Abstract
Liver safety concerns were raised in randomized controlled trials of cannabidiol (CBD) in patients with Lennox‐Gastaut and Dravet syndromes, but the relevance of these concerns to healthy adults consuming CBD is unclear. The objective of this manuscript is to report on liver safety findings from healthy adults who received therapeutic daily doses of CBD for ~ 3.5 weeks and to investigate any correlation between transaminase elevations and baseline characteristics, pharmacogenetic, and pharmacokinetic data. Sixteen healthy adults were enrolled in a phase I, open‐label, fixed single‐sequence drug–drug interaction trial to investigate the effect of repeated dose administration of CBD (1,500 mg/day) on cytochrome P450 (CYP) 1A2 activity. Seven (44%) participants experienced peak serum alanine aminotransferase (ALT) values greater than the upper limit of normal (ULN). For five (31%) participants, the value exceeded 5 × ULN, therefore meeting the international consensus criteria for drug‐induced liver injury. There was no correlation between transaminase elevations and baseline characteristics, CYP2C19 genotype, or CBD plasma concentrations. All ALT elevations above the ULN began within 2–4 weeks of initial exposure to CBD. Among the six participants with ALT elevations who were discontinued from the protocol, some had symptoms consistent with hepatitis or hypersensitivity. We conclude that healthy adults consuming CBD may experience elevations in serum ALT consistent with drug‐induced liver injury. Given the demonstrated interindividual variation in susceptibility, clinicians should be alert to this potential effect from CBD, which is increasingly available in various nonprescription forms and doses to consumers.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Frequent serum aminotransferase elevations were observed in randomized controlled trials with cannabidiol in patients with Lennox‐Gastaut and Dravet syndromes, raising liver safety concerns. However, the relevance of these safety concerns to healthy adults taking cannabidiol is unclear.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Do therapeutic doses of cannabidiol cause serum chemistry abnormalities consistent with liver injury in healthy adults?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ In 16 healthy adult participants receiving 1,500 mg/day cannabidiol in an open‐label drug–drug interaction trial, peak serum alanine aminotransferase values were above the upper limit of normal in 7 (44%) participants and exceeded international criteria for drug‐induced liver injury in 5 (31%) participants, some of whom had symptoms consistent with hepatitis.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Clinicians should be aware that cannabidiol can cause abnormalities in serum chemistries consistent with liver injury in healthy adults and be on the lookout for association with clinically important liver injury.
Highly purified cannabidiol (CBD) (approved as Epidiolex in the United States; Epidyolex in the European Union, in conjunction with clobazam) has demonstrated efficacy, with an acceptable safety profile in patients with Dravet syndrome (DS) or Lennox‐Gastaut syndrome (LGS) in four randomized controlled trials (RCTs), 1 , 2 , 3 , 4 and has also been studied at higher doses in an RCT in patients with tuberous sclerosis complex. 5 In all the CBD RCTs, liver safety concerns arose following the frequent occurrence of elevations in serum alanine aminotransferase (ALT). 1 , 2 , 3 , 4 , 5 These events typically first occurred in the first 2 months of treatment initiation. 6 Serum alkaline phosphatase (ALP) elevations were uncommon, 1 , 2 , 3 , 4 consistent with a hepatocellular injury. 7 There was a clear dose dependence, with transaminase elevations observed in 4–5% of patients with DS/LGS treated with 10 mg/kg/day CBD 2 , 4 vs. 13–23% of patients with DS/LGS treated with 20 mg/kg/day. 1 , 2 , 3 , 4
No cases of significant liver dysfunction were observed in the pivotal DS/LGS RCTs. 1 , 2 , 3 , 4 During the follow‐on open‐label extension (OLE) trial, one child who received placebo during the RCT and began taking CBD in the OLE experienced a marked rise in serum aminotransferases followed by an upward drift in total serum bilirubin (TBL; although within normal limits), suggestive of early liver dysfunction (Figure 1 ). Routine monitoring of serum liver tests (LTs) resulted in early discontinuation of CBD treatment in 2–7% of patients in the pivotal DS/LGS RCTs, 1 , 2 , 3 , 4 which may have prevented liver injury progression. CBD labeling has liver safety warnings and recommends routine monitoring of LTs in all treated patients. 6
Figure 1.
