Abstract
Background and objective
Anti‐IL‐17A IgG/κ monoclonal antibody CJM112 binds both IL‐17A and IL‐17AF. The purpose of this First‐in‐Human study was to assess CJM112 effects on safety and efficacy in patients with moderate to severe plaque psoriasis.
Methods
This study had two parts: single ascending doses of 5–450 mg subcutaneous (s.c.) CJM112 (SAD) and multi‐dose parallel groups of CJM112 15 mg, 50 mg and 150 mg s.c. low frequency or high frequency (MD). SAD/MD were double‐blind, randomized and placebo‐controlled; MD also included a secukinumab 150 mg s.c. arm as an active comparator. Patients 18–65 years with moderate to severe psoriasis were included in this study. The efficacy outcome was the change in Psoriasis Area Severity Index (PASI) from baseline to Week 4 in the SAD part of the study, and from baseline to Week 12 in the MD part.
Results
96 patients were enrolled in this study (SAD, n = 42; MD, n = 54). In SAD, CJM112 doses from 15 mg and above demonstrated higher PASI responses compared with placebo at Week 12. CJM112 450 mg did not add further efficacy, but efficacy duration was prolonged compared with CJM112 150 mg. CJM112 MD resulted in a dose‐dependent decrease in PASI over time to Week 12. CJM112 150 mg high frequency did not exceed the effect of CJM112 150 mg low frequency and had similar efficacy to secukinumab 150 mg. The safety profile of CJM112 was as expected for an antibody targeting IL‐17A/IL‐17AF.
Conclusions
CJM112 had clinical efficacy in moderate to severe psoriasis and was generally safe and well tolerated in the doses tested. Additional neutralization of IL‐17AF did not translate to increased clinical efficacy compared with secukinumab.
Introduction
Plaque psoriasis (PsO) is a chronic systemic inflammatory disease characterized by erythematous and scaly skin plaques. PsO is associated with physical and psychosocial comorbidities, including cardiovascular disease, metabolic syndrome and depression. 1 , 2 , 3 , 4 , 5 , 6 , 7 Interleukin (IL)‐17A has been identified as a cornerstone cytokine involved in the development of psoriatic disease, and clinical trials have demonstrated the direct effects of inhibition of the IL‐17 pathway on PsO. 8 , 9 , 10
To date, three monoclonal antibodies targeting the IL‐17 pathway have been approved for the treatment of PsO, with numerous other compounds in clinical development. Both secukinumab and ixekizumab directly target IL‐17A, and brodalumab targets the IL‐17A receptor (IL‐17RA) blocking the biological activity of IL‐17A, IL‐17C, IL‐17F, IL‐17E and IL‐17AF. 11 , 12 Ixekizumab has specificity for both the IL‐17A homodimers and IL‐17AF heterodimers. 13 , 14 , 15 Bimekizumab, currently in development, selectively neutralizes both IL‐17A and IL‐17F. 16
CJM112 is a novel fully human anti‐IL‐17A IgG1/κ monoclonal antibody that, compared with secukinumab, targets a different epitope. CJM112 exhibits favourable physicochemical properties and has demonstrated a more than 10‐fold higher affinity to IL‐17A and a more than 200‐fold higher affinity to IL‐17AF in vitro (data on file, Novartis). In contrast to secukinumab, and similar to ixekizumab, CJM112 binds with similar affinity to both human IL‐17A and IL17AF. 13 CJM112 has been developed for the potential treatment of autoimmune and inflammatory conditions. The purpose of this First‐in‐Human (FIH) study was to generate safety, pharmacokinetics/pharmacodynamics (PK/PD), efficacy and dose–response data of single and multiple CJM112 dosing regimens in patients with PsO and directly compare it to secukinumab.
Methods
The generation and characterization of anti‐IL‐17 antibody CJM112 are detailed in Methods S1.
Study design
This study was a FIH study of CJM112 in moderate to severe PsO patients. This study was conducted in two parts, a single ascending dose part and a multi‐dose part conducted in a parallel group design. Both parts were double‐blind, randomized and placebo‐controlled, with Part II also including an active comparator arm (registered at ClinicalTrials.gov, NCT01828086 and Eudract, 2012‐004507‐12). Further details on study design (patient assignment/blinding, randomization, sample size calculations and power analysis) are given in Methods S2.
Part I Study Design (Single Ascending Doses, SAD)
Part I evaluated five sequential single ascending doses (SAD) between 5 and 450 mg of subcutaneous (s.c.) CJM112 compared with placebo for each dose group (Fig. 1a). Each dose cohort had 6 patients randomized in a 2:1 ratio to active dose vs. placebo. This study also included an Expansion Cohort in which an additional 12 patients (8 CJM112, 4 placebo) were added to the 50 mg cohort to ensure sufficient data was available for assessing efficacy. Therefore, a total of 12 patients were dosed with CJM112 50 mg. At evaluation, all patients from placebo groups were pooled into a single group for analysis. All cohorts underwent a screening period, baseline evaluations, randomization, a one‐day single dose treatment (Day 1), a follow‐up period (minimum 12 weeks) and an end of study (EOS) visit. After completion of SAD, an Interim Analysis (IA) was carried out to fully evaluate safety and efficacy of single doses of CJM112 in PsO patients and to determine which doses of CJM112 should be assessed in Part II of the study. The escalation of the doses in the SAD part occurred only after the majority of the patients in the previous cohort reached Day 15, to allow inspection of available 2‐week safety data before starting the next cohort.
Figure 1.
