Abstract
The main objectives of this study were to model various scenarios of African swine fever (ASF) virus transmission among farms in Vietnam and to evaluate the impact of control strategies using North American Animal Disease Spread Model (NAADSM). A total of 7,882 pig farms in the Red River Delta (RRD) region were obtained from the General Statistics Office, and then, random points corresponding to the number of farms in each province were generated as exact farm locations were not available. A total of 10 models were developed, including movement control scenarios. In addition, we conducted sensitivity analysis to assess the impact of indirect contact transmission probability (TP). Overall, the indirect contact exhibited an important role in transmitting the ASF virus. In order to minimize ASF transmission between farms, we found that movement restriction needed to reach a certain level (approximately between 50% and 75%) and that the restriction had to be applied in a timely manner. This study offers valuable insight into how ASF virus can be transmitted via direct and indirect contact and controlled among farms under the various simulation scenarios. Our results suggest that the enforcement of movement restriction was an effective control measure as soon as the outbreaks were reported. In addition, this study provided evidence that high standards of biosecurity can contribute to the reduction of disease spread.
Keywords: African swine fever, epidemiology, simulation model, transmission, Vietnam
1. INTRODUCTION
African swine fever (ASF) is a highly contagious virus and classified as a notifiable disease by the World Organization for Animal health (OIE) (Tulman, Delhon, Ku, & Rock, 2009). It is a double‐stranded DNA virus of the Asfarviridae family, genus Asfivirus (Dixon et al., 2005). The disease causes acute haemorrhagic fever with mortality of up to 100% depending on the virulence of the isolate, dose and route of exposure to the virus (Costard, Mur, Lubroth, Sanchez‐Vizcaino, & Pfeiffer, 2013). Pigs are infected via contact with infected animals (including free‐ranging and wild pigs), fomites, premises, vehicles, clothes, consumption of contaminated feed and bites of infected ticks (OIE, 2020a). The disease can have serious economic consequences, through reduced international trade and a decrease in pig populations, which can in turn pose a huge threat to food security (Blome, Gabriel, & Beer, 2013). The disease is endemic in sub‐Saharan African countries, Caucasus, Eastern Europe and Baltic countries (OIE, 2020b). In Asia, the first outbreak was confirmed in northeastern China in August 2018 (Zhou et al., 2018), and then, the virus rapidly spread to other Asian countries (Dixon, Sun, & Roberts, 2019; FAO, 2020). In Vietnam, the first ASF outbreak was reported in February 2019 in backyard pig farms in Hung Yen province (Van Phan Le et al., 2019). Since then, ASF outbreaks have been reported in all 63 provinces, resulting in approximately 6 million pigs (20% of pig production) that have been either culled or killed by the disease (FAO, 2020). As of May 2020, outbreaks have not been reported for more than 30 days in 35 of the 63 provinces.
The use of simulation models for infectious diseases is an important tool for decision‐makers to evaluate the impact of outbreaks and to identify cost‐effective control strategies (e.g. vaccination, movement control and depopulation) (Francis, Klotz, Harvey, & Stacey, 2010; Keeling, 2005; Morris, Wilesmith, Stern, Sanson, & Stevenson, 2001). The North American Animal Disease Spread Model (NAADSM) is a computer software used to develop simulation models of the spread of highly infectious animal diseases (Harvey et al., 2007). It provides a flexible framework with user‐established parameters to define the disease spread by direct, indirect contact, airborne and local spread as well as assess the impact of various control measures.
In Vietnam, the first study using NAADSM explored porcine reproductive and respiratory syndrome (PRRS) transmission via direct and indirect contacts across different farm types (Lee et al., 2019). With the ongoing outbreak and impact of ASF on the pig sector, the main objectives of this study were to model various scenarios of ASF virus transmission among farms and to evaluate the impact of control strategies using NAADSM.
2. MATERIALS AND METHODS
2.1. Study location and population
The Red River Delta (RRD) region is the smallest region in Vietnam, located in the north (Figure 1), yet it has the highest concentration of human population (22 million) in the country (GSO, 2020). It also has the largest pig population (7.2 million), which accounts for 28%–29% of the pig population in Vietnam (GSO, 2018). In order to develop the ASF transmission models, farm locations and characteristics were necessary. The number of livestock farms at provincial level was obtained from the General Statistics Office of Vietnam. As exact farm locations were not available, random points corresponding to the number of farms in each province were generated using QGIS (Quantum GIS development 2012, QGIS version 3.12.2) (Figure 1). The coordinates were then extracted and imported into NAADSM. A total of 7,882 farms were recorded and used for the simulation model. These farms were categorized into 3 production types: a) small (<100 pigs), b) medium (≥100 pigs) and c) large (>1,000 pigs) (Nga, Ninh, Van Hung, & Lapar, 2014). The proportion of each production type was 70% (a), 25% (b) and 5% (c), respectively (Lapar, Binh, & Ehui, 2003), Table 1.