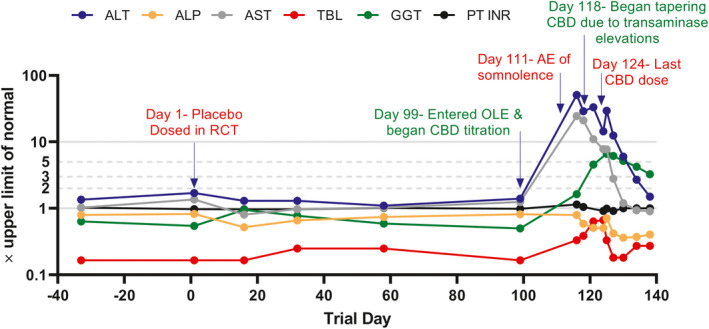
Serial liver chemistries in a patient with DS on a semilogarithmic scale are shown. This was a 10‐year‐old patient with DS who had been receiving treatment with valproate for >1 year when they entered the double‐blind placebo‐controlled trial. The patient was randomized to placebo treatment for the first 3 months during the RCT, then commenced treatment with CBD at 20 mg/kg/day during the OLE trial (after an 11‐day up‐titration). The patient was noted to be somnolent about 2 weeks after starting CBD treatment, and 19 days after starting CBD treatment was found to have a high serum ALT level (50 × the upper limit of normal). Serum valproate levels were not elevated at this time. The patient began a tapered withdrawal of CBD 2 days after their peak serum ALT value and had completely stopped CBD treatment 1 week later. The patient had no clear symptoms during the event other than somnolence which resolved off CBD. They remained on valproate treatment throughout their recovery. The mild rise in serum total bilirubin following the peak serum ALT is consistent with mild liver dysfunction secondary to hepatocellular injury, although the value did not exceed the reference range. Evaluation for alternate etiologies was unrevealing and this event was judged to be probably due to CBD by the site investigator and P.B.W. AE, treatment‐emergent adverse event; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBD, cannabidiol; DS, Dravet syndrome; GGT, gamma‐glutamyl transferase; OLE, open‐label extension; PT INR, prothrombin time international normalized ratio; RCT, randomized controlled trial; TBL, total bilirubin.
Nonprescription CBD‐containing products are widely available and are being touted as safe treatment for many health conditions. 8 The relevance of the liver events noted in the pivotal DS/LGS RCTs for the liver safety of CBD‐containing products in other populations is unclear. A remarkable observation in the pivotal DS/LGS RCTs was that the incidence of aminotransferase elevations was markedly higher in those patients concomitantly treated with valproate; 1 , 2 , 3 , 4 the incidence of ALT elevations > 3× the upper limit of normal (ULN) was 21% in patients taking CBD with concomitant valproate (without clobazam) compared with 3% of patients not taking valproate. 6 Additionally, patients taking 20 mg/kg/day CBD with elevated baseline serum ALT had almost a threefold greater incidence of significant ALT elevations (> 3× ULN) than patients starting treatment with normal aminotransferase levels. 6 These observations suggest that people without baseline liver conditions, not receiving valproate, and taking lower CBD doses should be at a much lower risk of hepatotoxicity than patients with DS/LGS.
We were therefore surprised to observe that in this phase I caffeine drug–drug interaction (DDI) trial involving 16 healthy adults receiving 1,500 mg/day CBD (~ 20 mg/kg/day in a 70 kg person), 5 (31%) participants experienced drug‐induced liver injury (DILI), as defined by international consensus criteria (serum ALT exceeding 5× ULN). 9 These events fit the characteristics of the liver events observed in the pivotal DS/LGS RCTs and reported in two healthy adult participants in a prior phase I trial. 10
While the full pharmacokinetic results from this trial are the subject of a separate publication, here we describe in detail the liver safety results.
Methods
Trial design
This trial was reviewed and approved by the Independent Ethics Committee for the investigational site and conducted in accordance with the Helsinki Declaration of 1975.
This was a phase I, open‐label, fixed single‐sequence DDI trial that enrolled healthy participants to investigate the effect of repeat‐dose CBD administration on cytochrome P450 (CYP)1A2 activity using caffeine as a probe CYP1A2 substrate. Participants received a pharmaceutical formulation of highly purified cannabidiol derived from Cannabis sativa (L.) plant (100 mg/ml oral solution; Epidiolex in the United States; Epidyolex in the European Union; GW Research Ltd., Cambridge, UK). The trial was performed at a specialist phase I unit (PRA Health Sciences' Early Development Services in The Netherlands) between April and July 2019.
Figure 2 presents the trial design. Potential participants were assessed for trial eligibility within 28 days before the first trial drug dose. Sixteen participants were enrolled and admitted to the trial site on Day − 1, which was the day before Day 1, the first day of trial drug administration (200 mg caffeine + CBD‐matched placebo). On Day 3, the first dose of 250 mg CBD was taken in the morning at the site. Participants were discharged on Day 3 and instructed to escalate their CBD doses at home from the next day, as follows:
Figure 2.
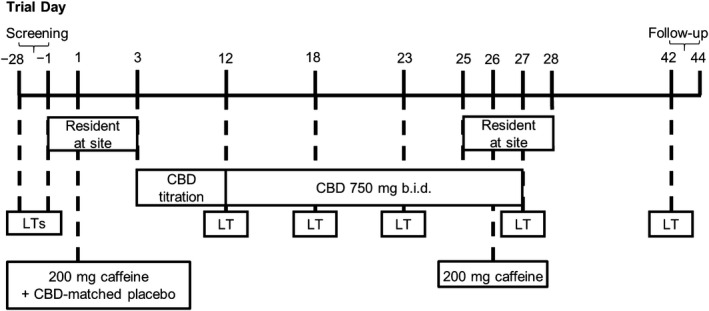
Trial design. b.i.d. twice daily; CBD, cannabidiol; LT, liver test.