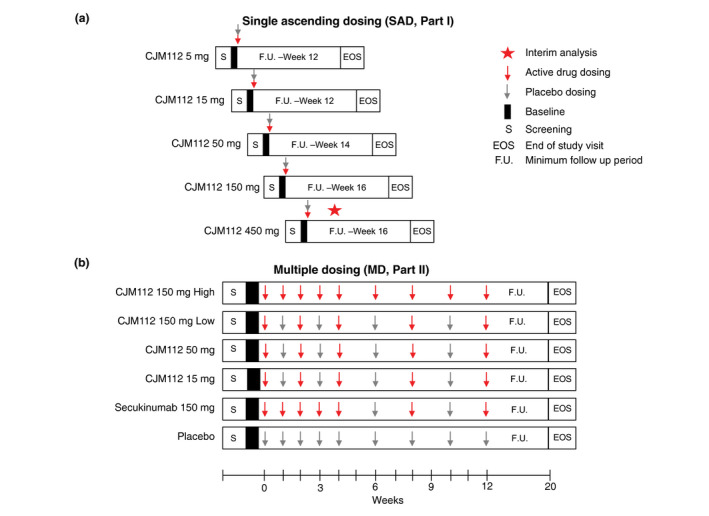
Study Design. Schematic describing study design for study Part I single ascending dosing (SAD) and Part II multiple dosing (MD). Results from the first interim analysis in SAD were used to determine both which cohort to expand and the dosing strategy for MD.
Part II Study Design (Randomized parallel‐group multi‐dose part, MD)
The MD study part included six parallel cohorts, with four active CJM112 treatment arms with different doses and schedules, an active control arm (secukinumab 150 mg s.c.) and placebo (Fig. 1b). The 150 mg secukinumab dose was selected at the time of the study based on its well‐known dose–response profile to allow better efficacy comparison with CJM112. The dosing arms for CJM112 consisted of multiple doses of 15 mg, 50 mg, 150 mg low frequency dosing and 150 mg high frequency dosing with the 15 mg, 50 mg and 150 mg low frequency dosing all following the same dosing regimen. The treatment period was 12 weeks, after which all patients entered an eight‐week follow‐up period before the EOS visit on Week 20 (Fig. 1b).
Patients
Patients (18‐65 years old), weighing ≥ 50 kg with moderate to severe chronic plaque PsO diagnosed at least 6 months prior to randomization, were included. Moderate to severe PsO was defined by a Psoriasis Area Severity Index (PASI) score ≥ 12, Investigator’s Global Assessment (IGA) score ≥ 3 and a Body Surface Area (BSA) score ≥ 10%. Patients were inadequately controlled by topical treatment and/or failed to respond to previous phototherapy or previous systemic therapies. Key exclusion criteria included forms of PsO other than chronic plaque‐type or previous treatment with IL‐17/IL‐17 receptor blocking agents. Previous treatment with biologics required a minimum washout period of six months. Concomitant use of other systemic immunomodulating treatments (e.g. methotrexate), phototherapy and topical treatment (e.g. corticosteroids) was also prohibited during the study period.
Study objectives and outcomes
The primary objectives of both study parts were to evaluate the safety and tolerability of CJM112 in PsO. The primary variable of interest was the incidence of related serious adverse events (SAEs) in CJM112 treatment arms. The frequency of adverse events (AEs) was also described. Safety analysis was conducted on all patients that received any study drug.
The key secondary objectives were to assess the efficacy in percentage change of the PASI score of ascending single doses of CJM112 from baseline to Week 4 (SAD) and Week 12 (MD). The efficacy analysis of CJM112 included all randomized patients with available data and no relevant protocol deviations. Within the SAD part, the efficacy endpoint was measured by the change from baseline in continuous PASI at Week 4. The MD efficacy endpoint was measured by both changes in PASI score and the proportion of PASI 75/90/100 responders at Week 12. Previous pivotal Phase 3 secukinumab studies 16 , 17 , 18 were used to provide estimates of PASI changes over time and PASI responder rates.
Additional secondary endpoints (PK/PD and immunogenicity profile) of CJM112 are detailed in Methods S2.
Results
Patient population
A total of 96 PsO patients were enrolled in this study and 89 (93%) completed it. 42 patients were enrolled into the SAD study. 95.2% of patients completed SAD; two patients (4.8%) were lost to follow up and discontinued the study (n = 1 CJM112 5 mg group, n = 1 CJM112 150 mg group). An additional 54 patients were enrolled into the MD study, with 49 (90.7%) completing the study and 5 (9.3%) patients discontinued. Two patients were lost to follow up (3.7%; n = 1 CJM112 150 mg high frequency; n = 1 CJM112 50 mg group), two were discontinued due to an AE (3.7%; n = 2 CJM112 150 mg high frequency group) and one patient was discontinued due to poor study attendance (1.9%; CJM112 150 mg low frequency group).
Baseline characteristics for both SAD and MD studies are described in Table 1. As expected for the small sample size of the individual cohorts, some patient characteristics were not evenly distributed between the groups which were considered in the interpretation of the results. In SAD, the average age was 44.7 years (range: 20–65 years) and weight was 99.3 kg (range 52.5–275 kg). There was one outlier with a very high bodyweight of reported 275 kg in the 50 mg SAD group. The weight of this patient was specifically confirmed by the study site. As the protocol did not have an upper bodyweight limit, inclusion of this patient was allowed in the study. The average age and weight of patients in MD was 44.5 years (range: 22–65 years) and 98.9 kg (range: 51–180.5 kg), respectively. All active treatment groups included patients with morbid obesity.
Table 1.
