Abstract
Laparoscopic living donor hepatectomy for transplantation has been well established over the past decade. This study aimed to assess its safety and feasibility in pediatric living donor liver transplantation (LDLT) by comparing the surgical and long‐term survival outcomes on both the donor and recipient sides between open and laparoscopic groups. The medical records of 100 patients (≤17 years old) who underwent ABO‐compatible LDLT using a left lateral liver graft between May 2008 and June 2016 were analyzed. A total of 31 donors who underwent pure laparoscopic hepatectomy and their corresponding recipients were included in the study; 69 patients who underwent open living donor hepatectomy during the same period were included as a comparison group. To overcome bias from the different distributions of covariables among the patients in the 2 study groups, a 1:1 propensity score matching analysis was performed. The mean follow‐up periods were 92.9 and 92.7 months in the open and laparoscopic groups, respectively. The mean postoperative hospital stay of the donors was significantly shorter in the laparoscopic group (8.1 days) than in the open group (10.6 days; P < 0.001). Overall, the surgical complications in the donors and overall survival rate of recipients did not differ between the groups. Our data suggest that the laparoscopic environment was not associated with long‐term graft survival during pediatric LDLT. In addition, the laparoscopic approach for the donors did not adversely affect the corresponding recipient's outcome. Laparoscopic left lateral sectionectomy for living donors is a safe, feasible, and reproducible procedure for pediatric liver transplantation.
Abbreviations
- BMI
body mass index
- BSA
body surface area
- HVOO
hepatic venous outflow obstruction
- INR
international normalized ratio
- LDH
laparoscopic donor hepatectomy
- LDLT
living donor liver transplantation
- LFT
liver function test
- LGA
left gastric artery
- LHA
left hepatic artery
- ODH
open donor hepatectomy
- PELD
pediatric end‐stage liver disease
- PSM
propensity score matching
- RHA
right hepatic artery
- SMA
superior mesenteric artery
Liver transplantation is the most definitive treatment for children with end‐stage liver disease. In general, the indications for liver transplantation in children include cholestatic disease, metabolic disorders, malignancy, chronic hepatitis, and acute liver failure attributed to viral infections or the effects of medication.
Progress in the management of immunosuppressive therapy suitable for children has been of key importance in improving survival rates after transplant procedures. The use of variant allografts has helped in overcoming the shortage of suitable donors for children. The evolution of donor methods and surgical techniques in graft implantation has also contributed to the success rate of pediatric living donor liver transplantation (LDLT).( 1 , 2 , 3 )
After the introduction of laparoscopic hepatectomy, a sufficient number of case studies have demonstrated that this procedure has similar survival outcomes as laparotomy. Thus, laparoscopic hepatectomy has been vigorously applied in the field of transplantation, beginning approximately 10 years ago until its current active implementation in various centers.( 4 , 5 , 6 )
Cherqui et al.( 7 ) first described laparoscopic left lateral sectionectomy for an adult‐to‐pediatric donation, and the procedure is now considered an acceptable practice. However, because pediatric LDLT is performed only at highly specialized centers, data on the safety of laparoscopic procedures for liver donation have not been accumulated. Therefore, we aimed to assess the safety of the procedure in children, including the surgical complications and survival outcomes after LDLT using a left lateral liver graft, to evaluate and compare pure laparoscopic donor hepatectomy (LDH) and open donor hepatectomy (ODH) in a high‐volume LDLT center. In addition, the long‐term graft survival outcome was also investigated to determine the effects of the laparoscopic environment represented by carbon dioxide pneumoperitoneum.
Patients and Methods
A retrospective, observational, and single‐center study was conducted after obtaining written informed consent from each patient and acquiring approval from the ethics committee of Asan Medical Center. We analyzed the medical records of 100 patients (≤17 years old) who underwent ABO‐compatible LDLT between May 2008 and June 2016. Of the 100 patients, 69 underwent ODH, and 31 underwent pure LDH.
Pediatric LDLT using an open approach was first performed at Asan Medical Center in December 1994. A total of 133 cases of pediatric LDLT using an open donor liver graft were performed from December 1994 to December 2007. Later, with the accumulation of experience with laparoscopic surgery, LDH began to be used for pediatric LDLT in 2008. Donors were provided a thorough explanation of the advantages and disadvantages of ODH and LDH, after which they decided on their preferred type of operation. No specific exclusion criteria have been established since then, and anatomical abnormalities such as a left hepatic artery arising from the left gastric artery and a right posterior bile duct draining into the left hepatic duct have been shown not to interfere with the laparoscopic approach.
To minimize time bias, we collected and compared data from 2008 (when LDH was initially implemented) onward. In addition, to overcome bias from the different distributions of covariables among the patients in the 2 study groups, a propensity score matching (PSM) analysis was performed in a 1:1 ratio using the nearest‐neighbor matching method for the following variables: diagnosis; recipient age, sex, body mass index (BMI), and body surface area (BSA); and donor sex, age, and BMI. Our study was approved by the Asan Medical Center institutional ethical board (approval no. 2020‐0723).
Donor Preparation
The donor's preoperative examination was performed twice in total. In the first stage, blood tests as well as a liver function test (LFT) and a computed tomography scan to measure the graft volume were performed. Subsequently, in the second stage, magnetic resonance cholangiography and the indocyanine green retention test after 15 minutes were performed to determine the detailed anatomy and accurate liver function. Liver biopsy was not performed if the donor's age was <30 years, the LFT result was normal, and mild fatty liver was suspected on the basis of imaging findings. When necessary, sonography‐guided percutaneous liver biopsy was performed to obtain liver tissue and performed twice on the right and left lobes. A hepatobiliary scan was performed routinely on the fifth day after the operation. According to International Study Group of Liver Surgery classification,( 8 ) grade B (bile leakage requiring a change in patient clinical management [additional diagnostic or interventional procedures] but manageable without relaparotomy or a grade A bile leakage lasting for >1 week) was considered a surgical complication.
