Abstract
Background:
Patients with treatment-naive advanced urothelial cancer (UC) Ineligible for cisplatin-based chemotherapy are typically older and have comorbidities, representing a difficult-to-treat population.
Objective:
To evaluate the safety and antitumor activity of first-line pembrolizumab in subgroups of cisplatin-ineligible older patients (aged ≥65 and ≥75 yr) with advanced UC in KEYNOTE-052 (NCT02335424), including those with poor performance status (Eastern Cooperative Oncology Group performance status score 2 [ECOG PS2]).
Design, setting, and participants:
Patients were cisplatin ineligible, had treatment-naive, histologically/cytologically confirmed, locally advanced/metastatic UC with measurable disease (Response Evaluation Criteria in Solid Tumors version 1.1 [RECIST v1.1]), and had ECOG PS0–2. Patient subgroups analyzed were aged ≥65 yr (n = 302), ≥75 yr (n = 179), ≥65 yr with ECOG PS2 (≥65 yr + ECOG PS2; n = 119), and ≥75 yr + ECOG PS2 (n = 78).
Intervention:
All patients received pembrolizumab 200 mg intravenously every 3 wk until confirmed progression, intolerable toxicity, patient withdrawal, or 24 mo of therapy.
Outcome measurements and statistical analysis:
The primary endpoint was objective response rate (ORR) as per RECIST v1.1. The key secondary endpoints were overall survival (OS), duration of response (DOR), and safety.
Results and limitations:
ORRs for the ≥65 yr, ≥75 yr, ≥65 yr + ECOG PS2, and ≥75 yr + ECOG PS2 subgroups were 29%, 27%, 29%, and 31%, respectively; rates of complete and partial responses were similar across subgroups (9%, 5%, 6%, and 6%, and 20%, 22%, 23%, and 24%, respectively). Median DOR and OS were also consistent across the ≥65 yr and ≥65 yr + ECOG PS2 subgroups and the ≥75 yr and ≥75 yr + ECOG PS2 subgroups. Study limitations included open-label design, lack of a comparator group, and nature of post hoc exploratory analysis.
Conclusions:
The clinical benefit of pembrolizumab in advanced UC appeared to be consistent regardless of age and/or poor performance status.
Keywords: Aged, Bladder cancer, Checkpoint inhibitor, Cisplatin ineligible, Immunotherapy, Programmed death 1, Pembrolizumab, Platinum ineligible, Poor performance status, Urothelial carcinoma
Patient summary:
This study looked at whether older age and poorer performance status affect how well patients with previously untreated advanced urothelial cancer ineligible for standard-of-care treatment respond to pembrolizumab. Outcomes with pembrolizumab were not affected by older age or poorer performance status, making it an effective option.
1. Introduction
Urothelial cancers (UCs) comprise a range of tumors arising from the urinary tract, including the bladder, which accounts for >90% of all urothelial tumors in the USA and Europe [1]. Bladder cancer ranks 10th in the world regarding incidence, with >500 000 cases estimated to have been diagnosed in 2018 [2]. Diagnosis peaks after age 70 yr, and approximately 20% of patients are older than 80 yr [3].
Approximately half of patients with newly diagnosed, advanced UC are ineligible for first-line cisplatin-based combination chemotherapy, in part because of age-related decline in performance status and the presence of medical comorbidities that can impact treatment-related toxicity [3–5]. Indeed, one criterion used to establish eligibility for cisplatin-based therapy is Eastern Cooperative Oncology Group performance status (ECOG PS) <2 [4]. Comorbidities in older patients include renal function impairment (chronic kidney disease with creatinine clearance level <50–60 ml/min), grade ≥2 hearing loss or neuropathy, and New York Heart Association (NYHA) class III–IV cardiac failure [4,6–8]. Treatment of UC in older patients may also be affected by potential interactions among drugs used to treat comorbidities and chemotherapy [7]. Survival with non-cisplatin-based therapies is usually short, with median progression-free (PFS) and overall (OS) survival of 4–5 and 8–10 mo, respectively [7].
Clinical benefit has been noted with gemcitabine plus carboplatin (GCa) as a first-line treatment; alternatives include gemcitabine or carboplatin monotherapy and gemcitabine plus paclitaxel [7,9]. However, shorter OS and severe toxicity in patients with both poor performance status and renal impairment (estimated glomerular filtration rate <60 ml/min) compared with those who had only one of these factors were reported with GCa [10]. Moreover, chemotherapy is not administered to approximately 20–52% of patients with UC, including those with advanced disease, because of the presence of comorbidities, poor overall health status, and concerns regarding chemotherapy-associated toxicities [3,9,11]; these untreated patients generally fare worse than those who receive chemotherapy and usually pursue only best supportive and palliative care [3]. The good tolerability profile of checkpoint inhibitors represents a new opportunity for therapeutic intervention in older patients who are chemotherapy ineligible.
Immune checkpoint blockade (anti–programmed death 1 [PD-1]/anti–programmed death ligand 1 [PD-L1]/anti-cytotoxic T-lymphocyte–associated protein 4) has demonstrated efficacy for a variety of malignancies, including UC [12,13]. The PD-1 inhibitor pembrolizumab is approved in the USA and Europe as first-line therapy for cisplatin-ineligible patients with UC, based on available efficacy and safety data from the open-label, single-arm, phase 2 KEYNOTE-052 trial (ClinicalTrials.gov, NCT02335424) evaluating the efficacy and safety of first-line pembrolizumab in cisplatin-ineligible patients with advanced UC [14–16]. A 2018 revision of these approvals for first-line pembrolizumab limited its use to cisplatin-ineligible patients with tumors exhibiting high PD-1 expression based on the companion assay (22C3; Agilent Technologies, Carpinteria, CA, USA) or patients ineligible for any platinum (cisplatin or carboplatin)-containing chemotherapy regardless of PD-L1 status [14,15]. These revisions are based on an early interim analysis that found that survival was shorter in patients with advanced UC and tumors with low PD-L1 expression who received pembrolizumab than in those who received chemotherapy alone in an ongoing phase III trial (KEYNOTE-361, ClinicalTrials.gov, NCT02853305) [17].
