Abstract
Cigarette smoking presents oral health professionals with a clinical and research conundrum: reduced periodontal vascular responsiveness to the oral biofilm accompanied by increased susceptibility to destructive periodontal diseases. This presents a significant problem, hampering diagnosis and complicating treatment planning. The aim of this review is to summarize contemporary hypotheses that help explain mechanistically the phenomenon of a suppressed bleeding response to dysbiotic plaque in the periodontia of smokers. The influence of smoke exposure on angiogenesis, innate cell function, the production of inflammatory mediators including cytokines and proteases, tobacco-bacterial interactions and potential genetic predisposition, are discussed.
Keywords: dental plaque, innate immunity, periodontal diseases, tobacco, vasculature
Periodontal diseases and cigarette smoking
Cigarette use is a, or perhaps the, major risk factor for destructive forms of periodontal disease1,2. Tobacco use has been associated with increased disease progression and severity, as well as with refractory disease3,4. Risk appears dose-related1,5,6 while environmental smoke exposure may also be associated with increased risk of destructive forms of periodontitis7–9. At the population level, smoking alone may account for most cases of periodontitis in adults in developed nations10–13. Indeed, smoking rates and sales each predict periodontitis prevalence10,14. In 2000, US NHANES data ascribed almost 80% of the attributable risk of destructive forms of periodontitis in cigarette users to smoking13. The 2016 NHANES data suggest that the prevalence of periodontal diseases in smokers and non-smokers is 62% and 32%, respectively 15,16, while severe periodontitis prevalence is 26% and 7%, respectively17. In Sweden, the population attributable fraction for smoking was 80% in 1970 and is presently close to 60%11. Similar tobacco-periodontitis relationships have now been reported at the population level in New Zealand12. In many developing nations, smoking rates are rising dramatically18, suggesting that an increase in the periodontitis burden in such countries should be expected. In Pakistan, for example, 55% of those with chronic periodontitis are smokers, while 82% of smokers have this form of disease19. Thus, epidemiological data from across the globe clearly indicates an intimate association between tobacco smoking and periodontal diseases.
Interpretation of the clinical signs of inflammation of the periodontal tissues is an important aid in the diagnosis and prognosis of plaque-associated periodontal diseases20. Indeed, key warning signs are spontaneous gingival bleeding or bleeding that occurs in response to tooth brushing or periodontal probing in the dental office. However, tobacco-related suppression of the periodontal vascular response to plaque in cigarette smokers is long established. Pindborg noted, in the 1940s, that many individuals with an aggressive form of periodontitis were smokers and suggested that nicotine-mediated “vascular spasms” may be a contributing factor21,22. McMurray et al later suggested a potential relationship between gingival bleeding and smoking status23. It is now accepted that tobacco smoking presents oral health professionals with a clinical conundrum: decreased gingival bleeding but increased disease susceptibility. This apparent paradox also represents a challenge to the research community as, although tobacco use may account for the majority cases of chronic periodontitis13, the underlying mechanisms of tobacco-induced vascular suppression and disease promotion remain unclear.
While smoking has effects on multiple biological systems that may predispose to chronic periodontitis, including amplification of cholinergic anti-inflammatory signaling24–26; a reduced antibody response27–31; suppressed immune cell function32–35; promotion of osteoclast/osteoblast imbalance36,37; a depleted antioxidant defense 38,39; and compromised tissue remodeling40–42, this review will focus specifically on the vascular response itself.
Suppressed gingival bleeding in tobacco smokers
Multiple studies have firmly established that cigarette smoking exerts a profound, potentially dose-dependent suppressive effect on the bleeding response to plaque in humans, as shown in Figure 1A and B and as evidenced in Table 1. As an example, Ditriech et al reported that, in those smoking more than 10 cigarettes/day, the odds ratio (O.R.) of bleeding at healthy periodontal sites was 0.6 (95% CI: 0.4–0.7) compared to non-smokers, and diseased sites were considerably more likely to bleed in non-smokers (O.R. 5.7; 95% CI, 4.3–7.6)43.
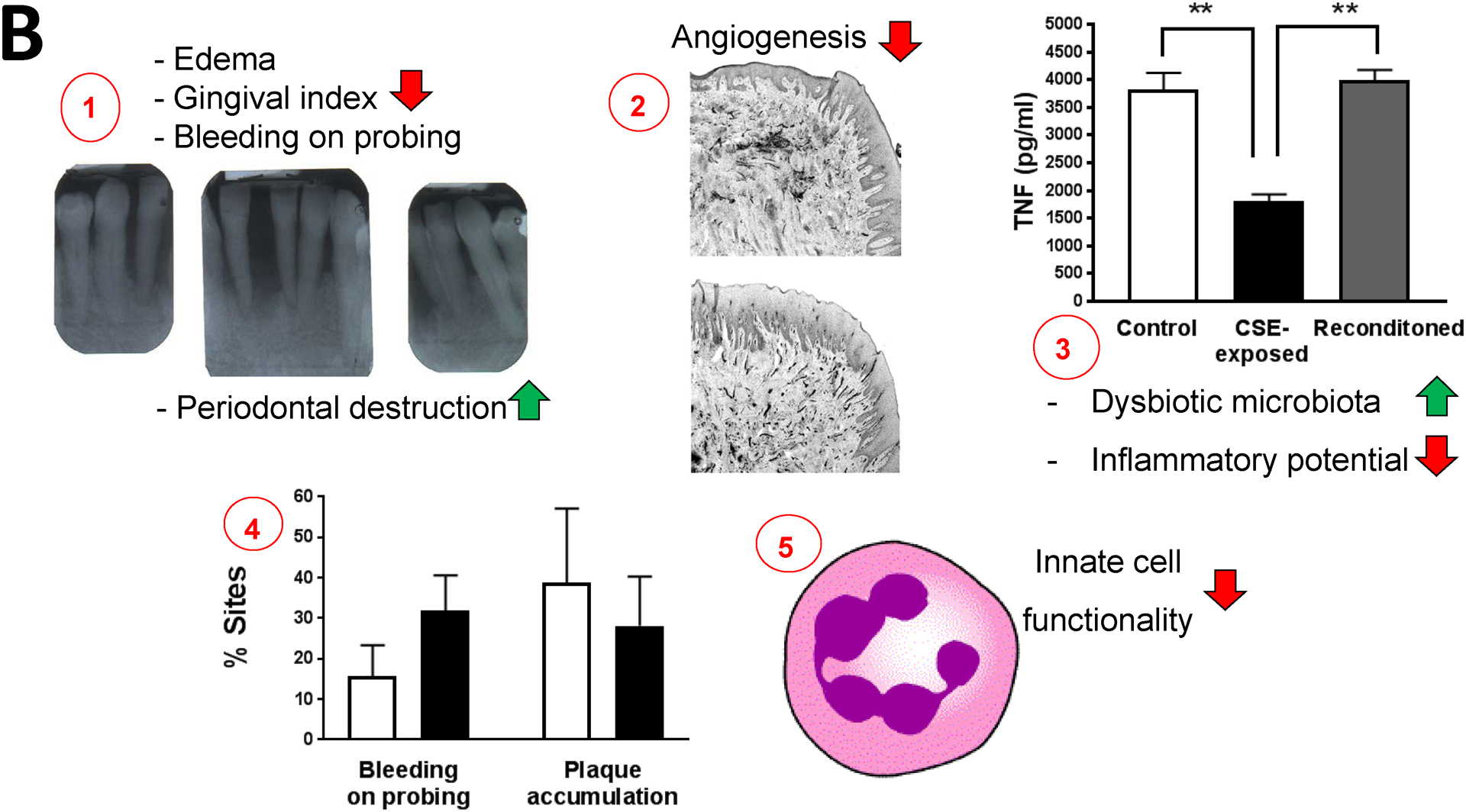
Figure 1: The clinical conundrum in smoking-related periodontitis.
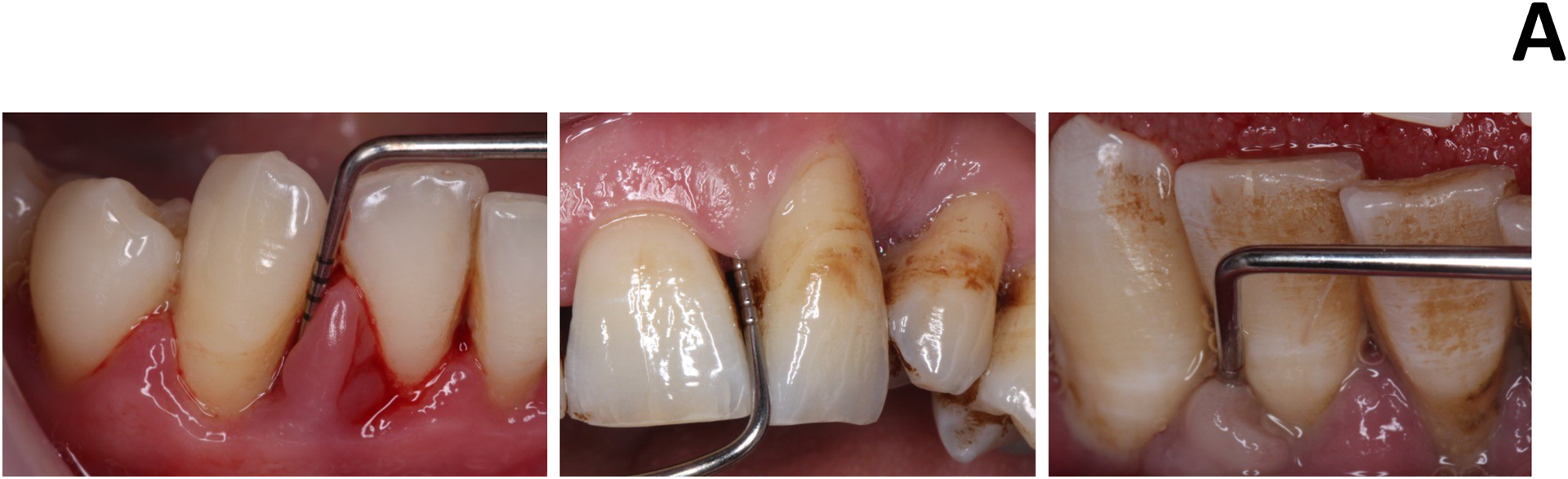
A. Typical bleeding response in a non-smoker (left) and smoker (middle and right) with generalized aggressive periodontitis.