Days 4–5: 250 mg twice daily (b.i.d.)
Days 6–7: 500 mg morning and 250 mg evening
Days 8–9: 500 mg b.i.d.
Days 10–11: 750 mg morning and 500 mg evening
Days 12–27: maintenance dosing with 750 mg b.i.d.
Ambulatory visits occurred on Days 12, 18, and 23. Participants were admitted again to the trial site on Day 25. On Day 26, participants received a single oral dose of 200 mg caffeine with the morning dose of CBD. Participants were discharged on Day 28. A follow‐up visit took place 14–16 days after the last CBD dose. Participants received standardized meals while resident at the trial site and were advised to eat as normal (with the exception of trial‐related prohibitions) while at home. Participants were instructed to take their CBD doses 30 minutes after meals. Food diaries were not employed.
Trial population
Healthy male and female participants aged 18–60 years with a body mass index (BMI) between 18–32 kg/m2, and weighing ≥ 50.0 kg were eligible for this trial. Participants had no clinically significant medical history, no history of substance abuse, and normal physical examination, 12‐lead electrocardiogram, vital signs, and clinical laboratory findings, as judged by the principal investigator. Female participants were nonpregnant and nonlactating, and participants of childbearing potential or with a partner of childbearing potential agreed to use effective contraception throughout the trial site and for 90 days after the follow‐up visit. The use of all prescribed medication and all over‐the‐counter medication, vitamin preparations and other food supplements, or herbal medications was prohibited from first admission to the trial site until the follow‐up visit. An exception was made for hormonal contraceptives and for acetaminophen (without caffeine; up to 2 g/day for up to 3 consecutive days).
Liver safety assessments
Clinical LT ULN values designated by the Contract Research Organization were: 68 IU/L for ALT, 45 IU/L for aspartate aminotransferase (AST), 129 IU/L for ALP, 29 µmol/L for TBL, and 49 IU/L (females) or 59 IU/L (males) for gamma‐glutamyl transferase (GGT). LTs were performed at screening, on Days −1, 12, 18, 23, 27, and at follow‐up. Anyone with Day − 1 serum ALT or AST > 1.5 × ULN, TBL above the ULN, or international normalized ratio > 1.27 was excluded from the trial.
During the trial, participants meeting any of the following criteria were discontinued:
ALT or AST > 3× ULN accompanied by fatigue, nausea, vomiting, right upper quadrant pain, or tenderness, fever, rash, and/or eosinophilia (> 5%)
ALT or AST > 5× ULN
ALT or AST > 3× ULN and (TBL > 2× baseline or international normalized ratio > 1.5)
CBD concentrations
CBD trough plasma concentrations were measured before dosing on Days 23, 25, and 26. CBD plasma concentrations in the published CBD withdrawal trial were listed in the clinical study report 10 and were determined for compliance monitoring on trial Days 7, 14, 21, 28, 35, and 42 at 4–5 hours after morning dose.
CYP2C19 genotyping
Blood for CYP2C19 genotyping was collected on Day − 1. See Supplementary Methods for methods relating to pharmacogenetic analysis.
Results
Participants
Sixteen healthy adults participated in this open‐label DDI trial. Participant baseline characteristics are summarized in Table 1 .
Table 1.
Baseline characteristics
| Parameter | CBD (N = 16) |
|---|---|
| Age, year | |
| Median (Q1, Q3) | 29 (23.5, 37.8) |
| Sex, n (%) | |
| Male | 6 (37.5) |
| Female | 10 (62.5) |
| Race, n (%) | |
| White | 13 (81.3) |
| Black or African American | 1 (6.3) |
| Asian | 1 (6.3) |
| American Indian or Alaska Native | 1 (6.3) |
| BMI, kg/m2 | |
| Median (Q1, Q3) | 22.7 (21.1, 24.6) |
BMI, body mass index; CBD, cannabidiol; Q, quartile.
Safety
There were treatment‐emergent adverse events (AEs) in 14 (88%) of the 16 participants. The most common all‐causality AEs by preferred term were gastrointestinal disorders, including diarrhea in eight (50%) participants and abdominal discomfort in five (31%) participants. Most of these AEs were first experienced during the CBD titration phase. AEs were generally mild (31%) or moderate (50%) in severity.
The most notable biochemical abnormalities were elevation in serum ALT, AST, and GGT (Table S1 ). Lesser elevations in serum ALP were also observed. In seven (44%) participants, peak serum ALT values were above the ULN. Of these, five (31%) participants had peak ALT values > 5× ULN, the international consensus criteria for DILI (Table 2 ). 9 The serial LTs observed in the five participants with peak ALT values > 5× ULN are displayed in Figure 3 . The five participants with peak ALT elevations > 5× ULN were discontinued from treatment in accordance with withdrawal criteria.