FIGURE 1.
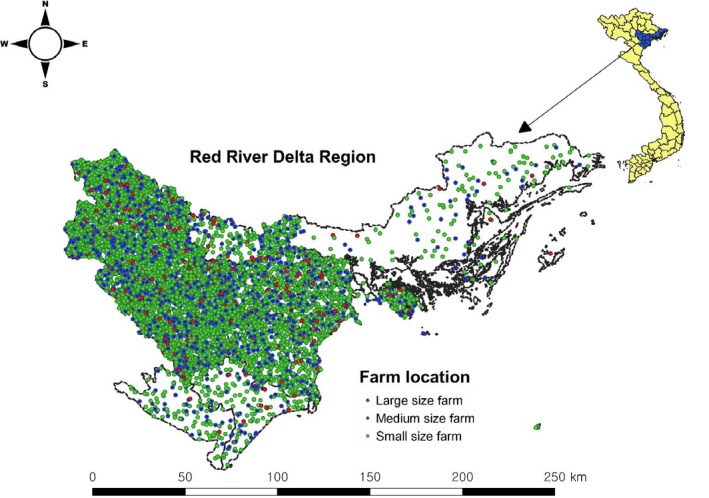
Spatial distribution of three pig farm types in the Red River delta region [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Model parameters used for simulation model between pig farm spread of ASF virus in the Red River Delta region, Vietnam
| Parameters | Value | Reference |
|---|---|---|
| Total farms (n) | 7,882 | General statistics office of Vietnam |
| Small | 5,499 (69.77%) | |
| Medium | 1,989 (25.23%) | |
| Large | 394 (5.00%) | |
| Transmission probability | ||
| Direct contact | 0.6 a | (Guinat et al., 2016) |
| Indirect contact to small/medium farms | 0.6 | Contaminated products (e.g. swill) and vehicle movements are the main source of infection which was determined as the same as direct contact |
| Indirect contact to large farms | 0.006 | Due to high biosecurity |
| Infectious duration | ||
| Small | 52 weeks | Assumptions |
| Medium | 10–12 weeks | Assumptions |
| Large | 4 weeks | Assumptions |
| Contact distances between farms (km) | BetaPERT (0.5, 30, 300) | |
The median value was used.
2.2. Model parameters
The NAADSM requires three key parameters: (a) transmission probability (TP) related to each contact (it is a probability between 0 and 1, and representing the likelihood of the contact herd will become infected given the exposure to an infected herd); (b) distance distribution associated with contact between farms; and (c) mean contact rates (estimated number of contacts per week) (Harvey et al., 2007). These parameters were estimated based on previous studies (Guinat et al., 2016; Lee et al., 2019) and assumptions (expert opinions) (Table 1). Transmission of ASF in Vietnam is mainly through indirect contact (e.g. swill feeding and human/vehicle movements). Therefore, TPs for direct and indirect contacts were considered as the same value (0.6) (expert opinions) for small and medium farms, whereas large farms were parametrized to have the value of 0.006 for indirect contact and 0.6 for direct contact TP due to comparatively higher levels of biosecurity (Table 1). These values were internally discussed among local experts. We assumed that the different infectious durations were determined by farm type (small farm: 52 weeks; medium farm: 10–12 weeks; and large farm: 4 weeks) as a result of different levels of biosecurity. Because, we assumed that a continuous flow (CF) system was used in small farms as they are replacing pigs continuously from different sources (with unknown disease status), while the all‐in‐all‐out system (AIAO) was followed in large and some medium farms where these farms introduce new pigs in batches and mostly from farms with high biosecurity and known infection status. Our estimates of infectious duration for the three farm types were based on these considerations, where large and medium farms were allowed to remain infectious for a relatively shorter duration. In contrast, small farms due to the continuous reintroduction of animals were allowed to remain infectious for the entire simulation duration.
A PERT distribution was defined for contact distances between farms, with a minimum of 0.5 km, a most likely value of 30 km and a maximum of 300 km (Table 1). The weekly mean contact rates (following a Poisson distribution) between different farm types were obtained from a previous study and plugged into simulation models (Lee et al., 2019) (Table 2).
TABLE 2.