Immunosuppression
The immunosuppressive protocol used at our institute includes tacrolimus, mycophenolate mofetil, and steroids. Basiliximab was administered at 12 mg/m2 on posttransplant days 0 and 4. Tacrolimus was given at 0.0375 mg/kg twice a day and started on posttransplant day 1. Mycophenolate mofetil therapy was started preoperatively at 15 mg/kg a day and was continued for 6 months. Steroid administration was started intraoperatively and continued for 3 months with a lower dose.
Open Left Lateral Donor Hepatectomy
ODH was performed under general anesthesia with the patients in the supine position. Exploratory laparotomy was performed using a J‐shaped or midline skin incision, and the left lateral section was drawn to the left of the round ligament after the dissection of the left triangular ligament. In a separate procedure on the hilar vasculature, the left hepatic artery and portal vein were exposed and isolated, with each vessel looped after dissection of the connective tissue. The hepatic parenchyma was divided along the right side of the falciform ligament by using an ultrasonic aspirator (Cavitron Ultrasonic Surgical Aspiratior [CUSA] Excel; Valleylab, Boulder, CO), and the pedicles to segment IV were divided without the Pringle maneuver. After the transection of the parenchyma, intraoperative cholangiography was used to divide the left hepatic duct between 2 radio‐opaque rubber bands, tagged transversely on the proposed dividing site of the left hepatic duct by holding sutures. After the infusion of 5000 units of heparin, the left hepatic vein was divided using a vascular clamp, and the stump was closed with a 5‐0 Prolene continuous suture (Ethicon, Johnson & Johnson, Somerville, NJ). Finally, the left lateral liver graft was procured at the completion of the recipient's surgery.
The graft was flushed on the back table with 1 or 2 L of a histidine‐tryptophan‐ketoglutarate solution (Odyssey Pharmaceuticals, East Hanover, NJ) through the left portal vein.
Laparoscopic Left Lateral Donor Hepatectomy
The surgical technique was similar to those described previously with few modifications.( 9 ) The left hepatic artery and portal vein were dissected and encircled with 2 vessel loops. The Pringle maneuver was used during the parenchymal transection. The liver was transected using an alternating combination of a laparoscopic ultrasonic aspirator (CUSA Excel) and THUNDERBEAT (Olympus, Tokyo, Japan). The hepatic parenchyma was divided along the right side of the falciform ligament, and the pedicles to segment IV were divided. When the liver division reached the left hepatic vein, the left lateral sector was surrounded by a Jackson‐Pratt drain line, which was passed under the left hepatic vein, portal vein, and hepatic artery, for a liver hanging maneuver.
The left bile duct was identified after intraoperative cholangiography using a mobile C‐arm in which contrast was infused via the cystic duct through a cobra tube (Torcon NB Advantage catheter; Cook Medical, Bloomington, IN). After Hem‐o‐Lok (Weck Closure Systems, Research Triangle Park, NC) clipping at the target divide level, cholangiography was performed again to check whether the right duct was intact, and then the duct was resected.( 10 ) Finally, after the infusion of 5000 units of heparin, the left hepatic artery and left portal vein were clipped and divided using Hem‐o‐Lok clips. A unilateral linear stapler (30‐mm Endo TA; US Surgical, Norwalk, CT) was used to cut the left hepatic vein. The graft was placed in an endo‐bag that was inserted through a 12‐mm trocar and retrieved through a 10‐cm suprapubic incision site. After the graft was taken out of the body, a leaking test was performed using a methylene blue solution injected through a cobra tube inserted in the cystic duct. The graft was flushed on the back table with 1 or 2 L of a histidine‐tryptophan‐ketoglutarate solution (Odyssey Pharmaceuticals) through the left portal vein.
Statistical Analyses
Data with a normal distribution are reported as mean ± standard deviation. Variables not fitting a normal distribution are presented in the Result section as median (range). Continuous variables were compared using the Student t test if normally distributed; otherwise, the Mann‐Whitney U test was used.
Categorical variables were compared using the chi‐square test. The patients' overall and death‐censored graft survival rates were estimated using the Kaplan‐Meier method and compared with log‐rank tests. Data were considered statistically significant at P values of <0.05. Multivariate models were manually built using a forward strategy. Statistical analyses were performed using SPSS version 22.0 for Windows (IBM Corp., Armonk, NY).
Results
Demographic Data and Surgical Outcomes of the Donors and Recipients Before PSM
In the ODH group, the ages, preoperative ventilator use, and preoperative pediatric end‐stage liver disease (PELD) scores of the recipients were significantly different from those in the LDH group. The total ischemic time, including the warm and cold ischemic times of the graft, was not significantly different between the 2 groups. The overall surgical complication rate of the recipients also did not significantly differ between the 2 groups (Table 1).
TABLE 1.
