1.
Study population.
Dependent variable
Newborn size was defined as appropriate for gestational age (between the 10th and 90th percentiles), SGA (<10th percentile), or LGA (>90th percentile), using sex-specific curves corresponding to singleton live births as established by the INTERGROWTH 21st Consortium (33) to classify weight at gestational age (24/0 to 42/0 gestational weeks). Gestational age was primarily measured according to the date of the mother's last menstrual period: the database contained 3,694,761 (62.8%) records with this information. Birth weight was recorded as the first live birth weight measurement in grams, and presents very high reliability as previously demonstrated by κ index values (34).
Independent variables
The following covariates were included in the analysis. Variables related to maternal and newborn characteristics were obtained from SINASC records: maternal age range (14–19 y, 20–34 y, or 35–49 y), sex of newborn (male or female), and number of prenatal visits (≥7 visits, 4–6 visits, 1–3 visits, or none). Socioeconomic characteristics were obtained from the 100M Cohort database: marital status (married: married or in a stable relationship; unmarried: single, divorced, or widowed); mother's level of education (illiterate, 1–3 y, 4–7 y, or ≥8 y of schooling); self-declared race/skin color [white/Asian descent, mixed-race (parda), black, or indigenous]; housing conditions (adequate, 1–2 inadequacies, or 3+ inadequacies); and urban/rural area of residence. The variable referencing housing conditions is represented as the sum of the following circumstances: house construction material (adequate: brick/masonry; inadequate: wattle and daub, wood, or other), water supply (adequate: public system; inadequate: well/spring or other), lighting (adequate: home with electricity meter; inadequate: no meter), garbage collection (adequate: public collection service; inadequate: not collected), sanitary drainage (adequate: sewage system connection; inappropriate: sewage pit, ditch, or other), and family density (number of individuals in household ÷ number of rooms: ≤2 adequate; >2 inadequate). In each circumstance, a value of zero indicates an “adequate” housing condition, whereas 1 is considered “inadequate.” A maximum value of 6 would indicate that all housing conditions were inadequate, whereas zero would signify that all were adequate. This variable was categorized as no inadequacy, 1–2 inadequacies, or 3+ inadequacies.
Statistical analysis
All the analysis conducted in this study was cross-sectional in nature. Socioeconomic, maternal, and live birth characteristics were summarized using frequency distributions. Collinearity was assessed for each independent variable by calculating changes in the estimation of the other covariates included in the model. Spearman rank correlation coefficients (ρ) were calculated for the quantitative variables birth order and maternal age. Multinomial (polytomous) logistic regression models were used to investigate factors associated with SGA and LGA. It is considered an appropriate technique when a response variable has >2 categories and does not assume a natural ordering. Birth weight according to gestational age was organized in 3 categories: appropriate-for-gestational-age (reference group), SGA, and LGA. Results are expressed as ORs with their respective 95% CIs.
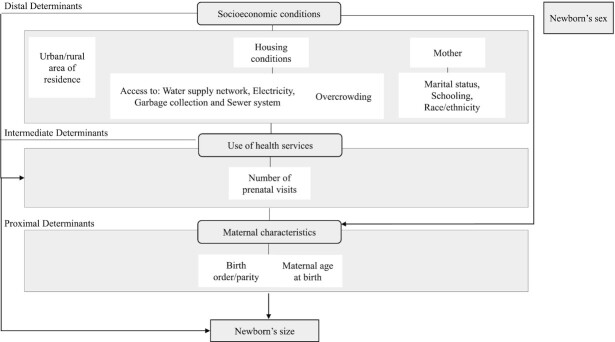
All analyses were conducted using the available covariates considered to be relevant and plausible in the literature (35–39). A conceptual hierarchy-based model was adopted for the introduction of variables (Figure 2). The initial model was adjusted for the following socioeconomic variables: education level, marital status, race/color, housing condition, urban/rural area of residence, in addition to the sex of the newborn and the year the mother entered into the cohort. In the second model, all variables contained in the previous model were maintained, with the inclusion of the number of prenatal visits. The final model included, in addition to the variables contained in the 2 previous models, the mother's age at the time of delivery. Given that inclusion in the cohort occurred dynamically, our analyses were also adjusted according to the year of the mother's entry in the cohort. Data were processed and analyzed using Stata version 15.1 (Stata Corp.) (40).
FIGURE 2.
Analytical model detailing determinants of small- and large-for-gestational-age.
Ethical considerations
The present research was approved by the institutional review board of the Collective Health Institute-Federal University of Bahia, and is a subproject under the umbrella of the main project entitled “Impacts of the Family Fund conditional cash-transfer program on mortality and hospitalization outcomes in Brazil” [Impactos do Bolsa Família em desfechos de mortalidade e hospitalizações no Brasil (in Portuguese)] (CAAE: 41,695,415.0.0000.5030).
Results
A total of 5,521,517 live births were included in the study, of which 428,949 (7.8%) and 944,448 (17.1%) were classified as SGA and LGA, respectively (Figure 1).