Table 2.
Observed peak serum ALT expressed in terms of the stated and consensus 15 reference ranges
| Reference ULN | > ULN | ≥2 × ULN | ≥3 × ULN | ≥5 × ULN | ≥10 × ULN | ≥20 × ULN |
|---|---|---|---|---|---|---|
| Stated ULN | 7 (44) | 6 (38) | 5 (31) | 5 (31) | 0 (0) | 0 (0) |
| Consensus ULN | 11 (69) | 7 (44) | 7 (44) | 6 (38) | 5 (31) | 1 (6) |
ALT, alanine aminotransferase; ULN, upper limit of normal.
Figure 3.
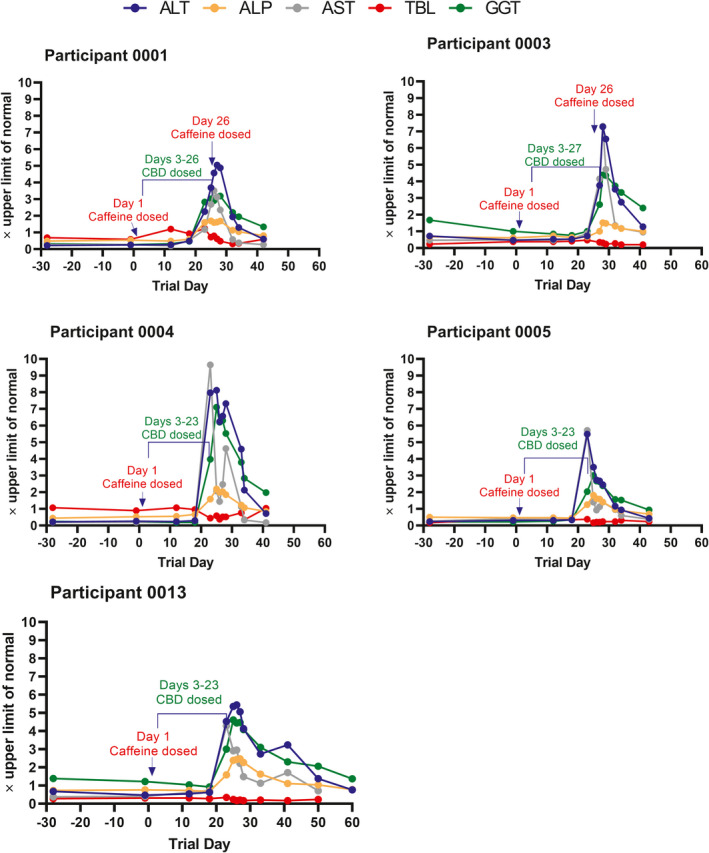
Serial liver chemistries for the five participants with ALT ≥ 5× the upper limit of normal. Acetaminophen was received by participant 0001 (single 500 mg dose on Day 20) for fever, headache, and myalgia; participant 0004 (single 1,000 mg dose on Day 23) for fever; and participant 0005 (1,000 mg as needed on Days 22–23) for abdominal cramps and backache. Participants 0003 and 0013 did not receive acetaminophen. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; TBL, total bilirubin.
When expressed as consensus ULNs for ALT which are different for males (33 IU/L) and females (25 IU/L), 11 six (38%) participants experienced peak ALT values > 5× ULN. Of these, five (31%) participants had peak ALT values > 10× ULN, and one (6%) of these participants experienced a peak ALT value > 20× ULN (Table 2 ).
In total, six (38%) participants with serum ALT elevations > 3× ULN were withdrawn from the trial prior to completion. Of these, three participants were discontinued solely due to AEs of elevated ALT and AST; two of these three participants also experienced concomitant fever: 38.5°C in one participant and 39.2°C in the other. The other three discontinued participants experienced additional AEs that contributed to their withdrawal. The first experienced eosinophilia, the second experienced vomiting, abdominal discomfort, and eosinophilia, and the third experienced nausea and syncope. In all six cases, the LT elevations were first detected between trial Days 23 and 27.
By international criteria, only one of the five participants with an ALT value > 5× ULN would be considered to have a hepatocellular liver injury (R value ≥ 5). 12 The remaining four participants would be considered to have a mixed hepatocellular/cholestatic injury (R value between 2 and 5). 12
Risk factors
Review of participant characteristics did not reveal an association between serum ALT elevations and sex, BMI, or genotype.