Contact structure of pig farms used by production type for simulation model
| Contact groups (Source–Destination) | Mean contact rate/week (Lee et al., 2019) | |
|---|---|---|
| Direct | Indirect | |
| Small farms → Small farms | Poisson 0.072 | Poisson 0.282 |
| Small farms → Medium farms | ‐ | Poisson 0.282 |
| Medium farms → Small farms | Poisson 0.072 | Poisson 0.282 |
| Medium farms → Medium farms | Poisson 0.073 | Poisson 0.271 |
| Medium farms → Large farms | ‐ | Poisson 3.5 |
| Large farms → Medium farms | Poisson 0.073 | Poisson 0.271 |
| Large farms → Large farms | ‐ | Poisson 3.5 |
2.3. Simulation model structure and sensitivity analysis
In Vietnam, most large farms are commercialized and contract farms, while medium farms are mainly suppliers and have high connectivity to small farms (Figure 2). It is very rare that pigs from small farms move to other sized farms locally. We assumed that none of the pigs had resistance to ASF virus. If a single pig became infected, then the whole farm was considered to be infectious. The baseline scenario was that one medium farm was infected and the same farm‐initiated infection in the following iterations. We assumed that the rest of the farms were susceptible at the beginning of the simulation and remained infectious until the end of the study period for small farms or remained infectious for a specified period for medium and large farms and became susceptible again (Table 1). Especially, medium and large farms were allowed to be infected multiple times during the simulation.
FIGURE 2.
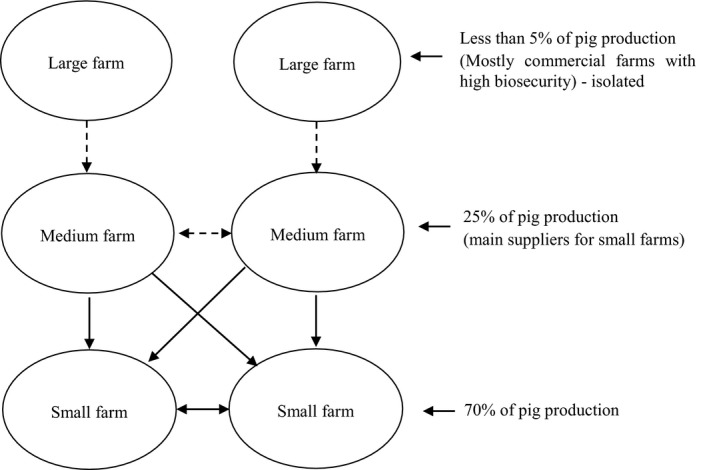
The simple diagram of network movement structure in Vietnam (dash arrow: rare movement)
The model was run over 500 iterations for 52 weeks, which was long enough to cover at least one complete pig production cycle (6–8 months in Vietnam). Since there is no vaccine for ASF, we evaluated the effectiveness of movement control on contact rates by 25%, 50%, 75% and 100% reduction, which was applied to both direct and indirect contact rates. It was hypothesized that movement restrictions were imposed for the baseline scenario within 4 weeks of detection of outbreaks. We conducted sensitivity analysis to assess the impact of infectious duration for small farms (basic: 52 weeks) and indirect contact TP for the small/medium farms (basic: 0.6) by −25%, −50% and −75%, respectively. In addition, the timing of movement restriction was imposed at 2 weeks, 6 weeks and 8 weeks after detection, respectively, under the 25% and 50% movement restriction scenarios.
3. RESULTS
The baseline scenarios (A1: including both direct and indirect contacts) showed that a total of 7,640 (5 and 95 percentiles: 6,729–7,790) median farms were infected, while A2 (only including indirect contact) presented a slightly lower median number of infected farms (7,544, 5 and 95 percentiles: 5,890–7,685) (Table 3). The epidemic peak was reached the earliest in scenarios (week 33) of A1 and A2 compared to A3 (no contact to large farms). Overall, the indirect contact exhibited an important role in transmitting the ASF virus. We evaluated the impact of movement restriction strategies on the number of infected farms. The MC1 scenario (25% reduction of contact rates) showed that the medium number of infected farms reduced by 19.28% compared to the baseline scenario (A1), while the large farms had a higher reduction of cases (−28.87%) compared to the small (−24.79%) and medium farms (−3.79%) (Table 4). The number of median infected farms for scenario MC3 (75% reduction of contact rates) dramatically decreased by 99.96% compared to the scenario A1, which was considered to be a very effective option. Overall, we found that the medium number of infected farms decreased as the contact rates reduced. Interestingly, in some iterations, the virus did not spread beyond the index farm under the M1, M2 and M3 scenarios. The proportion of such iterations with no infection spread for scenarios M1‐3 were 8.9%, 26.7% and 39.7% of the total 500 simulated iterations, respectively. We found that 4.9% and 9.2% of total infected large and medium farms had more than one outbreak, respectively, during the simulation.
TABLE 3.