Table 1 lists the characteristics of the study population. Both SGA and LGA were slightly more common in rural areas, in households with ≥3 inadequacies, and in mothers who were indigenous or had fewer years of formal education. SGA was more frequent in babies born to single mothers compared with LGA.
TABLE 1.
Small-for-gestational-age (SGA) and large-for-gestational-age (LGA) in full-term births from 2012 to 2015 according to variables related to mothers, live births, prenatal care, and socioeconomic conditions.
| n Missing | SGA1 | LGA1 | ||
|---|---|---|---|---|
| Variables | (%) | n (%) | n (%) | n (%) |
| Urban/rural area of residence | 168,270 (4.1) | |||
| Urban | 3,957,206 (74.7) | 303,196 (7.7) | 665,888 (16.8) | |
| Rural | 1,342,669 (25.3) | 109,154 (8.1) | 241,787 (18.0) | |
| Housing conditions | 362,848 (8.7) | |||
| No inadequacy | 1,482,365 (29.4) | 110,929 (7.5) | 238,318 (16.1) | |
| 1–2 inadequacies | 2,016,007 (40.0) | 153,357 (7.6) | 347,024 (17.2) | |
| 3+ inadequacies | 1,539,243 (30.6) | 128,001 (8.3) | 274,714 (17.8) | |
| Maternal race/ethnicity | 315,086 (7.6) | |||
| White/Asian descent | 1,583,226 (31.0) | 114,173 (7.2) | 260,175 (16.4) | |
| Mixed-race (parda) | 3,075,059 (60.2) | 244,098 (7.9) | 537,974 (17.5) | |
| Black | 403,060 (7.9) | 35,848 (8.9) | 66,192 (16.4) | |
| Indigenous | 42,916 (0.8) | 3763 (8.8) | 9004 (21.0) | |
| Marital status | 48,775 (1.2) | |||
| Married, civil union | 2,909,389 (53.3) | 212,615 (7.3) | 521,053 (17.9) | |
| Single, divorced, widowed | 2,545,579 (46.7) | 210,901 (8.3) | 411,054 (16.1) | |
| Maternal schooling | 69,295 (1.7) | |||
| ≥8 y of study | 3,704,186 (68.3) | 270,873 (7.3) | 614,328 (16.6) | |
| 4–7 y of study | 1,438,325 (26.5) | 123,758 (8.6) | 254,257 (17.7) | |
| 1–3 y of study | 243,154 (4.5) | 22,149 (9.1) | 49,651 (20.4) | |
| Illiterate | 41,209 (0.8) | 4449 (10.8) | 8584 (20.8) | |
| Number of prenatal visits | 23,982 (0.6) | |||
| ≥7 visits | 3,351,441 (61.1) | 242,741 (7.2) | 558,962 (16.7) | |
| 4–6 visits | 1,625,029 (29.6) | 132,690 (8.2) | 288,707 (17.8) | |
| 1–3 visits | 413,571 (7.5) | 38,510 (9.3) | 76,740 (18.6) | |
| None | 99,412 (1.8) | 11,567 (11.6) | 15,398 (15.5) | |
| Maternal age at birth | 12 (0.0) | |||
| 20–35 y | 3,780,015 (68.5) | 273,117 (7.2) | 670,804 (17.7) | |
| 14–20 y | 1,253,184 (22.7) | 117,136 (9.3) | 168,508 (13.4) | |
| 35–49 y | 488,301 (8.8) | 38,694 (7.9) | 105,133 (21.5) | |
| Birth order | 244,990 (5.9) | |||
| 2nd–4th child | 2,891,438 (55.6) | 188,274 (6.5) | 563,712 (19.5) | |
| ≥5th child | 343,156 (6.6) | 27,476 (8.0) | 78,149 (22.8) | |
| 1st child | 1,964,808 (37.8) | 182,939 (9.3) | 255,722 (13.0) | |
| Newborn sex | 0 (0.0) | |||
| Male | 2,825,961 (51.2) | 215,168 (7.6) | 486,291 (17.2) | |
| Female | 2,695,556 (48.8) | 213,781 (7.9) | 458,157 (17.0) | |
SGA/LGA frequencies calculated by row. Adequate-for-gestational-age (AGA) has been omitted, but can be calculated from the information in Table 1. For example, %SGA in each category = (number of SGA individuals/number of individuals in the category) × 100.