In this trial, 5 of 10 women (50%) and 2 of 6 men (33%) developed ALT above the ULN. The median BMI in participants with a peak serum ALT above the ULN was 23 (1st and 3rd quartiles: 22 and 25, respectively) compared with a median BMI of 22 in participants with normal ALT throughout (1st and 3rd quartiles: 21 and 23, respectively). The major route of phase I metabolism for CBD is CYP2C19, a polymorphic enzyme. 13 , 14 Based on CYP2C19 genotype, among the seven (44%) participants with elevations in serum ALT above the ULN, two participants were considered intermediate metabolizers (genotype *1/*2 or *2/*17), four were considered extensive metabolizers (genotype *1/*1), and one was considered an ultrarapid metabolizer (*1/*17) (Figure 4 ). Only one of the six participants considered ultrarapid metabolizers experienced ALT elevations, but this elevation exceeded 5 × ULN.
Figure 4.
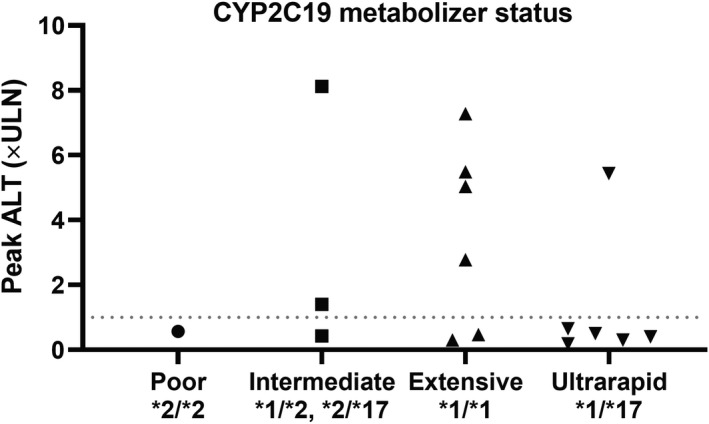
Relationship between CYP2C19 genotype and peak ALT × the upper limit of normal. Dotted line is drawn at 1 × the upper limit of normal. ALT, alanine aminotransferase; CYP, cytochrome P450; ULN, upper limit of normal.
When analyzing data from the current trial and a published trial investigating CBD withdrawal, 10 there was no correlation between systemic exposure to CBD and peak ALT elevations (Figure 5 ).
Figure 5.
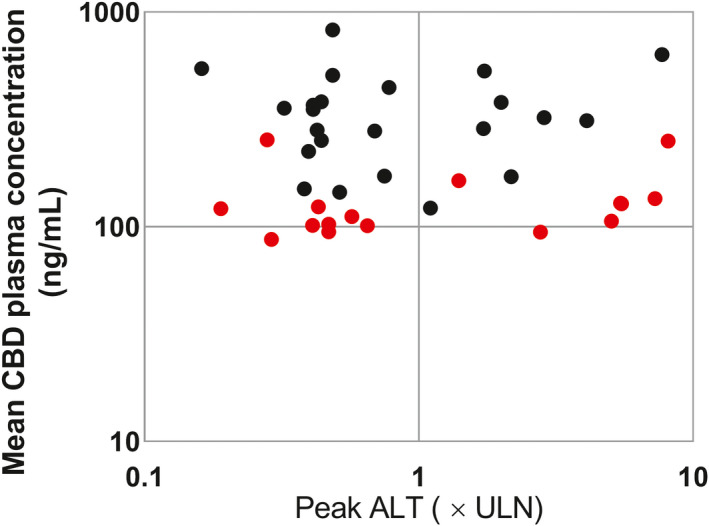
Peak serum ALT and the mean CBD concentrations (when multiple concentration measurements were available (five participants had only one concentration)) observed in the current trial (red) and another published trial in healthy adult volunteers that received comparable doses of CBD for ≥ 3 weeks (black). 10 CBD plasma concentrations in the caffeine trial were measured before dosing (trough), collected sporadically on days when CBD had reached steady state. CBD plasma concentrations in the other published trial represent samples collected around 4–5 hours after dosing on sporadic days after CBD had reached steady state. No significant correlation was observed. ALT, alanine aminotransferase; CBD, cannabidiol; ULN, upper limit of normal.
Experience in healthy participants receiving lower daily CBD doses
To determine whether lower CBD doses than we studied may cause ALT elevations in healthy adults, we reviewed the entire phase I clinical trial experience with Epidiolex. In one trial examining the effects of steady‐state CBD on the pharmacokinetics of clobazam, ALT elevations above the ULN were observed in five healthy (42%) adults receiving 5 mg/kg/day. Although no elevations exceeded 2 × ULN, the characteristic biochemical profile and latency was observed (Figure S1 ).