Median number of infected pig farms and time required to reach the peak epidemic under assumptions of various direct and indirect contacts
| Scenario | Contact information | No. of mean infected farms (5 and 95 percentiles) | Week to peak epidemic | |||
|---|---|---|---|---|---|---|
| Overall | Small | Medium | Large | |||
| A1 | Direct and indirect contact | 7,640 (6,729–7,790) | 5,231 (4,433–5,358) | 2,084 (2,018–2,102) | 324 (276–345) | 33 |
| A2 | Indirect contact | 7,544 (5,890–7,686) | 5,144 (3,722–5,279) | 2,079 (1,945–2,100) | 323 (238–348) | 33 |
| A3 |
Indirect contact (no contact to large farms) |
7,153 (5,801–7371) | 5,074 (3,831–5,278) | 2,077 (1,970–2,101) | 0 | 35 |
TABLE 4.
Median number of infected pig farms under the different movement restrictions on the contact rates
| Scenario | Movement control | No. of mean infected farms (5 and 95 percentiles) | % change in the number of median infected farm | |||
|---|---|---|---|---|---|---|
| Overall | Small | Medium | Large | |||
| A1 | Baseline | 7,640 (6,729–7,790) | 5,231 (4,433–5,358) | 2,084 (2,018–2,102) | 324 (276–345) | NA |
| MC1 | 25% | 6,171 (0–6,950) | 3,934 (0–4,615) | 2,005 (0–2,075) | 231 (0–273) | −19.23% |
| MC2 | 50% | 1,231 (0–3,071) | 639 (0–1,679) | 560 (0–1,298) | 35 (0–93) | −83.89% |
| MC3 | 75% | 30 (0–159) | 15 (0–79) | 14 (0–77) | 1 (0–4) | −99.62% |
| MC4 | 100% | 1 (0–3) | 0 (0–2) | 0 (0–2) | 0 (0–1) | −99.99% |
The sensitivity analysis was implemented to evaluate on infectious duration (small farms; 52, 39, 26 and 13 weeks), TPs for indirect contact (small and medium farms), movement restrictions and the timing of restrictions compared to the baseline scenario. We found that the infectious duration did not have an impact on the results (less than 2% increase, not shown). The number of medium infected farms for scenario IC3 had a sharp reduction by 99.99% compared to the baseline scenario (A1) (Table 5). Overall, we found that the median number of infected farms had reduced as the TPs for indirect rates decreased. The timing of control measure did not have much impact on the median number of infected farms compared to the baseline (MC1) (Table 6). In addition, in the movement restriction scenario by 50%, it showed that TC6 had the relatively large increase in the median number of infected farms (30.38%). Overall, in order to minimize ASF transmission between farms, we found that movement restriction needed to reach a certain level (approximately between 50% and 75%) and that the restriction had to be applied in a timely manner.
TABLE 5.
Sensitivity analysis of the median epidemic size of simulated ASF outbreaks to indirect contact transmission probability in a population of 7,882 pig farms
| Scenarios | Parameters | ±% change of parameters |
Epidemic size median (5 and 95 percentile) |
% change in median outcome compared to baseline | ||
|---|---|---|---|---|---|---|
| DC Transmission probability |
IC Transmission probability |
DC Transmission probability |
IC Transmission probability |
|||
| Baseline | 0.6 | 0.6 | N/A | N/A | 7,640 (6,729–7,790) | N/A |
| IC1 | 0.6 | 0.45 | N/A | −25% | 6,201 (1,699–7,015) | −18.84% |
| IC2 | 0.6 | 0.3 | N/A | −50% | 1,106 (0–3,152) | −85.52% |
| IC3 | 0.6 | 0.15 | N/A | −75% | 10 (0–152) | −99.99% |
Abbreviation: DC, direct contact; IC (small and medium farms): indirect contact.
TABLE 6.
Sensitivity analysis of the median epidemic size of simulated ASF outbreaks to timing of movement restriction under the reduction of contact rate by 25% and 50%
| Scenarios | Parameters | ±% change of parameters |
Epidemic size median (5 and 95 percentile) |
% change in median outcome compared to baseline | ||
|---|---|---|---|---|---|---|
| Movement restriction | Timing | Movement restriction | Timing | |||
| MC1 | 25% | 4 weeks | N/A | N/A | 6,171 (0–6,950) | N/A |
| TC1 | 25% | 2 weeks | N/A | −50% | 6,145 (16–6,913) | −0.42% |
| TC2 | 25% | 6 weeks | N/A | 50% | 6,172 (611–6,980) | 0.02% |
| TC3 | 25% | 8 weeks | N/A | 100% | 6,276 (1,379–7,026) | 1.70% |
| MC2 | 50% | 4 weeks | N/A | N/A | 1,231 (0–3,071) | N/A |
| TC4 | 50% | 2 weeks | N/A | −50% | 1,180 (0–2,933) | −4.14% |
| TC5 | 50% | 6 weeks | N/A | 50% | 1,440 (0–3313) | 16.98% |
| TC6 | 50% | 8 weeks | N/A | 100% | 1605 (0–3477) | 30.38% |
4. DISCUSSION
This was the first study in Vietnam to assess the transmission of ASF virus among swine farms using NAADSM. The weekly mean contact rates by farm types were obtained from the previous study in Vietnam (Lee et al., 2019), which made our model more realistic in terms of applicability to local farms. In the model, indirect contact had a predominant role in the transmission of the ASF virus between farms. It has been suggested that the various means of indirect contact (e.g. swill feeding, human/transport‐associated routes and improper disinfection) account for 70%–80% of the transmission of ASF virus among farms in Vietnam (DAH, 2020). In fact, it is still a common practice to give swill feeding in small pig holders in Vietnam even after ASF outbreaks have occurred. In addition, it is well known that wild boars and soft tickets could be the main source of infection in other countries (Galindo & Alonso, 2017; Thomson, 1985). In Asia, infected wild boars have been reported in China and South Korea (FAO, 2020; Li et al., 2019). However, no studies have been conducted to assess the roles of wild boars and soft tickets for virus spread in Vietnam. Therefore, further thorough investigation is necessary to identify the transmission route of ASF virus at farm level.