Baseline characteristics in SAD (Part I) and MD (Part II)
| SAD |
CJM112 5 mg N = 4 |
CJM112 15 mg N = 4 |
CJM112 50 mg N = 12 |
CJM112 150 mg N = 4 |
CJM112 450 mg N = 4 |
Placebo N = 14 |
Total N = 42 |
|---|---|---|---|---|---|---|---|
| Age, years | 43.0 ± 10.6 | 41.8 ± 7.8 | 41.0 ± 11.0 | 47.5 ± 18.7 | 43.0 ± 13.4 | 48.9 ± 7.1 | 44.7 ± 10.6 |
| Sex, male | 3 (75.0) | 3 (75.0) | 6 (50.0) | 2 (50.0) | 4 (100.0) | 10 (71.4) | 28 (66.7) |
| Race | |||||||
| Caucasian | 3 (75) | 3 (75) | 9 (75) | 3 (75) | 4 (100) | 14 (100.0) | 36 (85.7) |
| Black | 0 | 0 | 1 (8.3) | 0 | 0 | 0 | 1 (2.4) |
| Asian | 1 (25) | 0 | 1 (8.3) | 1 (25) | 0 | 0 | 3 (7.1) |
| Other | 0 | 1 (25) | 1 (8.3) | 0 | 0 | 0 | 2 (4.8) |
| Weight, kg | 95.8 ± 11.8 | 98.8 ± 12.7 | 113.7 ± 56.2 | 75.6 ± 19.4 | 90.5 ± 21.9 | 97.5 ± 15.1 | 99.3 ± 33.7 |
| BMI, kg/m2 | 33.3 ± 1.2 | 32.8 ± 5.7 | 39.8 ± 18.9 | 26.3 ± 4.3 | 28.0 ± 4.4 | 32.1 ± 5.5 | 33.5 ± 11.4 |
| PASI | 17.2 ± 8.8 | 17.1 ± 6.2 | 18.2 ± 4.3 | 23.1 ± 9.7 | 14.2 ± 1.3 | 21.9 ± 9.7 | 19.3 ± 7.6 |
| DLQI | 11.0 ± 5.4 | 10.8 ± 9.0 | 12.2 ± 8.4 | 14.0 ± 7.6 | 8.8 ± 4.9 | 13.6 ± 8.8 | 12.2 ± 7.7 |
| Time since diagnosis, years | 5.3 ± 3.7 | 17.5 ± 15.4 | 14.6 ± 8.3 | 11.7 ± 10.7 | 10.8 ± 2.5 | 15.5 ± 12.3 | 13.3 ± 10.0 |
| PsA present | 1 (25.0) | 1 (25.0) | 1 (8.3) | 0 | 0 | 3 (21.4) | 6 (14.3) |
| MD |
CJM112 15 mg N = 11 |
CJM112 50 mg N = 10 |
CJM112 150 mg low N = 10 |
CJM112 150 mg high N = 11 |
Secukinumab 150 mg N = 6 |
Placebo N = 6 |
Total N = 54 |
|---|---|---|---|---|---|---|---|
| Age, years | 45.3 ± 9.8 | 41.5 ± 9.6 | 44.4 ± 8.0 | 44.3 ± 11.8 | 51.7 ± 6.4 | 41.7 ± 14.7 | 44.5 ± 10.2 |
| Sex, male | 9 (81.8) | 7 (70.0) | 7 (70.0) | 7 (63.6) | 3 (50.0) | 2 (33.3) | 35 (64.8) |
| Race | |||||||
| Caucasian | 9 (81.8) | 8 (80.0) | 9 (90.0) | 10 (90.9) | 6 (100.0) | 6 (100.0) | 48 (88.9) |
| Black | 1 (9.1) | 1 (10.0) | 1 (10.0) | 1 (10.0) | 0 | 0 | 4 (7.4) |
| Asian | 1 (9.1) | 0 | 0 | 0 | 0 | 0 | 1 (1.9) |
| Other | 0 | 1 (10.0) | 0 | 0 | 0 | 0 | 1 (1.9) |
| Weight, kg | 103.0 ± 33.2 | 111.6 ± 33.8 | 93.8 ± 14.0 | 105.7 ± 29.1 | 90.6 ± 13.8 | 74.5 ± 20.2 | 98.9 ± 27.8 |
| BMI, kg/m2 | 35.9 ± 12.2 | 37.0 ± 9.6 | 30.7 ± 6.2 | 36.3 ± 8.4 | 33.5 ± 5.8 | 26.9 ± 5.0 | 34.0 ± 9.0 |
| PASI | 24.8 ± 12.4 | 18.4 ± 9.7 | 21.9 ± 8.7 | 18.4 ± 5.1 | 17.5 ± 6.1 | 22.8 ± 9.4 | 20.8 ± 9.1 |
| DLQI | 16.7 ± 8.5 | 11.9 ± 5.2 | 16.2 ± 6.3 | 12.6 ± 5.5 | 14.0 ± 7.7 | 14.0 ± 5.0 | 14.3 ± 6.5 |
| Time since diagnosis, years | 14.6 ± 13.1 | 12.2 ± 7.6 | 13.5 ± 7.4 | 21.8 ± 13.7 | 9.7 ± 5.4 | 16.9 ± 13.3 | 15.1 ± 11.0 |
| PsA present | 4 (36.4) | 2 (20.0) | 3 (30.0) | 3 (27.3) | 2 ( 33.3) | 1 ( 16.7) | 15 (27.8) |
Data are expressed as mean ± SD or n (%), where n = number of patients per cohort. BMI, Body Mass Index; DLQI, Dermatology Life Quality Index; Kg, kilogram; MD, multiple dosing; PASI, Psoriasis Area Severity Index; PsA, psoriatic arthritis; SAD, single ascending dose.
Efficacy
The mean percentage of PASI change from baseline over time following a single dose of CJM112 is presented in Fig. 2. All doses of CJM112 from 15 mg and above demonstrated a higher response compared with placebo at all timepoints measured, from baseline to Week 12. At Week 4, the percentage change in PASI from baseline (secondary endpoint) was 0%, −36%, −55%, −81% and −62% for CJM112 5 mg, 15 mg, 50 mg, 150 mg and 450 mg, compared with −19% in the placebo cohort. Despite CJM112 150 mg achieving a greater PASI reduction by Week 4, the highest dose of CJM112 had a relatively longer duration of efficacy over time, with patients achieving an average percentage reduction of −84% 12 weeks after treatment with CJM112 450 mg.