B. 1. Cigarette smoking is associated with reduced edema, gingival index and bleeding response in humans but increased periodontal destruction. Photo kindly provided by Dr. Richard Palmer, King’s College London, England. 2. This may be due to a chronically suppressed angiogenic response accompanied by suppressed endothelial activation rather than an acute vasoactive phenomenon. Figure and legend reproduced, with permission, from159#. 3. Tobacco also induces a dysbiotic microbiota, with phenotypically tobacco-altered P. gingivalis interacting with the immune response in a profoundly different manner than unexposed bacteria. Figure and legend reproduced, with permission, from111##. 4. The inflammatory and bleeding response to plaque, however, rebound within weeks of smoking cessation. Figure extrapolated, with permission, from 72. The mean (s.d.) proportion of sites exhibiting bleeding on probing and plaque at baseline (white bars) and at 4–6 weeks after quitting smoking (black bars). 5. These anti-inflammatory events are accompanied by an increased local (periodontal) and systemic proteolytic burden and dysregulated innate cell function.
# Typical histological sections of gingival tissue from a smoker (upper) and a non-smoker (lower) with chronic periodontitis. Tissue sections were labelled with monoclonal antibodies to ICAM-1 and secondarily labelled. The percentage of ICAM-1-positive vessels of the gingival microvasculature was determined following immunostaining of von Willebrand factor, an endothelial marker, in adjacent sections. ICAM-1 expression microvascular development was compromised in smokers.
## Pro-inflammatory TNF release by primary human monocytes (0.5 × 106) stimulated for 20 h with control P. gingivalis (107); CSE-treated P. gingivalis; or P. gingivalis cells that were first grown in CSE-treated medium for two passages then reconditioned in untreated medium for 2 passages (107 P. gingivalis cells). Error bars represent the mean (s.d.) of 3 experiments.
Table 1:
Comparison of direct vascular indices in the periodontal tissues and GCF of smokers and non-smokers.
| Vascular Index | Vascular relevance | Smokers vs Non-smokers |
|---|---|---|
| GCF flow/volume | Serum-derived trans-periodontal tissue exudate |
124
CP n.s.d. 125 ↓ LCP 126 ↓ CPs 127 ↓ H 128 ↓ H 129 ↓ P 129 ↓ H |
| Gingival / sulcular bleeding | Spontaneous or provoked (probing; brushing) vascular response to plaque |
78
↓ P 130 ↓ P 131 ↓ P 132 ↓ P 133 ↓ P 86 ↓ H 134 ↓ H 125 ↓ LCP 79 ↓ CP 43 ↓ H, P 135 ↓ SPT 136 n.s.d. CP 137 n.s.d. CP 138 n.s.d. CP    ↓ CS ↓ CS   ↓ AP ↓ AP
|
| Microvascular density | Density of small blood vessels, reflecting angiogenesis |
44
↓ P 141 n.s.d. P |
| Gingival index | Measure of the clinical severity of gingival inflammation |
125
↓ LCP 47 n.s.d P 141 n.s.d. P 126 ↓ CP |
| Neutrophil numbers | Bacterial killing, promotion of inflammation, reflection of vascular activation |
141
n.s.d. CP 86 ↓, ↑ H |
| sICAM-1 | Marker of vascular activation | 68 ↓ P |
| VE-cadherin | Endothelial cell junction promotion | 125 n.s.d. LCP |
| VEGF | Potent promoter of angiogenesis | 125 n.s.d. LCP |
↓, significantly reduced in smokers; ↑, significantly enhanced in smokers compared to non-smokers; n.s.d., no significant difference.
AP, aggressive periodontitis; CP, chronic periodontitis; CS, cross-sectional; GCF, gingival crevicular fluid; H, periodontally healthy; sICAM-1, soluble intracellular adhesion molecule-1; LCP, localized chronic periodontitis; P, periodontitis; SPT, supportive periodontal therapy subjects; VE-vadherin, vascular endothelial cadherin; VEGF, vascular endothelial growth factor.
While healthy and diseased periodontal tissues in smokers exhibit greater junctional and marginal epithelial thickness than do non-smokers44,45, the relevance of this phenomenon to gingival bleeding, if any, remains unclear. Smoking contributes to a lowered oxygen tension in the periodontal pocket46,47. The lack of oxygen sufficiency in gingival pockets could influence gingival bleeding directly or indirectly. For example, it is possible that vascular responsiveness, which occurs primarily beneath the junctional epithelium adjacent to plaque accumulation, may be less efficient in anoxic conditions and upon exposure to the composite insult of chemicals found in cigarette smoke. It is also clear that smoking leads to altered dental plaque composition, with the accumulating oral microbiome the key driver of gingival inflammation and bleeding. We shall address tobacco-bacterial interactions in more detail later.
There has been some debate as to whether cigarette consumption reduces gingival bleeding by suppression of angiogenesis or due to the influence of vasoactive smoke components or metabolites on the existing gingival microvasculature. The evidence remains somewhat ambiguous, particularly for acute vascular events, and it is possible that both phenomena occur concomitantly.
As we and others have reviewed previously20,48,49, the reduced bleeding response in smokers was once attributed primarily to nicotine-mediated vasoactivity. In support of this theory, intraartererial nicotine infusion in rabbits has been shown to induce gingival ischemia50. Also in rabbits, the same group later determined the influence of epinephrine and nicotine on gingival blood flow, using thermal diffusion. Initial, transitory dilatory effects were observed with the reverse, considered a reflection of vasoconstriction, noted within ten minutes51. Oral or systemic nicotine administration to dogs has been associated with increased blood flow to the anterior gingiva, relative to untreated controls, as assessed using radiolabeled microspheres 52. However, central, intracerebroventricular injection of nicotine reduced gingival blood flow in rats in a dose-related manner53.
In humans, nicotine can influence variant vascular beds differentially48,54–58. Grudianov and Kemulariia have reported that microvascular blood flow in humans with periodontitis, as measured by laser doppler flowmetery (LDF), is reduced immediately upon smoking a cigarette and that the recovery period is longer, the more severe the periodontal disease59. However, Mavropoulos et al showed a minor increase in blood flow to the gingiva upon smoking in 13 periodontally healthy, casual tobacco users (1 self-reported cigarette per week up to 5 per day), as determined by LDF. Importantly, this gingival hyperemic response was minor compared to the major vasoconstriction observed in the peripheral (thumb) vasculature and was overcome by arterial perfusion pressure58. The same group later reported, in patients with periodontitis, that resting gingival blood flow did not differ between smokers and non-smokers, again as determined by LDF60. The LDF study of Meekin et al also concluded that smoking did not impair gingival blood flow in humans, although an acute cutaneous forehead vasoactive phenomenon was apparent55. More recently, Molnar et al have shown that the gingiva of smokers are less responsive to a heat provocation test, which assesses vascular reactivity, than non-smokers, as measured by induction of gingival crevicular fluid flow61. Interestingly, the authors reported that, independent of periodontal disease classification, the volume of GCF produced is significantly lower in smokers compared to non-smokers. The combined evidence that gingival vasoconstriction is promoted by tobacco smoke is, at most, limited in comparison with other areas of the body in humans48,55. The use of LDF to monitor gingival blood flow in humans has been reviewed recently by Kouadio et al62.
A lack of bleeding response to plaque accumulation could reflect suppressed angiogenesis rather than vasoactivity. In a study of 8 young smokers and 8 non-smokers, in whom gingivitis was promoted by the cessation of oral hygiene measures, Bergstrom et al noted that, although dental plaque accumulated in both groups, the intensity of the vascular reaction, i.e., the number of gingival vessels determined sterophotometrically, was reduced by approximately 50% in the smokers63. In a small number of smokers (n = 4), Mirbod et al reported that, compared to non-smokers, cigarette consumption was associated with a higher number of microvessels with low internal circumference in the gingiva and a lower number of high internal circumference microvessels64. Scardina and Messina further observed that while, compared to non-smokers, the gingival microvasculature of smokers exhibited a larger number of capillaries, the capillaries were of smaller caliber, as determined by videomicroscopy65. Lindeboom et al, though, found no difference in gingival microvascular density, as examined by spectroscopically, in a young and periodontally healthy set of smokers and non-smokers66.
When looking specifically at inflamed areas of the periodontium in subjects with chronic periodontitis, Rezavandi et al noted an increased number of vessels, as determined by Von Willebrand Factor staining, in non-smokers compared to smokers67, suggesting tobacco-induced angiogenic suppression. While the number of activated, ICAM-1 and E-selectin expressing small vessels was greater in sites with inflammation compared to non-inflamed sites in smokers and non-smokers alike, reduced ICAM-1 expression was noted in the non-inflamed tissues of the smokers. A reduction in ICAM-1 expression could reflect a lack of gene activity. However, the substantive increase in soluble ICAM-1 in smokers hints at vascular ICAM-1 shedding, a risk factor for vascular diseases68,69. These data are supportive of dysregulated activation of the periodontal vascular endothelium in smokers.