In terms of control measures, we evaluated the impact of movement restriction on the number of infected farms compared to the baseline scenario. Our scenarios suggest that strict movement control should be imposed to prevent the onwards disease transmission, which is consistent with other studies (Nielen, Jalvingh, Meuwissen, Horst, & Dijkhuizen, 1999; Turner, Bowers, & Baylis, 2012). In addition, the TC1‐6 scenarios showed that the timing of movement control was not an important factor under the loosen movement restriction scenarios. In Vietnam, when the first outbreak was confirmed in February 2019, the prime minister issued a directive to all provinces to apply all necessary control measures, including strict movement restrictions of pigs and pig products from infected provinces to other parts of the country, especially the south. In spite of this, within 8–9 months, ASF outbreaks were reported in all provinces. Monitoring of movement was poor mainly due to lack of management capacity and the low density of quarantine checkpoints, which were only set up at national highways and major routes across the provinces. Pig traders used alternative routes to avoid the quarantine checkpoints. In addition, some farmers who thought the ASF virus was a zoonotic disease urgently sold pigs through illegal means during the outbreaks, especially during the Tet holiday period (Vietnamese New Year in February).
Our simulation models showed that a decrease of indirect contact for TP resulted in a reduction of the number of infected farms when it reached a certain low level; otherwise, it was not effective. The main implication was that strict enforcement of high levels of biosecurity measures was the effective way to prevent the introduction of disease into pig farms. In Vietnam, poor biosecurity in small‐ and medium‐scale farms has already been identified as the main risk factor for disease transmission (Lee et al., 2020). Indeed, the absence of disinfection mattresses, no or rare use of protective boots and clothes, irregular disinfection of farm premises and the use of left‐over food for feedings are very common. One study showed that the biosecurity scores (it evaluates both external biosecurity [reduce the introduction of diseases] and internal biosecurity [reduce the spread of diseases]) in pig farms were between 53.68% and 55.05% based on percentage grade (0%–100%) (Tuan, Dewulf, Postma, Cuc, & Dinh, 2019). It is therefore very important to establish regular training programmes to educate farmers on biosecurity practices.
However, we acknowledge that in the absence of available data, the indirect contact TP, for small‐ and medium‐scale farms (0.6), is used in the baseline scenario and other movement control scenarios, which was based on our assumption owing to the above considerations. Our indirect contact TP is larger than the probabilities used for this parameter in ASF spread models in different jurisdictions. Our sensitivity analysis supported our assumption that indirect contact had a larger role in ASF spread in Vietnam as smaller indirect contact TP had resulted in nominal spread of the virus, contrary to what had been observed during the initial ASF outbreaks (much rapid/wider spread) in Vietnam. Although our simulation provided some guidance on the probable range of this probability, the uncertainty in this parameter estimate was still not resolved, and future field studies may help to provide better estimates.
It was assumed that the ASF virus was introduced from China as the virus strain was 100% identical to China strains (Van Phan Le et al., 2019). The most probable route of transmission was through the importation of pork products through illegal channels from China to Vietnam. In fact, the first outbreak was detected in the northern part of Vietnam where illegal animal/meat product movements are commonly reported (FAO, 2018). From then on, it was likely that the virus had spread to central and south provinces from the RDD (north part of Vietnam). This pattern was very similar when highly pathogenic porcine reproductive and respiratory syndrome (HP‐PRRS) outbreaks occurred in 2007 (Metwally et al., 2010). Another study also found similar pig movement patterns (Baudon et al., 2017).