Table 2 illustrates the results of the multivariate analysis (final model). All steps up to this final model can be found in Supplemental Table 1. Due to collinearity between maternal age and parity/birth order (ρ = 0.56), the latter variable was removed from the adjusted model. The adjusted odds for SGA were higher in children born to women who self-reported as black (OR: 1.21; 95% CI: 1.19, 1.22), indigenous (OR: 1.11; 95% CI: 1.06, 1.15), or were unmarried (OR: 1.08; 95% CI: 1.07, 1.08). ORs were progressively higher with regard to fewer years of schooling (ORilliterate: 1.47; 95% CI: 1.42, 1.52), number of prenatal visits (ORnone: 1.57; 95% CI: 1.53, 1.60), and for women in the upper and lower age ranges. Considering LGA children, higher odds were found in those born to women living in households with ≥3 inadequate housing conditions (OR: 1.11; 95% CI: 1.10, 1.12), in those who were indigenous (OR: 1.22; 95% CI: 1.19, 1.25), reported 1–3 y of schooling (OR: 1.18; 95% CI: 1.17, 1.19), attended 1–3 prenatal visits (OR: 1.16; 95% CI: 1.14, 1.17), or were older (OR: 1.26; 95% CI: 1.25, 1.27). The chance of LGA was not observed to progressively increase with fewer prenatal consultations (ORnone: 0.95; 95% CI: 0.93, 0.97). In addition, younger women were found to have a much lower chance of giving birth to LGA newborns (OR: 0.72; 95% CI: 0.72, 0.73).
TABLE 2.
Final model of the determinants of small-for-gestational-age (SGA) and large-for-gestational-age (LGA)1
| SGA | LGA | |
|---|---|---|
| Variables | OR (95% CI) | OR (95% CI) |
| Urban/rural area of residence | ||
| Urban | Ref | Ref |
| Rural | 1.01 (1.00, 1.01) | 1.02 (1.01, 1.02) |
| Housing conditions | ||
| No inadequacy | Ref | Ref |
| 1–2 inadequacies | 1.01 (1.00, 1.01) | 1.08 (1.08, 1.09) |
| 3+ inadequacies | 1.06 (1.05, 1.07) | 1.11 (1.10, 1.12) |
| Maternal race/ethnicity | ||
| White/Asian descent | Ref | Ref |
| Mixed-race (parda) | 1.08 (1.07, 1.09) | 1.07 (1.06, 1.07) |
| Black | 1.21 (1.19, 1.22) | 0.99 (0.98, 1.00) |
| Indigenous | 1.11 (1.06, 1.15) | 1.22 (1.19, 1.25) |
| Marital status | ||
| Married, civil union | Ref | Ref |
| Single, divorced, widowed | 1.08 (1.07, 1.08) | 0.93 (0.92, 0.93) |
| Maternal schooling | ||
| ≥8 y of study | Ref | Ref |
| 4–7 y of study | 1.14 (1.13, 1.15) | 1.09 (1.08, 1.09) |
| 1–3 y of study | 1.26 (1.24, 1.28) | 1.18 (1.17, 1.19) |
| Illiterate | 1.47 (1.42, 1.52) | 1.14 (1.11, 1.18) |
| Number of prenatal visits | ||
| ≥7 visits | Ref | Ref |
| 4–6 visits | 1.11 (1.10, 1.12) | 1.09 (1.09, 1.10) |
| 1–3 visits | 1.26 (1.24, 1.27) | 1.16 (1.14, 1.17) |
| None | 1.57 (1.53, 1.60) | 0.95 (0.93, 0.97) |
| Maternal age at birth | ||
| 20–35 y | Ref | Ref |
| 14–20 y | 1.21 (1.20, 1.22) | 0.72 (0.72, 0.73) |
| 35–49 y | 1.12 (1.10, 1.13) | 1.26 (1.25, 1.27) |
| Newborn's sex | ||
| Male | Ref | Ref |
| Female | 1.04 (1.04, 1.05) | 0.99 (0.98, 0.99) |
Multinomial (polytomous) adjusted logistic regression was applied to all modeled variables and year of cohort entry. Ref, reference.
Discussion
Our study aimed to identify frequencies and associated factors relative to SGA and LGA live births in poor and extremely poor women in Brazil. SGA occurrence was higher in mothers who were younger and older, mixed-race (parda), black or indigenous, single, divorced, or widowed, less educated, and who attended fewer prenatal visits. The occurrence of LGA was higher in households with inadequate housing conditions, in indigenous women, those who had less formal education, and in mothers who were older. In addition, LGA was less commonly observed in the absence of prenatal visits.
Our findings are consistent with data in the literature on disparities in birth outcomes due to socioeconomic conditions (8, 38, 39, 41–43), highlighting the influence of demographic, socioeconomic, and health service access factors on pregnancy outcomes. Our results indicate that the socioeconomic conditions of the households where the mothers lived were associated with LGA and SGA. Indeed, some of the poorest regions in Brazil are becoming progressively more impacted by emerging noncommunicable diseases, for example, obesity, diabetes, and hypertension, which increasingly affect women of reproductive age (44). In Brazil, the prevalence of overweight and obesity in adult women increased from 33.8% to 46.3% between 2008 and 2015 (44). Given this scenario, similar increases in LGA and fetal macrosomia are likely to occur (45–49). Similarly to the findings herein, previous studies have reported substantial disparities in the prevalence of SGA in women of different racial and ethnic backgrounds (50–52). Our results indicate that babies born to mixed-race (parda), black, or indigenous women were more likely to be SGA, and that mixed-race or indigenous women were more likely to give birth to LGA newborns compared with white mothers or those of Asian descent. Regarding the mechanisms of how maternal race impacts pregnancy-related outcomes, it is important to recognize how the experiences of racism and segregation can influence the outcome (53). This is especially true in cases where racism and residential segregation occur simultaneously, placing vulnerable women at a higher risk of facing a lack of investment and inferior conditions, which isolates them from amenities, opportunities, and resources, resulting in stressful conditions and/or the promotion of behaviors considered harmful to well-being, thus negatively affecting their reproductive health (46, 52–55). Other studies have demonstrated that unmarried (single) mothers are more likely to give birth to newborns with SGA than married mothers (56, 57). Being unmarried is increasingly recognized as a risk factor that can influence adverse results in perinatal health, potentially due to a lack of social support or increased stress (55, 57). However, the exact nature of such stressors remains unelucidated.