Finally, there is evidence for potentially thermally-induced nerve damage in the oral cavities of smokers70,71. While this has not been directly examined in the gingiva, it is possible that another consequence of smoking is compromise of local innervation networks that can influence the microvascular response not least through the release of vasoactive neuropeptides.
Importantly, within weeks of biochemically validated (expired air CO) cessation of cigarette smoking, bleeding on probing is increased. Recovery in bleeding occurred concomitantly with improved plaque control, presumably representing more rigorous oral hygiene prompted by participation in a dental research trial72. Similarly, Morozumi et al later reported that gingival bleeding and GCF volume increased shortly after validated smoking cessation73.
Smoking clearly suppresses the bleeding response to plaque in human smokers. While the mechanistic data are somewhat inconsistent, we hypothesize that smoking leads to a reduced angiogenic response to plaque but appropriate periodontal inflammation, reflected in increased bleeding, is quick to recover upon quitting cigarette use.
Inflammatory mediator imbalance in tobacco smokers
Inflammation encompasses manifold processes that occur simultaneously. Some aspects of the inflammatory response will be upregulated by components of cigarette smoke, or their metabolites, e.g., production of some proteolytic enzymes. Other inflammation-related events will be suppressed, e.g. pro-inflammatory cytokine production and angiogenesis. Still other aspects of the host response to oral biofilms will be unaffected. While it is clear that the systemic levels of multiple inflammatory mediators and biomarkers, e.g. CRP, sICAM-1, MMP-9, myeloperoxidase and neutrophil elastase, are increased in cigarette smokers, compared to non-smokers (e.g.68,74–76), the inflammatory burden has also been extensively examined in the local periodontal environment. Studies on gingival crevicular fluid (GCF) content have been particularly enlightening. GCF is a trans-periodontal, serum-derived exudate that contains multiple inflammation-related molecules garnered on its journey across the gingival tissues. As such, it is considered to reflect the disease process across the periodontium and has been extensively characterized in order to better understand periodontal disease progression. A comparison of key inflammatory indices and mediators in the GCF of smokers and non-smokers is presented in Tables 1–3. While certain bioactive molecules noted in Tables 1–3 will have direct effects of angiogenesis and vasoactivity, such as VEGF, IL-8 and prostaglandins, the overall balance and intensity of pro- versus anti-inflammatory mediators is critically important.
Table 1 summarizes direct vascular indices. There is a clear suppression in smokers, relative to non-smokers, of gingival bleeding, GCF production and gingival index (a clinically evaluated combined inflammation and bleeding scale, summarized in Scott and Singer20; alterations to the microvasculature architecture; while reports on intravascular gingival blood flow provide varied results. Interestingly, the systemic neutrophilia and vascular activation (sICAM-1) seen systemically in smokers are not reflected in the GCF68,77,78, indicating a limitation in vascularity and/or diapedesis. Table 2 addresses cytokines, chemokines and related molecules, with the weight of evidence suggesting a reduced GCF content of pro-inflammatory mediators, such as IL-1α, IL-1β IL-8, MCP-1 and MIP-1. Table 3 (Supplemental Material) considers GCF concentrations of proteases, anti-proteases and other inflammatory biomolecules involved in tissue remodeling. Overall, there appears to be a cigarette-induced protease-antiprotease imbalance, at least as determined by comparing MMP-8, MMP-9 (proteases) and α1-antitrypsin, α2-macroglobulin levels (protease inhibitors). Indeed, serum derived from smokers with chronic periodontitis has been shown to enhance MMP-1 and -9, but suppress TIMP-1 (a key MMP inhibitor), release in P. gingivalis-exposed epithelial cells compared to serum from non-smokers79. Further, Bondy-Carey et al have shown that cigarette smoke influences cytokine and protease production in a profound and complex manner in a tripartite (epithelial cell-neutrophil-P. gingivalis) model of the gingival crevice33. The enhanced proteolytic burden is likely to contribute to the connective tissue destruction that helps define chronic periodontitis.
Table 2:
Comparison of cytokines, chemokines and related inflammatory mediators in the GCF of smokers and non-smokers.
| Mediator | Vascular relevance | Smokers vs Non-smokers |
|---|---|---|
| IL-1α | Pro-inflammatory (NF-κB activator); endothelial activation and vasoactive amine release |
141
↓ CP 142 ↓ SP 143 n.s.d. H 143 ↓ P |
| II-1β | Pro-inflammatory (NF-κB activator); endothelial activation and vasoactive amine release |
144
n.s.d., ↑*
CS 145 ↓ P 141 ↓ CP 133 n.s.d. P 146 n.s.d. CP |
| IL-1Ra | Anti-inflammatory (IL-1α and β inhibitor) |
145
↓ P 133 n.s.d. P |
| IL-6 | Pro-inflammatory (STAT-3 pathway, promotion of acute phase response) and potential anti-inflammatory (PI3 kinase pathway) functions |
147
n.s.d. CP n.s.d. 143 ↓ H, P 140 ↑ H, AP |
| CXCL8 (IL-8) | Leukocyte chemoattractant, promoter of angiogenesis and vasoactive amine release |
143
↓ H, P 140 ↓ H, AP |
| IL-10 | Anti-inflammatory (NF-κB activator inhibitor) |
146
n.s.d. CP 79 ↑ CP |
| IL-12 | Innate-adaptive immune response bridging cytokine; antagonist of angiogenesis |
148
n.s.d. CP 143 ↓ H, P |
| IL-16 | Pro-inflammatory (monocyte chemoattractant) | 148 n.s.d. |
| CCL2 (MCP-1) | Leukocyte chemoattractant | 143 ↓ H, P |
| CCL4 (MIP-1) | Leukocyte chemoattractant |
143
↓ H, P ↓ P |
| TNF | Pro-inflammatory (NF-κB activator) |
147
n.s.d. CP 149 ↑ P 149 ↑ CP 79 ↓ CP 150 n.s.d. CP |
| PGE2 | Pro-inflammatory (COX pathway; direct vasodilator) |
144
↓ CS 151 n.s.d. CP |
| CCL5 (RANTES) | Leukocyte chemoattractant | 143 ↓ H, P |
| TGF-β | Suppressor of leukocyte activation; extracelluar matrix stimulation |
152
↑ CP 153 n.s.d. SP |
| PAI-2 | Anti-inflammatory (blocks plasmin-C3a axis) | 154 H ↓ |
↓, significantly reduced in smokers compared to non-smokers; ↑, significantly enhanced in smokers compared to non-smokers; nsd, no significant difference.
AP, aggressive periodontitis; COX, cyclooxygenase; CP, chronic periodontitis; CS, cross-sectional; GCF, gingival crevicular fluid; H, periodontally healthy; IL-1Ra, IL-1 receptor antagonist; P, periodontitis; PAI-2, plasminogen activator inhibitor-2; PGE2, prostaglandin E2; SP, severe periodontitis; SPT, supportive periodontal therapy subjects; STAT-3, signal transducer and activator of transcription-3; TNF, tumor necrosis factor (formerly TNF-α).
n.s.d. for former and current smokers with a pack year history of less than 20 years.
The response of innate cells to plaque in tobacco smokers
Innate cells activated in the host response to plaque release large amounts of factors that drive inflammation, angiogenesis and tissue remodeling. The primary innate cells found in the gingival crevice are neutrophils, where they attempt to form a barrier between the junctional epithelium and the accumulating microbiome80.
A detrimental effect of cigarette smoking on oral neutrophil function has been suspected for at least 40 years81,82. Tobacco-related cytoskeletal, chemotactic, oxidative burst, phagocytic, bactericidal and other functional defects, as well as inefficient neutrophil differentiation and compromised viability, have all been noted48,83–87.
While smoking induces a chronic systemic neutrophilia88, the crevicular neutrophil count in smokers and non-smokers in disease-matched subjects appear similar78, which, again, suggests compromised vascular-neutrophil interactions controlling diapedesis into the gingival tissues and crevice.
Multiple research avenues suggest that neutrophil interactions with oral microbes are compromised by smoking, although how this may influence gingival bleeding is not entirely clear. For example, nicotine has been shown to diminish elements of the oxidative burst and inhibit the ability of human neutrophils to kill multiple bacteria, including Actinomyces naeslundii, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Porphyromonas gingivalis and Staphylococcus aureus34,35,85.
The function of other myeloid cell types may be similarly compromised by cigarette smoke. For example, Yanagita et al reported that nicotine exposure during differentiation suppressed subsequent production of multiple cytokines (TNF, IL-10, IL-12 p40 and p70, RANTES/CCL5) by P. gingivalis-derived LPS-stimulated monocyte-derived dendritic cells, while MCP-1/CCL2 and CCL-22 (macrophage-derived chemokine, MDC) release was enhanced89. Ryder et al have observed a profound influence of tobacco smoke on the monocytic transcriptome90. A reduced IL-12 (p70) response to oral bacteria (Prevotella intermedia and Fusobacterium nucleatum) in PBMCs isolated from smokers with generalized aggressive periodontitis, as compared to innate cells isolated from diseased non-smokers, has also been noted91.
Interestingly, in vitro exposure of endothelial cells to high levels of nicotine (10 mM) resulted in reduced production of the leukocyte chemoattractant, IL-8, and a suppression of endothelial migration in response to stimulation with P. gingivalis-derived lipopolysaccharide (LPS)92. Such evidence is supportive of the anti-angiogenesis hypothesis.
