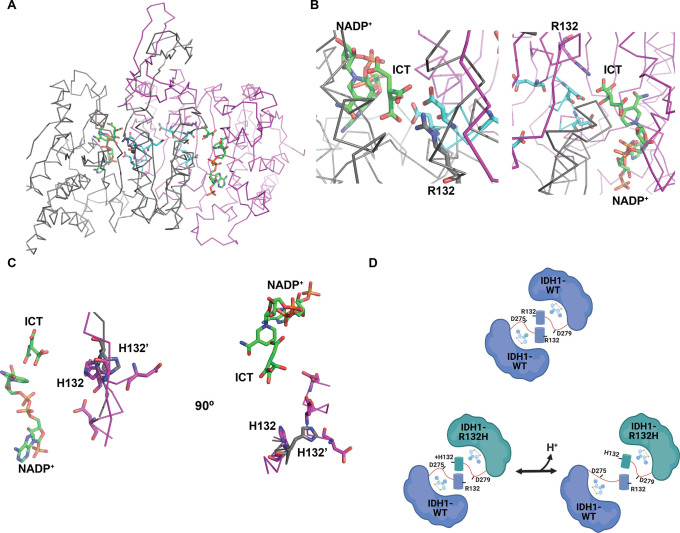
Figure 7.
Proposed mechanism of pH sensitive heterodimer formation. (A) Crystal structure of the WT:WT homodimer (PDB entry 1T0L).30 Monomer A is colored gray, and monomer B magenta. Regions containing key conserved aspartate residues are colored cyan. Shown as sticks in each monomer: Arg132, Asp273, Asp275, Asp279, with bound NADP+ and isocitrate (ICT) in green stick. (B) Close-up of monomer A (left) and monomer B (right) active sites. Shown as sticks in each monomer are Arg132 (gray in monomer A, magenta in monomer B), Asp273, Asp275, and Asp279 in cyan and bound NADP+ and ICT in green. (C) Crystal structure overlay of an R132H:R132H homodimer at high crystallization pH (gray, PDB entry 3MAP) and at low crystallization pH (magenta, PDB entry 4KZO). Shown as sticks are His132 and key conserved aspartate residues (Asp273, Asp275, and Asp279) that are only resolved in the low-pH structure (magenta). NADP+ and ICT are shown as green stick from the 3MAP structure. (D) Model for pH sensitive heterodimer formation, mediated by the ability of His132 to coordinate key aspartate residues in the heterodimer to promote the quasi-open conformation specifically at low pHi (left).