Abstract
Background
Fatty acid‐binding protein 3 (FABP3) is a cytosolic carrier protein of polyunsaturated fatty acids (PUFAs) and regulates cellular metabolism. However, the physiological functions of FABP3 in immune cells and how FABP3 regulates inflammatory responses remain unclear.
Methods
Contact hypersensitivity (CHS) induced by 2,4‐dinitrofluorobenzene (DNFB) and fluorescein isothiocyanate was applied to the skin wild‐type and Fabp3 −/− mice. Skin inflammation was assessed using FACS, histological, and qPCR analyses. The development of γ/δ T cells was evaluated by a co‐culture system with OP9/Dll1 cells in the presence or absence of transgene of FABP3.
Results
Fabp3‐deficient mice exhibit a more severe phenotype of contact hypersensitivity (CHS) accompanied by infiltration of IL‐17‐producing Vγ4+ γ/δ T cells that critically control skin inflammation. In Fabp3 −/− mice, we found a larger proportion of Vγ4+ γ/δ T cells in the skin, even though the percentage of total γ/δ T cells did not change at steady state. Similarly, juvenile Fabp3 −/− mice also contained a higher amount of Vγ4+ γ/δ T cells not only in the skin but in the thymus when compared with wild‐type mice. Furthermore, thymic double‐negative (DN) cells expressed FABP3, and FABP3 negatively regulates the development of Vγ4+ γ/δ T cells in the thymus.
Conclusions
These findings suggest that FABP3 functions as a negative regulator of skin inflammation through limiting pathogenic Vγ4+ γ/δ T‐cell generation in the thymus.
Keywords: contact hypersensitivity, FABP3, skin inflammation, γ/δ T‐cell development
FABP3 negatively regulates the differentiation of thymic DN2 cells into Vγ4+ γ/δ T cells. FABP3‐deficient mice exhibit higher frequency of Vγ4+ γ/δ T cells in skin of both juvenile and adults. FABP3 deficiency in Vγ4+ γ/δ T cells enhances production of high amounts of IL‐17A, which contributes to the pathogenesis of contact hypersensitivity. DN2, double negative cell; FABP3, fatty acid‐binding protein 3; KO, knockout.
Abbreviations: DN2, double negative cell; FABP3, fatty acid‐binding protein 3; KO, knockout.
Abbreviations
- CHS
contact hypersensitivity
- DN cell
double‐negative cells
- DNFB
2,4‐dinitrofluorobenzene
- FA
fatty acid
- FABP
fatty acid‐binding protein
- FITC
fluorescein isothiocyanate
- LN
lymph node
- PUFA
polyunsaturated fatty acid
- Tc
cytotoxic T cell
- TF
transcription factor
- Th
helper T cell
1. INTRODUCTION
The skin harbors various immune cells 1 and function as a frontier barrier that protects the host from external threats. γ/δ T cells express TCR γ/δ, but not TCR α/β, and these cells are divided into some subsets including Vγ5 or Vγ4 expressing γ/δ T cells (Vγ5+ γ/δ T cells and Vγ4+ γ/δ T cells, respectively). 2 Although γ/δ T cells are a small population among circulating T cells, some certain subsets of γ/δ T cells are more presented in epithelial tissues, such as skin and intestine. 3 The skin is divided into two major compartments: the epidermis and the dermis. In mice, while epidermal compartment predominantly contains Vγ5+ γ/δ T cells, Vγ4+ γ/δ T cells abundantly reside in dermal compartment. 4 More than half of Vγ4+ γ/δ T cells are IL‐17‐producing cells, and these cells are a major source of IL‐17 production in the early stages of skin inflammation. 5 It has been shown that γ/δ T cells promote inflammation and insulin resistance during high‐fat diet‐induced obesity 6 and are involved in the pathology of psoriatic dermatitis. 7 However, it remains elusive how systemic and/or cellular lipid metabolism affects the activation of γ/δ T cells and consequently the pathology of skin inflammation.
T cell precursors go through the positive and negative selection in the thymus and differentiate into CD4+, CD8+, and γ/δ T cells. These precursors display a CD4−CD8− (double negative, DN) phenotype and are further classified into CD44+CD25− (DN1), CD44+CD25+ (DN2), CD44−CD25+ (DN3), and CD44−CD25− (DN4). 8 Mouse γ/δ T‐cell development initiates in the thymus at the embryonic stage, with some subsets of γ/δ T cells developing in an overlapping sequence. Unlike α/β T cells that are functionally differentiated to IL‐17‐producing effector cells only in the periphery, γ/δ T cells produce IL‐17 even in the early fetal thymus. 9 Transcription factor (TF) Sox4, Sox13, and HEB have been shown to be a master regulator of γ/δ T‐cell differentiation in the thymus and to pivotally participate in the differentiation of IL‐17‐producing γ/δ T cells. 10 , 11 , 12 Although TFs and cytokines that regulate γ/δ T cells have been identified, 13 the effects of lipid metabolism on T‐cell differentiation have not been fully elucidated.
Fatty acids (FAs) are a critical source of energy for maintaining activation, proliferation, and function of lymphocytes. 14 Although the immunological effects of polyunsaturated fatty acids (PUFAs) have been studied, the molecular mechanisms underlying these effects are still unknown. Fatty acid‐binding proteins (FABPs) can bind to long‐chain FAs including PUFAs and control signal transduction and transcription. 15 FABP3 that preferably binds to ω‐6 PUFA is widely expressed throughout the tissues and organs 16 , 17 , 18 and recently shown to be expressed in helper T subsets. 19 Regarding the function of FABPs in the immune cells, it is noted that tissue‐resident memory T cells can survive for a long time by modifying their intracellular lipid environment in accordance with expressing FABP4 and FABP5 at the differentiation stages. 20 On the other hand, FABP4 and FABP5 have been reported to be involved in the regulation of asthma and skin inflammation, 21 , 22 , 23 suggesting that FABP molecules regulate allergic diseases through immune regulation linked to lipid metabolism. Therefore, it seems likely that FABP3 plays a critical role in the immunological function of lymphocytes and is involved in the regulation of allergic diseases, but the roles of FABP3 in immune cells or regulation of allergic diseases are ill‐defined.
In this study, we investigated the effects of FABP3‐mediated changes in lipid metabolism of γ/δ T cells. We demonstrate a novel function of FABP3 as a negative regulator of Vγ4+ γ/δ T cells development from thymic DN2 precursors.
2. MATERIALS AND METHODS
2.1. Cell culture
OP9/Dll1 cells were kindly gifted by Dr Akihiko Yoshimura (Keio University, Japan). OP9 cells were cultured in MEM‐alpha supplemented with 20% Fetal calf serum, 50 μmol/L 2‐mercaptoethanol, 2 mmol/L L‐glutamine, and 1% penicillin/streptomycin. OP9/Dll1 co‐cultures were conducted in accordance with minor modifications of the previous protocol. 24 For DN cell development, thymic DN2 cells were placed on monolayers of OP9/Dll1, supplemented with 5 ng/mL IL‐7 and Flt3 ligand. After 4 days, the medium was replaced, and then, the cells were further incubated for 4 days more. For in vitro T‐cell culture, naive (CD44loCD62Lhi) CD4+ T cells were purified from spleens of wild‐type or Fabp3 −/− mice with a naive CD4+ T‐cell isolation kit II (130‐093‐227; Miltenyi Biotec). Naive CD4+ T cells were cultured with plate‐bound anti‐CD3 (1 μg/mL) and soluble anti‐CD28 (1 μg/mL), with the addition of cytokines shown in Table S1.