Figure 2.
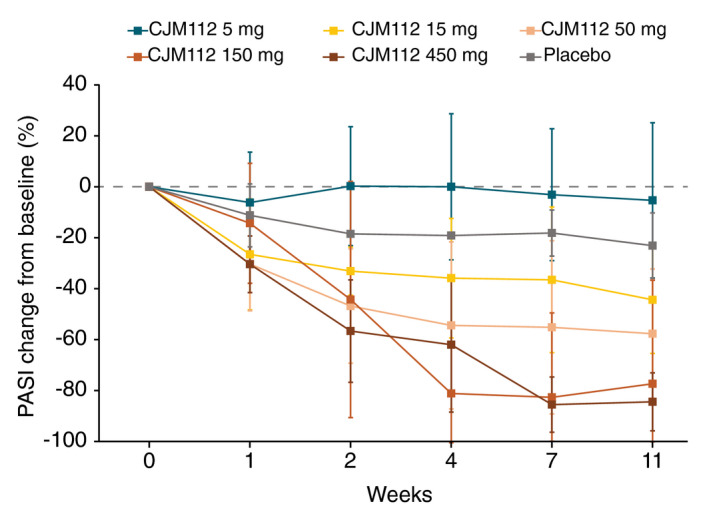
CJM112 single ascending dose (SAD) reduction in PASI over time. Line graph demonstrating mean percentage change of PASI from baseline to Week 12 following a single dose of CJM112 compared with placebo. PASI, Psoriasis Area Severity Index.
Mean percentage change in PASI following multiple CJM112 dosing is presented in Fig. 3. Similarly to the effects in SAD, CJM112 multiple dosing results in a dose‐dependent decrease in PASI over time, clearly lower than placebo. The greatest change from baseline at Week 12 was recorded in the CJM112 150 mg low frequency group (−89%), compared with −3% in the placebo group. The fact that the highest dose group (CJM112 150 mg high frequency) did not exceed the effect of the CJM112 150 mg low frequency dose group demonstrates that the maximal possible effect has probably been reached (referred to as ‘ceiling effect’). CJM112 150 mg high frequency demonstrated similar efficacy compared with secukinumab 150 mg, with a reduction of −76% and −78% at Week 12, respectively.
Figure 3.
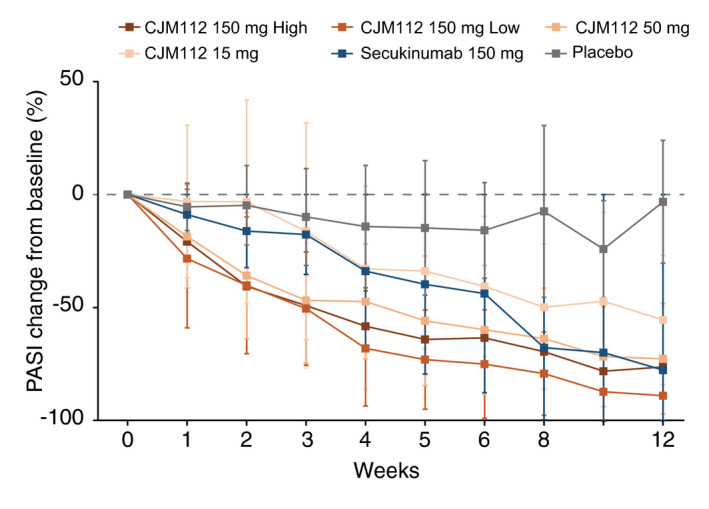
CJM112 multiple dosing (MD) reduction in PASI over time. Line graph demonstrating mean percentage change of PASI from baseline to Week 12 following multiple dosing of CJM112 compared with placebo and secukinumab. PASI, Psoriasis Area Severity Index.
The CJM112 150 mg low frequency group also showed the greatest PASI responses, with 90% (n = 9), 60% (n = 6) and 30% (n = 3) of patients achieving PASI 75/90/100, respectively. CJM112 150 mg high frequency and secukinumab 150 mg had similar rates of PASI response. 63.6% (n = 7), 36.4% (n = 4) and 9.1% (n = 1) of patients receiving CJM112 150 mg high frequency achieved PASI 75/90/100 compared with 66.7% (n = 4), 33.3% (n = 2) and 16.7% (n = 1) of patients receiving secukinumab 150 mg. No patients in the placebo group achieved PASI 75.
Pharmacokinetics and Pharmacodynamics
Following SAD and as expected for a human IgG1 isotype antibody targeting a soluble cytokine, the mean apparent total body clearance (CL/F) for CJM112 in PsO patients was low, ranging from 0.28 to 0.59 L/day, which was fairly consistent across the dose range and indicative of linear pharmacokinetics for CJM112. The apparent volume of distribution (Vz/F) was low, ranging from 10.6‐12.7 L, with no dose dependency. Overall, the mean elimination half‐life for CJM112 ranged between 17 and 19 days after a single dose and maximum CJM112 concentrations (observed Cmax) were generally observed between 3.0 and 7.1 days. Following MD, after an induction period in each cohort (up to 4 weeks), mean trough steady state CJM112 concentrations at Day 85 (Week 12) were reported as 18.4 µg/mL, 7.14 µg/mL, 2.14 µg/mL and 0.782 µg/mL in the CJM112 150 mg high frequency, 150 mg low frequency, 50 mg and 15 mg cohorts, respectively. Total CJM112 serum concentration profiles for SAD and MD are shown in Figure S3A and B, respectively.