In KEYNOTE-052, first-line treatment of 370 patients with pembrolizumab yielded an objective response rate (ORR) of 29%, with 33 (9%) complete responses (CRs) and 73 (20%) partial responses (PRs) across the entire trial population [18]. Responses were usually rapid and durable, and pembrolizumab was well tolerated overall. It was hypothesized that pembrolizumab activity and tolerability are independent of age and performance status. An exploratory post hoc analysis of KEYNOTE-052 was conducted to evaluate the antitumor activity and safety of pembrolizumab in the subgroup of cisplatin-ineligible patients who were considered older (aged ≥65 and ≥75 yr) and/or had poor performance status (ie, ECOG PS2).
2. Patients and methods
2.1. Study design and patient population
This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines, and in compliance with local and institutional regulations. All patients provided written informed consent to participate. Study design and methods are described in detail elsewhere [16]. In brief, adults with treatment-naive, histologically/cytologically confirmed, locally advanced (unresectable) or metastatic UC of the renal pelvis, ureter, bladder, or urethra, who were ineligible for cisplatin-based chemotherapy, had centrally confirmed measurable disease (as per Response Evaluation Criteria in Solid Tumors version 1.1 [RECIST v1.1]), and had ECOG PS 0–2 were enrolled. Cisplatin ineligibility was defined by having one or more of the following factors: ECOG PS2, creatinine clearance level 30–59 ml/min, grade ≥2 neuropathy/hearing loss, or NYHA class III heart failure.
2.2. Treatment and assessments
All enrolled patients were administered pembrolizumab 200 mg intravenously every 3 wk until documented disease progression, intolerable toxicity, physician/patient decision to withdraw, or completion of 24 mo of treatment. Tumor response was assessed by computed tomography or magnetic resonance imaging at 9 wk after the first dose of pembrolizumab, every 6 wk thereafter for the 1st year, and then every 12 wk through year 2.
PD-L1 expression (assessed using the PD-L1 IHC 22C3 pharmDx assay; Agilent Technologies) was determined using combined positive score (CPS; number of PD-L1–positive cells [tumor cells, lymphocytes, and macrophages]/total number of tumor cells × 100). A CPS cutoff of 10 was used to define tumors expressing PD-L1 and was validated by determining the ORR among all subsequently enrolled patients with CPS ≥ 10 (referred to as the validation set) [16,19].
Safety was assessed by reporting adverse events (AEs) using Common Terminology Criteria for Adverse Events, version 4.0. AEs and serious AEs (SAEs) were monitored throughout the study and for 30 and 90 d, respectively, after the last dose.
2.3. Study endpoints
The primary endpoint was the ORR as per RECIST v1.1 by an independent central imaging review. Secondary efficacy endpoints were duration of response (DOR), OS, and PFS as per RECIST v1.1 by an independent central imaging review. The primary safety objective was characterization of the safety and tolerability of pembrolizumab, which was achieved by AE reporting, and included SAEs, fatal AEs, and immune-mediated AEs.
2.4. Statistical analysis
Data for the following patient subgroups were analyzed: age ≥65 yr (≥65yr), age ≥75 yr (≥75 yr), age ≥65 yr with ECOG PS2 (≥65 yr + ECOG PS2), and age ≥75 yr with ECOG PS2 (≥75 yr + ECOG PS2). ORR was summarized using point estimates with 95% confidence intervals (CIs) based on the binomial exact method. Secondary efficacy endpoints were evaluated using the Kaplan-Meier method to estimate summary statistics, including medians. Data cutoff date for these analyses was September 26, 2018.
3. Results
3.1. Baseline patient characteristics
Median follow-up for all trial patients was 11.4 mo (range, 0.1–41.2 mo). Of 370 patients, 302 (82%) were aged ≥65 yr, 179 (48%) were aged ≥75 yr, 119 (32%) were aged ≥65 yr with ECOG PS2, and 78 (21%) were aged ≥75 yr with ECOG PS2. Baseline patient characteristics were generally comparable across groups (Table 1).
Table 1 -.
Patient baseline characteristics and study disposition.