Thus, a profound influence of cigarette consumption on innate cell function is apparent. What is less clear is how cigarette-innate cell interactions may be mechanistically linked to a reduced vascular response to dental plaque. Further research in this area seems warranted.
Tobacco-microbe interactions
It has now been firmly established that smoking profoundly influences the composition of the subgingival biofilm93–102; that the microbiome of smokers is more diverse, pathogen rich and commensal poor94,95,97–100; that it is more difficult to eradicate key pathogens from smokers, compared to non-smokers93,103; and that smokers are more susceptible to the re-establishment of a pathogenic subgingival biofilm than are non-smokers93. The elegant study by Joshi et al, showed that smokers exhibit early colonization by, and more abundant infection with, pathogens relative to non-smokers as established in an experimental gingivitis setting94.
While the relevance of an altered microbiome to a reduced bleeding response in smokers is not transparent, certain clues are available. Indeed, the cigarette-altered oral microbiota may have less pro-inflammatory potential than plaque in non-smokers. For example, key drivers of gingival inflammation are the lipopolysaccharide (LPS) molecules produced by Gram-negative bacteria. Variant LPS structures have differential capacities to engage the Toll-like receptors (TLRs) that drive inflammatory mediator release from epithelial and immune cells. Certainly, the overall LPS profile in smokers, as measured in saliva, is composed of lower levels of LPS with the optimal TLR-activating structure than that of non-smokers104.
The best studied periodontal pathogen is P. gingivalis. Smokers are more likely to be infected with, and to harbor higher numbers of, Porphyromonas gingivalis, relative to non-smokers105–109. In other words, the periodontium of smokers is a preferred niche for P. gingivalis. Indeed, P. gingivalis is resistant to very high doses of cigarette smoke and tobacco constituents110–113. Yet, tobacco-pathogen interactions generally, and P. gingivalis specifically, remain poorly understood.
Recent work has established that smoke exposure exerts a profound influence on P. gingivalis physiology resulting in dramatically altered pathogen-innate immune interactions. For example, the whole blood leukocyte response to P. gingivalis, other bacteria or bacterial products, as monitored by cytokine release (IL-1β), has been shown to be suppressed in smokers compared to non-smokers114. In keeping with de Heens et al, P. gingivalis cells exposed to cigarette smoke extract (CSE) have been shown induce a lower pro-inflammatory response (TNF, IL-6, IL-12 p40) from monocytes and peripheral blood mononuclear cells than do unexposed bacteria111. Further, CSE leads to differential regulation of a large number of P. gingivalis genes111, the products of several having the potential to regulate the inflammatory response in the periodontium. For example, the production of bacterial capsule, highly inflammatory in most strains, is down-regulated in CSE-exposed P. gingivalis cells115. At the same time, FimA, the primary component of the major fimbriae, is upregulated. FimA protein can induce TLR hyposensitivity in immune cells as monitored by pro-inflammatory cytokine production, yet efficiently induce anti-inflammatory IL-10 release111,115. CSE exposure can also promote mono- and dual species P. gingivalis biofilms110. This phenomenon is likely to promote P. gingivalis persistence. Persistence coupled with lower inflammatory potential may, therefore, contribute to a chronic, low-grade infection and, subsequently, the reduced bleeding response noted in smokers relative to non-smokers.
Animal models
We discussed the influence of tobacco or nicotine on gingival blood flow earlier in, rabbits and rodents. There are, perhaps surprisingly, only a few other studies that have employed animals to model tobacco-induced periodontal diseases directly. For example, in 1977, Kraal et al observed that a solution of tobacco smoke applied to the gingiva of dogs could suppress subsequent crevicular innate cell migration in neutrophils isolated from both healthy and inflamed sites116. In nicotine-exposed rats, both capillary length and height were noted to be reduced, compared to untreated controls, in the maxillary gingiva117. Breivik et al, 2009 reported that, in ligature-induced periodontitis in rats, nicotine administration enhanced alveolar bone loss concomitant with a reduced pro- and anti-acute inflammatory cytokine response to LPS (TNF, TGF-β, IL-10)118. It is unclear why anti-inflammatory cytokines, which were not influenced by pre-treatment of the non-selective nicotinic receptor antagonist, mecamylamine, should be suppressed in this model. Animal studies, therefore, reflect the reduced inflammatory burden noted in diseased adults. Further, the obvious in vivo model, where animals are exposed to direct or indirect cigarette smoke in an exposure chamber is lacking but likely to be particularly informative.
Genetic predisposition to tobacco-related periodontal diseases
With the profound negative influence of cigarette use on periodontal health well-established, it is unfortunate that some studies examining potential genetic links to disease susceptibility do not consider this key factor. Limited evidence, though, does suggest gene-tobacco interactions that may predispose to destructive periodontal diseases.
For example, Yoshihara et al119 reported that smoking enhances the progression of periodontal tissue destruction in elderly people with a FcγRIIIb (CD16b)-neutrophil antigen (NA) 2 polymorphism which determines neutrophil responses to IgG antibody (O.R., 3.0; 95% CI, 1.1–8.3). However, the bleeding response was not reported. CCL2, the leukocyte chemoattractant, is reduced in the GCF of smokers with periodontitis (Table 1). In a study of generalized aggressive periodontitis, the authors reported an increased disease risk for males who smoked in combination with either a particular CCL2 polymorphism (G+ genotype; O.R. 4.9) or VV polymorphism in the CCL2 receptor, CCR2, (O.R., 7.2; 95% CI, 1.3–41.1)120. Again, the influence of such gene-tobacco interactions on specific components of the inflammatory response was not the study focus. In a study of chronic and aggressive periodontitis in the context of smoking and a variant vitamin D receptor (VDR) sequence, both the presence and the progression of disease was associated with the VDR-Taq-I TT polymorphism121. Bleeding on probing was measured, but not reported. Recently, others have reported an association between smoking, TNF-308 GA/AA genotypes and peri-implantitis122.
Thus, while increasing data are suggestive of genetic predisposition to destructive periodontal diseases in smokers, the influence of such tobacco-gene interactions on the vascular response to plaque remain unclear.
Conclusions
Tobacco use is a, or the, leading risk factor for chronic periodontitis. Cigarette smoking leads to a suppression of overt gingival inflammation, manifested as reduced angiogenesis and a compromised bleeding response to plaque (Figure 1), but simultaneously promotes periodontal tissue destruction relative to non-smokers. The bleeding response recovers rapidly following smoking cessation. The mechanisms underlying vascular suppression in cigarette users are yet to be fully elucidated. While there may be a genetic element to susceptibility, tobacco use profoundly influences the biological mediators that control the angiogenic and bleeding responses to bacterial challenges (Tables 1–3); promotes oral bacterial dysbiosis; compromises innate cell function; and promotes a protease-antiprotease imbalance in the gingival tissues. It may be possible that specific preventive and therapeutic approaches to the control of chronic periodontitis, and other destructive plaque-induced oral disease, may need to be tailored for smokers.
Review provisos
It is important to note that biochemical confirmation of smoking status and the level of smoke exposure, such as biofluid cotinine analysis or expired-air CO monitoring, are clearly preferable to self-report. However, the studies cited herein contain a variety of classification systems for smoking status. Additionally, while many contemporary studies delineate periodontal diseases using the 1999 Consensus Classification of Periodontal Diseases123, those cited herein use a wide variety of definitions for “periodontitis”. It is not practical to note such nuances herein. The manuscript by Grudianov and Kemulariia59 is in Russian with interpretation reliant on the English abstract. As a final proviso, because the profound negative influence of cigarette use on oral health is accepted, the exclusion of smokers from research studies is a not uncommon strategy. Therefore, smokers may be under-represented in the knowledge base.
Supplementary Material
Funding and acknowledgments
The tobacco-related research in D.A. Scott’s lab is funded by the U.S. Department of Health and Human Services via the National Institute for Dental and Craniofacial Research (R01DE026963 [DAS]; R01DE017680 [DAS]; and R01DE026963 [P.I., H. Wang]); and via the National Institute for General Medical Sciences / National Institute for Dental and Craniofacial Research (P20GM125504 [P.I., R.J. Lamont]).
Footnotes
Declaration of Interests
The authors have no competing interests to declare.