In both SAD and MD parts of the study, target engagement was demonstrated in all cohorts through a slow and saturable accumulation of total IL‐17A concentration in serum (accumulation of the CJM112‐IL‐17A complexes). These profiles were characteristic of a slow elimination of the CJM112‐IL‐17A complex (which is taking on the elimination of CJM112), a slow turnover of IL‐17A and a long duration of IL‐17A capture. As expected, increasing the dose led to an increase in the duration of target capture by CJM112 (SAD) (Figure S4a). In MD, sustained target engagement was demonstrated in all cohorts receiving CJM112 for the entire treatment period (Figure S4b). The inter‐individual variability in the total IL‐17A profiles within each cohort was likely due to a combination between variability in drug exposure due to bodyweight and variability in individual production rate of IL‐17A.
Target engagement of secukinumab with IL‐17A has been published previously. 17
Immunogenicity
Immunogenicity was determined based on the presence of anti‐CJM112 antibodies in serum. In SAD, four patients (14.3%) treated with CJM112 were anti‐drug antibody (ADA)‐positive (at least one treatment induced or boosted ADA‐positive sample at any time during the treatment or follow‐up observation period; no pre‐existing antibodies). In MD, six patients (14.3%) treated with CJM112 were considered ADA‐positive and, while the number of patients was quite low, the frequency of immunogenicity positive patients appeared to correlate negatively with the dose (5/11, 0/10, 1/10 and 0/11 patients were ADA‐positive in the 15 mg, 50 mg, 150 mg low frequency and 150 mg high frequency cohort, respectively). None of the patients treated with secukinumab had ADAs. While it cannot be excluded that in some cases the ADA response may have had a negative impact on PASI reduction, the presence of ADAs in these patients was not associated with an immune‐related safety signal.
Safety
Overall, 21/42 patients (50%) reported at least one AE during SAD, with the overall incidence varying between different treatment arms (Table 2). The most frequent AE by system organ class (SOC) was infections/infestations with 6/28 (21.4%) for CJM112‐treated patients, an effect which was higher than in the placebo group (1/14; 7.1%). Although the incidence amongst CJM112 groups was greatest in the highest dose group (CJM112 450 mg, 2/4; 50%), there was no clear dose–response relationship. Three serious AEs (SAEs) were reported during SAD. One patient had a Grade 4 hypertensive crisis (CJM112 150 mg group), one patient reported a right distal radius fracture (CJM112 450 mg group) and one reported an exacerbation of mild thoracic back pain secondary to a degenerative disc (placebo group). None of these SAEs were suspected as related to the study drug or resulted in study discontinuation.
Table 2.
Adverse events in SAD (Part I) by system organ class
|
CJM112 5 mg (n = 4) |
CJM112 15 mg (n = 4) |
CJM112 50 mg (n = 12) |
CJM112 150 mg (n = 4) |
CJM112 450 mg (n = 4) |
CJM112 Total (n = 28) |
Placebo (n = 14) |
|
|---|---|---|---|---|---|---|---|
| Patients with AE | 1 (25) | 2 (50) | 5 (41.7) | 2 (50) | 3 (75) | 13 (46.4) | 8 (57.1) |
| System organ class (SOC) | |||||||
| Infections and infestations † | 0 | 1 (25) | 3 (25) | 0 | 2 (50) | 6 (21.4) | 1 (7.1) |
| Skin and subcutaneous tissue disorders | 0 | 0 | 0 | 1 (25) | 1 (25) | 2 (7.1) | 3 (21.4) |
| Gastrointestinal disorders†‡ | 0 | 0 | 1 (8.3) | 1 (25) | 1 (25) | 3 (10.7) | 1 (7.1) |
| Nervous system disorders | 0 | 1 (25) | 2 (16.7) | 0 | 1 (25) | 4 (14.3) | 0 |
| General disorders and administration site conditions | 0 | 0 | 1 (8.3) | 1 (25) | 0 | 2 (7.1) | 1 (7.1) |
| Investigations | 0 | 0 | 1 (8.3) | 0 | 1 (25) | 2 (7.1) | 1 (7.1) |
| Musculoskeletal and connective tissue disorders | 0 | 0 | 0 | 0 | 1 (25) | 1 (3.6) | 0 |
| Respiratory, thoracic and mediastinal disorders | 1 (25) | 1 (25) | 0 | 0 | 0 | 2 (7.1) | 1 (7.1) |
| Injury, poisoning and procedural complications | 0 | 0 | 0 | 0 | 1 (25) | 1 (3.6) | 1 (7.1) |
| Psychiatric disorders | 0 | 0 | 0 | 1 (25) | 0 | 1 (3.6) | 1 (7.1) |
| Blood and lymphatic system disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) |
| Cardiac disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) |
| Immune system disorders | 0 | 0 | 0 | 1 (25) | 0 | 1 (3.6) | 0 |
| Metabolism and nutrition disorders | 0 | 0 | 0 | 1 (25) | 0 | 1 (3.6) | 0 |
| Reproductive system and breast disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) |
| Vascular disorders | 0 | 0 | 0 | 1 (25) | 0 | 1 (3.6) | 0 |
Infections and infestations include cellulitis (n = 1), folliculitis (n = 1), gastroenteritis (n = 2), pneumonia (n = 1) and upper respiratory tract infection (n = 4).
Gastrointestinal disorders include flatulence (n = 1), lip pain (n = 1) and toothache (n = 1). Subjects with multiple adverse events within a body system are counted only once in the table above.