| Characteristic | Age subgroups |
Age/ECOG PS2 subgroups |
||
|---|---|---|---|---|
| Age ≥65 yr (n = 302) | Age ≥75 yr (n = 179) | Age ≥65 yr + ECOG PS2 (n = 119) | Age ≥75 yr + ECOG PS2 (n = 78) | |
| Baseline characteristics | ||||
| Age (yr), median (range) | 76 (65–94) | 81 (75–94) | 78 (65–91) | 81 (75–91) |
| Sex (men), n (%) | 230 (76) | 137 (77) | 93 (78) | 57 (73) |
| ECOG PS, n (%) | ||||
| 0 or 1 | 183 (61) | 101 (56) | 0 | 0 |
| 2 | 119 (39) | 78 (44) | 119 (100) | 78 (100) |
| 3 | 0 | 0 | 0 | 0 |
| Upper tract primary tumora, n (%) | 57 (19) | 27 (15) | 23 (19) | 14 (18) |
| Metastasis locationb, n (%) | ||||
| Visceral disease | 257 (85) | 154 (86) | 98 (82) | 64 (82) |
| Lymph node disease only | 41 (14) | 21 (12) | 19 (16) | 12 (15) |
| Liver metastases, n (%) | 65 (22) | 43 (24) | 30 (25) | 22 (28) |
| Hemoglobin <10 g/dl, n (%) | 31 (10) | 21 (12) | 15 (13) | 11 (14) |
| Prior chemotherapy, n (%) | 54 (18) | 27 (15) | 22 (19) | 10 (13) |
| Reasons for cisplatin ineligibility, n (%) | ||||
| Renal dysfunction | 154 (51) | 90 (50) | 9 (8) | 7 (9) |
| ECOG PS2 | 87 (29) | 54 (30) | 81 (68) | 49 (63) |
| ECOG PS2 + renal dysfunction | 31 (10) | 22 (12) | 27 (23) | 20 (26) |
| Otherc | 30 (10) | 13 (7) | 2 (2) | 2 (3) |
| Study disposition | ||||
| Completed, n (%) | 34 (11) | 8 (5) | 12 (10) | 5 (6) |
| Discontinued, n (%) | 268 (89) | 171 (96) | 107 (90) | 73 (94) |
| Adverse event | 50 (17) | 34 (19) | 22 (19) | 15 (19) |
| Clinical progression | 36 (12) | 22 (12) | 15 (13) | 9 (12) |
| Complete response | 11 (4) | 7 (4) | 5 (4) | 4 (5) |
| Physician decision | 10 (3) | 8 (5) | 5 (4) | 4 (5) |
| Progressive disease | 142 (47) | 89 (50) | 51 (43) | 36 (46) |
| Withdrawal by patient | 18 (6) | 11 (6) | 8 (7) | 5 (6) |
| Noncompliance with study drug | 1 (<1) | 0 | 1 (1) | 0 |
| Treatment ongoing, n (%) | 0 | 0 | 0 | 0 |
ECOG PS = Eastern Cooperative Oncology Group performance status.
Unknown for one patient.
Unknown for four patients.
Other reasons include New York Heart Association Class III heart failure, grade ≥2 peripheral neuropathy, and grade ≥2 hearing loss.
3.2. Efficacy
ORRs were 29%, 27%, 29%, and 31% for the ≥65 yr, ≥75 yr, ≥65 yr + ECOG PS2, and ≥75 yr + ECOG PS2 subgroups, respectively (Table 2). The best response of CR was achieved by 9%, 5%, 6%, and 6%, respectively, and that of PR was achieved by 20%, 22%, 23%, and 24%, respectively. A supportive analysis of responses in patients aged ≥85 yr (n = 40) yielded an ORR of 28% (11 PRs). The ORRs among patients with a tumor CPS of ≥10 were 52% (32/62), 50% (17/34), 52% (17/33), and 55% (12/22) for the ≥65 yr, ≥75 yr, ≥65 yr + ECOG PS2, and ≥75 yr + ECOG PS2 subgroups, respectively (Fig. 1). For patients who were <65 yr of age (n = 68) and those who were <65 yr of age with ECOG PS 2 (n = 37), ORRs were 29% and 19%, respectively.
Table 2 -.
Best confirmed objective response and response duration based on RECIST v1.1 per central imaging.
| Age subgroups | Age/ECOG PS2 subgroups | |||
|---|---|---|---|---|
| Age ≥65 yr (n = 302) | Age ≥75 yr (n = 179) | Age ≥65 yr + ECOG PS2 (n = 119) | Age ≥75 yr + ECOG PS2 (n = 78) | |
| Objective response rate, n (%) | 86 (29) | 48 (27) | 34 (29) | 24 (31) |
| Complete response | 27 (9) | 9 (5) | 7 (6) | 5 (6) |
| Partial response | 59 (20) | 39 (22) | 27 (23) | 19 (24) |
| Stable disease | 56 (19) | 32 (18) | 17 (14) | 10 (13) |
| Progressive disease | 125 (41) | 78 (44) | 48 (40) | 34 (44) |
| Not evaluable | 9 (3) | 6 (3) | 5 (4) | 4 (5) |
| No assessment | 26 (9) | 15 (8) | 15 (13) | 6 (8) |
| Time to response (mo)a, median (range) | 2.1 (1.3–9.0) | 2.1 (1.3–4.7) | 2.1 (1.3–5.0) | 2.1 (1.3–4.7) |
| Duration of response (mo)a,b, median (range) | 30.1 (1.6+ to 35.9+) | 12.5 (1.6+ to 33.4+) | 30.1 (1.6+ to 34.3+) | 11.8 (1.6+ to 33.4+) |
| Proportion of patients with responses lasting ≥24 mo (%)a,b | 53 | 35 | 51 | 45 |
ECOG PS2 = Eastern Cooperative Oncology Group performance status 2; RECIST v1.1 = Response Evaluation Criteria in Solid Tumors version 1.1.
In patients achieving complete or partial responses only.
Based on the Kaplan-Meier method.
Fig. 1 –

Objective response rate as per RECIST v1.1 by central imaging review in patients with CPS ≥ 10 (validation set) for each of the patient subgroups. The striped bar represents CR, while the solid bar represents PR. CI = confidence interval; CPS = combined positive score; CR = complete response; ECOG PS2 = Eastern Cooperative Oncology Group performance status 2; ORR = objective response rate; PR = partial response; RECIST v1.1 = Response Evaluation Criteria in Solid Tumors version 1.1.