Our findings indicate that low levels of maternal education are associated with an increased chance of SGA/LGA in newborns. Education represents one of the most important dimensions of socioeconomic status in predicting the health of mothers and their children (58–60). Maternal education is a representative variable of social insertion in relation to access to material goods and information, and is an important factor in overcoming challenges to the health and social progress of vulnerable women (61). It is known that more highly educated women tend to seek out more information during pregnancy and solicit medical attention when appropriate (6, 62).
With regard to prenatal care, the chance of SGA at birth was observed to be higher in mothers with reduced numbers or no prenatal visits. The opposite was found with respect to LGA. A high number of prenatal visits might not necessarily be an indicator of high-quality prenatal care, and could be related to high-risk pregnancies requiring additional care (63). Accordingly, it is possible that women who had fewer prenatal consultations could have had fewer comorbidities, such as diabetes and obesity, and thus faced a reduced risk of having a baby with LGA. Another explanation could be due to the low occurrence of zero prenatal visits in LGA babies, which could lead to a spurious inverse association; however, this variable was found to be consistent in SGA. Because the SINASC database does not contain information related to comorbidities during pregnancy, we were not able to explore this hypothesis.
As we identified in our study, large-scale epidemiological studies also identified an increased risk of SGA in much younger and older mothers (64, 65). Advanced maternal age has been reported as a risk factor for LGA (37, 66, 67) whereas younger age has been associated with lower incidence of LGA (68).
Some of the variables associated with SGA and LGA are considered modifiable factors, and they could theoretically reduce the prevalence of these outcomes. Thus, socioeconomic and health interventions, such as financial support for the poorest pregnant women, equality policies, and access to education and high-quality health services, might improve maternal and child health to reduce the occurrence of SGA and LGA.
Strengths and limitations
With respect to strengths, the use of a large dataset allowed the analysis of factors associated with growth curve deviations (SGA/LGA). Moreover, the concurrent analysis of SGA and LGA allowed the identification of common factors. Regarding limitations, it was not possible to investigate some classical biological risk factors associated with SGA or LGA, such as diabetes or obesity. The study was further limited by the fact that multiple methods of assessing gestational age could have led to measurement errors. However, a previous study evaluated the reliability of gestational age in the SINASC database and reported fair reliability (κ = 0.46) (34). Nonetheless, it is important to emphasize that the conclusions stated herein are not solely based on significant associations; the magnitude of effect size, as well as supporting data in the literature, further bolster the present findings. Importantly, the variables analyzed in this study were restricted to sociodemographic factors and the frequency of prenatal care visits. Because our results pertain to mothers living in poverty and extreme poverty, any attempts to generalize the present findings beyond the scope of the population considered herein must be made with due caution.
Conclusion
In summary, the present large-scale retrospective study of adverse pregnancy outcomes (SGA and LGA) in poor and extremely poor Brazilian mothers identified associations with sociodemographic factors and prenatal care visits, which highlights the importance of monitoring birth weight in vulnerable populations. Although socioeconomic and maternal characteristics were observed to be consistently strongly associated with SGA births, it is necessary to further investigate relations between LGA and schooling and number of prenatal visits. We emphasize the importance of maintaining financial support for vulnerable mothers and social protections for pregnant workers, as well as the promotion and implementation of policies fostering equity among genders and races. Finally, it is essential to improve access to high-quality primary services, such as education and health, in addition to increasing efforts focused on preventing teenage pregnancy. We further recommend the undertaking of longitudinal studies designed to elucidate the long-term consequences of SGA and LGA.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—IRF, RdCR-S: conceptualized and designed the study, drafted the initial manuscript, carried out the analyses, and reviewed and revised the manuscript; MFdA, RLF: conceptualized and designed the study and critically reviewed the intellectual content of the manuscript; MLB, LCR, MYI: conceptualized and designed the study, acquired data, contributed to data interpretation, and critically reviewed the intellectual content of the manuscript; NJS, ESP: drafted the initial manuscript and contributed to data analysis, interpretation, and the critical review of the manuscript; and all authors: read and approved the final manuscript.