2.2. Mice
Fabp3 −/− mice on a C57BL/6 background have been described. 25 CD45.2+ wild‐type and Fabp3 −/− mice of the same genetic background were used in this study. All mice were bred and maintained under specific pathogen‐free conditions at the Institute for Animal Experimentation, Tohoku University Graduate School of Medicine, and were used at the age of 5 days old or 8‐12 weeks old. All procedures were approved by the Institutional Committee for the Use and Care of Laboratory Animals of Tohoku University.
2.3. Induction of contact hypersensitivity
Two days after shaving the back hair with electrical clippers, the back skin was treated with 25 µL of 0.5% DNFB in a 4:1 mixture of acetone and olive oil or 150 µL of 1.0% FITC isomer I in a 1:1 mixture of acetone and dibutyl phthalate. Five days later, the respective mice were challenged with 40 µL of 0.2% DNFB or 0.5% FITC isomer I (each surface of the left ear) and 40 µL of the vehicle alone (each surface of the right ear). The ear thickness was measured before and after DNFB or FITC challenge using dial thickness gauges.
2.4. RNA extraction and Real‐time RT‐PCR
Total RNA was extracted from whole skin tissues or sorted Vγ4+ γ/δ T cells using RNeasy Micro Kit (QIAGEN) and reverse‐transcribed with SuperScript III Reverse Transcriptase (Thermo Fisher Scientific). Real‐time PCR was performed utilizing SYBR Premix Ex Tag (Takara Bio) on a 7500 real‐time PCR system (Thermo Fisher Scientific). Relative gene expression was calculated by the ΔCt method and normalized to the amount of Gapdh. Primers were shown in Table S2.
2.5. Flow cytometry
Cells were incubated with anti‐CD16/CD32 (2.4G2) before being stained with the following antibodies to cell‐surface and intracellular antigens. For intracellular staining, after cell activation with PMA/Ionomycin and staining of surface markers, cells were fixed and permeabilized with Cytofix/Cytoperm and Perm/Wash buffer (BD Biosciences) according to the manufacturer's instructions: CD3 (17A2), CD4 (GK1.5), CD8 (53‐6.7), CD25 (PC61), CD44 (IM7), c‐kit (2B8), γ/δ TCR (GL3), Vγ4 (UC3‐10A6), Vγ5 (536), IL‐17 (TC11‐18H10.1), and IFN‐γ (XMG1.2) (BioLegend). Data were acquired on a FACSCanto II (BD Biosciences) and were analyzed with FlowJo software (Tree Star).
2.6. Retroviral transfection
Retroviral transduction was previously described 26 and was modified slightly. The cDNA that encodes FABP3 was cloned into pMY‐IRES‐EGFP. Recombinant retroviruses were prepared by transfecting the Plat‐E packaging cells. Virus‐containing supernatants were concentrated by centrifugation at 6000 × g at 4°C for 16 hours, and then, the concentrated virus particles were added into sorted DN2 cells from wild‐type and Fabp3 −/− mice in the presence of 20 μg/mL polybrene. Virus‐containing media were replaced by fresh media, and the cells were used for experiments 24‐48 hours later. In some experiments, FABP3‐transducted DN2 cells were cultured over OP9/Dll1 cells and were analyzed the capacity of differentiation into γ/δ T cells.
2.7. Histochemistry and Immunofluorescence staining
For HE staining, ear tissues were fixed by ALTFIX and embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin. For immunofluorescence staining, the thymus was fixed with ALTFIX and embedded in OCT compound. Sections were stained with antibodies as follows. The first and secondary antibodies were FABP3 (mouse polyclonal antibody) and goat anti‐mouse IgG‐Alexa Fluor 488 (Life Technologies), respectively. Nuclei were stained by DAPI (Life Technologies).
2.8. Statistical analysis
To test differences among more than two groups, one‐way analysis of variance (ANOVA) was used to establish statistical significance. In all other instances, an unpaired and two‐tailed Student's t test was applied. P values of <.05 were considered statistical significance. *P < .05, **P < .01, ***P < .001.
3. RESULTS
3.1. FABP3‐deficient mice display augmented contact hypersensitivity
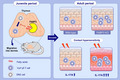
To understand whether FABP3 regulates skin inflammatory responses, we first evaluated ear swelling after eliciting DNFB‐induced CHS Fabp3 −/− mice exhibited more severe ear swelling when compared with wild‐type mice (Figure 1A). Histological analysis revealed hyperplasia of both epidermal and dermal skin in Fabp3 −/− mice (Figure 1B). Similarly, FITC‐challenged Fabp3 −/− mice exhibited more severe ear swelling (Figure 1C). Moreover, FACS analysis showed that eosinophils and neutrophils, but not CD4+ T cells and CD8+ T cells, were more highly accumulated in Fabp3−/− skin than wild‐type (Figure 1D). CHS is regulated by adaptive immunity such as CD4+ helper T cells (Th) and CD8+ cytotoxic T cells (Tc). 27 , 28 To examine the direct effect of FABP3‐deficient pathogenic T cells, we performed CHS to congenic wild‐type mice (CD45.1), which were transferred with sensitized wild‐type or Fabp3 −/− α/β T cells (CD45.2). No difference in ear swelling between the two groups was observed (Figure 1E), suggesting adaptive immunity has no major role in the enhanced CHS phenotype observed in Fabp −/− mice. Additionally, mRNA expression of Ifnγ and Il17a, which play critical roles for skin inflammation, 29 was elevated in the inflamed skin of Fabp3 −/− mice (Figure 1F). However, there was no significant difference in the expression of Il4, Il13, Cxcl1, Cxcl2, Ccl2, and Ccl20 mRNA between Fabp3 −/− mice and wild‐type mice (Figure 1F). These results suggested that FABP3 negatively regulates CHS probably by modulating inflammatory responses.
FIGURE 1.
