Abstract
Aim
Anastomotic leakage is a severe complication after low anterior resection (LAR) for rectal cancer and occurs in up to 20% of patients. Most research focuses on reducing its incidence and finding predictive factors for anastomotic leakage. There are no robust data on severity and treatment strategies with associated outcomes. The aims of this work were (1) to investigate the factors that contribute to severity of anastomotic leakage and to compose an anastomotic leakage severity score and (2) to evaluate the effects of different treatment approaches on prespecified outcome parameters, stratified for severity score and other leakage characteristics.
Method
TENTACLE–Rectum is an international multicentre retrospective cohort study. Patients with anastomotic leakage after LAR for primary rectal cancer between 1 January 2014 and 31 December 2018 will be included by each centre. We aim to include 1246 patients in this study. The primary outcome is 1‐year stoma‐free survival (i.e. patients alive at 1 year without a stoma). Secondary outcomes include number of reinterventions and unplanned readmissions within 1 year, total length of hospital stay, total time with a stoma, the type of stoma present at 1 year (defunctioning, permanent), complications related to secondary leakage and mortality. For aim (1) regression models will be used to create an anastomotic leakage severity score. For aim (2) the effectiveness of different treatment strategies for leakage will be tested after correction for severity score and leakage characteristics, in addition to other potential related confounders.
Conclusion
TENTACLE–Rectum will be an important step towards drawing up evidence‐based recommendations and improving outcomes for patients who experience severe treatment‐related morbidity.
Keywords: rectal cancer, anastomotic leakage, treatment
INTRODUCTION
Anastomotic leakage occurs in up to 20% of patients after low anterior resection (LAR) for rectal cancer [1, 2, 3]. It comprises a wide range of clinical entities at first presentation, from occult leakage below a defunctioning stoma to faecal peritonitis with multiple organ failure. Initially silent leaks can develop into chronic pelvic sepsis. In general, it is a severe complication associated with high morbidity, admission to intensive care (ICU), prolonged hospital stay and the need for reinterventions and readmissions. Permanent stoma rates of around 20% have been reported and this is associated with a significant impact on quality of life [4]. In addition, anastomotic leakage is reported to be independently associated with an increased risk of local recurrence and reduced long‐term survival [5].
Conventional treatment of anastomotic leakage after LAR consists of faecal diversion, achieved by a primary or secondary defunctioning stoma, and less frequently breakdown of the anastomosis with an end colostomy. Additionally, antibiotics are given and the pelvic abscess is often drained. However, up to 50% of leaks do not heal with such passive management, especially not in an irradiated field [1]. Pelvic sepsis might even persist after construction of an end colostomy. A competent sphincter, impeding adequate drainage of a presacral abscess, probably contributes to nonhealing of a leak. For this reason, management of leakage in more recent years has shifted towards more active treatment strategies [6, 7, 8, 9].
Remarkably, there is no robust evidence comparing different treatments for anastomotic leakage after LAR and there are no evidence‐based treatment algorithms. An important reason for this lack of good‐quality evidence is the clinical heterogeneity of patients with anastomotic leakage after LAR, which complicates the performance of meaningful studies. In addition, there is no generally accepted pretreatment classification of anastomotic leakage and leakage severity is currently graded according to how it is treated [10]. Although scoring anastomotic leakage by how it is treated is useful for reporting the consequences of anastomotic leakage, it is by definition unsuitable for research comparing different treatment strategies for anastomotic leakage. Moreover, it cannot be used to guide decision making when anastomotic leakage is diagnosed in a clinical setting.
Therefore, the aims of this study are (1) to investigate which factors contribute to the severity of anastomotic leakage and to use these data to compose an anastomotic leakage severity score, and (2) to evaluate the effectiveness of different treatment approaches on different prespecified outcome parameters, stratified by severity score, anatomical characteristics of the leak and timing of diagnosis.
METHOD
Study design
The TENTACLE–Rectum study is an international multicentre retrospective cohort study in which all consecutive patients who underwent LAR for rectal cancer between 1 January 2014 and 31 December 2018 and subsequently developed anastomotic leakage after LAR for rectal cancer will be included from each participating centre. Follow‐up of included patients will be for at least 1 year. The study opened in April 2020 and will recruit until March 2021. The study timeline is presented in Figure 1.
Figure 1.