According to local policy, all pigs in infected small farms must be culled, while neighbouring pigs in small farms without any suspected infections are not culled but are closely monitored until the outbreak is resolved. After culling, farmers receive different compensation rates based on the weight of breeding sows/boars and other pigs. However, farmers have a tendency to hide or postpone the reporting of suspicious cases (dead or alive pigs) to authorities. There are several reasons for this. Firstly, the symptoms of ASF are not clearly distinguishable from other diseases (e.g. Classical swine fever), particularly at the early clinical stages. Most smallholder farmers also do not fully vaccinate their pigs; therefore, it is easy for them to assume that their pigs are ill or have died from diseases other than ASF. Secondly, farmers fear the loss of all their pigs once a single pig becomes infected with ASF virus, because all pigs kept in the same pen should be depopulated. Thirdly, compensation procedures are complicated, with low compensation rates and long waiting periods, varying from several months to years depending on the availability of funds from the local authority. Lastly, farmers with infected pigs are concerned about the negative impact on their farm's reputation within their local community.
This model assumed that the whole farm became infectious if one pig was infected within the herd, which is realistic because of the highly contagious nature of the virus. There is a low probability that the ASF virus would fade out without onward transmission to other pigs in the farms. Some studies have suggested that R0 of ASF virus was estimated more than 1 in other countries (Barongo et al., 2015; Guinat et al., 2016; Iglesias et al., 2016). The limitation of this study was that individual pig farms have their own contact structures. However, in the model, contacts with the pre‐determined combination of farm types were random, within the given distance distribution, which may have resulted in an over estimation of outbreak size (especially in large farm types). Our model showed that quite a number of large farms were infected, whereas in reality, only few large farms were reported to have been affected up to now. The local‐area spread was not considered in the model. In particular, water, air and rodents may also contribute to the introduction and spread of the disease, given the fact that keeping pigs in open housing systems is a common practice of pig farms in Vietnam. Some studies have suggested that the ASF virus transmission was associated with aerosols, pest and rodent (de Carvalho Ferreira, Weesendorp, Quak, Stegeman, & Loeffen, 2013; Fasina et al., 2012; Olesen et al., 2017). In addition, our results may be influenced by the local‐area spread if some farms are in closer proximity. Therefore, in order to evaluate the impact of randomly created farm locations using QGIS, two more data were generated to make a comparison. We found that their impacts on the outcomes were negligible. In the study, the actual number of farms (especially, smallholders) in the RDD region may be much higher than the national data. Indeed, it is not easy to identify the number of smallholder farms (e.g. less than 10 pigs) in very remote rural and high mountainous areas unless farmers are willing to register. Therefore, it may be possible that the transmission of ASF virus was much faster than it was in our models.
This study offers valuable insight into how ASF virus can be transmitted via direct and indirect contact and controlled among farms under the various simulation scenarios. Our results suggest that the enforcement of movement restriction was an effective control measure as soon as the outbreaks were reported. In addition, this study provided evidence that high standards of biosecurity can contribute to the reduction of disease spread. This simulation model can be applied to other regions or countries with modified parameters. In addition, it may be useful for assessing the cost‐effective infection control and prevention strategies in the Vietnamese context through running the ‘what‐if’ scenarios related to ASF virus transmission.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
H.S.L, K.K.T and B.W. designed research. H.S.L performed research. H.S.L and K.K.T. analysed data. H.S.L, K.K.T. VNB, TLP, ANB, TDD, VTT and B.W. wrote the paper.
ETHICAL APPROVAL
Ethics approval was not required for this study as any sample collection or questionnaires from animal/human has not been gathered.
ACKNOWLEDGEMENTS
This study was funded by the CGIAR Research Program on Livestock and we thank donors who support its work through their contributions to the CGIAR Trust Fund. CGIAR is a global research partnership for a food‐secure future. Its science is carried out by 15 Research Centers in close collaboration with hundreds of partners across the globe. www.cgiar.org. We also would like to give special thanks to Mireille Ferrari for English editing.
Lee HS, Thakur KK, Bui VN, et al. A stochastic simulation model of African swine fever transmission in domestic pig farms in the Red River Delta region in Vietnam. Transbound Emerg Dis.2021;68:1384–1391. 10.1111/tbed.13802
DATA AVAILABILITY STATEMENT
All datasets supporting our findings are available from the corresponding author on reasonable request.