Median time to response was similar among all subgroups (median, 2.1 mo overall). Median DOR was 30.1 mo for both the ≥65 yr and the ≥65 yr + ECOG PS2 subgroup, 12.5 mo for the ≥75 yr subgroup, and 11.8 mo for the ≥75 yr + ECOG PS2 subgroup (Table 2). Proportions of patients who maintained response for ≥24 mo were 53% and 51% for the ≥65 yr and ≥65 yr + ECOG PS2 subgroups, respectively, and 35% and 45% for the ≥75 yr and ≥75 yr + ECOG PS2 subgroups, respectively. Findings for patients with CPS ≥ 10 were similar (median time to response, 2.1 mo overall; median DOR was not reached for all but the ≥75 yr + ECOG PS2 subgroup, in which it was 13 mo; Supplementary Table 1). For responders who were <65 yr of age (n = 20) and those who were <65 yr of age with ECOG PS 2 (n = 7), median (range) DOR was 18.1 (1.4+to 31.9+), which was not reached (5.6 to 26.3+), respectively. Similar proportions of patients in each subgroup experienced a reduction in tumor size from baseline: 59% of the ≥65 yr, 58% of the ≥75 yr, 60% of the ≥65 yr + ECOG PS2, and 57% of the ≥75yr + ECOG PS2 subgroup (Fig. 2).
Fig. 2 –
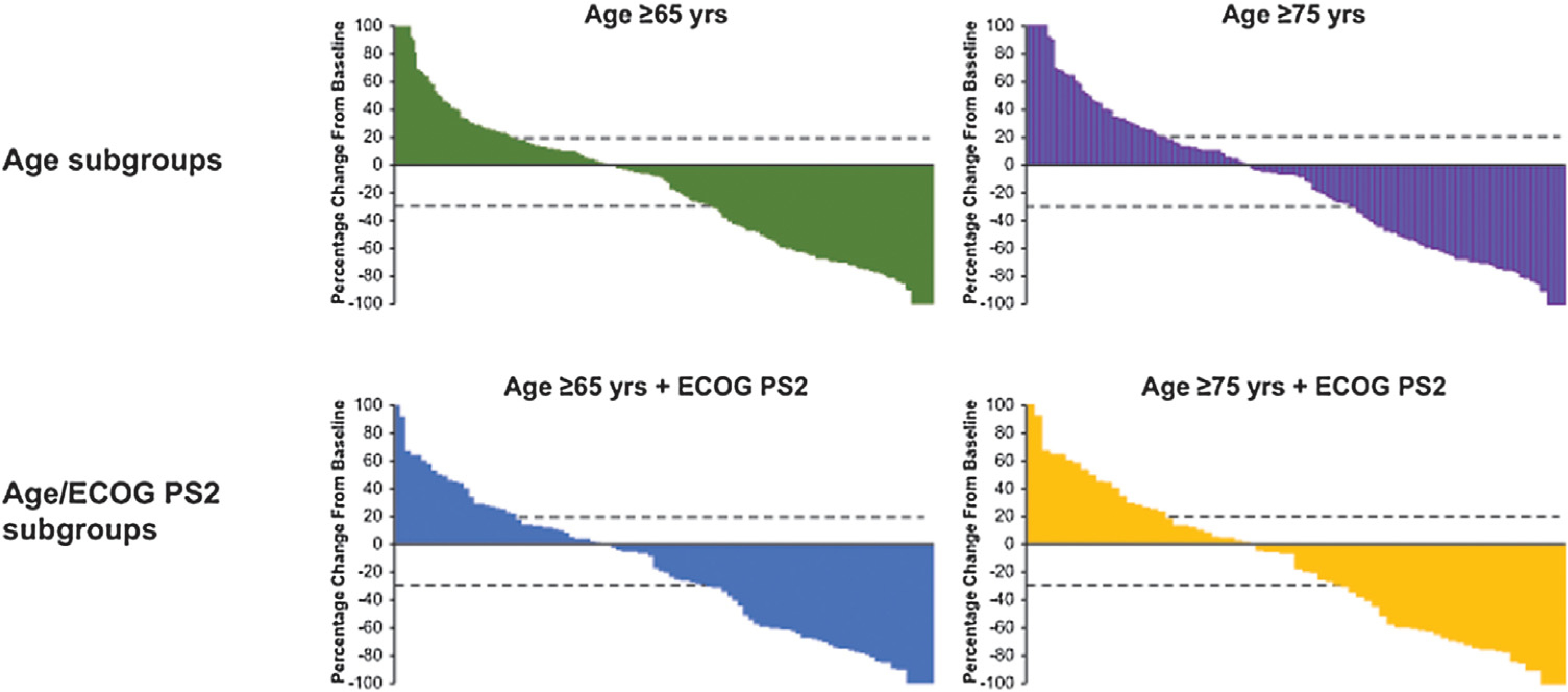
Best percentage change from baseline in the sum of the longest diameters of target lesions as per RECIST v1.1 by a central imaging review in the subgroups of patients aged ≥65yr (n = 270), ≥75 yr (n = 158), ≥65yr with ECOG PS2 (n = 102), and ≥75 yr with ECOG PS2 (n = 70). Dotted lines correspond to patients with a 20% increase in tumor burden and a 30% decrease in tumor burden. ECOG PS2 = Eastern Cooperative Oncology Group performance status 2; RECIST v1.1 = Response Evaluation Criteria in Solid Tumors version 1.1.