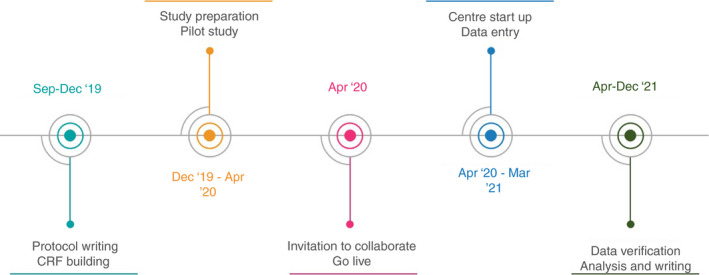
Tentacle–Rectum study timeline
TENTACLE–Rectum is open to participation by all centres that perform rectal cancer surgery. All centres are asked to fill out a questionnaire about their practice. This includes questions on hospital type, rectal cancer case‐load, LAR case‐load and available diagnostic and treatment modalities. International research networks will be asked to support this study in order to increase inclusion of patients and optimize the chance of obtaining robust results. The TENTACLE–Rectum study is an investigator‐initiated study which receives financial support from Medtronic Inc. The company is not involved in the conduct of the study or the analysis and interpretation of the data.
Study population
Inclusion criteria are as follows: (1) aged 18 years or older; (2) primary tumour with the lower border below the level of the sigmoid take‐off according to the international consensus definition of the rectum [11]; (3) LAR with primary anastomosis with or without defunctioning loop ileostomy for either (a) primary cancer, (b) completion total mesorectal excision after local excision or (c) salvage resection for regrowth after watch and wait or (d) after local excision; (4) anastomotic leakage according to the following definition: ‘a breach in a surgical join between two hollow viscera, with or without active leak of luminal contents’, being diagnosed at any time point during the first postoperative year [12].
Exclusion criteria are as follows: (1) rectal resection for benign disease; (2) rectal resection for recurrent rectal cancer after previous low anterior resection or other primary malignancies; (3) emergency resection.
Collaborators from the participating centres are provided with instructions on patient selection and data entry to ensure homogeneity in the inclusion process and data entry and to ensure that any anastomotic leakage occurring within 1 year postoperatively will be captured during screening of the medical files, including occult leaks and late leaks developing after ileostomy closure.
Study parameters
Hospital characteristics
The following hospital characteristics will be collected through a questionnaire that is sent to the coordinating investigator of all sites: hospital type (academic, nonacademic teaching, specialist); annual volume of rectal cancer resections, annual volume of restorative LARs and number of restorative LARs for rectal cancer with the lower border below the sigmoid take‐off on sagittal MRI during the period 2014–2018; number of hospital beds; diagnosis and treatment strategy depending on surgeon on call (general or colorectal surgeon); ward facilities (e.g. dedicated colorectal nurse/physician assistant); and types of diagnostic and treatment modalities that are available in the hospital.
Patient, cancer treatment and index operation characteristics
Collected patient characteristics are sex, age, height, weight, American Society of Anesthesiologists classification, Charlson comorbidity index, tumour location (distance from the anorectal junction to the lower border of the tumour on sagittal MRI), preoperative T‐stage, preoperative N‐stage, preoperative M‐stage and year of surgery. Cancer treatment characteristics include type of neoadjuvant therapy, surgical approach (e.g. minimally invasive versus open), extent of resection, level of vascular ligation and splenic flexure mobilization, type of anastomosis [e.g. configuration, hand‐sewn versus (single/double) stapled] and distance from the anal verge, and primary defunctioning stoma.
Anastomotic leakage characteristics
The following characteristics regarding diagnosis of anastomotic leakage will be recorded: time from surgery to diagnosis of the leakage (days) and modality used to diagnose anastomotic leakage. At the time of diagnosis, vital signs, leukocyte count, C‐reactive protein, serum albumin and creatinine will be recorded (parameters within 24 h of the test that first diagnosed the leak are used).
Characteristics of anastomotic leakage are as follows: location of the leak (e.g. dorsal versus ventral, blind loop versus anastomosis), fistulation (e.g. vagina, perineum), estimated proportion of the circumference, presence of ischaemia and retraction of the afferent colon, contaminated spaces and degree of contamination, and drains in place at diagnosis with their corresponding location.
Anastomotic leakage treatment characteristics
Characteristics of treatment for anastomotic leakage include admission to intensive care or high‐dependency care, the need for emergency surgery, reoperation approach (e.g. minimally invasive or open), type of reoperation (e.g. secondary defunctioning stoma, drainage, suturing of the anastomosis, breakdown of the anastomosis), endoscopic vacuum‐assisted drainage, percutaneous drainage and transanal drainage.