REFERENCES
- Barongo, M. B. , Ståhl, K. , Bett, B. , Bishop, R. P. , Fevre, E. M. , Aliro, T. , … Ssematimba, A. (2015). Estimating the basic reproductive number (R0) for African swine fever virus (ASFV) transmission between pig herds in Uganda. PLoS One, 10(5), e0125842. 10.1371/journal.pone.0125842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudon, E. , Fournié, G. , Hiep, D. T. , Pham, T. T. H. , Duboz, R. , Gély, M. , … Peyre, M. (2017). Analysis of swine movements in a province in Northern Vietnam and application in the design of surveillance strategies for infectious diseases. Transboundary and Emerging Diseases, 64, 411–424. 10.1111/tbed.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blome, S. , Gabriel, C. , & Beer, M. (2013). Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Research, 173, 122–130. 10.1016/j.virusres.2012.10.026 [DOI] [PubMed] [Google Scholar]
- Costard, S. , Mur, L. , Lubroth, J. , Sanchez‐Vizcaino, J. M. , & Pfeiffer, D. U. (2013). Epidemiology of African swine fever virus. Virus Research, 173, 191–197. 10.1016/j.virusres.2012.10.030 [DOI] [PubMed] [Google Scholar]
- DAH, V. (2020). AFRICAN SWINE FEVER IN VIETNAM Lessons learnt. [Google Scholar]
- de Carvalho Ferreira, H. C. , Weesendorp, E. , Quak, S. , Stegeman, J. A. , & Loeffen, W. L. A. (2013). Quantification of airborne African swine fever virus after experimental infection. Veterinary Microbiology, 165, 243–251. 10.1016/j.vetmic.2013.03.007 [DOI] [PubMed] [Google Scholar]
- Dixon, L. K. , Escribano, J. M. , Martins, C. , Rock, D. L. , Salas, M. L. , & Wilkinson, P. J. (2005). Asfarviridae. In Fauquet C. M., Mayo M. A., Maniloff J., Desselberger U., & Ball L. A. (Eds.). Virus Taxon. eighth Rep. Int. Comm. Taxon. Viruses (pp. 135–143). London, UK: Elsevier, Acad. Press. [Google Scholar]
- Dixon, L. K. , Sun, H. , & Roberts, H. (2019). African swine fever. Antiviral Research, 165, 34–41. [DOI] [PubMed] [Google Scholar]
- FAO (2018). African swine fever threatens people's Republic of China. [Google Scholar]
- FAO (2020). ASF situation in Asia update [Online] Retrieved from http://www.fao.org/ag/againfo/programmes/en/empres/ASF/situation_update.html [Google Scholar]
- Fasina, F. O. , Agbaje, M. , Ajani, F. L. , Talabi, O. A. , Lazarus, D. D. , Gallardo, C. , … Bastos, A. D. S. (2012). Risk factors for farm‐level African swine fever infection in major pig‐producing areas in Nigeria, 1997–2011. Preventive Veterinary Medicine, 107, 65–75. 10.1016/j.prevetmed.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Francis, J. , Klotz, G. , Harvey, N. , & Stacey, D. (2010). Modeling and support tools for studying disease spread in livestock using networks. [Google Scholar]
- Galindo, I. , & Alonso, C. (2017). African swine fever virus: A review. Viruses, 9, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GSO (2018). Pig population by province. [Google Scholar]
- GSO (2020). General statistics office of Viet Nam: Statistical data: Administrative unit, land and climate. http://www.gso.gov.vn [Online] Retrieved from https://www.gso.gov.vn/default_en.aspx?tabid=778 [Google Scholar]
- Guinat, C. , Gubbins, S. , Vergne, T. , Gonzales, J. L. , Dixon, L. , & Pfeiffer, D. U. (2016). Experimental pig‐to‐pig transmission dynamics for African swine fever virus, Georgia 2007/1 strain. Epidemiology and Infection, 144, 25–34. 10.1017/S0950268815000862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, N. , Reeves, A. , Schoenbaum, M. A. , Zagmutt‐Vergara, F. J. , Dubé, C. , Hill, A. E. , … Salman, M. D. (2007). The North American animal disease spread model: A simulation model to assist decision making in evaluating animal disease incursions. Preventive Veterinary Medicine, 82, 176–197. 10.1016/j.prevetmed.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Iglesias, I. , Muñoz, M. J. , Montes, F. , Perez, A. , Gogin, A. , Kolbasov, D. , & De la Torre, A. (2016). Reproductive ratio for the local spread of African swine fever in wild boars in the Russian Federation. Transboundary and Emerging Diseases, 63, e237–e245. 10.1111/tbed.12337 [DOI] [PubMed] [Google Scholar]
- Keeling, M. J. (2005). Models of foot‐and‐mouth disease. Proceedings of the Royal Society B: Biological Sciences, 272, 1195–1202. 10.1098/rspb.2004.3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapar, M. L. , Binh, V. T. , & Ehui, S. K. (2003). Identifying barriers to entry to livestock input and output markets in Southeast Asia. Rome, Italy: FAO. [Google Scholar]