FABP3 limits contact hypersensitivity. (A) Wild‐type (WT) and Fabp3 −/− (KO) mice were sensitized and challenged with DNFB. The ear skin thickness of the mice was measured at the indicated time points after challenge with DNFB or vehicle alone. *P < .05 vs. corresponding values for vehicle‐treated mice, and †P < .05 vs. hapten‐challenged wild‐type mice. (B) Representative histological views (H&E) of ear skin from WT and KO mice at 24 h after challenge with DNFB or vehicle alone. Scale bar = 50 μm. (n = 8) (C) WT and KO mice were sensitized and challenged with FITC. The ear skin thickness of the mice was measured at the indicated time points after challenge with FITC or vehicle alone. *P < .05 vs. corresponding values for vehicle‐treated mice, and †P < .05 vs. hapten‐challenged wild‐type mice. (D) The absolute number of eosinophils, neutrophils, CD4+ T cells, and CD8+ T cells in indicated skin samples at 48 h after challenge. (E) WT and KO mice (CD45.2) were sensitized with DNFB. Five days after sensitization, purified α/β CD3+ T cells (5.0 × 106 cells) from DNFB‐sensitized mice were transferred to naive congenic WT mice (CD45.1), and these mice were challenged with DNFB or vehicle alone 24 h after cell transfer. The ear skin thickness of the mice was measured at the indicated time points after challenge with DNFB or vehicle alone. *P < .05 vs. corresponding values for vehicle‐treated mice. (F) Ifnγ, Il17a, Il4, Il13, Cxcl1, Cxcl2, Ccl2, and Ccl20 mRNA expression in whole ear skin. Results are normalized to those of Gapdh and are presented relative to those of vehicle‐challenged WT mice. *P < .05, ***P < .001 (Student's t test). Data are from one experiment representative of three independent experiments with similar results (A, C, D) (mean ± SEM of total 12 mice per genotype (A, C, E); mean ± SEM of three replicates (D, F))
3.2. FABP3‐deficient mice exhibited normal T‐cell development
Although Figure 1 results showed there was no difference between the two groups in the number of infiltrating T cells into the inflamed ear tissues, it is unclear whether there is a functional difference between wild‐type and Fabp3 −/− T cells. As a result, Fabp3−/− mice display normal T‐cell populations in spleen, LNs, and thymus (Figure S1A‐C). Although Th1 and Th17 highly expressed FABP3 when compared with naive CD4+ T cells, 19 Fabp3 −/− naive CD4+ T cells could normally differentiate into T‐bet+ and IFNγ+ (Th1), GATA3+, IL‐5+ and IL‐13+ (Th2), RORγt+ and IL‐17A+ (Th17), and Foxp3+ (Treg) T cells when compared with wild‐type naive CD4+ T cells (Figure S1D‐G). These results suggest that FABP3 has no critical effect on the differentiation and function of α/β T cells.
3.3. Increase in γ/δ T cells in inflamed ear tissues of FABP3‐deficient mice
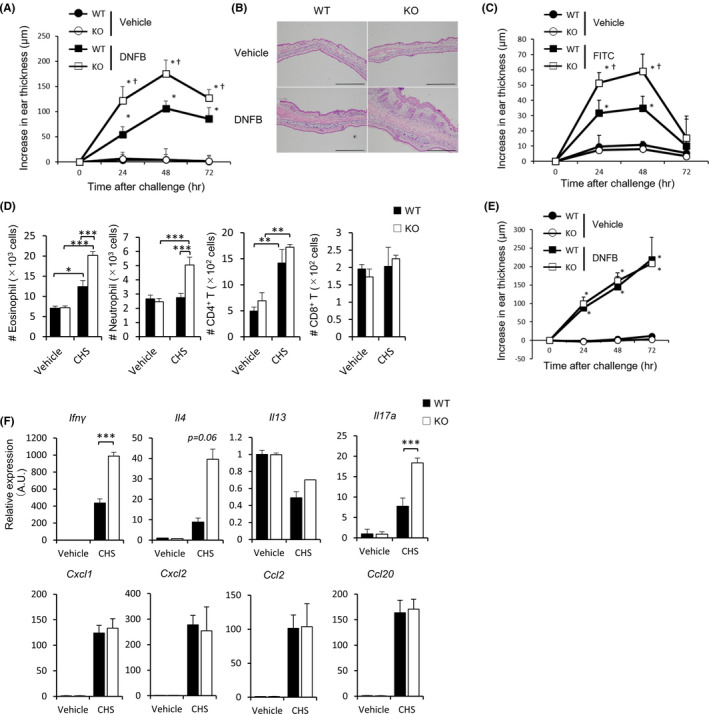
In the skin, the epidermal layer exclusively contains Vγ5+ γ/δ T cells, while the dermal layer enriches Vγ4+ and other V chain positive γ/δ T cells. 30 , 31 IL‐17‐producing γ/δ T cells in dermis mainly comprise of Vγ4+ γ/δ T cells, which are attributed to CHS regulation. 5 , 32 To clarify whether dermal Vγ4+ γ/δ T cells are responsible for the augmented skin inflammation in Fabp −/− mice, we analyzed inflamed ear tissues by flow cytometry. We found that there were significantly higher numbers of Vγ5+ and Vγ4+ γ/δ T cells accumulated in the skin of Fabp3 −/− mice when compared with that of wild‐type mice after DNFB‐challenge (Figure 2A, B). Furthermore, we found that Fabp3 −/− mice exhibited a more infiltration of IL‐17‐producing Vγ4+ γ/δ T cells in the inflamed ear tissues (Figure 2C). In accordance with intracellular cytokine staining, Fabp3 −/− Vγ4+ γ/δ T cells produced a high amount of IL‐17A compared with wild‐type cells (Figure S2). On the contrary, there was no difference in the number of IFN‐γ‐producing Vγ5+ γ/δ T cells between the two groups (Figure S3).
FIGURE 2.
Exacerbation of CHS is associated with skin Vγ4 + γ/δ T cells increment in Fabp3 −/− mice. (A, B) Frequency (left) and number (right) of CD3+ γ/δ TCR+, Vγ5+ and Vγ4+ γ/δ T cells in the inflamed (DNFB) ears (A) and normal (vehicle) ears (B) of WT and Fabp3 −/− mice at 24 h after challenge. (C) Frequency (left) and number (right) of IL‐17A+ Vγ4+ γ/δ T cells in the inflamed ears of WT and KO mice. (D, E) Ccr6 and Ccr2 (D) and Heb, Sox4, Sox13, Rorc, and Maf (E) mRNA expression of sorted dermal Vγ4+ γ/δ T cells in the inflamed and normal skin. Results are normalized to those of Gapdh and are presented relative to those of sorted dermal Vγ4+ γ/δ T cells in vehicle‐challenged WT mice. *P < .05 and ***P < .001 (Student's t test). Data are from one experiment representative of three independent experiments with similar results (A‐E) (mean ± SEM of three replicates (A‐E))
While CCR6 is involved in the positioning of IL‐17‐producing γ/δ T cell in the skin at steady state, CCR2 controls the migration of these cells into inflamed skin tissues. 33 , 34 In this process, activated γ/δ T cells downregulate CCR6 while upregulating CCR2. 33 To evaluate the effect of FABP3 deficiency on these chemokine receptors, we analyzed Ccr6 and Ccr2 mRNA expression in Vγ4+ γ/δ T cells by quantitative PCR. Interestingly, sorted dermal Fabp3 −/− Vγ4+ γ/δ T cells expressed higher levels of Ccr6 and Ccr2 when compared with wild‐type cells (Figure 2D). In addition, sorted dermal Fabp3 −/− Vγ4+ γ/δ T cells also exhibited higher levels of Heb, Sox4, Sox13, Rorc, and Maf (Figure 2E). Previous studies have shown that Heb, Sox4, Sox13, Rorc, and Maf interact with each other to regulate mutually these genes expression in γ/δ T cells, 12 , 31 , 35 and that Rorc expression regulates IL‐17A production and CCR6 expression, while Maf potentially controls CCR2 expression. 36 , 37 Thus, our results, in line with these findings, suggest FABP3 regulates γ/δ T‐cell activity through probably regulating the expression of their essential genes.