References
- 1.Gonzalez YM, De Nardin A, Grossi SG, Machtei EE, Genco RJ, De Nardin E. Serum cotinine levels, smoking, and periodontal attachment loss. J Dent Res. 1996;75(2):796–802. [DOI] [PubMed] [Google Scholar]
- 2.Rheu GB, Ji S, Ryu JJ, et al. Risk assessment for clinical attachment loss of periodontal tissue in Korean adults. J Adv Prosthodont. 2011;3(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacFarlane GD, Herzberg MC, Wolff LF, Hardie NA. Refractory periodontitis associated with abnormal polymorphonuclear leukocyte phagocytosis and cigarette smoking. J Periodontol. 1992;63(11):908–913. [DOI] [PubMed] [Google Scholar]
- 4.Soder B, Nedlich U, Jin LJ. Longitudinal effect of non-surgical treatment and systemic metronidazole for 1 week in smokers and non-smokers with refractory periodontitis: a 5-year study. J Periodontol. 1999;70(7):761–771. [DOI] [PubMed] [Google Scholar]
- 5.Machtei EE, Dunford R, Hausmann E, et al. Longitudinal study of prognostic factors in established periodontitis patients. J Clin Periodontol. 1997;24(2):102–109. [DOI] [PubMed] [Google Scholar]
- 6.Grossi SG, Zambon J, Machtei EE, et al. Effects of smoking and smoking cessation on healing after mechanical periodontal therapy. J Am Dent Assoc. 1997;128(5):599–607. [DOI] [PubMed] [Google Scholar]
- 7.Sutton JD, Ranney LM, Wilder RS, Sanders AE. Environmental tobacco smoke and periodontitis in U.S. non-smokers. Journal of dental hygiene : JDH / American Dental Hygienists’ Association. 2012;86(3):185–194. [PubMed] [Google Scholar]
- 8.Akinkugbe AA, Slade GD, Divaris K, Poole C. Systematic Review and Meta-analysis of the Association Between Exposure to Environmental Tobacco Smoke and Periodontitis Endpoints Among Nonsmokers. Nicotine Tob Res. 2016;18(11):2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton JD, Salas Martinez ML, Gerkovich MM. Environmental Tobacco Smoke and Periodontitis in US Non-smokers, 2009 to 2012. J Periodontol. 2017:1–14. [DOI] [PubMed] [Google Scholar]
- 10.Sanders A, Slade G. State cigarette excise tax, secondhand smoke exposure, and periodontitis in US nonsmokers. American journal of public health. 2013;103(4):740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergstrom J Smoking rate and periodontal disease prevalence: 40-year trends in Sweden 1970–2010. J Clin Periodontol. 2014;41(10):952–957. [DOI] [PubMed] [Google Scholar]
- 12.Haisman-Welsh RJ, Thomson WM. Changes in periodontitis prevalence over two decades in New Zealand: evidence from the 1988 and 2009 national surveys. The New Zealand dental journal. 2012;108(4):134–138. [PubMed] [Google Scholar]
- 13.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71(5):743–751. [DOI] [PubMed] [Google Scholar]
- 14.Eke PI, Zhang X, Lu H, et al. Predicting Periodontitis at State and Local Levels in the United States. J Dent Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogtmann E, Graubard B, Loftfield E, et al. Contemporary impact of tobacco use on periodontal disease in the USA. Tob Control. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eke PI, Wei L, Thornton-Evans GO, et al. Risk Indicators for Periodontitis in US Adults: National Health and Nutrition Examination Survey (NHANES) 2009 – 2012. J Periodontol. 2016:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eke PI, Dye BA, Wei L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.W.H.O. WHO global report on trends in prevalence of tobacco smoking. 2015.
- 19.Khan S, Khalid T, Awan KH. Chronic periodontitis and smoking. Prevalence and dose-response relationship. Saudi Med J. 2016;37(8):889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott DA, Singer DL. Suppression of overt gingival inflammation in tobacco smokers - clinical and mechanistic considerations. Int J Dent Hyg. 2004;2(3):104–110. [DOI] [PubMed] [Google Scholar]
- 21.Pindborg JJ. Tobacco and gingivitis: statistical examination of the significance of tobacco in the development of ulceromembranous gingivitis and in the formation of calculus. J Dent Res. 1947;26(3):261–264. [DOI] [PubMed] [Google Scholar]
- 22.Pindborg JJ. Tobacco and gingivitis; correlation between consumption of tobacco, ulceromembranous gingiivitis and calculus. J Dent Res. 1949;28(5):460–463. [DOI] [PubMed] [Google Scholar]
- 23.McMurray CM, Moore GE, Vincent R. Gingival bleeding after cessation of smoking. J Oral Med. 1969;24(3):131. [PubMed] [Google Scholar]
- 24.Adamowicz K, Wang H, Jotwani R, Zeller I, Potempa J, Scott D. Inhibition of GSK3 abolishes bacterial-induced periodontal bone loss in mice. Mol Med. 2012(18):1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagaitkar J, Zeller I, Renaud DE, Scott DA. Cotinine inhibits the pro-inflammatory response initiated by multiple cell surface Toll-like receptors in monocytic THP cells. Tob Induc Dis. 2012;10(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehani K, Scott DA, Renaud D, et al. Cotinine-induced convergence of the cholinergic and PI3 kinase-dependent anti-inflammatory pathways in innate immune cells. Biochim Biophys Acta. 2008;1783(3):375–382. [DOI] [PubMed] [Google Scholar]
- 27.Gunsolley JC, Pandey JP, Quinn SM, Tew J, Schenkein HA. The effect of race, smoking and immunoglobulin allotypes on IgG subclass concentrations. J Periodontal Res. 1997;32(4):381–387. [DOI] [PubMed] [Google Scholar]
- 28.Quinn SM, Zhang JB, Gunsolley JC, Schenkein HA, Tew JG. The influence of smoking and race on adult periodontitis and serum IgG2 levels. J Periodontol. 1998;69(2):171–177. [DOI] [PubMed] [Google Scholar]
- 29.Tebloeva LM, Revazova ZE, Fabrikant KG, Dmitrieva LA, Gurevich KG. Differences in immune response to Porphyromonas gingivalis. J Contemp Dent Pract. 2014;15(5):573–575. [DOI] [PubMed] [Google Scholar]
- 30.Zeller I, Hutcherson JA, Lamont RJ, et al. Altered Antigenic Profiling and Infectivity of Porphyromonas Gingivalis in Smokers and Non-Smokers With Periodontitis. J Periodontol. 2014;85:837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mooney J, Hodge PJ, Kinane DF. Humoral immune response in early-onset periodontitis: influence of smoking. J Periodontal Res. 2001;36(4):227–232. [DOI] [PubMed] [Google Scholar]
- 32.Archana MS, Bagewadi A, Keluskar V. Assessment and comparison of phagocytic function and viability of polymorphonuclear leukocytes in saliva of smokers and non-smokers. Arch Oral Biol. 2015;60(2):229–233. [DOI] [PubMed] [Google Scholar]
- 33.Bondy-Carey JL, Galicia J, Bagaitkar J, et al. Neutrophils alter epithelial response to Porphyromonas gingivalis in a gingival crevice model. Mol Oral Microbiol. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pabst MJ, Pabst KM, Collier JA, et al. Inhibition of neutrophil and monocyte defensive functions by nicotine. J Periodontol. 1995;66(12):1047–1055. [DOI] [PubMed] [Google Scholar]
- 35.Guzik K, Skret J, Smagur J, et al. Cigarette smoke-exposed neutrophils die unconventionally but are rapidly phagocytosed by macrophages. Cell Death Dis. 2011;2:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang D, Wang KJ, Tang ZQ, et al. Effects of nicotine on the metabolism and gene expression profile of SpragueDawley rat primary osteoblasts. Mol Med Rep. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirschneck C, Maurer M, Wolf M, Reicheneder C, Proff P. Regular nicotine intake increased tooth movement velocity, osteoclastogenesis and orthodontically induced dental root resorptions in a rat model. Int J Oral Sci. 2017;9(3):174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dodington DW, Fritz PC, Sullivan PJ, Ward WE. Higher Intakes of Fruits and Vegetables, beta-Carotene, Vitamin C, alpha-Tocopherol, EPA, and DHA Are Positively Associated with Periodontal Healing after Nonsurgical Periodontal Therapy in Nonsmokers but Not in Smokers. J Nutr. 2015;145(11):2512–2519. [DOI] [PubMed] [Google Scholar]
- 39.Mathias TM, Silva JF, Sapata VM, Marson FC, Zanoni JN, Silva CO. Evaluation of the effects of periodontal treatment on levels of ascorbic acid in smokers. J Int Acad Periodontol. 2014;16(4):109–114. [PubMed] [Google Scholar]
- 40.Romero A, Caceres M, Arancibia R, et al. Cigarette smoke condensate inhibits collagen gel contraction and prostaglandin E2 production in human gingival fibroblasts. J Periodontal Res. 2015;50(3):371–379. [DOI] [PubMed] [Google Scholar]
- 41.Bunaes DF, Mustafa M, Mohamed HG, Lie SA, Leknes KN. The effect of smoking on inflammatory and bone remodeling markers in gingival crevicular fluid and subgingival microbiota following periodontal therapy. J Periodontal Res. 2017. [DOI] [PubMed] [Google Scholar]