Demographic Data and Surgical Outcomes of Recipients Before PSM
| ODH (n = 69) | LDH (n = 31) | Total (n = 100) | P Value | |
|---|---|---|---|---|
| Sex | 0.332 | |||
| Male | 36 (52.1) | 12 (38.7) | 48 (48.0) | |
| Female | 33 (47.9) | 19 (61.3) | 52 (52.0) | |
| Age, months | 51.6 ± 53.3 | 17.6 ± 27.3 | 41.8 ± 49.6 | <0.001 |
| BMI, kg/m2 | 17.2 ± 3.2 | 17.6 ± 7.1 | 17.3 ± 4.6 | 0.729 |
| BSA, m2 | 0.7 ± 0.4 | 0.5 ± 0.2 | 0.6 ± 0.4 | <0.001 |
| Prothrombin time, INR | 2.3 ± 1.3 | 1.4 ± 0.5 | 2.0 ± 1.2 | <0.001 |
| Total bilirubin, mg/dL | 15.8 ± 11.4 | 13.2 ± 9.3 | 15.0 ± 10.9 | 0.267 |
| PELD score | 21.0 ± 11.0 | 14.0 ± 7.4 | 18.9 ± 10.5 | <0.001 |
| Indication for transplant | <0.001 | |||
| Biliary atresia | 17 (27.6) | 23 (74.2) | ||
| Primary sclerosing cholangitis | 1 (1.3) | 0 (0.0) | ||
| Hepatoblastoma | 7 (13.2) | 2 (6.5) | ||
| Wilson’s disease | 6 (7.9) | 0 (0.0) | ||
| Hepatocellular carcinoma | 0 (0.0) | 1 (3.2) | ||
| Fulminant | 28 (36.8) | 4 (12.9) | ||
| Others | 10 (13.2) | 1 (3.2) | ||
| Length of operation time, minutes | 573.4 ± 100.4 | 581.2 ± 93.7 | 575.6 ± 98.2 | 0.710 |
| Surgical complication | ||||
| Biliary stricture | 0.702 | |||
| No | 67 (97.1) | 29 (93.5) | 96 (96.0) | |
| Yes | 2 (2.9) | 2 (6.5) | 4 (4.0) | |
| Biliary leakage | 0.684 | |||
| No | 66 (95.6) | 28 (90.3) | 94 (94.0) | |
| Yes | 3 (4.4) | 3 (9.7) | 6 (6.0) | |
| Hepatic artery thrombosis | 0.901 | |||
| No | 68 (98.5) | 31 (100.0) | 99 (99.0) | |
| Yes | 1 (1.5) | 0 (0.0) | 1 (1.0) | |
| HVOO | 0.459 | |||
| No | 65 (94.2) | 31 (100.0) | 96 (96.0) | |
| Yes | 4 (5.8) | 0 (0.0) | 4 (4.0) | |
| Portal vein stenosis | 0.186 | |||
| No | 64 (92.7) | 27 (87.1) | 91 (91.0) | |
| Yes | 5 (7.3) | 4 (12.9) | 9 (9.0) | |
| Rejection episode | 1.000 | |||
| No | 57 (82.6) | 24 (77.4) | 81 (81.0) | |
| Yes | 12 (17.4) | 7 (22.6) | 19 (19.0) |
Data are provided as n (%) or mean ± standard deviation.
For the donors, those in the LDH group were younger than those in the ODH group, and the postoperative hospital stay was shorter in the LDH group. The donor operation time was longer in the LDH group. The postoperative peak levels of aspartate aminotransferase, alanine aminotransferase, and total bilirubin showed no significant differences between the 2 groups. The overall postoperative complication rate was higher in the ODH group (Table 2).
TABLE 2.
Demographic Data and Surgical Outcomes of Donors and Grafts Before PSM
| ODH (n = 69) | LDH (n = 31) | Total (n = 100) | P Value | |
|---|---|---|---|---|
| Sex | 0.031 | |||
| Male | 32 (46.3) | 7 (22.6) | 39 (39.0) | |
| Female | 37 (53.7) | 24 (77.4) | 61 (61.0) | |
| Age, years | 34.8 ± 6.7 | 31.7 ± 6.0 | 33.9 ± 6.6 | 0.031 |
| BMI, kg/m2 | 23.4 ± 3.4 | 22.8 ± 3.7 | 23.2 ± 3.4 | 0.433 |
| Graft weight, g | 311.1 ± 125.1 | 265.1 ± 78.9 | 297.8 ± 115.2 | 0.025 |
| Artery anatomy | 0.088 | |||
| Classic | 59 (85.5) | 27 (87.1) | 86 (86.0) | |
| Replaced RHA from SMA | 2 (2.9) | 3 (9.7) | 5 (5.0) | |
| Replaced LHA from LGA | 5 (7.2) | 1 (3.2) | 6 (6.0) | |
| Accessory LHA from LGA | 3 (4.3) | 0 (0.0) | 3 (3.0) | |
| Portal vein anatomy | 0.422 | |||
| Type 1 | 61 (88.4) | 29 (93.5) | 90 (90.0) | |
| Type 2 | 4 (5.8) | 2 (6.5) | 6 (6.0) | |
| Type 3 | 4 (5.8) | 0 (0.0) | 4 (4.0) | |
| Bile duct anatomy* | 0.447 | |||
| Type 1 | 57 (82.6) | 28 (90.3) | 85 (85.0) | |
| Type 2 | 3 (4.3) | 0 (0.0) | 3 (3.0) | |
| Type 3b | 6 (8.6) | 3 (9.7) | 9 (9.0) | |
| Type 4b | 3 (4.3) | 0 (0.0) | 3 (3.0) | |
| Cold ischemic time, minutes | 44.6 ± 22.8 | 50.2 ± 24.1 | 46.2 ± 23.2 | 0.262 |
| Warm ischemic time, minutes † | 5.6 ± 1.9 | 6.8 ± 3.6 | 6.2 ± 2.8 | 0.780 |
| Total ischemic time, minutes | 75.2 ± 26.1 | 81.3 ± 23.2 | 77.0 ± 25.3 | 0.266 |
| Number of arteries | 0.508 | |||
| 1 | 67 (97.1) | 30 (96.8) | 97 (97.0) | |
| 2 | 2 (2.9) | 1 (3.2) | 3 (3.0) | |
| Number of bile duct openings | 0.409 | |||
| 1 | 60 (86.9) | 29 (93.5) | 89 (89.0) | |
| 2 | 9 (13.1) | 2 (6.5) | 11 (11.0) | |
| Length of operation time, minutes | 349.4 ± 55.1 | 383.0 ± 64.0 | 359.2 ± 59.5 | 0.008 |
| Length of postoperative hospital stay, days | 11.3 ± 2.4 | 8.1 ± 2.7 | 10.3 ± 2.9 | <0.001 |
| Surgical complication | 0.025 | |||
| Wound problem | 2 (2.8) | 0 (0.0) | 2 (2.0) | |
| Bile leakage | 1 (1.4) | 0 (0.0) | 1 (1.0) | |
| Pleural effusion | 2 (2.6) | 0 (0.0) | 2 (2.0) | |
| Fluid collection | 3 (3.9) | 0 (0.0) | 3 (3.0) | |
| None | 61 (88.4) | 31 (100.0) | 92 (92.0) |
Data are provided as n (%) or mean ± standard deviation.