3.4. FABP3 deficiency affects γ/δ T‐cell distribution in the periphery
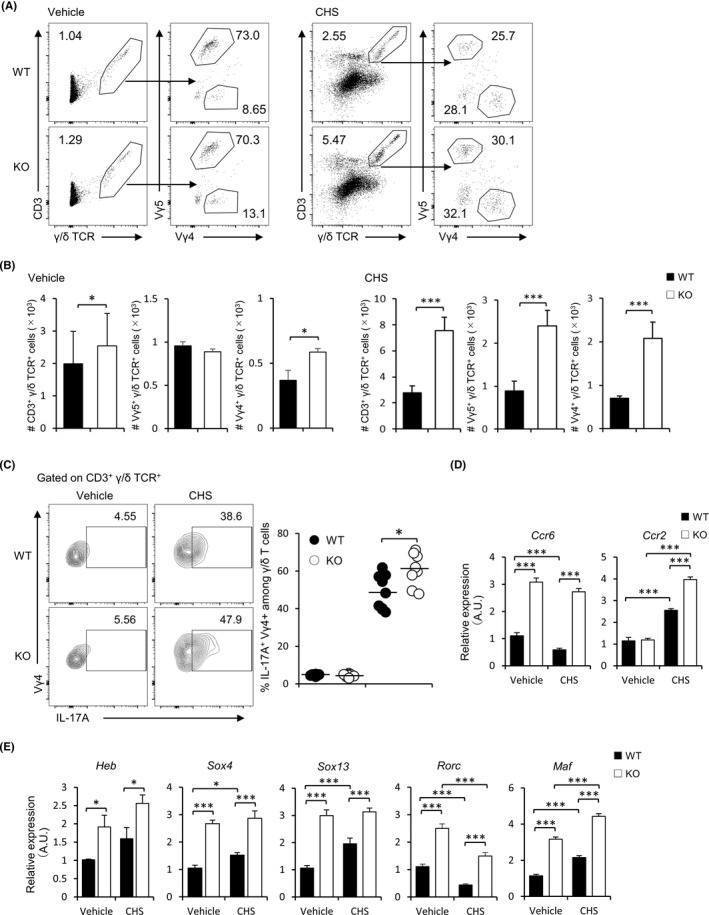
Since we have shown that FABP3 is involved in the expression of molecules that control the dynamics of Vγ4+ γ/δ T cells, we next evaluated the distribution of this cell type in various FABP3‐deficient organs. Although the percentage of total γ/δ T cells was no difference between two groups (Figure 3A‐E), there was a higher percentage of Vγ4+ γ/δ T cells in the skin but significant lower percentages of the cell type in the thymus, spleen, and inguinal LNs (iLNs) in FABP3‐deficient mice when compared with wild‐type mice (Figure 3A, F). Nevertheless, Vγ4+ γ/δ T cells in peripheral blood were comparable between WT and Fabp3 −/− mice (Figure 3F). γ/δ T cells arise from common multipotent double‐negative (DN) precursors in the thymus, which can be further dissected into four subsets based on CD44 and CD25 expression. 38 , 39 Contingent on the previous findings in adult mice, we hypothesized that FABP3 deficiency might affect the generation of Vγ4+ γ/δ T cells at the fetal stage. By using juvenile mice at five days old, we identified that juvenile Fabp3 −/− mice displayed more Vγ4+ γ/δ T cells than wild‐type counterparts in thymus and skin tissues (Figure 4A, B), while no difference in the number of total thymocytes (Figure 4C). Moreover, sorted dermal Vγ4+ γ/δ T cells in juvenile Fabp3 −/− mice expressed high levels of Ccr6, Ccr2, Heb, Sox4, Sox13, Rorc, and Maf, which is also observed in adult mice (Figure 4D, E). These results suggest that Fabp3 −/− mice had increased generation of Vγ4+ γ/δ T cells highly expressed these genes in the thymus during at least neonatal stage, leading to an increased proportion of Vγ4+ γ/δ T cells in the skin.
FIGURE 3.
FABP3‐deficient adult mice displayed a high percentage of Vγ4 + γ/δ T cells in the skin, but not thymus, spleen, inguinal lymph nodes, and peripheral blood. (A) Percentages of CD3+ γ/δ TCR+ cells, Vγ5+, and Vγ4+ γ/δ T cells in the ear skin of adult WT and KO mice. (B‐E) Total CD3+ γ/δ T cell profiles in the spleen (B), iLNs (C), thymus (D), and peripheral blood (E) from WT and KO mice. Numbers adjacent to outlined areas (left) indicate percentages of CD3+ and γ/δ TCR+ T cells. (F) Percentages of Vγ4+ γ/δ T cells in various tissues of WT and KO mice. *P < .05, ***P < .001 (Student's t test). Data are one experiment representative of at least three independent experiments with similar results (mean ± SEM of three replicates (A‐F))
FIGURE 4.
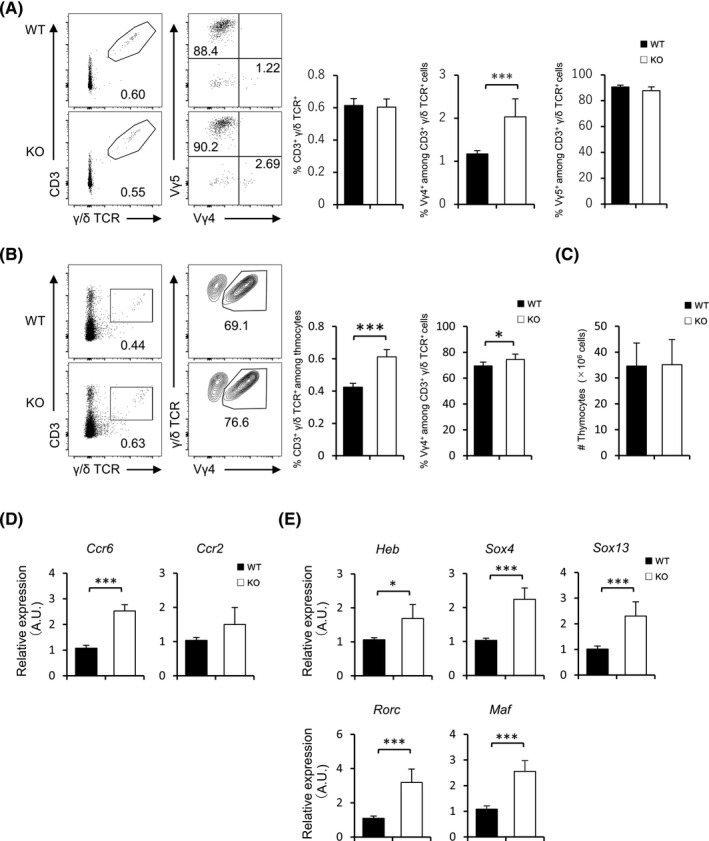
Juvenile FABP3‐deficient mice showed more abundant Vγ4 + γ/δ T cells. (A, B) Frequency (left) and number (right) of CD3+ γ/δ TCR+, Vγ5+, and Vγ4+ γ/δ T cells in the skin (A) and thymus (B) of juvenile WT and Fabp3 −/− mice. (C) The number of thymocytes in juvenile WT and Fabp3 −/− mice. (D, E) Ccr6 and Ccr2 (D) and Heb, Sox4, Sox13, Rorc, and Maf (E) mRNA expression of sorted dermal Vγ4+ γ/δ T cells in juvenile mice. Results are normalized to those of Gapdh and are presented relative to those of WT Vγ4+ γ/δ T cells. *P < .05, ***P < .001 (Student's t test). Data are from one experiment representative of three independent experiments with similar results (A‐E) (mean ± SEM of three replicates (A‐E))
3.5. FABP3 is expressed by thymic DN2 precursors and regulates its differentiation into Vγ4+ γ/δ T cell