Median PFS was 2.3 mo for the ≥65 yr subgroup and 2.1 mo for the other three subgroups (Table 3). PFS rates at 6 mo in the ≥65 yr, ≥75 yr, ≥65 yr + ECOG PS2, and ≥75 yr + ECOG PS2 subgroups were 34%, 31%, 33%, and 31%, respectively. For patients who were <65 yr of age (n = 68) and were <65 yr of age with ECOG PS 2 (n = 37), median (95% CI) PFS was 2.2 (2.0–31.9+) and 2.1 (1.9–3.6) mo, respectively. Median (95% CI) OS was 11.0 (9.5–12.5), 9.7 (7.8–11.5), 8.7 (5.2–10.6), and 8.2 (4.4–10.8) mo, respectively, and OS rates at 24 mo were 29%, 21%, 24%, and 23%, respectively. In patients with CPS ≥ 10, median OS was 16.6 mo (95% CI, 11.5–27.6) in ≥65 yr, 13.6 mo (95% CI, 10.0–27.6) in ≥65 yr + ECOG PS2,11.5 mo (95% CI, 5.8–17.1) in ≥75 yr, and 10.6 mo (95% CI, 4.4–27.0) in ≥75 yr + ECOG PS2 patients. The same pattern was observed for the OS rates at 24 mo (Supplementary Table 1). For patients who were <65 yr of age (n = 68) and those who were <65 yr of age with ECOG PS 2 (n = 37), median (95% CI) OS was 15.7 (6.9–24.2) and 14.1 (5.2–24.2), respectively.
Table 3 -.
Summary of PFS and OS according to age and age/ECOG PS2 subgroups.
| Survival | Age subgroups |
Age/ECOG PS2 subgroups |
||
|---|---|---|---|---|
| Age ≥65 yr (n = 302) | Age ≥75 yr (n = 179) | Age ≥65 yr + ECOG PS 2 (n = 119) | Age ≥75 yr + ECOG PS 2 (n = 78) | |
| PFSa, mo (95% CI) | ||||
| Median | 2.3 (2.1–3.4) | 2.1 (2.0–3.4) | 2.1 (2.0–3.5) | 2.1 (2.0–4.7) |
| PFS rate at 6 mo | 33.6 (28.3–39.0) | 31.3 (24.6–38.1) | 32.8 (24.5–41.2) | 30.8 (20.9–41.1) |
| PFS rate at 12 mo | 22.2 (17.7–27.1) | 17.7 (12.5–23.6) | 20.0 (13.3–27.6) | 18.9 (11.1–28.3) |
| OSa, mo (95% CI) | ||||
| Median | 11.0 (9.5–12.5) | 9.7 (7.8–11.5) | 8.7 (5.2–10.6) | 8.2 (4.4–10.8) |
| OS rate at 12 mo | 45.6 (39.9–51.1) | 39.1 (32.0–46.2) | 36.6 (28.0–45.2) | 35.9 (25.5–46.4) |
| OS rate at 18 mo | 34.9 (29.6–40.3) | 25.7 (19.6–32.3) | 27.2 (19.6–35.5) | 26.9 (17.7–37.0) |
| OS rate at 24 mo | 28.9 (23.9–34.1) | 21.2 (15.5–27.4) | 23.8 (16.6–31.8) | 23.0 (14.4–32.8) |
CI = confidence interval; ECOG PS2 = Eastern Cooperative Oncology Group performance status 2; OS = overall survival; PFS = progression-free survival.
From the product-limit (Kaplan-Meier) method for censored data.
3.3. Safety
Treatment-related AEs (TRAEs) of any grade were reported by 210 (70%), 125 (71%), 72 (61%), and 50 (64%) of the ≥65 yr, ≥75 yr, ≥65yr + ECOG PS2, and ≥75 yr + ECOG PS2 subgroups, respectively (Table 4). Rates of grade 3–5 TRAEs were 22%, 20%, 19%, and 17%, respectively. The most common TRAEs were similar between the subgroups, with both fatigue and pruritus occurring in ≥10% of patients in any subgroup. Other TRAEs occurring in ≥10% of patients were decreased appetite, hypothyroidism, and rash in the ≥65 yr group and decreased appetite in the ≥75 yr group. Treatment-related SAEs ranged from 9% to 12% across the four subgroups. Rates of study discontinuations attributable to a TRAE or a treatment-related SAE were also consistent among subgroups (8–10% and 3–5%, respectively). Four grade 4 treatment-related AEs (myocarditis, asthenia, decreased appetite, and hypercalcemia) occurred in two patients who were ≥75 yr old but did not have ECOG PS 2. Only one death was considered attributable to pembrolizumab (myositis in a patient aged ≥75 yr but not with ECOG PS2). Only three immune-mediated AEs occurred in >2% of patients (across subgroups): hyperthyroidism, hypothyroidism, and pneumonitis (Table 4). Hepatitis and pruritus were also observed in >2% of patients in the ≥75 yr and ≥75 yr + ECOG PS2 subgroups, respectively. Hypothyroidism occurred in 11% of the ≥65 yr, 7% of the ≥75 yr, 5% of the ≥65 yr + ECOG PS2, and 8% of the ≥75 yr + ECOG PS2 subgroups. The rate of pneumonitis—at 5%, 6%, 7%, and 6%, respectively—was similar across the four subgroups.
Table 4 -.