Classification of biliary tree anatomy variation according to Varotti et al.( 26 )
Time to retrieve a graft from the abdomen.
Clinicopathological Features and Surgical Outcomes of the Recipients After PSM
After PSM, the ODH and LDH groups were well balanced (31 cases each). The mean follow‐up period was 92.9 months in the ODH group and 92.7 months in the LDH group. The most common cause of surgical treatment in both groups was biliary atresia. The mean PELD score was 18.2 in the ODH group and 14.0 in the LDH group. The cold ischemic time was 42.2 ± 19.1 minutes in the ODH group and 50.2 ± 24.1 minutes in the LDH group. The warm ischemic time was 5.6 ± 1.9 minutes in the ODH group and 6.8 ± 3.6 minutes in the LDH group.
Portal vein stenting was performed because of postoperative portal vein stenosis in 5 recipients in the ODH group and 4 recipients in the LDH group. During the follow‐up period, 5 recipients in the ODH group and 7 recipients in the LDH group experienced rejection episodes. The overall surgical complication rate of the recipients did not differ between the 2 groups (Table 3).
TABLE 3.
Clinicopathological Features and Surgical Outcomes of Recipients After PSM
| ODH (n = 31) | LDH (n = 31) | P Value | |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 12 (38.7) | 12 (38.7) | |
| Female | 19 (61.3) | 19 (61.3) | |
| Age, months | 29.7 ± 38.0 | 17.6 ± 27.3 | 0.157 |
| BMI, kg/m2 | 17.7 ± 3.1 | 17.6 ± 7.1 | 0.929 |
| BSA, m2 | 0.6 ± 0.4 | 0.5 ± 0.2 | 0.052 |
| Prothrombin time, INR | 1.9 ± 1.0 | 1.4 ± 0.5 | 0.065 |
| Total bilirubin, mg/dL | 15.0 ± 8.9 | 13.2 ± 9.3 | 0.447 |
| PELD score | 18.2 ± 11.0 | 14.0 ± 7.4 | 0.082 |
| Indication for transplant | 0.059 | ||
| Biliary atresia | 13 (41.9) | 23 (74.2) | |
| Primary sclerosing cholangitis | 0 (0.0) | 0 (0.0) | |
| Hepatoblastoma | 6 (19.4) | 2 (6.5) | |
| Wilson’s disease | 1 (3.2) | 0 (0.0) | |
| Hepatocellular carcinoma | 0 (0.0) | 1 (3.2) | |
| Fulminant | 7 (22.6) | 4 (12.9) | |
| Others | 4 (12.9) | 1 (3.2) | |
| Length of operation time, minutes | 577.8 ± 87.2 | 581.2 ± 93.7 | 0.884 |
| Surgical complication | |||
| Biliary stricture | 0.902 | ||
| No | 30 (96.8) | 29 (93.5) | |
| Yes | 1 (3.2) | 2 (6.5) | |
| Biliary leakage | 0.237 | ||
| No | 31 (100.0) | 28 (90.3) | |
| Yes | 0 (0.0) | 3 (9.7) | |
| Hepatic artery thrombosis | 1.000 | ||
| No | 31 (100.0) | 31 (100.0) | |
| Yes | 0 (0.0) | 0 (0.0) | |
| HVOO | 1.000 | ||
| No | 31 (100.0) | 31 (100.0) | |
| Yes | 0 (0.0) | 0 (0.0) | |
| Portal vein stenosis | 0.906 | ||
| No | 26 (83.9) | 27 (87.1) | |
| Yes | 5 (16.1) | 4 (12.9) | |
| Rejection episode | 0.748 | ||
| No | 26 (83.9) | 24 (77.4) | |
| Yes | 5 (16.1) | 7 (22.6) |
Data are provided as n (%) or mean ± standard deviation.