Discussion
There has been a large increase in the availability of nonprescription CBD preparations. 15 The US Food and Drug Administration has recently stated that it “cannot conclude that CBD is generally recognized as safe” and has sent letters to multiple CBD manufacturers warning them they are breaking the law. 16 Nonetheless, these products remain widely available and are projected to achieve $16 billion a year in sales by 2025. 17
The frequency and height of elevations in serum ALT observed in patients with DS/LGS during CBD treatment at 10 or 20 mg/kg/day has raised liver safety concerns and prompted recommendations for routine LT monitoring in these patients. Although the liver events observed in the pivotal DS/LGS RCTs were reversible, at least one patient (data presented in Figure 1 ) appeared to experience a mild degree of liver dysfunction attributed to CBD after commencing CBD treatment during the OLE trial. Furthermore, protocol‐driven discontinuation of treatment based on LT monitoring may have prevented more significant liver injuries in the pivotal DS/LGS RCTs.
The relevance of the CBD liver safety data obtained in patients with DS/LGS to healthy adults is unclear. Patients with DS/LGS often receive potentially hepatotoxic concomitant medications and appear to have a high background frequency of LT abnormalities suggesting preexisting liver conditions. Patients with DS/LGS who had normal baseline LTs and were not treated with valproate experienced a low incidence of treatment‐emergent LT abnormalities, and few of these patients discontinued CBD on this basis. 1 , 2 , 3 , 4 , 6
It was therefore unexpected that 31% of healthy adults would experience LT elevations consistent with DILI (ALT > 5× ULN) 9 in this trial, while receiving doses of CBD commonly administered to patients with DS/LGS (~ 20 mg/kg/day). It should also be noted that the ULNs for the laboratory analyzing the serum samples (ALT 68 IU/L for men and women) are not consistent with recent recommendations which are ALT 33 IU/L for men and 25 IU/L for women. 11 Applying these consensus reference ranges would result in a marked increase in the incidence of ALT elevations at each cutoff, including one participant with an elevation > 20× ULN.
In the current trial, all elevations began within 2–4 weeks of initial CBD exposure. While ALT and AST represented the predominant elevations observed in the healthy participants, similar to observations in patients with DS/LGS, there was a more prominent cholestatic component in the five cases with ALT elevations > 5× ULN, as evidenced by the concomitant rise in serum ALP and GGT, which was unique to the healthy adults.
Interestingly, CBD‐related ALT elevations were reported in another phase I trial in healthy adults examining withdrawal effects from CBD (20 mg/kg/day for 4 weeks). 10 The participants had inclusion/exclusion criteria similar to the current trial and only received CBD treatment. In this trial, 2 of 30 healthy participants were discontinued because of elevated LTs (peak ALT > 3× ULN in 1 participant and > 5× ULN in another participant). One participant experienced eosinophilia, fatigue, and nausea in addition to elevated LTs and the second participant experienced abdominal pain and pyrexia along with raised LTs. No elevated TBL levels were detected in either participant, and both participants recovered from these AEs after withdrawal of CBD. An additional 12 participants experienced ALT elevations that did not exceed 3 × ULN.
The high rate of serum ALT elevations we observed does not necessarily indicate potential for CBD to cause serious liver injury. Elevations in serum ALT can result from treatment with drugs that have minimal significant liver safety liability, including heparins, 18 cholestyramine, 19 statins, 20 and tacrine. 21 These drugs cause transient and asymptomatic elevations in serum ALT that generally reverse even with continued treatment. The phenomenon was observed for lower‐level serum ALT elevations in the pivotal DS/LGS RCTs. 1 , 2 , 3 , 4 However, even drugs capable of progressive and serious liver injury often produce elevations in serum LTs that resolve despite continued treatment. 22
Importantly, when mean CBD plasma concentrations were plotted against ALT elevations using data from this and the published trial investigating CBD withdrawal effects, 10 there was no correlation between systemic exposure to CBD and peak ALT elevations. This suggests there is marked interparticipant variability in susceptibility to transaminase elevations. We were unable to identify patient risk factors for ALT elevations, including CYP2C19 metabolizer status. It is unlikely that caffeine contributed to the elevations in the current trial due to the lack of hepatotoxic potential of this drug and that the ALT elevations > 5× ULN occurred more than 2 weeks after the first caffeine dose was administered and had started before the second dose in the two participants who received a second dose.
The very high elevations in serum ALT that were sometimes observed in the pivotal DS and LGS RCTs and the suggestion of mild liver dysfunction in one patient that received CBD in an OLE are potential cause for concern. In the current trial, three healthy adults developed signs and symptoms that included eosinophilia, abdominal pain, nausea, and vomiting, and two participants experienced fever that occurred within the same time frame as the ALT elevations. While gastrointestinal AEs were common in this trial, they generally occurred during the titration phase. These symptoms are also associated with hepatitis 23 and are not expected with benign ALT elevations.