The authors report no conflicts of interest.
Notes
This study was funded by MCTI/CNPq/MS/SCTIE/Decit/Bill & Melinda Gates Foundation's Grandes Desafios Brasil—Desenvolvimento Saudável para Todas as Crianças (call number 47/2014). CIDACS and the 100 Million Cohort received core support from the Wellcome Trust (grant number 202912/Z/16/Z); Health Surveillance Secretariat, Ministry of Health, Brazil, Bahia State; Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB); Financiadora de Estudos e Projetos-FINEP, Secretariat of Science and Technology of the State of Bahia-SECTI. IRF received a doctoral scholarship from Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) (grant number BOL2330 / 2016). ESP is a fellow supported by the Wellcome Trust (grant number 213589/Z/18/Z).
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CIDACS, Centre for Data and Knowledge Integration for Health; LGA, large-for-gestational-age; LMIC, low- and middle-income countries; SGA, small-for-gestational-age; SINASC, National System of Information on Live Births.
Contributor Information
Ila R Falcão, The School of Nutrition, Federal University of Bahia, Salvador, Brazil; Centre for Data and Knowledge Integration for Health (CIDACS), Oswaldo Cruz Foundation, Salvador, Brazil.
Rita de Cássia Ribeiro-Silva, The School of Nutrition, Federal University of Bahia, Salvador, Brazil; Centre for Data and Knowledge Integration for Health (CIDACS), Oswaldo Cruz Foundation, Salvador, Brazil.
Marcia Furquim de Almeida, School of Public Health, University of São Paulo, São Paulo, Brazil.
Rosemeire L Fiaccone, Centre for Data and Knowledge Integration for Health (CIDACS), Oswaldo Cruz Foundation, Salvador, Brazil; Department of Statistics, Federal University of Bahia, Salvador, Brazil.
Natanael J Silva, Centre for Data and Knowledge Integration for Health (CIDACS), Oswaldo Cruz Foundation, Salvador, Brazil.
Enny S Paixao, Centre for Data and Knowledge Integration for Health (CIDACS), Oswaldo Cruz Foundation, Salvador, Brazil; Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Maria Yury Ichihara, Centre for Data and Knowledge Integration for Health (CIDACS), Oswaldo Cruz Foundation, Salvador, Brazil; Institute of Collective Health, Federal University of Bahia, Salvador, Bahia, Brazil.
Laura C Rodrigues, Centre for Data and Knowledge Integration for Health (CIDACS), Oswaldo Cruz Foundation, Salvador, Brazil; Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Mauricio L Barreto, Centre for Data and Knowledge Integration for Health (CIDACS), Oswaldo Cruz Foundation, Salvador, Brazil; Institute of Collective Health, Federal University of Bahia, Salvador, Bahia, Brazil.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request.
References
- 1. de Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. 1996;64(4):650–8. [DOI] [PubMed] [Google Scholar]
- 2. Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, Adair L, Baqui AH, Bhutta ZA, Caulfield LEet al. . National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1(1):e26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gigante DP, Horta BL, Matijasevich A, Loret de Mola C, Barros AJD, Santos IS, Barros FC, Victora CG. Gestational age and newborn size according to parental social mobility: an intergenerational cohort study. J Epidemiol Community Health. 2015;69(10):944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Norris T, Johnson W, Farrar D, Tuffnell D, Wright J, Cameron N. Small-for-gestational age and large-for-gestational age thresholds to predict infants at risk of adverse delivery and neonatal outcomes: are current charts adequate? An observational study from the Born in Bradford cohort. BMJ Open. 2015;5(3):e006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Certain conditions originating in the perinatal period. [Internet]. In:International classification of diseases, 11th ed. Geneva, Switzerland: WHO; 2019; [cited December 6, 2019]. Available from: https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1786398813 [Google Scholar]
- 6. Ruiz M, Goldblatt P, Morrison J, Kukla L, Svancara J, Riitta-Jarvelin M, Taanila A, Saurel-Cubizolles MJ, Lioret S, Bakoula Cet al. . Mother's education and the risk of preterm and small for gestational age birth: a DRIVERS meta-analysis of 12 European cohorts. J Epidemiol Community Health. 2015;69(9):826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henriksen T. The macrosomic fetus: a challenge in current obstetrics. Acta Obstet Gynecol Scand. 2008;87(2):134–45. [DOI] [PubMed] [Google Scholar]