- 42.Preber H, Linder L, Bergstrom J. Periodontal healing and periopathogenic microflora in smokers and non-smokers. J Clin Periodontol. 1995;22(12):946–952. [DOI] [PubMed] [Google Scholar]
- 43.Dietrich T, Bernimoulin JP, Glynn RJ. The effect of cigarette smoking on gingival bleeding. J Periodontol. 2004;75(1):16–22. [DOI] [PubMed] [Google Scholar]
- 44.Prakash P, Rath S, Mukherjee M, et al. Comparative evaluation of the marginal gingival epithelium in smokers and nonsmokers: a histomorphometric and immunohistochemical study. Int J Periodontics Restorative Dent. 2014;34(6):781–786. [DOI] [PubMed] [Google Scholar]
- 45.Villar CC, de Lima AF. Smoking influences on the thickness of marginal gingival epithelium. Pesqui Odontol Bras. 2003;17(1):41–45. [DOI] [PubMed] [Google Scholar]
- 46.Hanioka T, Tanaka M, Ojima M, Takaya K, Matsumori Y, Shizukuishi S. Oxygen sufficiency in the gingiva of smokers and non-smokers with periodontal disease. J Periodontol. 2000;71(12):1846–1851. [DOI] [PubMed] [Google Scholar]
- 47.Hanioka T, Tanaka M, Takaya K, Matsumori Y, Shizukuishi S. Pocket oxygen tension in smokers and non-smokers with periodontal disease. J Periodontol. 2000;71(4):550–554. [DOI] [PubMed] [Google Scholar]
- 48.Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors-- tobacco smoking. J Clin Periodontol. 2005;32 Suppl 6:180–195. [DOI] [PubMed] [Google Scholar]
- 49.Rivera-Hidalgo F Smoking and periodontal disease. Periodontol 2000. 2003;32:50–58. [DOI] [PubMed] [Google Scholar]
- 50.Clarke NG, Shephard BC, Hirsch RS. The effects of intra-arterial epinephrine and nicotine on gingival circulation. Oral Surg Oral Med Oral Pathol. 1981;52(6):577–582. [DOI] [PubMed] [Google Scholar]
- 51.Clarke NG, Shephard BC. The effects of epinephrine and nicotine on gingival blood flow in the rabbit. Arch Oral Biol. 1984;29(10):789–793. [DOI] [PubMed] [Google Scholar]
- 52.Johnson GK, Todd GL, Johnson WT, Fung YK, Dubois LM. Effects of topical and systemic nicotine on gingival blood flow in dogs. J Dent Res. 1991;70(5):906–909. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura T, Ono K, Honda E, Yokota M, Inenaga K. Central nicotinic stimulation reduces vascular conductance in the gingiva in anesthetized rats. J Periodontal Res. 2005;40(1):67–72. [DOI] [PubMed] [Google Scholar]
- 54.Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. [DOI] [PubMed] [Google Scholar]
- 55.Meekin TN, Wilson RF, Scott DA, Ide M, Palmer RM. Laser Doppler flowmeter measurement of relative gingival and forehead skin blood flow in light and heavy smokers during and after smoking. J Clin Periodontol. 2000;27(4):236–242. [DOI] [PubMed] [Google Scholar]
- 56.Baab DA, Oberg PA. The effect of cigarette smoking on gingival blood flow in humans. J Clin Periodontol. 1987;14(7):418–424. [DOI] [PubMed] [Google Scholar]
- 57.Bornmyr S, Svensson H. Thermography and laser-Doppler flowmetry for monitoring changes in finger skin blood flow upon cigarette smoking. Clin Physiol. 1991;11(2):135–141. [DOI] [PubMed] [Google Scholar]
- 58.Mavropoulos A, Aars H, Brodin P. Hyperaemic response to cigarette smoking in healthy gingiva. J Clin Periodontol. 2003;30(3):214–221. [DOI] [PubMed] [Google Scholar]
- 59.Grudianov AI, Kemulariia IV. [Laser doppler estimation of the influence of tobacco-smoking on the blood microcirculation in the periodont at the patients with the different stages of periodontal diseases]. Stomatologiia (Mosk). 2010;89(6):10–14. [PubMed] [Google Scholar]
- 60.Mavropoulos A, Brodin P, Rosing CK, Aass AM, Aars H. Gingival blood flow in periodontitis patients before and after periodontal surgery assessed in smokers and non-smokers. J Periodontol. 2007;78(9):1774–1782. [DOI] [PubMed] [Google Scholar]
- 61.Molnar E, Lohinai Z, Demeter A, Mikecs B, Toth Z, Vag J. Assessment of heat provocation tests on the human gingiva: the effect of periodontal disease and smoking. Acta Physiol Hung. 2015;102(2):176–188. [DOI] [PubMed] [Google Scholar]
- 62.Kouadio AA, Jordana F, Koffi NJ, Le Bars P, Soueidan A. The use of laser Doppler flowmetry to evaluate oral soft tissue blood flow in humans: A review. Arch Oral Biol. 2018;86:58–71. [DOI] [PubMed] [Google Scholar]
- 63.Bergstrom J, Persson L, Preber H. Influence of cigarette smoking on vascular reaction during experimental gingivitis. Scand J Dent Res. 1988;96(1):34–39. [DOI] [PubMed] [Google Scholar]
- 64.Mirbod SM, Ahing SI, Pruthi VK. Immunohistochemical study of vestibular gingival blood vessel density and internal circumference in smokers and non-smokers. J Periodontol. 2001;72(10):1318–1323. [DOI] [PubMed] [Google Scholar]
- 65.Scardina GA, Messina P. Morphologic changes in the microcirculation induced by chronic smoking habit: a videocapillaroscopic study on the human gingival mucosa. Am J Dent. 2005;18(4):301–304. [PubMed] [Google Scholar]
- 66.Lindeboom JA, Mathura KR, Harkisoen S, van den Akker HP, Ince C. Effect of smoking on the gingival capillary density: assessment of gingival capillary density with orthogonal polarization spectral imaging. J Clin Periodontol. 2005;32(12):1208–1212. [DOI] [PubMed] [Google Scholar]
- 67.Rezavandi K, Palmer RM, Odell EW, Scott DA, Wilson RF. Expression of ICAM-1 and E-selectin in gingival tissues of smokers and non-smokers with periodontitis. J Oral Pathol Med. 2002;31(1):59–64. [DOI] [PubMed] [Google Scholar]
- 68.Fraser HS, Palmer RM, Wilson RF, Coward PY, Scott DA. Elevated systemic concentrations of soluble ICAM-1 (sICAM) are not reflected in the gingival crevicular fluid of smokers with periodontitis. J Dent Res. 2001;80(7):1643–1647. [DOI] [PubMed] [Google Scholar]
- 69.Palmer RM, Stapleton JA, Sutherland G, Coward PY, Wilson RF, Scott DA. Effect of nicotine replacement and quitting smoking on circulating adhesion molecule profiles (sICAM-1, sCD44v5, sCD44v6). Eur J Clin Invest. 2002;32(11):852–857. [DOI] [PubMed] [Google Scholar]
- 70.Rittich AB, Ellrich J, Said Yekta-Michael S. Assessment of lingual nerve functions after smoking cessation. Acta Odontol Scand. 2017;75(5):338–344. [DOI] [PubMed] [Google Scholar]
- 71.Yekta SS, Luckhoff A, Ristic D, Lampert F, Ellrich J. Impaired somatosensation in tongue mucosa of smokers. Clin Oral Investig. 2012;16(1):39–44. [DOI] [PubMed] [Google Scholar]
- 72.Nair P, Sutherland G, Palmer RM, Wilson RF, Scott DA. Gingival bleeding on probing increases after quitting smoking. J Clin Periodontol. 2003;30(5):435–437. [DOI] [PubMed] [Google Scholar]
- 73.Morozumi T, Kubota T, Sato T, Okuda K, Yoshie H. Smoking cessation increases gingival blood flow and gingival crevicular fluid. J Clin Periodontol. 2004;31(4):267–272. [DOI] [PubMed] [Google Scholar]
- 74.Ozcaka O, Bicakci N, Pussinen P, Sorsa T, Kose T, Buduneli N. Smoking and matrix metalloproteinases, neutrophil elastase and myeloperoxidase in chronic periodontitis. Oral Dis. 2011;17(1):68–76. [DOI] [PubMed] [Google Scholar]
- 75.Nakamura T, Ebihara I, Shimada N, Koide H. Effect of cigarette smoking on plasma metalloproteinase-9 concentration. Clin Chim Acta. 1998;276(2):173–177. [DOI] [PubMed] [Google Scholar]
- 76.Crook MA, Scott DA, Stapleton JA, Palmer RM, Wilson RF, Sutherland G. Circulating concentrations of C-reactive protein and total sialic acid in tobacco smokers remain unchanged following one year of validated smoking cessation. Eur J Clin Invest. 2000;30(10):861–865. [DOI] [PubMed] [Google Scholar]
- 77.Miki K, Miki M, Okano Y, et al. Cigarette smoke-induced acute eosinophilic pneumonia accompanied with neutrophilia in the blood. Intern Med. 2002;41(11):993–996. [DOI] [PubMed] [Google Scholar]
- 78.Alavi AL, Palmer RM, Odell EW, Coward PY, Wilson RF. Elastase in gingival crevicular fluid from smokers and non-smokers with chronic inflammatory periodontal disease. Oral Dis. 1995;1(3):110–114. [DOI] [PubMed] [Google Scholar]
- 79.He CY, Gao XQ, Jiang LP. The impact of smoking on levels of chronic periodontitis-associated biomarkers. Exp Mol Pathol. 2016;101(1):110–115. [DOI] [PubMed] [Google Scholar]
- 80.Scott DA, Krauss J. Neutrophils in periodontal inflammation. Front Oral Biol. 2012;15:56–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kenney EB, Kraal JH, Saxe SR, Jones J. The effect of cigarette smoke on human oral polymorphonuclear leukocytes. J Periodontal Res. 1977;12(4):227–234. [DOI] [PubMed] [Google Scholar]
- 82.Kraal JH, Kenney EB. The response of polymorphonuclear leukocytes to chemotactic stimulation for smokers and non-smokers. J Periodontal Res. 1979;14(5):383–389. [DOI] [PubMed] [Google Scholar]
- 83.Ryder MI. The influence of smoking on host responses in periodontal infections. Periodontol 2000. 2007;43:267–277. [DOI] [PubMed] [Google Scholar]