Two children in the ODH group and 1 child in the LDH group died during the follow‐up period. In the ODH group, both children died after graft dysfunction secondary to a rejection episode. In the mortality case in the LDH group, the patient died of uncontrolled sepsis. The overall recipient mortality rates were 6.4% (n = 2) and 3.2% (n = 1) in the ODH and LDH groups, respectively. The 1‐, 3‐, and 5‐year overall survival rates were consistently 93.6% for all time points in the ODH group, and 96.8%, 93.6%, and 93.6% in the LDH group (P = 0.557). The 1‐, 3‐, and 5‐year death‐censored graft survival rates were 93.6%, 93.6%, and 93.6% in the ODH group and 100%, 100%, and 100% in the LDH group, respectively (P = 0.157; Fig. 1).
FIG. 1.
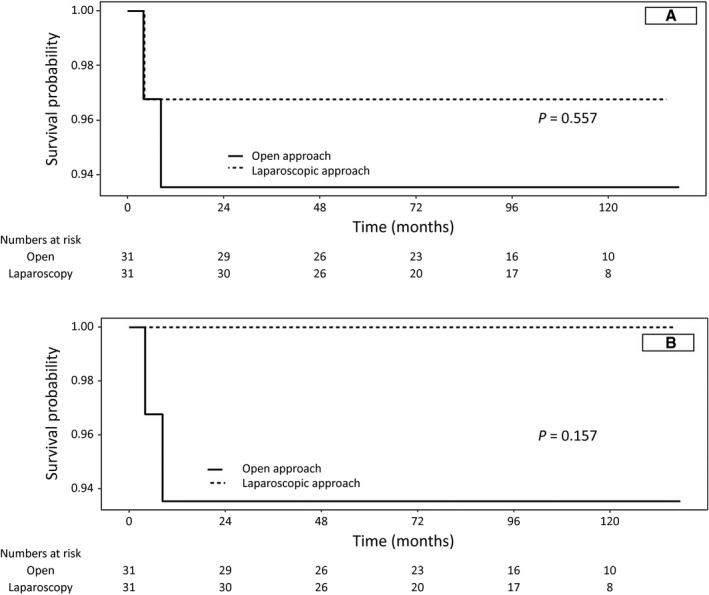
Overall (A) and graft survival rates (B) in the 2 groups.
Perioperative and Postoperative Surgical Outcomes of the Donors and Grafts After PSM
A replaced right hepatic artery arising from the superior mesentery artery was observed in 1 patient in the ODH group and 3 patients in the LDH group. In contrast, a replaced left hepatic artery arising from the left gastric artery was observed in 2 patients in the ODH group and 1 patient in the LDH group. A type 2 portal vein was observed in 3 patients in the ODH group and 3 patients in the LDH group, whereas a type 3 portal vein was observed in 2 patients in the ODH group and in 1 patient in the LDH group. In terms of the biliary system, types 2 (trifurcation) and 4b branching (the right posterior duct into the common hepatic duct) were observed in 2 cases and 1 case in the ODH group, respectively, whereas type 3b branching (the right posterior duct into the left hepatic duct) was observed in 3 cases in each group.
The mean operation time was 360.1 minutes in the ODH group and 383.0 minutes in the LDH group, with no significant difference between the 2 groups (P = 0.146).
The postoperative peak aspartate aminotransferase, alanine aminotransferase, and bilirubin levels were not different between the 2 groups. However, the postoperative hospital stay was significantly shorter in the LDH group (8.1 days) than in the ODH group (10.6 days; P < 0.001).
Donor complications with a Clavien‐Dindo grade higher than 3 were observed in 2 cases in the ODH group, whereas no such complications were observed in the LDH group. One case involved a wound problem treated with wound repair under local anesthesia, and the other case needed percutaneous drainage performed with fluid collection at the resection site after the operation (Table 4).
TABLE 4.
Perioperative and Postoperative Surgical Outcomes of Donors and Grafts After PSM
| ODH (n = 31) | LDH (n = 31) | P Value | |
|---|---|---|---|
| Sex | 0.271 | ||
| Male | 12 (38.7) | 7 (22.6) | |
| Female | 19 (61.3) | 24 (77.4) | |
| Age, years | 34.1 ± 4.7 | 31.7 ± 6.0 | 0.089 |
| BMI, kg/m2 | 23.4 ± 3.4 | 22.8 ± 3.7 | 0.495 |
| Graft weight, g | 269.9 ± 87.3 | 265.1 ± 78.9 | 0.821 |
| Artery anatomy | 0.509 | ||
| Classic | 28 (90.3) | 27 (87.1) | |
| Replaced RHA from SMA | 1 (3.2) | 3 (9.7) | |
| Replaced LHA from LGA | 2 (6.5) | 1 (3.2) | |
| Accessory LHA from LGA | 0 (0.0) | 0 (0.0) | |
| Portal vein anatomy | 0.530 | ||
| Type 1 | 27 (87.1) | 29 (93.5) | |
| Type 2 | 3 (9.7) | 2 (6.5) | |
| Type 3 | 1 (3.2) | 0 (0.0) | |
| Bile duct anatomy | 0.366 | ||
| Type 1 | 25 (80.6) | 28 (90.3) | |
| Type 2 | 2 (6.5) | 0 (0.0) | |
| Type 3b | 3 (9.7) | 3 (9.7) | |
| Type 4b | 1 (3.2) | 0 (0.0) | |
| Cold ischemic time, minutes | 42.2 ± 19.1 | 50.2 ± 24.1 | 0.156 |
| Warm ischemic time, minutes* | 5.5 ± 1.8 | 6.8 ± 3.6 | 0.518 |
| Total ischemic time, minutes | 72.1 ± 20.4 | 81.3 ± 23.2 | 0.105 |
| Number of arteries | 0.508 | ||
| 1 | 28 (90.3) | 30 (96.8) | |
| 2 | 3 (9.7) | 1 (3.2) | |
| Number of bile duct openings | 0.988 | ||
| 1 | 28 (90.3) | 29 (93.5) | |
| 2 | 3 (9.7) | 2 (6.5) | |
| Length of operation time | 360.1 ± 58.3 | 383.0 ± 64.0 | 0.146 |
| Length of postoperative hospital stay, days | 10.6 ± 1.6 | 8.1 ± 2.7 | <0.001 |
| Surgical complication | 0.152 | ||
| Wound problem | 1 (3.2) | 0 (0.0) | |
| Bile leakage | 0 (0.0) | 0 (0.0) | |
| Pleural effusion | 1 (3.2) | 0 (0.0) | |
| Fluid collection | 1 (3.2) | 0 (0.0) | |
| None | 28 (90.4) | 31 (100.0) | |
| Clavien‐Dindo classification | |||
| Grades 1 and 2 | 1 | 0 | |
| Grades 3 and 4 | 2 | 0 |
Data are provided as n (%) or mean ± standard deviation.