Given that FABP3 deficiency has an impact on γ/δ T‐cell distribution, we sought to assess the expression of FABP3 in the thymus. IL‐17‐producing γ/δ T cells (mainly Vγ4+) have shown to be derived from thymic DN2 cells. 40 Fluorescent immunostaining demonstrated that FABP3 localizes in the thymic cortex (Figure 5A). In line with that, thymic DN2 cells abundantly express Fabp3 when compared with thymic cells (Figure 5B). Vγ4+ γ/δ T cells are known to be produced from DN2 precursors in the thymus during the fetal/neonatal stage and mainly distribute to the skin dermal layer. 31 Juvenile Fabp5 −/− mice showed a higher frequency of DN2 cells in the thymus (Figure 5C), suggesting that FABP3 controls differentiation in DN stages. To further evaluate the intrinsic developmental potential of DN precursors under the same conditions, we next conducted the OP9‐DL1 co‐culture system. 41 Double‐negative 2 cells were sorted and placed in the OP9‐DL1 co‐culture with recombinant IL‐7 and Flt3 ligand. Interestingly, there was a significantly higher percentage of Vγ4+ γ/δ T cells differentiated from FABP3‐deficient DN2 when compared with wild‐type DN2 cells (Figure 5D). Moreover, to obtain the direct evidence of FABP3 in intrinsically controlling the differentiation of DN2 cells into Vγ4+ γ/δ, we infected Fabp3 −/− DN2 cells with the FABP3‐encoding retrovirus vector. We found that FABP3‐transgene thymic Fabp3 −/− DN2 cells illustrated a normal differentiation into Vγ4+ γ/δ T cells (Figure 5E). Finally, we observed that Fabp3 −/− DN2 cells displayed a higher expression of Heb, Sox4, and Sox13, when compared with wild‐type DN2, and transgene of FABP3 to Fabp3 −/− DN2 cells normalized its abnormal expression (Figure 5F). These results suggest FABP3 in thymic DN2 cells intrinsically plays an important role in appropriate commitment of γ/δ T cells.
FIGURE 5.
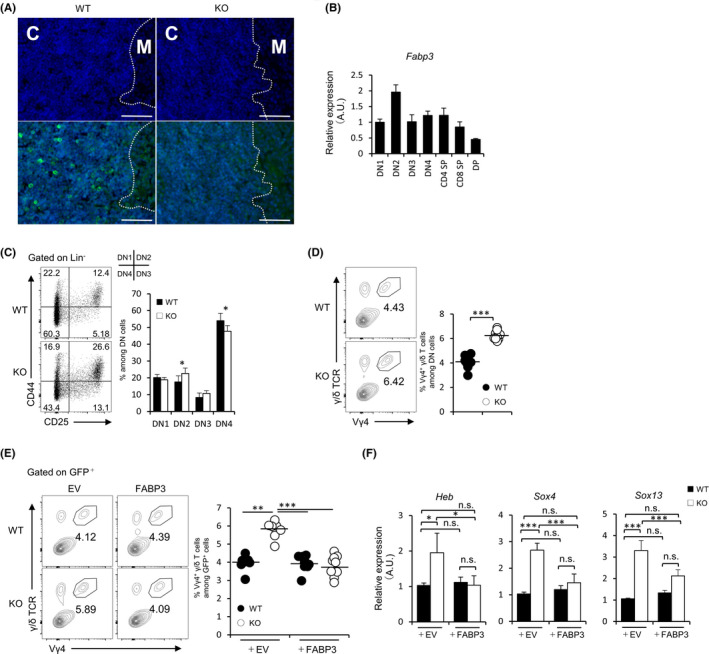
FABP3 regulates differentiation thymic DN2 cells into Vγ4 + γ/δT cells. (A) Immunofluorescence staining of FABP3 (green) and DAPI (blue) in the thymus. C: cortex, M: medulla, Scale bar: 50 μm. (B) Fabp3 mRNA expression of thymic double‐negative cells (DN), CD4 single‐positive thymocyte (CD4 SP), CD8 single‐positive thymocyte (CD8 SP), and double‐positive cell (DP) (C) Frequency (left) and number (right) of thymic DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−) cells in juvenile WT and KO mice. (D) Sorted DN2 cells from juvenile WT and KO mice were co‐cultured with OP9/Dll1 cells in the presence of IL‐7 (5 ng/mL) and Flt3 ligand (5 ng/mL). Representative dot plots show the frequency of Vγ4+ γ/δ T cells while graphs indicate the number of Vγ4+ γ/δ T cells. (E) WT and KO DN2 cells were retrovirally transduced with FABP3, and GFP+ DN2 cells were co‐cultured on OP9/Dll1 cells with IL‐7 (5 ng/mL) and Flt3 ligand (5 ng/mL). Representative dot plots show the frequency of GFP+ Vγ4+ γ/δ T cells while graphs indicate the number of GFP+ Vγ4+ γ/δ T cells. (F) Heb, Sox4, and Sox13 mRNA expression of thymic DN2 cells in juvenile mice. Results are normalized to those of Gapdh and are presented relative to those of WT transducing EV. EV: empty vector, *P < .05, ***P < .001 (Student's t test). n.s. not significant. Data are from one experiment representative of three independent experiments with similar results (A‐F) (mean ± SEM of three replicates (B‐F))
4. DISCUSSION
The skin resident immune cells work together closely to maintain homeostasis of skin tissues. 42 , 43 Obesity has been suspected as a major risk factor of allergic diseases, including asthma, atopic dermatitis, CHS, and psoriasis. 44 , 45 , 46 , 47 Previous reports provide many pieces of evidence that obesity is tightly involved in skin inflammation through functional changes in immune cells. 23 , 48 As described above, γ/δ T cells also are reported to regulate the skin inflammation. 7 , 49 In line with previous reports, Fabp3 −/− mice exhibited a severe phenotype of CHS accompanied by hyper‐activation of IL‐17‐producing Vγ4+ γ/δ T cells. Surprisingly, our results revealed that thymic DN2 cells were found to express FABP3 abundantly, and FABP3 regulates the development of Vγ4+ γ/δ T cells from these cells in the thymus. Together these findings clarify a previously unknown function of FABP3 in pathogenic Vγ4+ γ/δ T‐cell development.