Outcome parameters
The primary outcome parameter is 1‐year stoma‐free survival. Secondary outcomes include the following: length of stay in ICU, mortality, comprehensive complications index [13], total number of reinterventions (surgical, radiological, endoscopic) within 1 year, total number of unplanned readmissions within 1 year, total length of hospital stay during 1 year, total time of having a stoma during the first year, stoma present at 1 year, type of stoma present at 1 year (defunctioning, permanent), secondary leakage‐related complications (extrapelvic abscess, cutaneous fistula, vaginal fistula, bladder fistula, small bowel fistula and ureteric fibrosis with hydronephrosis).
Sample size calculation
For study aim (1), creation of a risk score with 20 candidate predictors with a 1‐year stoma‐free survival rate of 70% and a Nagelkerke R 2 of 0.15 requires a total of 1097 patients with anastomotic leakage. Tor compare the effectiveness of different treatment strategies (study aim 2), a relative difference of 25% in 1‐year stoma‐free survival is considered clinically significant, which corresponds to an absolute difference of 7.5% based on an expected 1‐year stoma‐free survival of 70%. With a power of 0.80 and a significance level of 0.05, a total of 1246 patients are needed to detect this difference.
Pilot study
After the study protocol and online case report form (CRF) were developed, we invited a panel of international experts on anastomotic leakage and rectal cancer surgery from eight countries to participate in a pilot (Table 1). The international steering committee was asked to contribute five patients to the online CRF and provide feedback on the protocol and CRF. The feedback was used to refine the protocol and CRF before finalizing these, to ensure international consensus and clarity on the use of the CRF and definitions. The feedback was evaluated by the study group and implemented if it was deemed relevant. The pilot was performed from January 2020 to April 2020 (Figure 1).
Table 1.
TENTACLE Rectum international steering committee
| Principal Investigator | Hospital | City | Country |
|---|---|---|---|
| Albert Wolthuis | UZ Leuven | Leuven | Belgium |
| F Borja de Lacy | Hospital Clinic de Barcelona | Barcelona | Spain |
| Hans de Wilt | Radboudumc | Nijmegen | The Netherlands |
| Jeremy Lefevre | Hopitaux de Paris | Paris | France |
| Michael Solomon | University of Sydney Central Clinical School | Sydney | Australia |
| Matteo Frasson | Valencia University Hospital La Fe | Valencia | Spain |
| Nicolas Rotholtz | Hospital Alemán, Buenos Aires | Buenos Aires | Argentina |
| Pieter Tanis | Amsterdam UMC | Amsterdam | The Netherlands |
| Quentin Denost | Bordeaux University Hospital | Bordeaux | France |
| Rodrigo Perez | University of Sao Paulo | Sao Paolo | Brazil |
| Tsuyoshi Konishi | Cancer Institute Hospital Tokyo | Tokyo | Japan |
| Yves Panis | Hôpital Beaujon | Paris | France |
Data handling and regulatory considerations
Data will be collected in an online CRF using the Castor database system (https://www.castoredc.com/). This online medical research database system is certified to meet international security standards and is compliant with all relevant regulations, amongst which are ICH‐GCP, GDPR, HIPAA, FDA 21 CFR part 11, ISO 27001 and ISO 9001. All entered data are only visible to collaborators from the same hospitals. Only the coordinating investigators, lead investigators and principal investigator will have access to data from the full study.
All pseudo‐anonymized patient data will be entered by or under the supervision of the treating physician(s). Each patient will be coded with a unique patient number before being entered into the database. Surgeons who participate in the TENTACLE–Rectum study keep a password‐coded file that can identify individual patients and which will be locked away in their practice. This file can be accessed by the local investigators if needed, for example in case a relevant new research question requires entry of additional data into the database. Up to four users per participating centre will receive a Castor account and can enter data into the database.
Access to data
Access to data may be requested by appropriately qualified researchers who have relevant questions. Proposals for additional research questions may be submitted by collaborators to the TENTACLE–Rectum steering group who will review their relevance and appropriateness. Data will only be transferred if appropriate ethical and data transfer agreements are in place.