The time to retrieve a graft from the abdomen.
Discussion
The general advantages of laparoscopic surgery are known for shortened rehabilitation period, improved cosmetic outcome, and high precision and visualization during the procedure.( 11 , 12 , 13 )
The first laparoscopic liver resection was reported nearly 25 years ago. The worldwide literature reports approximately 3000 laparoscopic liver resections, three‐quarters of which were performed from 2006 onward. The results suggest that in experienced centers, laparoscopic liver resection can be performed with equal safety and oncological efficacy as open surgery.( 14 , 15 , 16 )
Despite the surgical difficulties, the implementation of LDH is expanding across high‐volume LDLT centers owing to the accumulation of experience and advances in instrument development. National and international registries could potentially provide meaningful data by allowing for a risk‐benefit analysis of laparoscopic procedures for pediatric liver transplantation. Well‐organized articles that discuss the technique have been published from various centers.( 17 , 18 , 19 )
Moreover, in 2011, we also published an initial study on the feasibility of laparoscopic donor left lateral sectionectomy.( 9 ) This study builds on the aforementioned study to validate long‐term patient and graft survival outcomes, including detailed surgical complications, through a larger number of patients. To the best of our knowledge, this study has the longest follow‐up period among the studies about pediatric LDLT using the laparoscopic technique.
In 2015, an interesting article that validated the usefulness of laparoscopic lateral suspension in pediatric LDLT by comparing postoperative outcomes with those of laparoscopic donor nephrectomy (a well‐standardized and globally accepted procedure) was published.( 19 ) In that article, Soubrane et al. reported 21 cases (16.9%) of surgical complications, including 6 cases of Clavien‐Dindo grade 3 or higher, in 124 liver donors who underwent laparoscopic lateral suspension. Among the cases, 4 reoperations (3%) were reported.
In our present study, we identified no donor complications in the LDH group. The mean postoperative hospital stay of the donors was significantly shorter in the laparoscopic group (8.1 days) than in the open group (10.6 days; P < 0.001). The reason for the relatively longer hospital stays in Korea than in other countries such as the United States is probably the Korean insurance system. Owing to the national health insurance system, Korean patients tend to be hospitalized for long periods with low medical expenses. This is thought to be a major factor in the long hospitalization period of donors without special complications.
The stability of laparoscopic graft applications in pediatric LDLT should be validated in terms of surgical outcomes in recipients, including long‐term graft outcomes. In 2018, Broering et al.( 20 ) reported that 2 portal vein stenoses (2.8%), 1 hepatic artery thrombus (1.4%), and 6 biliary strictures (8.3%) occurred in the laparoscopic technique group (n = 72). In addition, 1 case of retransplantation attributed to chronic rejection episodes was reported in each of the open and laparoscopic technique groups.
In our study, 4 portal vein stenoses (12.9%), 2 biliary strictures (6.5%), and 3 episodes of biliary leakage (9.7%) occurred in the LDH group, with no significant differences from those in the ODH group. Our center does not routinely perform portal vein widening as the graft portal vein is considered a "no‐touch" area. Alternatively, we performed wedge‐patch venoplasty in cases with an exceptionally narrow graft portal vein to cope with the portal vein size mismatch in LDLT.( 18 )
Most centers that perform pediatric liver transplantation have been challenged by the fatal complication of hepatic venous outflow obstruction (HVOO). The ideal techniques for hepatic vein anastomosis minimize the possibility of the vein acting as an axis for torsion. Tannuri et al.( 21 ) changed their techniques to a large longitudinal incision on the recipient inferior vena cava after the closure of the hepatic vein orifice to create a widely patent anastomosis. Fukuda et al.( 22 ) proposed a modified triangular technique and reported no incidence of HVOO in 122 patients who received transplants with this technique. Our center also performs patchplasty routinely to ensure a wide opening and proper vein height using cold‐preserved fresh iliac vein tissue at the recipient and graft sides. This procedure also aids in the ease of the surgical procedure by securing the field of anastomosis. This venoplasty is particularly useful when an anomaly is present in the left hepatic vein of the donor (Fig. 2).
FIG. 2.