Summary of TRAEs and list of TRAEs at any grade occurring in≥5% of patients.
| Age subgroups |
Age/ECOG PS2 subgroups |
|||
|---|---|---|---|---|
| Age ≥65 yr (n = 302) | Age ≥75 yr (n = 179) | Age ≥65 yr + ECOG PS2 (n = 119) | Age ≥75 yr + ECOG PS2 (n = 78) | |
| AE summary, n (%) | ||||
| TRAE, any grade | 210 (70) | 125 (70) | 72 (61) | 50 (64) |
| TRAE, grades 3–5 | 66 (22) | 36 (20) | 23 (19) | 13 (17) |
| Serious TRAEs | 34 (11) | 21 (12) | 13 (11) | 7 (9) |
| Immune-mediated AE a | 78 (26) | 39 (22) | 23 (19) | 18 (23) |
| Discontinuations because of a TRAE | 28 (9) | 17 (10) | 10 (8) | 6 (8) |
| Discontinuations because of a serious TRAE | 12 (4) | 9 (5) | 4 (3) | 2 (3) |
| Deaths because of a TRAE | 1 (<1) | 1 (<1) | 0 | 0 |
| TRAEs (any grade) occurring in ≥5% of patients in any subgroup, n (%) | ||||
| Fatigue | 57 (19) | 32 (18) | 12 (10) | 8 (10) |
| Pruritus | 56 (19) | 33 (18) | 15 (13) | 12 (15) |
| Rash | 35 (12) | 16 (9) | 9 (8) | 6 (8) |
| Decreased appetite | 35 (12) | 23 (13) | 9 (8) | 7 (9) |
| Hypothyroidism | 30 (10) | 11 (6) | 6 (5) | 6 (8) |
| Diarrhea | 28 (9) | 13 (7) | 11 (9) | 6 (8) |
| Nausea | 25 (8) | 11 (6) | 8 (7) | 3 (4) |
| Asthenia | 14 (5) | 9 (5) | 8 (7) | 5 (6) |
| ALT level increased | 13 (4) | 9 (5) | 3 (3) | 3 (4) |
| AST level increased | 14 (5) | 11 (6) | 4 (3) | 4 (5) |
| Pneumonitis | 14 (5) | 9 (5) | 7 (6) | 4 (5) |
| Pyrexia | 11 (4) | 6 (3) | 6 (5) | 3 (4) |
| Dysgeusia | 11 (4) | 7 (4) | 4 (3) | 4 (5) |
| TRAEs (grades 3–5) occurring in ≥2 patients in any subgroup, n (%) | ||||
| All | 66 (22) | 36 (20) | 23 (19) | 13 (17) |
| Myocarditis | 2 (1) | 2 (1) | 1 (1) | 1 (1) |
| Colitis | 4 (1) | 1 (1) | 0 | 0 |
| Diarrhea | 3 (1) | 1 (1) | 2 (2) | 1 (1) |
| Asthenia | 3 (1) | 2 (1) | 2 (2) | 1 (1) |
| Fatigue | 8 (3) | 6 (3) | 2 (2) | 1 (1) |
| Autoimmune hepatitis | 2 (1) | 1 (1) | 1 (1) | 1 (1) |
| Hepatitis | 5 (2) | 4 (2) | 1 (1) | 1 (1) |
| ALT level increased | 2 (1) | 2 (1) | 0 | 0 |
| AST level increased | 4 (1) | 4 (2) | 1 (1) | 1 (1) |
| ALP level increased | 6 (2) | 3 (2) | 2 (2) | 1 (1) |
| Decreased appetite | 2 (1) | 2 (1) | 1 (1) | 1 (1) |
| Dehydration | 2 (1) | 0 | 1 (1) | 0 |
| Hyperglycemia | 2 (1) | 1 (1) | 0 | 0 |
| Type 1 diabetes mellitus | 2 (1) | 1 (1) | 0 | 0 |
| Muscular weakness | 4 (1) | 3 (2) | 1 (1) | 1 (1) |
| Pneumonitis | 4 (1) | 2 (1) | 3 (3) | 1 (1) |
| Pruritus | 2 (1) | 2 (1) | 2 (2) | 2 (3) |
| Immune-mediated AEs (any grade) occurring in ≥2% of patients in any subgroup, n (%) | ||||
| Hypothyroidism | 34 (11) | 13 (7) | 6 (5) | 6 (8) |
| Pneumonitis | 15 (5) | 10 (6) | 8 (7) | 5 (6) |
| Hyperthyroidism | 10 (3) | 7 (4) | 3 (3) | 3 (4) |
| Pruritus | 2 (1) | 2 (1) | 2 (2) | 2 (3) |
| Hepatitis | 5 (2) | 4 (2) | 1 (1) | 1 (1) |
AE = adverse event; ECOG PS = Eastern Cooperative Oncology Group performance status; TRAE = treatment-related adverse event.
AEs of potentially drug-related immunologic causes reported regardless of attribution by the investigator.
4. Discussion
Patients with advanced UC who are ineligible for cisplatin-based therapy and, in particular, those who are older and/or have poor performance status represent a population for whom systemic chemotherapy may be challenging and palliative care is often recommended instead [3,4]. In this population, chemotherapeutic alternatives to cisplatin-based chemotherapy tend to be associated with higher levels of toxicity, lower response rates, and inferior outcomes [10,20,21]. In the first-line setting, median OS was 7–10 mo with GCa, 13 mo with paclitaxel + gemcitabine, and 8 mo with single-agent gemcitabine; toxicity was particularly problematic with combination therapies, such as GCa and gemcitabine + paclitaxel [4,7,20].