Data verification and data validation
After the inclusion of new cases closes, data verification aims to increase the quality and completeness of the data. Data verification includes checking the data for inconsistencies and flagging parameters with substantial missing data that are deemed likely to be recorded in the medical files by the study team. This is fed back to the local investigators and they will have the opportunity to complete or adjust their data. After data verification, we aim to validate a core parameter set for 10–20% of the inclusions using local data validators who will be recruited independently of the original study team.
Statistical analysis
Statistical protocols have been drafted with a biostatistician (GH) with experience in setting up international multicentre collaborative studies. Prespecified analytical plans have been drafted for each of the main aims of the study.
Main study aim (1)
First, univariable analysis will be performed on variables that are considered to be potentially relevant for an anastomotic leakage severity score, i.e. parameters that are available at the time of diagnosis. Anastomotic leakage characteristics and patient characteristics are of particular interest for this. Second, factors that are considered to be clinically relevant based on literature and/or expert opinion will be selected for multivariable analysis. Backwards selection will be used to exclude values of P ≥ 0.05 from the competing risks model. Results will be presented as odds ratio (OR) with 95% confidence intervals (CIs). Third, the multivariable competing risks model will be internally validated by bootstrapping, using 5000 bootstrap resamples. Finally, a nomogram will be constructed based on the final bootstrapped multivariable regression analysis.
If the casemix is found to be very strongly associated with outcome relative to the severity score (to the extent that the severity score is of limited additional value in the regression model), latent class analysis will be used [14]. The parameters included in the anastomotic leakage severity score will be used to create casemix‐corrected classes of anastomotic leakage severity.
Main study aim (2)
In this analysis, the different treatment modalities are the exposures. The association between anastomotic index operation characteristics, leakage characteristics and outcome parameters will be evaluated for the exposures in regression analysis. Correction for patient characteristics, tumour characteristics and anastomotic leakage severity score will be performed.
Based on the results of this first analysis, subgroups of patients will be created based on their individual index operation and leakage characteristics or based on a combination of characteristics. The effectiveness of treatment strategies for anastomotic leakage will be assessed in regression models for the different outcome parameters and corrected for patient characteristics, tumour characteristics and anastomotic leakage severity score, if appropriate. Comparison of the primary outcome parameter and secondary outcome parameters will be expressed in terms of a OR and corresponding 95% CIs.
Ethical considerations
This study will be conducted in compliance with the principles of the Declaration of Helsinki. The study protocol and relevant documents have been approved by the medical ethical committee of the Radboud University Medical Center, Nijmegen, The Netherlands. All participating centres are provided with the study protocol and relevant documents. The need for ethical approval is underlined by the study coordinators to each of the participating centres, but because of the international study design, the exact format of local ethical approval is left to the discretion of the participating centre as this may vary. The TENTACLE–Rectum study has been registered on Clinicaltrials.gov (NCT 04127734). The full study protocol can be accessed at https://www.tentaclestudy.com/.
Publications
We aim to publish two main manuscripts covering the results for the main aims of our study. These will be submitted to peer‐reviewed journals. The TENTACLE–Rectum study embraces corporate authorship, and a maximum of four collaborators per centre that contributes to this study will form the TENTACLE–Rectum collaborative study group. This group will be part of all publications in which TENTACLE–Rectum study data are used.
The protocol writing committee is fully involved in conducting this study and will be included as authors in the main publications in which the TENTACLE–Rectum study data are used.
DISCUSSION
Anastomotic leakage remains a frequent and severe complication after rectal cancer surgery. Although previous research has mainly focused on incidence and has established (amendable) risk factors for anastomotic leakage [15, 16, 17], the optimal treatment for anastomotic leakage after LAR is unknown. There are several explanations for this observation. Treatment of anastomotic leakage often takes place in the emergency setting, is chosen on a case‐by‐case basis depending on several patient and surgical factors and is influenced by the preferences and expertise of the surgeon involved. In addition, the number of patients per centre is relatively small, despite the fact that anastomotic leakage is one of the most frequent complications after LAR. Actually, anastomotic leakage is a low‐volume heterogeneous disease entity with high complexity. This has probably hampered the initiation of standardized institutional treatment protocols and the design of prospective studies. The clinical heterogeneity of patients with anastomotic leakage and the wide variety of treatment approaches results in several clinically relevant subgroups, and this complicates the interpretation and generalizability of the small underpowered individual studies. Finally, some misperceptions might have contributed to the paucity of available evidence in this field. These misperceptions include overestimation of spontaneous healing of an anastomotic leakage and underestimation of late anastomotic problems as a consequence of a longstanding sealed abscess.