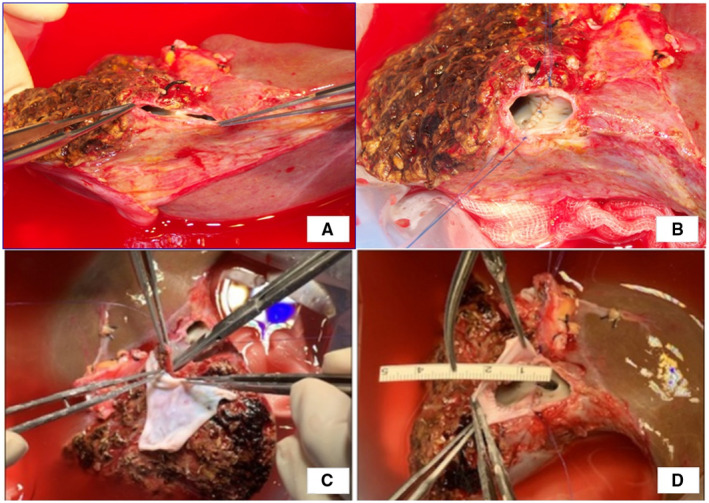
Operative photographs of left hepatic vein venoplasty. Two openings were identified and unified (A and B). Additional crossing septotomy can be performed to deepen the common channel portion, and patch venoplasty is performed using a cold‐preserved fresh iliac vein patch (C and D).
In our study, HVOOs did not occur in the laparoscopic technique group, which is a reasonable outcome compared with that in other studies. The surgical outcome data, including the overall survival rate of the recipients, showed no significant difference between the open and laparoscopic technique groups. These results suggest that the laparoscopic technique did not adversely affect the outcome of the recipients.
The secure division of the bile duct is also an important factor associated with complications on the donor and recipient sides. For safe bile duct division, cholangiography using C‐arm and indocyanine green fluorescence imaging are the most representative modalities used. Our institute prefers to use cholangiography because it not only captures images but also enables a bile leak test using methylene blue injected through the cholangiography tube.
Lastly, some authors argue that the laparoscopic environment represented by carbon dioxide pneumoperitoneum adversely affects the living donor graft in cases of kidney transplantation( 23 , 24 ); however, we identified that it did not adversely affect any clinical outcome, including the long‐term survival of recipients and graft rejection rates.
At the Second International Consensus Conference on laparoscopic liver resection held in October 2014 in Morioka, Japan, laparoscopic donor hepatectomy for pediatric LDLT was categorized as IDEAL 2b according to the Balliol group classification.( 25 )
The IDEAL freamwork, a model for the implementation of surgery with defined stages for innovation of a pariticular surgical technique. IDEAL comprises Idea (stage 1), Development (stage 2a), Exploration (stage 2b), Assessment (stage 3), and Long‐term study (stage 4).
On the basis of the research in our institute and other studies so far, we agree with this level of recommendation and expect to progress to laparoscopic minor hepatic resection (IDEAL 3).
Several limitations of our study must be addressed. As a single‐center study, this study was limited to center‐specific populations and treatment practices. From the indications and donor anatomies described in Tables 1 and 2, more open approaches have been implemented in cases of fulminant hepatic failure and complex donor anatomical characteristics. In other words, although no special exclusion criteria were used for the study period, some selection bias was present. Furthermore, the retrospective nature of our study limited our ability to directly control for confounding variables that could have affected the outcomes despite performing PSM.
In conclusion, on the basis of the analysis of 100 cases of pediatric LDLT, the laparoscopic approach does not increase the donor operation time as compared with the open approach and is associated with shorter postoperative hospital stays. The surgical approach (open versus laparoscopic) does not influence the ischemic time and surgical complication rates in donors or recipients. The present study shows that laparoscopic left lateral donor hepatectomy could be beneficial for the donor and that the use of the laparoscopic technique in donors does not adversely affect the long‐term outcomes of the recipients. Thus, laparoscopic left lateral sectionectomy for the living donor is a safe, feasible, and reproducible procedure for pediatric liver transplantation.
Supporting information
Table S1
Table S2
Table S3
Table S4
Potential conflict of interest: Nothing to report.