- 8. Lee ACC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, Adair L, Baqui AH, Bhutta ZA, Caulfield LEet al. . National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1(1):e26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koyanagi A, Zhang J, Dagvadorj A, Hirayama F, Shibuya K, Souza JP, Gülmezoglu AM. Macrosomia in 23 developing countries: an analysis of a multicountry, facility-based, cross-sectional survey. Lancet. 2013;381(9865):476–83. [DOI] [PubMed] [Google Scholar]
- 10. Chiavaroli V, Castorani V, Guidone P, Derraik JG, Liberati M, Chiarelli F, Mohn A. Incidence of infants born small- and large-for-gestational-age in an Italian cohort over a 20-year period and associated risk factors. Ital J Pediatr. 2016;42:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Black RE. Global prevalence of small for gestational age births. Nestle Nutr Inst Workshop Ser. 2015;81:1–7. [DOI] [PubMed] [Google Scholar]
- 12. Kuhle S, Maguire B, Zhang H, Hamilton D, Allen AC, Joseph KS, Allen AM. Comparison of logistic regression with machine learning methods for the prediction of fetal growth abnormalities: a retrospective cohort study. BMC Pregnancy Childbirth. 2018;18(1):333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang X, Lee NL, Burstyn I. Smoking and use of electronic cigarettes (vaping) in relation to preterm birth and small-for-gestational-age in a 2016 U.S. national sample. Prev Med. 2020;134:106041. [DOI] [PubMed] [Google Scholar]
- 14. Wang X, Zhang X, Zhou M, Juan J, Wang X. Association of prepregnancy body mass index, rate of gestational weight gain with pregnancy outcomes in Chinese urban women. Nutr Metab (Lond). 2019;16:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferguson KK, Rosario Z, McElrath TF, Velez Vega C, Cordero JF, Alshawabkeh A, Meeker JD. Demographic risk factors for adverse birth outcomes in Puerto Rico in the PROTECT cohort. PLoS One. 2019;14(6):e0217770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. AlSeaidan M, Al Wotayan R, Christophi CA, Al-Makhseed M, Abu Awad Y, Nassan F, Ahmed A, Abraham S, Boley RB, James-Todd Tet al. . Birth outcomes in a prospective pregnancy-birth cohort study of environmental risk factors in Kuwait: the TRACER study. Paediatr Perinat Epidemiol. 2016;30(4):408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng W, Huang W, Zhang Z, Zhang L, Tian Z, Li G, Zhang W. Patterns of gestational weight gain in women with overweight or obesity and risk of large for gestational age. Obes Facts. 2019;12(4):407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weschenfelder F, Lehmann T, Schleussner E, Groten T. Gestational weight gain particularly affects the risk of large for gestational age infants in non-obese mothers. Geburtshilfe Frauenheilkd. 2019;79(11):1183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langer O. Fetal macrosomia: etiologic factors. Clin Obstet Gynecol. 2000;43(2):283–97. [DOI] [PubMed] [Google Scholar]
- 20. Alberico S, Montico M, Barresi V, Monasta L, Businelli C, Soini V, Erenbourg A, Ronfani L, Maso G; Multicentre Study Group on Mode of Delivery in Friuli Venezia Giulia . The role of gestational diabetes, pre-pregnancy body mass index and gestational weight gain on the risk of newborn macrosomia: results from a prospective multicentre study. BMC Pregnancy Childbirth. 2014;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joseph KS, Liston RM, Dodds L, Dahlgren L, Allen AC. Socioeconomic status and perinatal outcomes in a setting with universal access to essential health care services. CMAJ. 2007;177(6):583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savitz DA, Kaufman JS, Dole N, Siega-Riz AM, Thorp JM Jr, Kaczor DT. Poverty, education, race, and pregnancy outcome. Ethn Dis. 2004;14(3):322–9. [PubMed] [Google Scholar]
- 23. Jacinto E, Aquino EML, Mota ELA. Mortalidade perinatal no município de Salvador, Bahia: evolução de 2000 a 2009. Rev Saúde Pública. 2013;47:846–53. [DOI] [PubMed] [Google Scholar]
- 24. Sousa A, Hill K, Dal Poz MR. Sub-national assessment of inequality trends in neonatal and child mortality in Brazil. Int J Equity Health. 2010;9(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barreto ML. Health inequalities: a global perspective. Ciên Saúde Colet. 2017;22:2097–108. [DOI] [PubMed] [Google Scholar]
- 26. Marmot M. Social determinants of health inequalities. Lancet. 2005;365:1099–104. [DOI] [PubMed] [Google Scholar]
- 27. Strully KW, Rehkopf DH, Xuan Z. Effects of prenatal poverty on infant health: state earned income tax credits and birth weight. Am Sociol Rev. 2010;75(4):534–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins JW Jr, David RJ, Rankin KM, Desireddi JR. Transgenerational effect of neighborhood poverty on low birth weight among African Americans in Cook County, Illinois. Am J Epidemiol. 2009;169(6):712–7. [DOI] [PubMed] [Google Scholar]