Anastomotic leakage is currently classified based on how it is treated [10, 18], but this classification cannot, by definition, be used for research on what treatments are most effective. Therefore, a classification of the severity of leakage should be based on pretreatment characteristics, such as the anastomotic leakage severity score that we aim to create. Such a score is needed to enable meaningful research on the effectiveness of treatment strategies for anastomotic leakage and support clinicians in deciding how to treat individual patients with anastomotic leakage.
The main strengths of the present study are the high level of detail of the collected data and the large number of patients we aim to include. This large number of patients is needed to perform regression analysis with a high number of factors, and this facilitates subgroup analyses for distinct clinical entities that we might identify. If more patients are included, even more detailed statistical models can be built to accommodate clinical heterogeneity. The inclusion of a large number of patients is made possible by the international collaborative nature of this study, which at the same time contributes to the generalizability of results to other populations. The pilot study that was performed with core collaborators from different continents ensured that the CRF also includes parameters that are important to other geographical regions and that definitions are clear for all collaborators worldwide.
Perhaps the most important limitation is the retrospective nature of the study. Because of the large number of patients needed to achieve our main aims a prospective study was considered to be unfeasible. The data that are generated in this study can be used to inform what factors it is important to incorporate in future prospective studies, preferably also including quality of life endpoints. Another limitation is that confounding by indication (i.e. patients who receive a particular type of treatment are inherently different from other patients) may occur. However, the absence of quality data on the effectiveness of treatments for anastomotic leakage has led to a wide variety of treatment options that can be used for any given patient. In this case, regression analysis of detailed parameters in a large cohort of patients is expected to accommodate most of this possible bias. Although we recognize that this study will not answer all the questions regarding treatment for anastomotic leakage we believe it will generate valuable data from a unique dataset and hope it will serve as a solid basis for future studies.
An important aspect of this study is investigating whether clinical leakage entities can be found for which some types of treatment are generally more effective than others. These hypothesis‐generating analyses could lead to a more personalized approach to treatment for anastomotic leakage. For example, a type of anastomotic leakage that was specifically addressed when designing this study is occult or minimal symptomatic leakage below a defunctioning stoma that was constructed at the index surgery. Such a leak often appears to be healed during assessment of the anastomosis a few months later but might subsequently reactivate after restoration of continuity. These leakages, the incidence of which is likely to be underreported [19, 20, 21], can ultimately have severe consequences (e.g. chronic pelvic sepsis, fistulation) despite initially presenting as ‘silent’ leaks. This probably needs more proactive management from the beginning, and probably a different type of treatment strategy in the case of persistent nonhealing later on. Conducting an international multicentre cohort study of the intended size may therefore also provide an opportunity to study several clinically rare, but important, subgroups.
CONCLUSION
The TENTACLE–Rectum study is a large international collaborative study that will investigate which factors contribute to severity of anastomotic leakage and evaluate treatment efficacy for different relevant subgroups, different clinical settings and different treatment modalities.
CONFLICTS OF INTEREST
The authors declare to have no conflicts of interest with regard to the present manuscript.
ETHICAL APPROVAL
The study protocol and relevant documents have been approved by the medical ethical committee of the Radboud University Medical Center, Nijmegen, The Netherlands. The need for central informed consent was waived, but local ethical requirements may differ according to geographical region, country or hospital.
FvW and KT contributed equally to this manuscript.
Study registration: this study is registered at clinicaltrials.gov (NCT 04127734).
FUNDING INFORMATIONThe TENTACLE–Rectum study is financially supported by Medtronic. The company is not involved in the conduct or analysis of the study or the interpretation of the data.
DATA AVAILABILITY STATEMENT
Access to data may be requested by appropriately qualified researchers who have relevant questions. Proposals for additional research questions may be submitted by collaborators to the TENTACLE–Rectum steering group who will review their relevance and appropriateness. Data will only be transferred if appropriate ethical and data transfer agreements are in place.