Although γ/δ T cells are a minor population compared to α/β T cells, these cells are involved in various inflammations. 50 Unlike αβ T cells, however, few reports are showing the relationship between lipid metabolism and γ/δ T cells. PUFA‐enriched diet‐fed mice exhibited a large number of splenic γ/δ T cells and high‐fat diet‐fed mice exhibited exacerbated dermatitis accompanied by accumulation of IL‐17‐producing Vγ4+ γ/δ T cells in the skin, 7 suggesting that changes in the lipid environment certainly have some effect on γ/δ T cells. Besides, γ/δ T cells were reported to contain a higher cholesterol level than α/β T cells, and the increase in intracellular cholesterol of γ/δ T cells affects the activation of these cells by enhancing the basal T‐cell receptor intensity. 51 In Fabp3 −/− γ/δ T cells, it is conceivable that downregulation of intracellular cholesterol level due to a disruption of the ω‐6/ω‐3 ratio by the decrease in intracellular ω‐6 PUFA, resulting in enhancing the activation of these cells. Meanwhile, γ/δ T cells are also known to be epigenetically regulated, as well as α/β T cells. 52 Thymus development of IL‐17‐producing γ/δ T cells is restricted to the embryonic and perinatal stages during embryonic development, 9 and it is demonstrated that these epigenetic regulations in γ/δ T cells have already occurred in the thymus. 53 Additionally, Malhotra et al have reported the histone modification profile of Rorc locus in immature Vγ4+ thymocytes was active condition. 12 This knowledge suggests that changes in the thymic lipid microenvironment caused by FABP3 deficiency affect the epigenetic regulation in early γ/δ T‐cell differentiation. Interestingly, we found that despite there was no difference in percentages of total skin γ/δ T cells between Fabp3 −/− mice and wild‐type mice at steady state, a higher percentage of Vγ4+ γ/δ T cells was present in Fabp3 −/− skin accompanied with high expression of chemokine receptors and TFs. Furthermore, since juvenile Fabp3 −/− skin also demonstrated a higher percentage of Vγ4+ γ/δ T cells, we speculated that FABP3 might involve in the early γ/δ T‐cell formation in thymus.
The thymus is an important organ for T‐cell development. 54 A body of reports revealed a requirement of IL‐7 in the proper IL‐17‐producing γ/δ T‐cell development. 55 , 56 Notch ligand expressed by thymic epithelial cells has also been suggested to be involved in IL‐17‐producing γ/δ T‐cell differentiation. 57 Additionally, IL‐17‐producing γ/δ T cells are considered to be generated from thymic T‐cell precursors which receive weak TCR signals during their development. 58 Although the mechanisms of γ/δ T‐cell commitment regulated by cytokines, stimulatory molecules, and TFs have been elucidated, how the lipid environment in thymus affects T‐cell development remains poorly unveiled. Many studies demonstrated that obesity promotes thymus senescence accompanied by affecting thymic epithelial cells and T‐cell precursors. 59 , 60 Moreover, aging alters the composition of γ/δ T cells in peripheral blood and LNs, resulting in an increase in IL‐17‐producing γ/δ T cells including Vγ4+ γ/δ T cells. 61 , 62 Our results revealed that Fabp3 −/− mice displayed a large number of Vγ4+ γ/δ T cells in the juvenile thymus. It is also noteworthy that the FA composition of the thymus has been shown to be rich in ω‐6 PUFA, 63 to which FABP3 prefers to bind. We previously showed that FABP3 is involved in epigenetic regulation in B cells. 64 Furthermore, PUFAs have been reported to be responsible for epigenetic modifications. 65 , 66 Our results from the OP9‐DL1 co‐culture system indicate that these events are caused by the intrinsic effects of FABP3 in DN2 cells. Considering above, changes in the thymic lipid microenvironment during fetal γ/δ T‐cell development in Fabp3 −/− mice affected the balance in γ/δ T‐cell commitment through modulating TF expression by epigenetic modifications in thymic DN2 cells due to some triggered induced by an abnormality in the suitable PUFA balance. Furthermore, our current results suggest that changes in the lipid microenvironment during the fetal period may regulate the commitment of pathogenic γ/δ T‐cell development in the thymus and affect the incidence of allergic dermatitis in adults. We hypothesize that abnormal maternal fatty acid intake causes disruption of lipid homeostasis during the fetal or neonatal period and promotes the production of pathogenic Vγ4+γ/δ T cells in the skin, which in turn influences the development of allergic skin inflammation in adulthood. Therefore, we also infer that proper regulation of fatty acid intake (or composition) in the motherhood may be able to control some of the onset and pathological exacerbations of skin allergic inflammation in the offspring.
Some studies reported that lipid metabolism in α/β T cells plays important role in generating memory T cells 67 , 68 and that the metabolic pattern dramatically changes when transitioning from naïve T cells to effector T cells and from effector T cells to memory T cells. 69 Recently, FABP3 was shown to be abundantly expressed by Th17 compared with naïve CD4+ T cells. 19 However, our current results demonstrated that FABP3‐deficient naive CD4+ T cells exhibited a comparable capacity of Th17 differentiation when compared with wild‐type counterparts. Since Th17 drastically changes its activity due to arachidonic acid and/or arachidonic acid metabolites, 70 , 71 it is possible that FABP3, which preferably binds to arachidonic acid, 16 is involved in functional expression of Th17 cells, including memory T cells formation, but not Th17 differentiation. Interestingly, since it has been reported that FABP4/5, which is highly homologous to FABP3, regulates longevity of tissue‐resident memory T cells, 20 FABP3 maybe participate in memory T‐cell regulation by linking with ω‐6 PUFA. Further consideration will be needed to yield any findings about this point.
In conclusion, we have revealed a critical role of FABP3 in regulating γ/δ T‐cell development and activity via modulating the expression of TFs in the thymus. This study demonstrates a novel mechanism in skin inflammation that illustrates a linkage between lipid metabolism and γ/δ T‐cell‐mediated responses and thus provides a new therapeutic approach to treat several immune diseases.
CONFLICT OF INTEREST
The authors have no financial conflicts of interest.
AUTHOR CONTRIBUTIONS
SK and YO conceived and directed this study, designed and performed experiments, analyzed the data, and contributed to the writing of the manuscript. HP, YK, HM, AA, and YT performed experiments and analyzed the data. TM, YA, and NI provided critical materials, discussion, participated in the interpretation of the data, and contributed to the writing of the manuscript.
Supporting information
Fig S1
Fig S2‐S3
Table S1‐S2
ACKNOWLEDGMENTS
We thank the Biomedical Research Core and the Institute for Animal Experimentation (Tohoku University Graduate School of Medicine) for technical support. This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (No. 19K24307 and 20K16392 to SK, 19H04026 to Y. O.) and by AMED under Grant Number JP17dm0107071 (to KF and YO).
Kobayashi S, Phung HT, Kagawa Y, et al. Fatty acid‐binding protein 3 controls contact hypersensitivity through regulating skin dermal Vγ4+ γ/δ T cell in a murine model. Allergy.2021;76:1776–1788. 10.1111/all.14630
Contributor Information
Shuhei Kobayashi, Email: s_kobayashi@med.tohoku.ac.jp.
Yuji Owada, Email: Owada@med.tohoku.ac.jp.