This exploratory post hoc analysis of KEYNOTE-052 demonstrated that pembrolizumab displays meaningful antitumor activity in the subset of cisplatin-ineligible patients with locally advanced (unresectable) or metastatic UC who are considered senior (aged ≥65 or ≥75 yr), including those with poor functional status (ie, ECOG PS2). Overall, neither age nor poor performance status appeared to have had an impact on the efficacy of pembrolizumab in this patient population; this lack of impact of age or poor performance status is also clinically relevant for patients who cannot tolerate any chemotherapy. ORR was 27–31% across the four subgroups, with 5–9% and 20–24% of the subgroups achieving CR and PR, respectively; in patients ≥85 yr (n = 40), the ORR was 28%. The data in these subgroups were comparable with those of the overall population [18]; in addition, given that most patients were aged ≥65 yr (82%), these patients appeared to be driving the results in the overall population. Percentages of patients with a response at ≥24 mo ranged from 35% to 53% across the four subgroups, whereas median DOR, PFS, OS, and overall efficacy were comparable with those in the original analysis of the entire population [18].
Under-representation and under-reporting of older patients in clinical trials render direct comparison with other studies problematic; the National Cancer Institute, along with several agencies and organizations, strongly recommends equal access to clinical trials regardless of age [5]. Pembrolizumab (indirectly) compares well with historical data with chemotherapy in the aged, poorly functioning population with advanced UC. Median OS was 11.3 mo with pembrolizumab (KEYNOTE-052 trial) and 9.3 mo with carboplatin/gemcitabine in the overall population in an earlier trial [10], but response duration appeared higher in a phase 2 international study of gemcitabine and paclitaxel (time to disease progression, 7.6 mo) [20]. However, comparisons among trials should always be interpreted with great caution because of selection, confounding, and other possible biases.
Although both GCa and methotrexate/carboplatin/vinblastine (M-CAVI) conferred antitumor activity in cisplatin-ineligible patients with advanced UC (N = 238), severe toxicity (defined as treatment-related death, grade 4 thrombocytopenia with bleeding, grade 3 or 4 renal toxicity, neutropenic fever, and mucositis) was experienced by 9.3% of patients receiving GCa and 21.2% receiving M-CAVI [10]. The current analysis demonstrates that pembrolizumab appears to be well tolerated, and its safety profile in these patient subsets, including more senior patients with poor performance status, was similar to that of the total trial population [18]. The data corroborate findings from the KEYNOTE 045 trial that showed higher tolerability and favorable patient-reported outcomes with pembrolizumab over cytotoxic chemotherapy in the platinum-refractory setting [22,23]. Additional information in this population will be available from the ongoing KEYNOTE-361 trial in which pembrolizumab, with or without platinum-based chemotherapy, is compared with platinum-based chemotherapy for advanced UC in the first-line setting (NCT02853305).
Study limitations include the exploratory nature of the subset analyses, open-label study design, and lack of a comparator arm (single-arm phase 2). The similarity in outcomes between the overall study population and the elderly subgroup was predictable, given that the majority of patients enrolled in this study were aged ≥65 yr (n = 302, 82%). These caveats can be addressed further in the ongoing KEYNOTE-361 (NCT02853305) trial.
5. Conclusions
Results from this subgroup analysis of older cisplatin-ineligible patients with advanced UC with or without poor performance status suggest that first-line pembrolizumab elicits clinically meaningful and durable responses consistent with those of the overall study population. No new safety signals were identified, consistent with prior reports. Pembrolizumab represents an established treatment option for patients with advanced UC who may not tolerate any platinum-based chemotherapy and are usually treated with best supportive care only.
Supplementary Material
Acknowledgments:
The authors thank the patients and their families and all investigators and site personnel. The authors also thank Markus Pulhmann, Jill Lindia, Steve Keefe, and Xiao Fang (employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA) for their contributions to the development of the manuscript. Medical writing and/or editorial assistance was provided by Matthew Grzywacz, PhD, of the ApotheCom pembrolizumab team (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA’s data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Funding/Support role of the sponsor: Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Representatives and academic advisors for Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, designed the study.
Financial disclosures: Petros Grivas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Petros Grivas: consultant/advisory role—Merck, Bristol-Myers Squibb, AstraZeneca, Clovis Oncology, EMD Serono, Seattle Genetics, Foundation Medicine, Driver, Pfizer, QED Therapeutics, Heron, Janssen, Bayer, Genzyme, Mirati Therapeutics, Exelixis, Roche, and GlaxoSmithKline; research funding (institution)—Pfizer, Clovis Oncology, Bavarian Nordic, Immunomedics, Bristol-Myers Squibb, and Debiopharm Group. Elizabeth R. Plimack: consultant—AstraZeneca, Bristol-Myers Squibb, Genentech/Roche, Merck, Novartis, Pfizer, Eli Lilly, Inc., Inovio, Clovis, Horizon Pharma, Exelixis, Incyte, Seattle Genetics, Janssen, and Flatiron Health; research funding (to institution)—AstraZeneca, Bristol-Myers Squibb, Merck, Peloton, and Pfizer; development of educational presentations—Bristol-Myers Squibb, Merck, Roche, Novartis, and Astellas; US patents 14/588,503 and 15/226,474. Arjun V. Balar: research funding and personal fees from Roche/Genentech, Merck, Seattle