A similar incidence of AEs were reported following MD, with 30/54 (55.6%) reporting at least one AE (Table 3). The overall incidence of AEs varied across different treatment arms, with the highest number of AEs reported in CJM112 150 mg high frequency group (n = 9; 81.8%). The most commonly affected SOC was again infections/infestations (mostly upper respiratory tract infections). This was the only SOC in which the AEs showed a possible tendency for a dose–response with most infections and infestations occurring in the highest CJM112 dose group (54.5%) with none reported in the lowest dose group. Overall, incidence of infections/infestations was slightly higher in the CJM112 group (mainly in CJM112 150 mg high frequency group, n = 6/11) as compared to placebo or secukinumab. One patient (CJM112 150 mg high frequency group) reported two SAEs during MD. On Day 126, this patient developed postoperative respiratory failure (Grade 4) following elective out‐patient surgery unrelated to study treatment. On the same day, the patient developed Grade 2 acute renal failure. Both events resolved and were not suspected to be related to the study drug. Two patients were discontinued from this study in MD, due to AEs (both CJM112 150 mg high frequency group). One patient developed a Grade 1 upper respiratory tract infection on Day 2. The event was resolved on Day 7 and the patient was discontinued from the study that day. A second patient developed worsening of PsO (Grade 2) on Day 85 which also led to study discontinuation. There were no cases of inflammatory bowel disease, candida infection or major adverse cardiovascular events in this study.
Table 3.
Adverse events in MD (Part II) by system organ class
| CJM112 15 mg (n = 11) | CJM112 50 mg (n = 10) | CJM112 150 mg low (n = 10) | CJM112 150 mg high (n = 11) | CJM112 Total (n = 42) | Secukinumab 150 mg (n = 6) | Placebo (n = 6) | |
|---|---|---|---|---|---|---|---|
| Patients with AE | 5 (45.5) | 5 (50) | 4 (40) | 9 (81.8) | 23 (54.8) | 2 (33.3) | 5 (83.3) |
| System organ class (SOC) | |||||||
| Infections and infestations† | 0 | 1 (10) | 2 (20) | 6 (54.5) | 9 (21.4) | 1 (16.7) | 2 (33.3) |
| Skin and subcutaneous tissue disorders | 2 (18.2) | 1 (10) | 3 (30) | 1 (9.1) | 7 (16.7) | 0 | 1 (16.7) |
| Gastrointestinal disorders † , ‡ | 1 (9.1) | 2 (20) | 1 (10) | 1 (9.1) | 5 (11.9) | 1 (16.7) | 0 |
| Nervous system disorders | 2 (18.2) | 2 (20) | 1 (10) | 0 | 5 (11.9) | 0 | 1 (16.7) |
| Investigations | 0 | 2 (20) | 2 (20) | 0 | 4 (9.5) | 0 | 1 (16.7) |
| Musculoskeletal and connective tissue disorders | 1 (9.1) | 1 (10) | 1 (10) | 1 (9.1) | 4 (9.5) | 0 | 1 (16.7) |
| Injury, poisoning and procedural complications | 0 | 0 | 1 (10) | 3 (27.3) | 4 (9.5) | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | 0 | 0 | 1 (10) | 1 (9.1) | 2 (4.8) | 0 | 1 (16.7) |
| Cardiac disorders | 0 | 1 (10) | 0 | 1 (9.1) | 2 (4.8) | 0 | 0 |
| General disorders and administration site conditions | 1 (9.1) | 0 | 0 | 1 (9.1) | 2 (4.8) | 0 | 0 |
| Neoplasms benign, malignant and unspecified | 1 (9.1) | 0 | 1 (10) | 0 | 2 (4.8) | 0 | 0 |
| Psychiatric disorders | 0 | 1 (10) | 0 | 0 | 1 (2.4) | 0 | 1 (16.7) |
| Eye disorders | 1 (9.1) | 0 | 0 | 0 | 1 (2.4) | 0 | 0 |
| Immune system disorders | 0 | 1 (10) | 0 | 0 | 1 (2.4) | 0 | 0 |
| Metabolism and nutrition disorders | 0 | 0 | 1 (10) | 0 | 1 (2.4) | 0 | 0 |
| Renal and urinary disorders | 0 | 0 | 0 | 1 (9.1) | 1 (2.4) | 0 | 0 |
| Reproductive system and breast disorders | 1 (9.1) | 0 | 0 | 0 | 1 (2.4) | 0 | 0 |
Infections and infestations include adenovirus (n = 1), conjunctivitis (1), cystitis (1), ear infection (1), gastroenteritis (3), nasopharyngitis (n = 1), otitis externa (n = 1), tinea infection (n = 2), upper respiratory tract infection (n = 4).
Gastrointestinal disorders include abdominal wall haematoma (n = 1), aphthous ulcer (n = 1), constipation (n = 1), diarrhoea (n = 2), dyspepsia (n = 1) and nausea (n = 1). Subjects with multiple adverse events within a body system are counted only once in the table above.
Discussion
This is the FIH study of CJM112, conducted in two parts (placebo‐controlled single ascending dose Part I and randomized placebo‐ and active‐controlled parallel‐group multi‐dose Part II) in patients with moderate to severe plaque PsO.
CJM112 demonstrated disposition characteristics that are typical for an IgG1 type antibody targeting a soluble target, and linear pharmacokinetics over the investigated dose range. Target engagement was demonstrated in all cohorts through a slow and saturable accumulation of total IL‐17A concentration in serum, and these profiles were characteristic of a slow elimination of the CJM112‐IL‐17A complexes, a slow turnover of the ligand and a long duration of IL‐17A capture.
In the SAD part of the study, the mean percentage decrease in PASI versus time profiles were better than placebo for the 15 mg and higher dose groups, indicating a clinical effect on PsO. Imbalances between the small cohorts, specifically regarding bodyweight limits the interpretation of the results and differences between the groups. The CJM112 50 mg group showed a 55% reduction in mean % PASI scores at Week 4. Considering that the 50 mg dose group had the highest bodyweight as a main potential confounding factor, this result is considered a true representation of the efficacy in psoriasis. The very positive outcome of the 150 mg dose group (−81% change from baseline) should, however, also be viewed with the background that this group had the lowest mean and median bodyweight. The CJM112 450 mg dose group did not add significant further efficacy benefit but seemed to prolong the efficacy duration. Thus, a single dose of CJM112 150 mg may have already achieved the ‘ceiling effect’ of maximal efficacy.