REFERENCES
- 1. Ho AW, Kupper TS. T cells and the skin: from protective immunity to inflammatory skin disorders. Nat Rev Immunol. 2019;19(8):490‐502. [DOI] [PubMed] [Google Scholar]
- 2. Sun LD, Qiao S, Wang Y, et al. Vγ4+ T Cells: A Novel IL‐17‐Producing γδ T Subsets during the Early Phase of Chlamydial Airway Infection in Mice. Mediators Inflamm. 2018;2018:6265746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chien YH, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Annu Rev Immunol. 2014;32:121‐155. [DOI] [PubMed] [Google Scholar]
- 4. Li Y, Wu J, Luo G, He W. Functions of Vγ4 T cells and dendritic epidermal T cells on skin wound healing. Front Immunol. 2018;9:1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cai Y, Shen X, Ding C, et al. Pivotal role of dermal IL‐17‐producing γδ T cells in skin inflammation. Immunity. 2011;35(4):596‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta P, Nuotio‐Antar AM, Smith CW. γδ T cells promote inflammation and insulin resistance during high fat diet‐induced obesity in mice. J Leukoc Biol. 2015;97(1):121‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakamizo S, Honda T, Adachi A, et al. High fat diet exacerbates murine psoriatic dermatitis by increasing the number of IL‐17‐producing γδ T cells. Sci Rep. 2017;7(1):14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Germain RN. T‐cell development and the CD4‐CD8 lineage decision. Nat Rev Immunol. 2002;2(5):309‐322. [DOI] [PubMed] [Google Scholar]
- 9. Haas JD, Ravens S, Düber S, et al. Development of interleukin‐17‐producing γδ T cells is restricted to a functional embryonic wave. Immunity. 2012;37(1):48‐59. [DOI] [PubMed] [Google Scholar]
- 10. Melichar HJ, Narayan K, Der SD, et al. Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science. 2007;315(5809):230‐233. [DOI] [PubMed] [Google Scholar]
- 11. Gray EE, Ramírez‐Valle F, Xu Y, et al. Deficiency in IL‐17‐committed Vγ4(+) γδ T cells in a spontaneous Sox13‐mutant CD45.1(+) congenic mouse substrain provides protection from dermatitis. Nat Immunol. 2013;14(6):584‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malhotra N, Narayan K, Cho OH, et al. A network of high‐mobility group box transcription factors programs innate interleukin‐17 production. Immunity. 2013;38(4):681‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baccala R, Witherden D, Gonzalez‐Quintial R, et al. Gamma delta T cell homeostasis is controlled by IL‐7 and IL‐15 together with subset‐specific factors. J Immunol. 2005;174(8):4606‐4612. [DOI] [PubMed] [Google Scholar]
- 14. Nicolaou A, Mauro C, Urquhart P, Marelli‐Berg F. Polyunsaturated Fatty Acid‐derived lipid mediators and T cell function. Front Immunol. 2014;5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanhoff T, Lücke C, Spener F. Insights into binding of fatty acids by fatty acid binding proteins. Mol Cell Biochem. 2002;239(1–2):45‐54. [PubMed] [Google Scholar]
- 16. Islam A, Kagawa Y, Sharifi K, et al. Fatty Acid Binding Protein 3 Is Involved in n‐3 and n‐6 PUFA transport in mouse trophoblasts. J Nutr. 2014;144(10):1509‐1516. [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto Y, Kida H, Kagawa Y, et al. FABP3 in the anterior cingulate cortex modulates the methylation status of the glutamic acid decarboxylase. J Neurosci. 2018;38(49):10411‐10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhuang L, Li C, Chen Q, et al. Fatty acid‐binding protein 3 contributes to ischemic heart injury by regulating cardiac myocyte apoptosis and MAPK pathways. Am J Physiol Heart Circ Physiol. 2019;316(5):H971‐H984. [DOI] [PubMed] [Google Scholar]
- 19. Field CS, Baixauli F, Kyle RL, et al. Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell‐Intrinsic Checkpoint for Treg Suppressive Function. Cell Metab. 2019.31(2):422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan Y, Tian T, Park CO, et al. Survival of tissue‐resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543(7644):252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shum BO, Mackay CR, Gorgun CZ, et al. The adipocyte fatty acid‐binding protein aP2 is required in allergic airway inflammation. J Clin Invest. 2006;116(8):2183‐2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suojalehto H, Kinaret P, Kilpeläinen M, et al. Level of Fatty Acid Binding Protein 5 (FABP5) Is Increased in Sputum of Allergic Asthmatics and Links to Airway Remodeling and Inflammation. PLoS One. 2015;10(5):e0127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Li Q, Rao E, et al. Epidermal Fatty Acid binding protein promotes skin inflammation induced by high‐fat diet. Immunity. 2015;42(5):953‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang H, Pierce LJ, Spangrude GJ. Distinct roles of IL‐7 and stem cell factor in the OP9‐DL1 T‐cell differentiation culture system. Exp Hematol. 2006;34(12):1730‐1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Binas B, Danneberg H, McWhir J, Mullins L, Clark AJ. Requirement for the heart‐type fatty acid binding protein in cardiac fatty acid utilization. FASEB J. 1999;13(8):805‐812. [DOI] [PubMed] [Google Scholar]
- 26. Simmons A, Alberola‐Ila J. Retroviral Transduction of T Cells and T Cell Precursors. Methods Mol Biol. 2016;1323:99‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saint‐Mezard P, Berard F, Dubois B, Kaiserlian D, Nicolas JF. The role of CD4+ and CD8+ T cells in contact hypersensitivity and allergic contact dermatitis. Eur J Dermatol. 2004;14(3):131‐138. [PubMed] [Google Scholar]
- 28. Akiba H, Kehren J, Ducluzeau MT, et al. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+ T cytotoxic 1 cells inducing keratinocyte apoptosis. J Immunol. 2002;168(6):3079‐3087. [DOI] [PubMed] [Google Scholar]
- 29. He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. IL‐17 and IFN‐gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J Immunol. 2009;183(2):1463‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Brien RL, Born WK. Dermal γδ T cells–What have we learned? Cell Immunol. 2015;296(1):62‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jouan Y, Patin EC, Hassane M, Si‐Tahar M, Baranek T, Paget C. Thymic Program Directing the Functional Development of γδT17 Cells. Front Immunol. 2018;9:981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang X, Park CO, Geddes Sweeney J, Yoo MJ, Gaide O, Kupper TS. Dermal γδ T Cells Do Not Freely Re‐Circulate Out of Skin and Produce IL‐17 to Promote Neutrophil Infiltration during Primary Contact Hypersensitivity. PLoS One. 2017;12(1):e0169397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McKenzie DR, Kara EE, Bastow CR, et al. IL‐17‐producing γδ T cells switch migratory patterns between resting and activated states. Nat Commun. 2017;8:15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramírez‐Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vγ4+ γδT17 memory‐like cells that travel to distant skin and accelerate secondary IL‐17‐driven responses. Proc Natl Acad Sci U S A. 2015;112(26):8046‐8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. In TSH, Trotman‐Grant A, Fahl S, et al. HEB is required for the specification of fetal IL‐17‐producing γδ T cells. Nat Commun. 2017;8(1):2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skepner J, Ramesh R, Trocha M, et al. Pharmacologic inhibition of RORγt regulates Th17 signature gene expression and suppresses cutaneous inflammation in vivo. J Immunol. 2014;192(6):2564‐2575. [DOI] [PubMed] [Google Scholar]