The mechanism underlying the elevations in serum ALT associated with CBD treatment has not been clarified. The dose‐dependency and relatively rapid resolution after discontinuing treatment is most consistent with a direct effect. Dose‐dependent hepatoxicity has been reported in mice and transcriptomics identified treatment‐induced changes in genes related to oxidative stress. 24 CBD has also been shown to inhibit mitochondrial function in cultured cells. 25 The concomitant symptoms and eosinophilia or fever, observed in some participants in both the current trial and the withdrawal trial 10 suggest immune activation; however, the rapid reversal upon discontinuing treatment is not characteristic of an immune response. It should also be noted that the > 2‐week latency to onset of the liver events is days after steady‐state blood levels of CBD and its two major metabolites have been attained, 26 which may indicate a role for a minor metabolite or possibly innate immune activation.
An important question is the liver safety implications of our trial for the use of nonprescription CBD products. The consumption of 1,500 mg CBD daily in our trial likely far exceeds that typically relevant for use of nonprescription products. 27 However, studies have documented ranges of CBD that are both well below or in excess of product labeling with one product reported to contain more than 650 mg/ml of CBD. 27 , 28
In reviewing the experience of Epidiolex in other phase I trials, it was noted that some healthy adults receiving 5 mg/kg/day experienced mild serum LT elevations with the characteristic biochemical profile and latency observed in the current trial. Moreover, it is possible that other drugs or conditions that may be operative in the general public will result in significantly enhanced susceptibilities to CBD liver effects. For instance, a recent study showed that pretreatment of mice with CBD increased hepatotoxicity due to acetaminophen. 29 It is therefore interesting that three of the five participants that developed CBD‐related ALT elevations > 5× ULN also took acetaminophen shortly before or concurrently with ALT elevations. However, a role for acetaminophen seems unlikely since two of these three participants received just a single dose (Figure 3 legend) and the timing and biochemical nature of the events were similar in all the participants. Given the large interparticipant variability in susceptibility, the possibility of unrecognized DDIs, and in our trial, the lack of correlation with CBD systemic exposure, it seems likely that some adults will experience ALT elevations at doses considerably lower than have been studied.
In summary, CBD administered to healthy adults at a dose of 1,500 mg/day can result in serum ALT elevations consistent with DILI, and the frequency of this event appears to be greater than in patients with DS/LGS not receiving valproate and with normal baseline LTs that took part in the pivotal RCTs. There exists large interparticipant variation in susceptibility and likely risk factors are yet to be identified. Clinicians should be alert to this effect of CBD to avoid needless diagnostic evaluations. They should also be on the lookout for clinically important liver injury associated with the rapidly expanding and unregulated use of this product.
Funding
This trial was sponsored by GW Research Ltd.
Conflict of Interest
P.B.W. and R.J.C. are paid consultants for GW Research Ltd. but were not compensated for the preparation of this manuscript. J.L. and V.K. are employees of Greenwich Biosciences.
Author Contributions
P.B.W., R.J.C., J.L., and V.K. wrote the manuscript contributed to the design and execution of this healthy volunteer study evaluation.
Supporting information
Figure S1
Supplementary Material
Table S1
Supplementary Methods S1
Acknowledgments
The authors thank Lesley Taylor and Shula Pollard for their assistance in editing the manuscript.
References
- 1. Devinsky, O. et al. Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. N. Engl. J. Med. 376, 2011–2020 (2017). [DOI] [PubMed] [Google Scholar]
- 2. Devinsky, O. et al. Effect of cannabidiol on drop seizures in the Lennox‐Gastaut syndrome. N. Engl. J. Med. 378, 1888–1897 (2018). [DOI] [PubMed] [Google Scholar]
- 3. Thiele, E.A. et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 391, 1085–1096 (2018). [DOI] [PubMed] [Google Scholar]
- 4. Miller, I. et al. Dose‐ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: a randomized clinical trial. JAMA. Neurol. 77, 613–621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thiele, E. et al.Cannabidiol (CBD) treatment in patients with seizures associated with tuberous sclerosis complex: a randomized, double‐blind, placebo‐controlled phase 3 trial (GWPCARE6). American Epilepsy Society (AES) Annual Meeting 2019 Conference, Baltimore, MD, December 6–10, 2019. Abstract 1.293. <https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/2421288>. Accessed July 10, 2020.
- 6. Epidiolex oral solution [prescribing information]. (Greenwich Biosciences, Carlsbad, CA, 92008). <https://www.epidiolex.com/sites/default/files/pdfs/EPIDIOLEX_Full_Prescribing_Information_04_16_2020.pdf>. Accessed June 22, 2020.