Genetics, and AstraZeneca; research funding from Bristol-Myers Squibb. Daniel Castellano: consultant/advisory role—Pfizer, Astellas, Janssen, Roche, Merck, Ipsen, Novartis, and Pierre Fabre. Peter H. O’Donnell: honoraria—Genetech/Roche, Novartis, Merck, AstraZeneca, Astellas Pharma, Seattle Genetics, Inovio, and Parexel; research funding—Boehringer Ingelheim, Merck, Genentech/Roche, AstraZeneca/Medimmune, Acerta Pharma, and Janssen. Joaquim Bellmunt: consultant/advisory role—Genentech, Merck & Co., Inc., Pfizer, Bristol-Myers Squibb, Pierre Fabre, and Janssen; honoraria—Genentech, Merck & Co., Inc., Pfizer, Bristol-Myers Squibb, Pierre Fabre, and Janssen; research funding—Takeda and Pfizer. Thomas Powles: honoraria—AstraZeneca, Bristol-Myers Squibb, Merck & Co., Inc., Roche, Pfizer, and Seattle Genetics; research funding—AstraZeneca and Roche. Noah M. Hahn: consultant/advisory role—AstraZeneca, Bristol-Myers Squibb, Champions Oncology, Ciclomed, Ferring Pharmaceuticals, Genentech Inc., Health Advances, Inovio Pharmaceuticals, Incyte Corporation, Janssen Pharmaceuticals Inc., Merck & Co., Inc., Pieris Pharmaceuticals, Principia Biopharma Inc., Rexahn Pharmaceuticals Inc., Seattle Genetics, TARIS BioMedical Inc., and TransMed; grant/research support from Astex Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Genentech Inc., Incyte Corporation, Merck & Co., Inc., Pieris Pharmaceuticals, Principia Biopharma Inc., and Seattle Genetics; honorarium from Bladder Cancer Academy. Ronald de Wit: advisory and speaker fees within the submitted work for Merck; advisory and speaker fees outside the submitted work for Sanofi, Roche, Janssen, and Clovis. Dean F. Bajorin: supported by Memorial Sloan Kettering Cancer Center (P30 CA008748); personal fees and other from Merck Sharp and Dome, Genentech, Bristol-Myers Squibb, Fidia Farmaceutici, Pfizer, AstraZeneca, and Eli Lilly, and grants and other from Merck and Novartis, during the conduct of the study. Misoo C. Ellison: employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Tara L. Frenkl: former employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, at the time of the analysis; is currently an employee of GlaxoSmithKline. James L. Godwin: employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Jacqueline Vuky: consultant/advisory role—Puma, BioTheranostics, and Agendia; research funding (institution)—Pfizer, Merck, Roche/Genentech, Celldex, and Eli-Lily.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euo.2020.02.009.
References
- [1].Wong MCS, Fung FDH, Leung C, Cheung WWL, Goggins WB, Ng CF. The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep 2018;8:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [3].Bellmunt J, Mottet N, De Santis M. Urothelial carcinoma management in elderly or unfit patients. EJC Suppl 2016;14:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol 2011;29:2432–8. [DOI] [PubMed] [Google Scholar]
- [5].Guancial EA, Roussel B, Bergsma DP, et al. Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging 2015;10:939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- [7].Shariat SF, Milowsky M, Droller MJ. Bladder cancer in the elderly. Urol Oncol 2009;27:653–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stern S, Behar S, Gottlieb S. Cardiology patient pages: aging and diseases of the heart. Circulation 2003;108:e99–101. [DOI] [PubMed] [Google Scholar]
- [9].National Comprehensive Cancer Network. NCCN clinical practice guidelines in?. 2019. Accessed March 10, 2020 https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf
- [10].De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012;30:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sonpavde G, Galsky MD, Latini D, Chen GJ. Cisplatin-ineligible and chemotherapy-ineligible patients should be the focus of new drug development in patients with advanced bladder cancer. Clin Genitourin Cancer 2014;12:71–3. [DOI] [PubMed] [Google Scholar]
- [12].Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 2018;62:29–39. [DOI] [PubMed] [Google Scholar]
- [13].Kim HS, Seo HK. Immune checkpoint inhibitors for urothelial carcinoma. Invest Clin Urol 2018;59:285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].KEYTRUDA® (pembrolizumab) injection, for intravenous use. Whitehouse Station, NJ, USA: Merck Sharp & Dohme Corp.; 2019,, July. [Google Scholar]
- [15].EMC. Keytruda (pembrolizumab) 50 mg powder for concentrate for solution for infusion (summary of product characteristics). Hoddesdon, UK: MSD Limited; 2019,, June 3. [Google Scholar]
- [16].Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92. [DOI] [PubMed] [Google Scholar]
- [17].Suzman DL, Agrawal S, Ning YM, et al. FDA approval summary: atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin-containing chemotherapy. Oncologist 2018;24:563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].O’Donnell PH, Balar AV, Vuky J, et al. KEYNOTE-052: Phase 2 study evaluating first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC)—Updated response and survival results. J Clin Oncol 2019;37:4546. [Google Scholar]
- [19].Balar A, Bellmunt J, O’Donnell PH, et al. Pembrolizumab (pembro) as first-line therapy for advanced/unresectable or metastatic urothelial cancer: preliminary results from the phase 2 KEYNOTE-052 study. Ann Oncol 2016;27:vi552–87. [Google Scholar]
- [20].Calabro F, Lorusso V, Rosati G, et al. Gemcitabine and paclitaxel every 2 weeks in patients with previously untreated urothelial carcinoma. Cancer 2009;115:2652–9. [DOI] [PubMed] [Google Scholar]
- [21].Stadler WM, Kuzel T, Roth B, Raghavan D, Dorr FA. Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol 1997;15:3394–8. [DOI] [PubMed] [Google Scholar]
- [22].Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vaughn DJ, Bellmunt J, Fradet Y, et al. Health-related quality-of-life analysis from KEYNOTE-045: a phase III study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. J Clin Oncol 2018;36:1579–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