- 29. Centro de Integração de Dados e Conhecimentos para a Saúde . Cohort of 100 million Brazilians. [Internet]. 2018 [cited December 6, 2019]. Available from: https://cidacs.bahia.fiocruz.br/en/platform/cohort-of-100-million-brazilians/ [Google Scholar]
- 30. Ali MS, Ichihara MY, Lopes LC, Barbosa GCG, Pita R, Carreiro RP, Dos Santos DB, Ramos D, Bispo N, Raynal Fet al. . Administrative data linkage in Brazil: potentials for health technology assessment. Front Pharmacol. 2019;10:984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Y, Li G, Ruan Y, Zou L, Wang X, Zhang W. An epidemiological survey on low birth weight infants in China and analysis of outcomes of full-term low birth weight infants. BMC Pregnancy Childbirth. 2013;13:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woodhouse C, Lopez Camelo J, Wehby GL. A comparative analysis of prenatal care and fetal growth in eight South American countries. PLoS One. 2014;9(3):e91292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, Lambert A, Papageorghiou AT, Carvalho M, Jaffer YAet al. . International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–68. [DOI] [PubMed] [Google Scholar]
- 34. Szwarcwald CL, do Carmo Leal M, Esteves-Pereira AP, da Silva de Almeida W, de Frias PG, Damacena GN, Borges de Souza PR Jr, Rocha NM, Mullachery PMH. Avaliação das informações do Sistema de Informações sobre Nascidos Vivos (SINASC), Brasil. Cad Saúde Pública. [Internet]2019;35(10). doi:org/10.1590/0102-311x00214918. [DOI] [PubMed] [Google Scholar]
- 35. Zambonato AMK, Pinheiro RT, Horta BL, Tomasi E. Fatores de risco para nascimento de crianças pequenas para idade gestacional. Rev Saúde Pública. 2004;38:24–9. [DOI] [PubMed] [Google Scholar]
- 36. Hadfield RM, Lain SJ, Simpson JM, Ford JB, Raynes-Greenow CH, Morris JM, Roberts CL. Are babies getting bigger? An analysis of birthweight trends in New South Wales, 1990–2005. Med J Aust. 2009;190(6):312–15. [DOI] [PubMed] [Google Scholar]
- 37. Agudelo-Espitia V, Parra-Sosa BE, Restrepo-Mesa SL. Factors associated with fetal macrosomia. Rev Saúde Pública. 2019;53:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mortensen LH, Lauridsen JT, Diderichsen F, Kaplan GA, Gissler M, Andersen AM. Income-related and educational inequality in small-for-gestational age and preterm birth in Denmark and Finland 1987–2003. Scand J Public Health. 2010;38(1):40–5. [DOI] [PubMed] [Google Scholar]
- 39. Sebayang SK, Dibley MJ, Kelly PJ, Shankar AV, Shankar AH. Determinants of low birthweight, small-for-gestational-age and preterm birth in Lombok, Indonesia: analyses of the birthweight cohort of the SUMMIT trial. Trop Med Int Health. 2012;17(8):938–50. [DOI] [PubMed] [Google Scholar]
- 40. StataCorp . Stata Statistical Software: Release 15. College Station, TX: StatCorp LLC; 2017. [Google Scholar]
- 41. Mahumud RA, Sultana M, Sarker AR. Distribution and determinants of low birth weight in developing countries. J Prev Med Public Health. 2017;50(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Slaughter-Acey JC, Holzman C, Calloway D, Tian Y. Movin' on up: socioeconomic mobility and the risk of delivering a small-for-gestational age infant. Matern Child Health J. 2016;20(3):613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Falcão IR, de Almeida MF, Fiaccone RL, Rocha ADS, Ortelan N, Silva NJ, Paixao ES, Ichihara MY, Rodrigues LCet al. . Factors associated with low birth weight at term: a population-based linkage study of the 100 million Brazilian cohort. BMC Pregnancy Childbirth. 2020;20(1):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Araujo FG, Velasquez-Melendez G, Felisbino-Mendes MS. Prevalence trends of overweight, obesity, diabetes and hypertension among Brazilian women of reproductive age based on sociodemographic characteristics. Health Care Women Int. 2019;40(4):386–406. [DOI] [PubMed] [Google Scholar]
- 45. Clayborne ZM, Giesbrecht GF, Bell RC, Tomfohr-Madsen LM. Relations between neighbourhood socioeconomic status and birth outcomes are mediated by maternal weight. Soc Sci Med. 2017;175:143–51. [DOI] [PubMed] [Google Scholar]
- 46. Catov JM, Lee M, Roberts JM, Xu J, Simhan HN. Race disparities and decreasing birth weight: are all babies getting smaller?. Am J Epidemiol. 2016;183(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ziauddeen N, Wilding S, Roderick PJ, Macklon NS, Alwan NA. Is maternal weight gain between pregnancies associated with risk of large-for-gestational age birth? Analysis of a UK population-based cohort. BMJ Open. 2019;9(7):e026220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kong L, Nilsson IAK, Gissler M, Lavebratt C. Associations of maternal diabetes and body mass index with offspring birth weight and prematurity. JAMA Pediatr. 2019;173(4):371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Czarnobay SA, Kroll C, Schultz LF, Malinovski J, Mastroeni S, Mastroeni MF. Predictors of excess birth weight in Brazil: a systematic review. J Pediatr (Rio J). 2019;95(2):128–54. [DOI] [PubMed] [Google Scholar]