REFERENCES
- 1. Borstlap WAA, Westerduin E, Aukema TS, Bemelman WA, Tanis PJ. Anastomotic leakage and chronic presacral sinus formation after low anterior resection: results from a large cross‐sectional study. Ann Surg. 2017;266(5):870–7. [DOI] [PubMed] [Google Scholar]
- 2. Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, et al. Incidence and risk factors for anastomotic failure in 1594 patients treated by transanal total mesorectal excision: results from the international TaTME registry. Ann Surg. 2019;269(4):700–11. [DOI] [PubMed] [Google Scholar]
- 3. Hain E, Maggiori L, Manceau G, Mongin C, Prost à la Denise J, Panis Y. Oncological impact of anastomotic leakage after laparoscopic mesorectal excision. Br J Surg. 2017;104(3):288–95. [DOI] [PubMed] [Google Scholar]
- 4. Vonk‐Klaassen SM, de Vocht HM, den Ouden ME, Eddes EH, Schuurmans MJ. Ostomy‐related problems and their impact on quality of life of colorectal cancer ostomates: a systematic review. Qual Life Res. 2016;25(1):125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kulu Y, Tarantio I, Warschkow R, Kny S, Schneider M, Schmied BM, et al. Anastomotic leakage is associated with impaired overall and disease‐free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg Oncol. 2015;22(6):2059–67. [DOI] [PubMed] [Google Scholar]
- 6. Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier‐Konrad B, Morel P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis. 2008;23(3):265–70. [DOI] [PubMed] [Google Scholar]
- 7. Blumetti J, Abcarian H. Management of low colorectal anastomotic leak: Preserving the anastomosis. World J Gastrointest Surg. 2015;7(12):378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boyce SA, Harris C, Stevenson A, Lumley J, Clark D. Management of low colorectal anastomotic leakage in the laparoscopic era: more than a decade of experience. Dis Colon Rectum. 2017;60(8):807–14. [DOI] [PubMed] [Google Scholar]
- 9. Creavin B, Ryan ÉJ, Kelly ME, Moynihan A, Redmond CE, Ahern D, et al. Minimally invasive approaches to the management of anastomotic leakage following restorative rectal cancer resection. Colorectal Dis. 2019;21(12):1364–71. [DOI] [PubMed] [Google Scholar]
- 10. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D'Souza N, de Neree tot Babberich MPM, d’Hoore A, Tiret E, Xynos E, Beets‐Tan RGH, et al. Definition of the rectum: an international, expert‐based Delphi consensus. Ann Surg. 2019;270(6):955–9. [DOI] [PubMed] [Google Scholar]
- 12. Peel AL, Taylor EW. Proposed definitions for the audit of postoperative infection: a discussion paper. Surgical Infection Study Group. Ann R Coll Surg Engl. 1991;73(6):385–8. [PMC free article] [PubMed] [Google Scholar]
- 13. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1–7. [DOI] [PubMed] [Google Scholar]
- 14. Rabe‐Hesketh S, Skrondal A. Classical latent variable models for medical research. Stat Methods Med Res. 2008;17(1):5–32. [DOI] [PubMed] [Google Scholar]
- 15. Qu H, Liu Y, Bi DS. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta‐analysis. Surg Endosc. 2015;29(12):3608–17. [DOI] [PubMed] [Google Scholar]
- 16. van Rooijen SJ, Huisman D, Stuijvenberg M, Stens J, Roumen RMH, Daams F, et al. Intraoperative modifiable risk factors of colorectal anastomotic leakage: why surgeons and anesthesiologists should act together. Int J Surg. 2016;36(Pt A):183–200. [DOI] [PubMed] [Google Scholar]
- 17. Sciuto A, Merola G, De Palma GD, Sodo M, Pirozzi F, Bracale UM, et al. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol. 2018;24(21):2247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH, et al. Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest Surg. 2016;20(12):2035–51. [DOI] [PubMed] [Google Scholar]
- 19. Arumainayagam N, Chadwick M, Roe A. The fate of anastomotic sinuses after total mesorectal excision for rectal cancer. Colorectal Dis. 2009;11(3):288–90. [DOI] [PubMed] [Google Scholar]
- 20. Fong SS, Chen K, Sim R. Chronic anastomotic sinus after low anterior resection: when can the defunctioning stoma be reversed? Colorectal Dis. 2011;13(6):644–9. [DOI] [PubMed] [Google Scholar]
- 21. Sloothaak DA, Buskens CJ, Bemelman WA, Tanis PJ. Treatment of chronic presacral sinus after low anterior resection. Colorectal Dis. 2013;15(6):727–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to data may be requested by appropriately qualified researchers who have relevant questions. Proposals for additional research questions may be submitted by collaborators to the TENTACLE–Rectum steering group who will review their relevance and appropriateness. Data will only be transferred if appropriate ethical and data transfer agreements are in place.