- 84.Ryder MI, Wu TC, Kallaos SS, Hyun W. Alterations of neutrophil f-actin kinetics by tobacco smoke: implications for periodontal diseases. J Periodontal Res. 2002;37(4):286–292. [DOI] [PubMed] [Google Scholar]
- 85.Xu M, Scott JE, Liu KZ, et al. The influence of nicotine on granulocytic differentiation - inhibition of the oxidative burst and bacterial killing and increased matrix metalloproteinase-9 release. BMC Cell Biol. 2008;9(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guntsch A, Erler M, Preshaw PM, Sigusch BW, Klinger G, Glockmann E. Effect of smoking on crevicular polymorphonuclear neutrophil function in periodontally healthy subjects. J Periodontal Res. 2006;41(3):184–188. [DOI] [PubMed] [Google Scholar]
- 87.Mariggio MA, Guida L, Laforgia A, et al. Nicotine effects on polymorphonuclear cell apoptosis and lipopolysaccharide-induced monocyte functions. A possible role in periodontal disease? J Periodontal Res. 2001;36(1):32–39. [DOI] [PubMed] [Google Scholar]
- 88.Fredriksson MI, Figueredo CM, Gustafsson A, Bergstrom KG, Asman BE. Effect of periodontitis and smoking on blood leukocytes and acute-phase proteins. J Periodontol. 1999;70(11):1355–1360. [DOI] [PubMed] [Google Scholar]
- 89.Yanagita M, Mori K, Kobayashi R, et al. Immunomodulation of dendritic cells differentiated in the presence of nicotine with lipopolysaccharide from Porphyromonas gingivalis. Eur J Oral Sci. 2012;120(5):408–414. [DOI] [PubMed] [Google Scholar]
- 90.Ryder MI, Hyun W, Loomer P, Haqq C. Alteration of gene expression profiles of peripheral mononuclear blood cells by tobacco smoke: implications for periodontal diseases. Oral Microbiol Immunol. 2004;19(1):39–49. [DOI] [PubMed] [Google Scholar]
- 91.Borch TS, Holmstrup P, Bendtzen K, Nielsen CH. In vitro cytokine responses to periodontal pathogens: generalized aggressive periodontitis is associated with increased IL-6 response to Porphyromonas gingivalis. Scand J Immunol. 2010;71(6):440–446. [DOI] [PubMed] [Google Scholar]
- 92.An N, Andrukhov O, Tang Y, et al. Effect of nicotine and porphyromonas gingivalis lipopolysaccharide on endothelial cells in vitro. PLoS One. 2014;9(5):e96942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feres M, Teacher H, Bernal MA, et al. Subgingival bacterial re-colonization after scaling and root planing in smokers with chronic periodontitis. Aust Dent J. 2014. [DOI] [PubMed] [Google Scholar]
- 94.Joshi V, Matthews C, Aspiras M, de Jager M, Ward M, Kumar P. Smoking decreases structural and functional resilience in the subgingival ecosystem. J Clin Periodontol. 2014. [DOI] [PubMed] [Google Scholar]
- 95.Moon JH, Lee JH, Lee JY. Subgingival microbiome in smokers and non-smokers in Korean chronic periodontitis patients. Mol Oral Microbiol. 2014. [DOI] [PubMed] [Google Scholar]
- 96.Guglielmetti MR, Rosa EF, Lourencao DS, et al. Detection and Quantification of Periodontal Pathogens in Smokers and Never-Smokers With Chronic Periodontitis by Real-Time Polymerase Chain Reaction. J Periodontol. 2014. [DOI] [PubMed] [Google Scholar]
- 97.Mason MR, Preshaw PM, Nagaraja HN, Dabdoub SM, Rahman A, Kumar PS. The subgingival microbiome of clinically healthy current and never smokers. The ISME journal. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar PS. Smoking and the subgingival ecosystem: a pathogen-enriched community. Future microbiology. 2012;7(8):917–919. [DOI] [PubMed] [Google Scholar]
- 99.Kumar PS, Matthews CR, Joshi V, de Jager M, Aspiras M. Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect Immun. 2011;79(11):4730–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matthews CR, Joshi V, de Jager M, Aspiras M, Kumar PS. Host-bacterial interactions during induction and resolution of experimental gingivitis in current smokers. J Periodontol. 2013;84(1):32–40. [DOI] [PubMed] [Google Scholar]
- 101.Shchipkova AY, Nagaraja HN, Kumar PS. Subgingival microbial profiles of smokers with periodontitis. J Dent Res. 2010;89(11):1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bizzarro S, Loos BG, Laine ML, Crielaard W, Zaura E. Subgingival microbiome in smokers and non-smokers in periodontitis: an exploratory study using traditional targeted techniques and a next-generation sequencing. J Clin Periodontol. 2013;40(5):483–492. [DOI] [PubMed] [Google Scholar]
- 103.Meulman T, Casarin RC, Peruzzo DC, et al. Impact of supragingival therapy on subgingival microbial profile in smokers versus non-smokers with severe chronic periodontitis. J Oral Microbiol. 2012;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buduneli N, Larsson L, Biyikoglu B, Renaud DE, Bagaitkar J, Scott DA. Fatty acid profiles in smokers with chronic periodontitis. J Dent Res. 2011;90(1):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zambon JJ, Grossi SG, Machtei EE, Ho AW, Dunford R, Genco RJ. Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J Periodontol. 1996;67(10 Suppl):1050–1054. [DOI] [PubMed] [Google Scholar]
- 106.Haffajee AD, Socransky SS. Relationship of cigarette smoking to the subgingival microbiota. J Clin Periodontol. 2001;28(5):377–388. [DOI] [PubMed] [Google Scholar]
- 107.Eggert FM, McLeod MH, Flowerdew G. Effects of smoking and treatment status on periodontal bacteria: evidence that smoking influences control of periodontal bacteria at the mucosal surface of the gingival crevice. J Periodontol. 2001;72(9):1210–1220. [DOI] [PubMed] [Google Scholar]
- 108.Kamma JJ, Nakou M, Baehni PC. Clinical and microbiological characteristics of smokers with early onset periodontitis. J Periodontal Res. 1999;34(1):25–33. [DOI] [PubMed] [Google Scholar]
- 109.Winning L, Patterson CC, Cullen KM, et al. The association between subgingival periodontal pathogens and systemic inflammation. J Clin Periodontol. 2015;42(9):799–806. [DOI] [PubMed] [Google Scholar]
- 110.Bagaitkar J, Daep CA, Patel CK, Renaud DE, Demuth DR, Scott DA. Tobacco smoke augments Porphyromonas gingivalis - Streptococcus gordonii biofilm formation. PLOS One. 2011;6(11):e27386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bagaitkar J, Scott DA, Williams LR, et al. Tobacco-induced alterations to Porphyromonas gingivalis-host interactions. Environmental Microbiology. 2009;11(5):1242–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cogo K, Calvi BM, Mariano FS, Franco GC, Goncalves RB, Groppo FC. The effects of nicotine and cotinine on Porphyromonas gingivalis colonisation of epithelial cells. Arch Oral Biol. 2009;54(11):1061–1067. [DOI] [PubMed] [Google Scholar]
- 113.Cogo K, Montan MF, Bergamaschi Cde C, E DA, Rosalen PL, Groppo FC. In vitro evaluation of the effect of nicotine, cotinine, and caffeine on oral microorganisms. Can J Microbiol. 2008;54(6):501–508. [DOI] [PubMed] [Google Scholar]
- 114.de Heens GL, Kikkert R, Aarden LA, van der Velden U, Loos BG. Effects of smoking on the ex vivo cytokine production in periodontitis. J Periodontal Res. 2009;44(1):28–34. [DOI] [PubMed] [Google Scholar]
- 115.Bagaitkar J, Demuth DR, Daep CA, Renaud DE, Pierce DL, Scott DA. Tobacco upregulates P. gingivalis fimbrial proteins which induce TLR2 hyposensitivity. PLoS One. 2010;5(5):e9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kraal JH, Chancellor MB, Bridges RB, Bemis KG, Hawke JE. Variations in the gingival polymorphonuclear leukocyte migration rate in dogs induced by chemotactic autologous serum and migration inhibitor from tobacco smoke. J Periodontal Res. 1977;12(4):242–249. [DOI] [PubMed] [Google Scholar]
- 117.Johnson GK, Fung YK, Squier CA. Effects of systemic administration of nicotine on capillaries in rat oral mucosa. J Oral Pathol Med. 1989;18(4):230–232. [DOI] [PubMed] [Google Scholar]
- 118.Breivik T, Gundersen Y, Gjermo P, von Horsten S, Opstad PK. Nicotinic acetylcholine receptor activation mediates nicotine-induced enhancement of experimental periodontitis. J Periodontal Res. 2009;44(1):110–116. [DOI] [PubMed] [Google Scholar]
- 119.Yoshihara A, Sugita N, Yamamoto K, et al. FcgammaRIIIb genotypes and smoking in periodontal disease progression among community-dwelling older adults in Japan. J Periodontol. 2005;76(2):250–255. [DOI] [PubMed] [Google Scholar]
- 120.Zhu XL, Meng HX, Xu L, et al. Combined association of CCR2-V64I and MCP-1–2518A/G polymorphisms with generalised aggressive periodontitis in Chinese. Chin J Dent Res. 2010;13(2):109–114. [PubMed] [Google Scholar]
- 121.Nibali L, Parkar M, D’Aiuto F, et al. Vitamin D receptor polymorphism (−1056 Taq-I) interacts with smoking for the presence and progression of periodontitis. J Clin Periodontol. 2008;35(7):561–567. [DOI] [PubMed] [Google Scholar]