In the MD part of the study, CJM112 showed clinical activity in all dose groups tested. All three higher CJM112 dose groups (CJM112 50 mg, CJM112 150 mg low and high frequency) appeared to have similar efficacy as the secukinumab group and similar to secukinumab data from pivotal Phase III trials, again hinting to a ‘ceiling effect’ for anti‐IL‐17A therapy in PsO. CJM112 has equal affinity to both the IL‐17A homodimer and IL‐17AF heterodimer, and has a potency towards IL‐17AF of > 400‐fold higher than that of secukinumab. This indicates that IL‐17A is the main effector cytokine and that additional blocking of IL‐17AF is not contributing to efficacy in PsO. The exact role of IL‐17F in the pathogenesis of PsO is not well understood. Although both IL‐17A and IL‐17F are highly expressed in psoriatic lesions, 18 , 19 preclinical models have demonstrated that IL‐17A plays a more important role in driving immunity compared with IL‐17F. 20 , 21 , 22 IL‐17F homodimer activation results in a weaker inflammatory response compared with IL‐17A, with IL‐17AF having intermediate potency. 22 Furthermore, serum IL‐17A correlated with disease activity in PsO, whereas IL‐17F did not. 23 . While all this points to IL‐17A as the main effector cytokine, emerging data on the safety and efficacy of bimekizumab (dual IL‐17A and IL‐17F inhibition) will help inform the relative importance of IL‐17A, IL‐17F and IL‐17AF in the pathophysiology and treatment of PsO.
CJM112 was safe and well tolerated in the doses and regimen tested in this study. Following SAD, no significant safety signals were detected. Although difficult to conclude given the low patient numbers, the overall AE frequency in CJM112‐dosed patients was similar to placebo with no specific AE or class of AEs occurring in a relevant higher frequency. Repeated dosing of CJM112 resulted in a slightly higher rate of non‐serious infections (mild or moderate severity) with a tendency for dose‐related increase. Since these results are based on a small number of patients, the potentially elevated risk for non‐serious infections with CJM112 therapy requires further investigation in larger patient cohorts.
Considering the fact that neither the higher neutralization capacity of CJM112 against IL‐17A nor the additional neutralization effects against IL‐17AF seem to translate into increased clinical efficacy in PsO patients compared with conventional IL‐17 blockers, the additional therapeutic value of CJM112 in PsO over already available therapies appears questionable. However, other indications sensitive to IL‐17 may have a greater benefit from additional features of CJM112 and therefore further clinical testing in other indications, such as hidradenitis suppurativa, are warranted.
Supporting information
Fig S1. Sequences of the heavy and light chain variable regions of CJM112. (A) Nucleotide and amino acid sequence of the heavy chain variable region of CJM112. (B) Nucleotide and amino acid sequence of the light chain variable region of CJM112. Differences to parental antibody are in bold and underlined
Fig S2. Cross‐reactivity of IL‐17 family members from different species Bar graph demonstrating mean±SEM O.D. values of IL‐17 family members from human, cynomolgus monkey, mouse and rat species
Fig S3. Total therapeutic antibody serum concentration following SAD and MD CJM112 treatment. Line graphs demonstrating total serum CJM112 concentrations over time following single ascending dosing (SAD) (A) and multiple dosing (MD) (B) Data is expressed as the arithmetic mean (semi‐logarithmic scale) ±SD
Fig S4. IL‐17A levels following SAD and MD CJM112 treatment. Line graphs demonstrating IL‐17A serum concentrations over time following single ascending dosing (SAD) (A) and multiple dosing (MD) (B) Data is expressed as the arithmetic mean±SD
Method S1 and S2. Supplementary Material
Table S1. Binding affinities to IL‐17A, IL‐17F and IL‐17AF from different species
Table S2. Recombinant proteins and antibodies used for binding assay
Acknowledgements
The authors would like to thank the following principal investigators for recruiting patients to this study: Teresa S. Sligh (Providence Clinical Research, USA), Pete J. Winkle (Anaheim Clinical Trials, USA), Gilber R. Weiner (Advanced Pharma CR, USA), Adnar Nasir (Carolina Phase I Research, USA), Tooraj Raoof (Ecino Research Center, USA), Melodie A. Armstrong (Vince & Associates Clinical, USA), Elizabeth H. Tichy (Clinical Trials of Texas, USA), Satinder Saini (Avant Research Associates, USA), Lawrence C. Parish (Paddington Testing Co. Inc., USA), Vishala L. Chindalore (Pinnacle Research Group, USA), Rafael Chiong (Kendall South Medical Center, USA), Dareen D. Siri (Sneeze, Wheeze & Itch, USA), Raymond L. Comelison (Lynn Health Science, USA), Thomas C. Marbury (Orlando Clinical Research, USA), Michael T. Jarratt (DermResearch, USA), Robert M. Fixler (Community Research, USA), Kenneth W. Dawes (Dawes Fretzin Clinical Research, USA) and Matthew Zook (Olympian Clinical Research, USA). The authors also thank Trudy McGarry, PhD, for providing medical writing support/editorial support, which was funded by Novartis Pharma AG, Basel, Switzerland, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding: The study was funded by Novartis Pharma AG, Basel, Switzerland.
Conflict of interest: Dr. Sligh has nothing to disclose. Dr. Aassi reports she is currently an employee of Novartis. Dr. Cebe reports he is currently an employee of Novartis. Dr. Calonder reports he is currently an employee of Novartis. Dr. DiPadova reports he is currently an employee of Novartis. Dr. Jarvis reports he is currently an employee of Novartis. Dr. Kolbinger reports he is currently an employee of Novartis. Dr. Mussmann reports he is currently an employee of Novartis. Dr. Rondeau reports he is currently an employee of Novartis. Dr. Rozenberg reports she is currently an employee of Novartis. Dr. Kaul reports he is currently an employee of Novartis. Dr. Huber reports he was employed by Novartis during study and holds Novartis shares. In addition, Dr. Huber has a patent US9193788B2 issued. Dr. Espie reports he was employed by Novartis during study.