- 50. Schempf AH, Kaufman JS, Messer LC, Mendola P. The neighborhood contribution to black-white perinatal disparities: an example from two north Carolina counties, 1999–2001. Am J Epidemiol. 2011;174(6):744–52. [DOI] [PubMed] [Google Scholar]
- 51. Collins JW Jr, Mariani A, Rankin K. African-American women's upward economic mobility and small for gestational age births: a population-based study. Matern Child Health J. 2018;22(8):1183–9. [DOI] [PubMed] [Google Scholar]
- 52. Goodman JM, Karasek D, Anderson E, Catalano RA. The contribution of attenuated selection in utero to small-for-gestational-age (SGA) among term African American male infants. Soc Sci Med. 2013;88:83–9. [DOI] [PubMed] [Google Scholar]
- 53. Grady SC. Racial disparities in low birthweight and the contribution of residential segregation: a multilevel analysis. Soc Sci Med. 2006;63(12):3013–29. [DOI] [PubMed] [Google Scholar]
- 54. Li X, Gao R, Dai X, Liu H, Zhang J, Liu X, Si D, Yuan Y, Li H. The association between symptoms of depression during pregnancy and low birth weight: a prospective study. BMC Pregnancy Childbirth. 2020;20(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khashan AS, Everard C, McCowan LM, Dekker G, Moss-Morris R, Baker PN, Poston L, Walker JJ, Kenny LC. Second-trimester maternal distress increases the risk of small for gestational age. Psychol Med. 2014;44(13):2799–810. [DOI] [PubMed] [Google Scholar]
- 56. Lurie S, Zalmanovitch A, Golan A, Sadan O. The effect of marital status on pregnancy outcome in Israel: a retrospective case-control study. J Obstet Gynaecol Res. 2010;36(6):1161–4. [DOI] [PubMed] [Google Scholar]
- 57. Shah PS, Zao J, Ali S, Knowledge Synthesis Group of Determinants of preterm/LBW births . Maternal marital status and birth outcomes: a systematic review and meta-analyses. Matern Child Health J. 2011;15(7):1097–109. [DOI] [PubMed] [Google Scholar]
- 58. Astone NM, Misra D, Lynch C. The effect of maternal socio-economic status throughout the lifespan on infant birthweight. Paediatr Perinat Epidemiol. 2007;21(4):310–18. [DOI] [PubMed] [Google Scholar]
- 59. Campbell EE, Gilliland J, Dworatzek PD, De Vrijer B, Penava D, Seabrook JA. Socioeconomic status and adverse birth outcomes: a population-based Canadian sample. J Biosoc Sci. 2018;50:102–13. [DOI] [PubMed] [Google Scholar]
- 60. Wohlfarth T. Socioeconomic inequality and psychopathology: are socioeconomic status and social class interchangeable?. Soc Sci Med. 1997;45(3):399–410. [DOI] [PubMed] [Google Scholar]
- 61. Barata RB. Como e por que as desigualdades sociais fazem mal à saúde. Rio de Janeiro: Fiocruz; 2009[cited December 6, 2019]. Available from: https://portal.fiocruz.br/livro/como-e-por-que-desigualdades-sociais-fazem-mal-saude-e-book-interativo. [Google Scholar]
- 62. Rai RK, Sudfeld CR, Barik A, Fawzi WW, Chowdhury A. Sociodemographic determinants of preterm birth and small for gestational age in rural West Bengal, India. J Trop Pediatr. 2019;65(6):537–46. [DOI] [PubMed] [Google Scholar]
- 63. Wehby GL, Murray JC, Castilla EE, Lopez-Camelo JS, Ohsfeldt RL. Prenatal care effectiveness and utilization in Brazil. Health Policy Plan. 2009;24(3):175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weng YH, Yang CY, Chiu YW. Risk assessment of adverse birth outcomes in relation to maternal age. PLoS One. 2014;9(12):e114843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Margerison-Zilko C. The contribution of maternal birth cohort to term small for gestational age in the United States 1989–2010: an age, period, and cohort analysis. Paediatr Perinat Epidemiol. 2014;28(4):312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hadfield RM, Lain SJ, Simpson JM, Ford JB, Raynes-Greenow CH, Morris JM, Roberts CL. Are babies getting bigger? An analysis of birthweight trends in New South Wales, 1990–2005. Med J Aust. 2009;190:312–15. [DOI] [PubMed] [Google Scholar]
- 67. Ng S-K, Olog A, Spinks AB, Cameron CM, Searle J, McClure RJ. Risk factors and obstetric complications of large for gestational age births with adjustments for community effects: results from a new cohort study. BMC Public Health. 2010;10:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Luangkwan S, Vetchapanpasat S, Panditpanitcha P, Yimsabai R, Subhaluksuksakom P, Loyd RA, Uengarporn N. Risk factors of small for gestational age and large for gestational age at Buriram Hospital. J Med Assoc Thai. 2015;98(Suppl 4):S71–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request.