- 37. Wheaton JD, Yeh CH, Ciofani M. Cutting Edge: c‐Maf Is Required for Regulatory T Cells To Adopt RORγt+ and Follicular Phenotypes. J Immunol. 2017;199(12):3931‐3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allman D, Sambandam A, Kim S, et al. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4(2):168‐174. [DOI] [PubMed] [Google Scholar]
- 39. Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zúñiga‐Pflücker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non‐T cell lineages. Immunity. 2004;20(6):735‐745. [DOI] [PubMed] [Google Scholar]
- 40. Shibata K, Yamada H, Nakamura M, et al. IFN‐γ‐producing and IL‐17‐producing γδ T cells differentiate at distinct developmental stages in murine fetal thymus. J Immunol. 2014;192(5):2210‐2218. [DOI] [PubMed] [Google Scholar]
- 41. Taghon T, Yui MA, Pant R, Diamond RA, Rothenberg EV. Developmental and molecular characterization of emerging beta‐ and gammadelta‐selected pre‐T cells in the adult mouse thymus. Immunity. 2006;24(1):53‐64. [DOI] [PubMed] [Google Scholar]
- 42. Kabashima K, Honda T, Ginhoux F, Egawa G. The immunological anatomy of the skin. Nat Rev Immunol. 2019;19(1):19‐30. [DOI] [PubMed] [Google Scholar]
- 43. Quaresma JAS. Organization of the skin immune system and compartmentalized immune responses in infectious diseases. Clin Microbiol Rev. 2019;32(4).e00034–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Silverberg JI, Kleiman E, Lev‐Tov H, et al. Association between obesity and atopic dermatitis in childhood: a case‐control study. J Allergy Clin Immunol. 2011;127(5):1180‐1186. [DOI] [PubMed] [Google Scholar]
- 45. Savetsky IL, Albano NJ, Cuzzone DA, et al. Lymphatic Function Regulates Contact Hypersensitivity Dermatitis in Obesity. J Invest Dermatol. 2015;135(11):2742‐2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sterry W, Strober BE, Menter A, Council IP. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br J Dermatol. 2007;157(4):649‐655. [DOI] [PubMed] [Google Scholar]
- 47. Miethe S, Guarino M, Alhamdan F, et al. Effects of obesity on asthma: immunometabolic links. Pol Arch Intern Med. 2018;128(7–8):469‐477. [DOI] [PubMed] [Google Scholar]
- 48. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T‐cell immune response and reverses starvation‐induced immunosuppression. Nature. 1998;394(6696):897‐901. [DOI] [PubMed] [Google Scholar]
- 49. Kitajima M, Kimura A, Suzuki H. Cutting edge: Nqo1 regulates irritant contact hypersensitivity against croton oil through maintenance of dendritic epidermal T cells. J Immunol. 2018;200(5):1555‐1559. [DOI] [PubMed] [Google Scholar]
- 50. Fay NS, Larson EC, Jameson JM. Chronic Inflammation and γδ T Cells. Front Immunol. 2016;7:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheng HY, Wu R, Gebre AK, et al. Increased cholesterol content in gammadelta (γδ) T lymphocytes differentially regulates their activation. PLoS One. 2013;8(5):e63746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmolka N, Wencker M, Hayday AC, Silva‐Santos B. Epigenetic and transcriptional regulation of γδ T cell differentiation: Programming cells for responses in time and space. Semin Immunol. 2015;27(1):19‐25.25726512 [Google Scholar]
- 53. Schmolka N, Serre K, Grosso AR, et al. Epigenetic and transcriptional signatures of stable versus plastic differentiation of proinflammatory γδ T cell subsets. Nat Immunol. 2013;14(10):1093‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takahama Y. Journey through the thymus: stromal guides for T‐cell development and selection. Nat Rev Immunol. 2006;6(2):127‐135. [DOI] [PubMed] [Google Scholar]
- 55. Nakamura M, Shibata K, Hatano S, et al. A genome‐wide analysis identifies a notch‐RBP‐Jκ‐IL‐7Rα axis that controls IL‐17‐producing γδ T cell homeostasis in mice. J Immunol. 2015;194(1):243‐251. [DOI] [PubMed] [Google Scholar]
- 56. Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC. Interleukin 7 (IL‐7) selectively promotes mouse and human IL‐17‐producing γδ cells. Proc Natl Acad Sci U S A. 2012;109(43):17549‐17554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shibata K, Yamada H, Sato T, et al. Notch‐Hes1 pathway is required for the development of IL‐17‐producing γδ T cells. Blood. 2011;118(3):586‐593. [DOI] [PubMed] [Google Scholar]
- 58. Wencker M, Turchinovich G, Di Marco BR, et al. Innate‐like T cells straddle innate and adaptive immunity by altering antigen‐receptor responsiveness. Nat Immunol. 2014;15(1):80‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang H, Youm YH, Vandanmagsar B, et al. Obesity accelerates thymic aging. Blood. 2009;114(18):3803‐3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Howard JK, Lord GM, Matarese G, et al. Leptin protects mice from starvation‐induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104(8):1051‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vasudev A, Ying CT, Ayyadhury S, et al. γ/δ T cell subsets in human aging using the classical α/β T cell model. J Leukoc Biol. 2014;96(4):647‐655. [DOI] [PubMed] [Google Scholar]
- 62. Prinz I, Sandrock I. Dangerous γδ T cells in aged mice. EMBO Rep. 2019;20(8):e48678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kimura F, Ito S, Endo Y, et al. Supplementation of DHA‐rich microalgal oil or fish oil during the suckling period in mildly n‐3 fatty acid‐deficient rat pups. Lipids. 2011;46(12):1101‐1110. [DOI] [PubMed] [Google Scholar]
- 64. Kobayashi S, Phung HT, Tayama S, et al. Fatty acid‐binding protein 3 regulates differentiation of IgM‐producing plasma cells. FEBS J. 2020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 65. Cinquina V, Calvigioni D, Farlik M, et al. Life‐long epigenetic programming of cortical architecture by maternal 'Western' diet during pregnancy. Mol Psychiatry. 2020;25(1):22‐36. [DOI] [PubMed] [Google Scholar]
- 66. Harb H, Irvine J, Amarasekera M, et al. The role of PKCζ in cord blood T‐cell maturation towards Th1 cytokine profile and its epigenetic regulation by fish oil. Biosci Rep. 2017;37(2).BSR20160485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. O'Sullivan D, van der Windt GJ, Huang SC, et al. Memory CD8(+) T cells use cell‐intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41(1):75‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ibitokou SA, Dillon BE, Sinha M, et al. Early Inhibition of Fatty Acid Synthesis Reduces Generation of Memory Precursor Effector T Cells in Chronic Infection. J Immunol. 2018;200(2):643‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T‐cell differentiation and memory development. Immunol Rev. 2012;249(1):27‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Monk JM, Turk HF, Fan YY, et al. Antagonizing arachidonic acid‐derived eicosanoids reduces inflammatory Th17 and Th1 cell‐mediated inflammation and colitis severity. Mediators Inflamm. 2014;2014:917149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee J, Aoki T, Thumkeo D, Siriwach R, Yao C, Narumiya S. T cell–intrinsic prostaglandin E2‐EP2/EP4 signaling is critical in pathogenic TH17 cell–driven inflammation. J Allergy Clin Immunol. 2019;143(2):631‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2‐S3
Table S1‐S2