- Lee, H. S. , Bui, V. N. , Nguyen, H. X. , Bui, A. N. , Hoang, T. D. , Nguyen‐Viet, H. , … Wieland, B. (2020). Seroprevalences of multi‐pathogen and description of farm movement in pigs in two provinces in Vietnam. BMC Veterinary Research, 16, 15. 10.1186/s12917-020-2236-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. S. , Thakur, K. K. , Bui, V. N. , Bui, A. N. , Van Dang, M. , & Wieland, B. (2019). Simulation of control scenarios of porcine reproductive and respiratory syndrome in Nghe An Province in Vietnam. Transboundary and Emerging Diseases, 66, 2279–2287. 10.1111/tbed.13278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Ren, Z. , Wang, Q. , Ge, S. , Liu, Y. , Liu, C. , … Bao, J. (2019). Infection of African swine fever in wild boar, China, 2018. Transboundary and Emerging Diseases, 66, 1395–1398. 10.1111/tbed.13114 [DOI] [PubMed] [Google Scholar]
- Metwally, S. , Mohamed, F. , Faaberg, K. , Burrage, T. , Prarat, M. , Moran, K. , … Carrillo, C. (2010). Pathogenicity and molecular characterization of emerging porcine reproductive and respiratory syndrome virus in Vietnam in 2007. Transboundary and Emerging Diseases, 57, 315–329. 10.1111/j.1865-1682.2010.01152.x [DOI] [PubMed] [Google Scholar]
- Morris, R. S. , Wilesmith, J. W. , Stern, M. W. , Sanson, R. L. , & Stevenson, M. A. (2001). Predictive spatial modelling of alternative control strategies for the foot‐and‐ mouth disease epidemic in great britain, 2001. Acta Horticulturae, 566, 337–347. 10.17660/ActaHortic.2001.566.43 [DOI] [PubMed] [Google Scholar]
- Nga, N. T. D. , Ninh, H. N. , Van Hung, P. , & Lapar, M. L. (2014). Smallholder pig value chain development in Vietnam: Situation analysis and trends. [Google Scholar]
- Nielen, M. , Jalvingh, A. W. , Meuwissen, M. P. M. , Horst, S. H. , & Dijkhuizen, A. A. (1999). Spatial and stochastic simulation to evaluate the impact of events and control measures on the 1997–1998 classical swine fever epidemic in The Netherlands.: II. Comparison of control strategies. Preventive Veterinary Medicine, 42, 297–317. 10.1016/S0167-5877(99)00081-1 [DOI] [PubMed] [Google Scholar]
- OIE (2020a). AFRICAN SWINE FEVER [Online] Retrieved from https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/AFRICAN_SWINE_FEVER.pdf [Google Scholar]
- OIE (2020b). Immediate notifications and follows‐up [Online] Retrieved from https://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/Diseasedistributionmap?disease_type_hidden=0&disease_id_hidden=12&selected_disease_name_hidden=African+swine+fever+%28‐+‐%29+&disease_type=0&disease_id_terrestrial=12&species_t=0&disease_id_a [Google Scholar]
- Olesen, A. S. , Lohse, L. , Boklund, A. , Halasa, T. , Gallardo, C. , Pejsak, Z. , … Bøtner, A. (2017). Transmission of African swine fever virus from infected pigs by direct contact and aerosol routes. Veterinary Microbiology, 211, 92–102. 10.1016/j.vetmic.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Thomson, G. R. (1985). The epidemiology of African swine fever: The role of free‐living hosts in Africa. [PubMed] [Google Scholar]
- Tuan, H. M. , Dewulf, J. , Postma, M. , Cuc, N. T. K. , & Dinh, N. C. (2019). Assessment of biosecurity level in pig and poultry production system in vietnam using bio‐check technology [Online] Retrieved from http://ap.fftc.agnet.org/ap_db.php?id=1069&print=1 [Google Scholar]
- Tulman, E. R. , Delhon, G. A. , Ku, B. K. , & Rock, D. L. (2009). African swine fever virus. Lesser known large dsDNA viruses (pp. 43–87). Berlin, Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Turner, J. , Bowers, R. G. , & Baylis, M. (2012). Modelling bluetongue virus transmission between farms using animal and vector movements. Scientific Reports, 2, 1–7. 10.1038/srep00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Phan Le, D. G. J. , Yoon, S.‐W. , Kwon, H.‐M. , Trinh, T. B. N. , Nguyen, T. L. , Bui, T. T. N. , … Van Tuyen, N. (2019). Outbreak of African swine fever, Vietnam, 2019. Emerging Infectious Diseases, 25, 1433. 10.3201/eid2507.190303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X. , Li, N. , Luo, Y. , Liu, Y. , Miao, F. , Chen, T. , … Tian, K. (2018). Emergence of African swine fever in China, 2018. Transboundary and Emerging Diseases, 65, 1482–1484. 10.1111/tbed.12989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets supporting our findings are available from the corresponding author on reasonable request.