References
- 1. Feier FH, Chapchap P, Pugliese R, da Fonseca ES, Carnevale FC, Moreira AM, et al. Diagnosis and management of biliary complications in pediatric living donor liver transplant recipients. Liver Transpl 2014;20:882‐892. [DOI] [PubMed] [Google Scholar]
- 2. Scatton O, Katsanos G, Boillot O, Goumard C, Bernard D, Stenard F, et al. Pure laparoscopic left lateral sectionectomy in living donors: from innovation to development in France. Ann Surg 2015;261:506‐512. [DOI] [PubMed] [Google Scholar]
- 3. Broering DC, Berardi G, El Sheikh Y, Spagnoli A, Troisi RI. Learning curve under proctorship of pure laparoscopic living donor left lateral sectionectomy for pediatric transplantation. Ann Surg 2020;271:542‐548. [DOI] [PubMed] [Google Scholar]
- 4. Soubrane O, Cherqui D, Scatton O, Stenard F, Bernard D, Branchereau S, et al. Laparoscopic left lateral sectionectomy in living donors: safety and reproducibility of the technique in a single center. Ann Surg 2006;244:815‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang S, Laurent A, Tayar C, Karoui M, Cherqui D. Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg 2007;94:58‐63. [DOI] [PubMed] [Google Scholar]
- 6. Troisi R, Debruyne R, Rogiers X. Laparoscopic living donor hepatectomy for pediatric liver transplantation. Acta Chir Belg 2009;109:559‐562. [DOI] [PubMed] [Google Scholar]
- 7. Cherqui D, Soubrane O, Husson E, Barshasz E, Vignaux O, Ghimouz M, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet 2002;359:392‐396. [DOI] [PubMed] [Google Scholar]
- 8. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:680‐688. [DOI] [PubMed] [Google Scholar]
- 9. Kim KH, Jung DH, Park KM, Lee YJ, Kim DY, Kim KM, Lee SG. Comparison of open and laparoscopic live donor left lateral sectionectomy. Br J Surg 2011;98:1302‐1308. [DOI] [PubMed] [Google Scholar]
- 10. Kim KH, Kang SH, Jung DH, Kim WJ, Yoon YI, Lee SG, et al. Initial outcomes of pure laparoscopic living donor right hepatectomy in an experienced adult living donor liver transplant center. Transplantation 2017;101:1106‐1110. [DOI] [PubMed] [Google Scholar]
- 11. Coelho FF, Bernardo WM, Kruger JAP, Jeismann VB, Fonseca GM, et al. Laparoscopy‐assisted versus open and pure laparoscopic approach for liver resection and living donor hepatectomy: a systematic review and meta‐analysis. HPB (Oxford) 2018;20:687‐694. [DOI] [PubMed] [Google Scholar]
- 12. Macacari RL, Coelho FF, Bernardo WM, Kruger JAP, Jeismann VB, Fonseca GM, et al. Laparoscopic vs. open left lateral sectionectomy: an update meta‐analysis of randomized and non‐randomized controlled trials. Int J Surg 2019;61:1‐10. [DOI] [PubMed] [Google Scholar]
- 13. Samstein B, Griesemer A, Halazun K, Kato T, Guarrera JV, Cherqui D, Emond JC. Pure laparoscopic donor hepatectomies: ready for widespread adoption? Ann Surg 2018;268:602‐609. [DOI] [PubMed] [Google Scholar]
- 14. Wakabayashi G, Nitta H, Takahara T, Shimazu M, Kitajima M, Sasaki A. Standardization of basic skills for laparoscopic liver surgery towards laparoscopic donor hepatectomy. J Hepatobiliary Pancreat Surg 2009;16:439‐444. [DOI] [PubMed] [Google Scholar]
- 15. Yu YD, Kim KH, Jung DH, Lee S‐G, Kim Y‐G, Hwang G‐S. Laparoscopic liver donor left lateral sectionectomy is safe and feasible for pediatric living donor liver transplantation. Hepatogastroenterology 2012;59:2445‐2449. [DOI] [PubMed] [Google Scholar]
- 16. Park JI, Kim KH, Lee SG. Laparoscopic living donor hepatectomy: a review of current status. J Hepatobiliary Pancreat Sci 2015;22:779‐788. [DOI] [PubMed] [Google Scholar]
- 17. Miura K, Sakamoto S, Shimata K, Honda M, Kobayashi T, Wakai T, et al. The outcomes of pediatric liver retransplantation from a living donor: a 17‐year single‐center experience. Surg Today 2017;47:1405‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kang SH, Namgoong JM, Hwang S, Jung D‐H, Kim K‐M. Wedged‐patch venoplasty of the left liver graft portal vein for size matching in pediatric living donor liver transplantation. Ann Hepatobiliary Pancreat Surg 2019;23:183‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soubrane O, de Rougemont O, Kim KH, Samstein B, Mamode N, Boillot O, et al. Laparoscopic living donor left lateral sectionectomy: a new standard practice for donor hepatectomy. Ann Surg 2015;262:757‐763; discussion 761‐763. [DOI] [PubMed] [Google Scholar]
- 20. Broering DC, Elsheikh Y, Shagrani M, Abaalkhail F, Troisi RI. Pure laparoscopic living donor left lateral sectionectomy in pediatric transplantation: a propensity score analysis on 220 consecutive patients. Liver Transpl 2018;24:1019‐1030. [DOI] [PubMed] [Google Scholar]
- 21. Tannuri U, Tannuri AC, Santos MM, Miyatani HT. Technique advance to avoid hepatic venous outflow obstruction in pediatric living‐donor liver transplantation. Pediatr Transplant 2015;19:261‐266. [DOI] [PubMed] [Google Scholar]
- 22. Fukuda A, Sakamoto S, Shigeta T, Uchida H, Hamano I, Sasaki K, et al. Clinical outcomes and evaluation of the quality of life of living donors for pediatric liver transplantation: a single‐center analysis of 100 donors. Transplant Proc 2014;46:1371‐1376. [DOI] [PubMed] [Google Scholar]
- 23. Troppmann C, Ormond DB, Perez RV. Laparoscopic (vs open) live donor nephrectomy: a UNOS database analysis of early graft function and survival. Am J Transplant 2003;3:1295‐1301. [DOI] [PubMed] [Google Scholar]
- 24. Wever KE, Bruintjes MH, Warle MC, Hooijmans CR. Renal perfusion and function during pneumoperitoneum: a systematic review and meta‐analysis of animal studies. PLoS One 2016;11:e0163419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wakabayashi G, Cherqui D, Geller D, Buell JF, Kaneko H, Han HS, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619‐629. [DOI] [PubMed] [Google Scholar]
- 26. Varotti G, Gondolesi G, Goldman J, WayneWayne M, Florman SS, Schwartz ME, et al. Anatomic variations in right liver living donors. J Am Coll Surg 2004;198:577‐582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4