- 7. Giannini, E.G. , Testa, R. & Savarino, V. Liver enzyme alteration: a guide for clinicians. CMAJ 172, 367–379 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White, C.M. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential. J. Clin. Pharmacol. 59, 923–934 (2019). [DOI] [PubMed] [Google Scholar]
- 9. Aithal, G.P. et al. Case definition and phenotype standardization in drug‐induced liver injury. Clin. Pharmacol. Ther. 89, 806–815 (2011). [DOI] [PubMed] [Google Scholar]
- 10. Taylor, L. , Crockett, J. , Tayo, B. , Checketts, D. & Sommerville, K. Abrupt withdrawal of cannabidiol (CBD): A randomized trial. Epilepsy. Behav. 104(Pt A), 106938 (2020). [DOI] [PubMed] [Google Scholar]
- 11. Kwo, P.Y. , Cohen, S.M. & Lim, J.K. ACG clinical guideline: evaluation of abnormal liver chemistries. Am. J. Gastroenterol. 112, 18–35 (2017). [DOI] [PubMed] [Google Scholar]
- 12. Xing, M. et al. Assessment of cholestasis in drug‐induced liver injury by different methods. Medicine (Baltimore). 98, e14399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang, R. , Yamaori, S. , Takeda, S. , Yamamoto, I. & Watanabe, K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life. Sci. 89, 165–170 (2011). [DOI] [PubMed] [Google Scholar]
- 14. Zendulka, O. et al. Cannabinoids and cytochrome P450 interactions. Curr. Drug. Metab. 17, 206–226 (2016). [DOI] [PubMed] [Google Scholar]
- 15. Hahn, S.M. US Food and Drug Administration. FDA Statement: FDA advances work related to cannabidiol products with focus on protecting public health, providing market clarity<https://www.fda.gov/news‐events/press‐announcements/fda‐advances‐work‐related‐cannabidiol‐products‐focus‐protecting‐public‐health‐providing‐market> (2020), Accessed April 9, 2020.
- 16. US Food and Drug Administration . FDA Statement: FDA warns 15 companies for illegally selling various products containing cannabidiol as agency details safety concerns <https://www.fda.gov/news‐events/press‐announcements/fda‐warns‐15‐companies‐illegally‐selling‐various‐products‐containing‐cannabidiol‐agency‐details> (2019). Accessed April 9, 2020.
- 17. Brodwin, E. Business Insider. Wall Street thinks CBD could be a $16 billion industry by 2025. Here’s what the cannabis compound does to your brain and body. <https://www.businessinsider.com/cbd‐effects‐physical‐mental‐benefits‐limits‐2019‐5?r=US&IR=T> (May 31, 2019). Accessed April 9, 2020.
- 18. Harrill, A.H. et al. The effects of heparins on the liver: application of mechanistic serum biomarkers in a randomized study in healthy volunteers. Clin. Pharmacol. Ther. 92, 214–220 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singhal, R. , Harrill, A.H. , Menguy‐Vacheron, F. , Jayyosi, Z. , Benzerdjeb, H. & Watkins, P.B. Benign elevations in serum aminotransferases and biomarkers of hepatotoxicity in healthy volunteers treated with cholestyramine. BMC. Pharmacol. Toxicol. 15, 42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thapar, M. , Russo, M.W. & Bonkovsky, H.L. Statins and liver injury. Gastroenterol. Hepatol. (NY) 9, 605–606 (2013). [PMC free article] [PubMed] [Google Scholar]
- 21. Gracon, S.I. et al. Safety of tacrine: clinical trials, treatment IND, and postmarketing experience. Alzheimer. Dis. Assoc. Disord. 12, 93–101 (1998). [DOI] [PubMed] [Google Scholar]
- 22. Andrade, R.J. et al. Drug‐induced liver injury. Nat. Rev. Dis. Primers 5, 58 (2019). [DOI] [PubMed] [Google Scholar]
- 23. Thuener, J. Hepatitis A and B infections. Prim. Care 44, 621–629 (2017). [DOI] [PubMed] [Google Scholar]
- 24. Ewing, L.E. et al. Hepatotoxicity of a cannabidiol‐rich cannabis extract in the mouse model. Molecules 24, 1694 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olivas‐Aguirre, M. , Torres‐López, L. , Valle‐Reyes, J.S. , Hernández‐Cruz, A. , Pottosin, I. & Dobrovinskaya, O. Cannabidiol directly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell. Death Dis. 10, 779 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor, L. , Gidal, B. , Blakey, G. , Tayo, B. & Morrison, G. A phase I, randomized, double‐blind, placebo‐controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs 32, 1053–1067 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bonn‐Miller, M.O. , Loflin, M.J.E. , Thomas, B.F. , Marcu, J.P. , Hyke, T. & Vandrey, R. Labeling accuracy of cannabidiol extracts sold online. JAMA 318, 1708–1709 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vandrey, R. , Raber, J.C. , Raber, M.E. , Douglass, B. , Miller, C. & Bonn‐Miller, M.O. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA 313, 2491–2493 (2015). [DOI] [PubMed] [Google Scholar]
- 29. Ewing, L.E. , McGill, M.R. , Yee, E.U. , Quick, C.M. , Skinner, C.M. , Kennon‐McGill, S. et al. Paradoxical patterns of sinusoidal obstruction syndrome‐like liver injury in aged female CD‐1 mice triggered by cannabidiol‐rich cannabis extract and acetaminophen co‐administration. Molecules 24, 2256 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Supplementary Material
Table S1
Supplementary Methods S1