- 122.Petkovic-Curcin A, Zeljic K, Cikota-Aleksic B, Dakovic D, Tatic Z, Magic Z. Association of Cytokine Gene Polymorphism with Peri-implantitis Risk. Int J Oral Maxillofac Implants. 2017;32(5):e241–e248. [DOI] [PubMed] [Google Scholar]
- 123.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1–6. [DOI] [PubMed] [Google Scholar]
- 124.Hendek MK, Erdemir EO, Kisa U, Ozcan G. Effect of initial periodontal therapy on oxidative stress markers in gingival crevicular fluid, saliva, and serum in smokers and non-smokers with chronic periodontitis. J Periodontol. 2015;86(2):273–282. [DOI] [PubMed] [Google Scholar]
- 125.Sakallioglu EE, Sakallioglu U, Lutfioglu M, Pamuk F, Kantarci A. Vascular endothelial cadherin and vascular endothelial growth factor in periodontitis and smoking. Oral Dis. 2015;21(2):263–269. [DOI] [PubMed] [Google Scholar]
- 126.Apatzidou DA, Riggio MP, Kinane DF. Impact of smoking on the clinical, microbiological and immunological parameters of adult patients with periodontitis. J Clin Periodontol. 2005;32(9):973–983. [DOI] [PubMed] [Google Scholar]
- 127.Rosa GM, Lucas GQ, Lucas ON. Study of the crevicular fluid flow rate in smokers. Acta Odontol Latinoam. 2000;13(1):51–60. [PubMed] [Google Scholar]
- 128.McLaughlin WS, Lovat FM, Macgregor ID, Kelly PJ. The immediate effects of smoking on gingival fluid flow. J Clin Periodontol. 1993;20(6):448–451. [DOI] [PubMed] [Google Scholar]
- 129.Persson L, Bergstrom J, Gustafsson A. Effect of tobacco smoking on neutrophil activity following periodontal surgery. J Periodontol. 2003;74(10):1475–1482. [DOI] [PubMed] [Google Scholar]
- 130.Ardais R, Mario Tde G, Boligon J, Kantorski KZ, Moreira CH. The effect of smoking on bleeding on probing after nonsurgical periodontal therapy: a quasi-experimental study. Braz Oral Res. 2014;28:1–7. [DOI] [PubMed] [Google Scholar]
- 131.Bergstrom J, Bostrom L. Tobacco smoking and periodontal hemorrhagic responsiveness. J Clin Periodontol. 2001;28(7):680–685. [DOI] [PubMed] [Google Scholar]
- 132.Biddle AJ, Palmer RM, Wilson RF, Watts TL. Comparison of the validity of periodontal probing measurements in smokers and non-smokers. J Clin Periodontol. 2001;28(8):806–812. [DOI] [PubMed] [Google Scholar]
- 133.Bostrom L, Linder LE, Bergstrom J. Smoking and GCF levels of IL-1beta and IL-1ra in periodontal disease. J Clin Periodontol. 2000;27(4):250–255. [DOI] [PubMed] [Google Scholar]
- 134.Javed F, Al-Askar M, Samaranayake LP, Al-Hezaimi K. Periodontal disease in habitual cigarette smokers and nonsmokers with and without prediabetes. The American journal of the medical sciences. 2013;345(2):94–98. [DOI] [PubMed] [Google Scholar]
- 135.Lang NP, Tonetti MS, Suter J, Sorrell J, Duff GW, Kornman KS. Effect of interleukin-1 gene polymorphisms on gingival inflammation assessed by bleeding on probing in a periodontal maintenance population. J Periodontal Res. 2000;35(2):102–107. [DOI] [PubMed] [Google Scholar]
- 136.Tonguc MO, Ozturk O, Sutcu R, et al. The impact of smoking status on antioxidant enzyme activity and malondialdehyde levels in chronic periodontitis. J Periodontol. 2011;82(9):1320–1328. [DOI] [PubMed] [Google Scholar]
- 137.Erdemir EO, Nalcaci R, Caglayan O. Evaluation of systemic markers related to anemia of chronic disease in the peripheral blood of smokers and non-smokers with chronic periodontitis. Eur J Dent. 2008;2(2):102–109. [PMC free article] [PubMed] [Google Scholar]
- 138.Erdemir EO, Bergstrom J. Relationship between smoking and folic acid, vitamin B12 and some haematological variables in patients with chronic periodontal disease. J Clin Periodontol. 2006;33(12):878–884. [DOI] [PubMed] [Google Scholar]
- 139.Sreedevi M, Ramesh A, Dwarakanath C. Periodontal status in smokers and nonsmokers: a clinical, microbiological, and histopathological study. Int J Dent. 2012;2012:571590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kamma JJ, Giannopoulou C, Vasdekis VG, Mombelli A. Cytokine profile in gingival crevicular fluid of aggressive periodontitis: influence of smoking and stress. J Clin Periodontol. 2004;31(10):894–902. [DOI] [PubMed] [Google Scholar]
- 141.Petropoulos G, McKay IJ, Hughes FJ. The association between neutrophil numbers and interleukin-1alpha concentrations in gingival crevicular fluid of smokers and non-smokers with periodontal disease. J Clin Periodontol. 2004;31(5):390–395. [DOI] [PubMed] [Google Scholar]
- 142.Shirodaria S, Smith J, McKay IJ, Kennett CN, Hughes FJ. Polymorphisms in the IL-1A gene are correlated with levels of interleukin-1alpha protein in gingival crevicular fluid of teeth with severe periodontal disease. J Dent Res. 2000;79(11):1864–1869. [DOI] [PubMed] [Google Scholar]
- 143.Tymkiw KD, Thunell DH, Johnson GK, et al. Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. J Clin Periodontol. 2011;38(3):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhong Y, Slade GD, Beck JD, Offenbacher S. Gingival crevicular fluid interleukin-1beta, prostaglandin E2 and periodontal status in a community population. J Clin Periodontol. 2007;34(4):285–293. [DOI] [PubMed] [Google Scholar]
- 145.Rawlinson A, Grummitt JM, Walsh TF, Ian Douglas CW. Interleukin 1 and receptor antagonist levels in gingival crevicular fluid in heavy smokers versus non-smokers. J Clin Periodontol. 2003;30(1):42–48. [DOI] [PubMed] [Google Scholar]
- 146.Goutoudi P, Diza E, Arvanitidou M. Effect of periodontal therapy on crevicular fluid interleukin-1beta and interleukin-10 levels in chronic periodontitis. J Dent. 2004;32(7):511–520. [DOI] [PubMed] [Google Scholar]
- 147.Erdemir EO, Duran I, Haliloglu S. Effects of smoking on clinical parameters and the gingival crevicular fluid levels of IL-6 and TNF-alpha in patients with chronic periodontitis. J Clin Periodontol. 2004;31(2):99–104. [DOI] [PubMed] [Google Scholar]
- 148.Tsai IS, Tsai CC, Ho YP, Ho KY, Wu YM, Hung CC. Interleukin-12 and interleukin-16 in periodontal disease. Cytokine. 2005;31(1):34–40. [DOI] [PubMed] [Google Scholar]
- 149.Bostrom L, Linder LE, Bergstrom J. Smoking and cervicular fluid levels of IL-6 and TNF-alpha in periodontal disease. J Clin Periodontol. 1999;26(6):352–357. [DOI] [PubMed] [Google Scholar]
- 150.Ikezawa-Suzuki I, Shimada Y, Tai H, Komatsu Y, Tanaka A, Yoshie H. Effects of treatment on soluble tumour necrosis factor receptor type 1 and 2 in chronic periodontitis. J Clin Periodontol. 2008;35(11):961–968. [DOI] [PubMed] [Google Scholar]
- 151.Kurtis B, Tuter G, Serdar M, Pinar S, Demirel I, Toyman U. Gingival crevicular fluid prostaglandin E(2) and thiobarbituric acid reactive substance levels in smokers and non-smokers with chronic periodontitis following phase I periodontal therapy and adjunctive use of flurbiprofen. J Periodontol. 2007;78(1):104–111. [DOI] [PubMed] [Google Scholar]
- 152.Stein SH, Green BE, Scarbecz M. Augmented transforming growth factor-beta1 in gingival crevicular fluid of smokers with chronic periodontitis. J Periodontol. 2004;75(12):1619–1626. [DOI] [PubMed] [Google Scholar]
- 153.Soder B, Jin LJ, Wickholm S. Granulocyte elastase, matrix metalloproteinase-8 and prostaglandin E2 in gingival crevicular fluid in matched clinical sites in smokers and non-smokers with persistent periodontitis. J Clin Periodontol. 2002;29(5):384–391. [DOI] [PubMed] [Google Scholar]
- 154.Buduneli N, Buduneli E, Kardesler L, Lappin D, Kinane DF. Plasminogen activator system in smokers and non-smokers with and without periodontal disease. J Clin Periodontol. 2005;32(4):417–424. [DOI] [PubMed] [Google Scholar]
- 155.Passoja A, Ylipalosaari M, Tervonen T, Raunio T, Knuuttila M. Matrix metalloproteinase-8 concentration in shallow crevices associated with the extent of periodontal disease. J Clin Periodontol. 2008;35(12):1027–1031. [DOI] [PubMed] [Google Scholar]
- 156.Mantyla P, Stenman M, Kinane D, et al. Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. J Periodontal Res. 2006;41(6):503–512. [DOI] [PubMed] [Google Scholar]
- 157.Victor DJ, Subramanian S, Gnana PP, Kolagani SP. Assessment of Matrix Metalloproteinases-8 and −9 in Gingival Crevicular Fluid of Smokers and Non-smokers with Chronic Periodontitis Using ELISA. J Int Oral Health. 2014;6(6):67–71. [PMC free article] [PubMed] [Google Scholar]
- 158.Soder B Neutrophil elastase activity, levels of prostaglandin E2, and matrix metalloproteinase-8 in refractory periodontitis sites in smokers and non-smokers. Acta Odontol Scand. 1999;57(2):77–82. [DOI] [PubMed] [Google Scholar]
- 159.Scott DA, Palmer RM. The influence of tobacco smoking on adhesion molecule profiles. Tobacco Induced Diseases. 2002;1(1):7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