Clinical Trial Registration Number: NCT01828086 (Clinicaltrials.gov) and 2012‐004507‐12 (Eudract).
Data availability statement
Novartis is committed to sharing with qualified external researchers, access to patient‐level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.
References
- 1. Dalgard FJ, Gieler U, Tomas‐Aragones L, Lien L, Poot F, Jemec GBE et al. The psychological burden of skin diseases: a cross‐sectional multicenter study among dermatological out‐patients in 13 European countries. J Invest Dermatol 2015; 135: 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta MA, Gupta AK. Depression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasis. Br J Dermatol 1998; 139: 846–850. [DOI] [PubMed] [Google Scholar]
- 3. Christophers E, Barker JN, Griffiths CE, Dauden E, Milligan G, Molta C et al. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European dermatology clinics. J Eur Acad Dermatol Venereol 2010; 24: 548–554. [DOI] [PubMed] [Google Scholar]
- 4. Di Meglio P, Villanova F, Nestle FO. Psoriasis. Cold Spring Harb Perspect Med 2014; 4: a015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gisondi P, Fostini AC, Fossa I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol 2018; 36: 21–28. [DOI] [PubMed] [Google Scholar]
- 6. Singh S, Young P, Armstrong AW. Relationship between psoriasis and metabolic syndrome: a systematic review. G Ital Dermatol Venereol 2016; 151: 663–677. [PubMed] [Google Scholar]
- 7. Boehncke WH. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol 2018; 9: 579.29675020 [Google Scholar]
- 8. Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol 2009; 129: 2175–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM et al. Interleukin 17‐producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005; 6: 1123–1132. [DOI] [PubMed] [Google Scholar]
- 10. Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL‐23/IL‐17 signaling pathway and the treatment of psoriasis. J Immunol 2018; 201: 1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med 2015; 373: 1318–1328. [DOI] [PubMed] [Google Scholar]
- 12. Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C et al. A prospective phase III, randomized, double‐blind, placebo‐controlled study of brodalumab in patients with moderate‐to‐severe plaque psoriasis. Br J Dermatol 2016; 175: 273–286. [DOI] [PubMed] [Google Scholar]
- 13. Liu L, Lu J, Allan BW, Tang Y, Tetreault J, Chow CK et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin‐17A. J Inflamm Res 2016; 9: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agency EM. EMEA/H/C/003943/0000 Assessment Report: Taltz. 2016. [Google Scholar]
- 15. Agency EM. EMEA/H/C/003729 Assessment Report: Cosentyx. 2014. [Google Scholar]
- 16. van der Heijde D, Gensler LS, Deodhar A, Baraliakos X, Poddubnyy D, Kivitz A et al. Dual neutralisation of interleukin‐17A and interleukin‐17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48‐week phase IIb, randomised, double‐blind, placebo‐controlled, dose‐ranging study. Ann Rheum Dis 2020; 79: 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruin G, Loesche C, Nyirady J, Sander O. Population pharmacokinetic modeling of secukinumab in patients with moderate to severe psoriasis. J Clin Pharmacol 2017; 57: 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soderstrom C, Berstein G, Zhang W, Valdez H, Fitz L, Kuhn M et al. Ultra‐sensitive measurement of IL‐17A and IL‐17F in psoriasis patient serum and skin. AAPS J 2017; 19: 1218–1222. [DOI] [PubMed] [Google Scholar]
- 19. Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin‐17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol 2009; 160: 319–324. [DOI] [PubMed] [Google Scholar]
- 20. Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L et al. Regulation of inflammatory responses by IL‐17F. J Exp Med 2008; 205: 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y et al. Differential roles of interleukin‐17A and ‐17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 2009; 30: 108–119. [DOI] [PubMed] [Google Scholar]
- 22. Gaffen SL. Structure and signalling in the IL‐17 receptor family. Nat Rev Immunol 2009; 9: 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kolbinger F, Loesche C, Valentin MA, Jiang X, Cheng Y, Jarvis P et al. beta‐Defensin 2 is a responsive biomarker of IL‐17A‐driven skin pathology in patients with psoriasis. J Allergy Clin Immunol 2017; 139: 923–932 e928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Sequences of the heavy and light chain variable regions of CJM112. (A) Nucleotide and amino acid sequence of the heavy chain variable region of CJM112. (B) Nucleotide and amino acid sequence of the light chain variable region of CJM112. Differences to parental antibody are in bold and underlined
Fig S2. Cross‐reactivity of IL‐17 family members from different species Bar graph demonstrating mean±SEM O.D. values of IL‐17 family members from human, cynomolgus monkey, mouse and rat species
Fig S3. Total therapeutic antibody serum concentration following SAD and MD CJM112 treatment. Line graphs demonstrating total serum CJM112 concentrations over time following single ascending dosing (SAD) (A) and multiple dosing (MD) (B) Data is expressed as the arithmetic mean (semi‐logarithmic scale) ±SD
Fig S4. IL‐17A levels following SAD and MD CJM112 treatment. Line graphs demonstrating IL‐17A serum concentrations over time following single ascending dosing (SAD) (A) and multiple dosing (MD) (B) Data is expressed as the arithmetic mean±SD
Method S1 and S2. Supplementary Material
Table S1. Binding affinities to IL‐17A, IL‐17F and IL‐17AF from different species
Table S2. Recombinant proteins and antibodies used for binding assay
Data Availability Statement
Novartis is committed to sharing with qualified external researchers, access to patient‐level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.


