Abstract
Objectives
To assess the risk of chronic kidney disease (CKD) associated with tenofovir disoproxil fumarate (TDF) use by baseline D:A:D CKD risk score.
Methods
Adult antiretroviral therapy (ART)‐naïve people living with HIV (PLWH) initiating treatment, with estimated glomerular filtration rate (eGFR) ≥ 60 mL/min/1.73 m2, were identified in the OPERA cohort. CKD was defined as two or more consecutive eGFR < 60 mL/min/1.73 m2, > 90 days apart. Associations between TDF use, baseline D:A:D CKD risk and incident CKD were assessed with incidence rates (IRs; Poisson regression) and adjusted pooled logistic regression. The impact of pharmacoenhancers on the observed association between TDF and CKD was also evaluated.
Results
Of 9802 PLWH included, 6222 initiated TDF and 3580 did not (76% and 79% low D:A:D CKD risk, respectively). Overall, 125 CKD events occurred over 24 382 person‐years of follow‐up. Within strata of D:A:D CKD risk score, IRs were similar across TDF exposure, with high baseline CKD risk associated with highest incidence. Compared with the low‐risk group without TDF, there was no statistical difference in odds of incident CKD in the low‐risk group with TDF (adjusted odds ratio = 0.55, 95% confidence interval: 0.19–1.54). Odds of incident CKD did not differ statistically significantly by pharmacoenhancer exposure, with or without TDF.
Conclusions
In this large cohort of ART‐naïve PLWH, incident CKD following ART initiation was infrequent and strongly associated with baseline CKD risk. TDF‐containing regimens did not increase the odds of CKD in those with a low baseline D:A:D CKD risk, the largest group of ART‐naïve PLWH, and may remain a viable treatment option in appropriate settings.
Keywords: antiretroviral therapy, chronic kidney disease, D:A:D CKD risk score, HIV, TDF
Introduction
International guidelines from the Kidney Disease: Improving Global Outcomes (KDIGO) Working Group classify individuals with glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 for 3 months or longer as having moderate to severe chronic kidney disease (CKD stage 3 or higher), even in the absence of structural or functional abnormalities [1]. Approximately 30 million people (15% of adults) in the US are estimated to have CKD, most (96%) with mild kidney damage [2], and the incidence of CKD is much higher in older individuals [3, 4]. The prevalence of CKD among people living with HIV (PLWH) in North America and Europe ranges from 4.7% to 9.7%, although it has been reported to be as high as 33% when including milder GFR reductions (60–90 mL/min/1.73 m2) or proteinuria [5]. In populations with access to care in resource‐rich countries, the CKD spectrum increasingly reflects the nephrotoxicity associated with antiretroviral therapy (ART) and the burden of comorbid disease in an ageing population of PLWH [6, 7].
Tenofovir disoproxil fumarate (TDF), a nucleotide reverse transcriptase inhibitor (NRTI), is widely recommended as a first‐line antiretroviral (ARV) agent for PLWH [8] and is considered to be safe and well‐tolerated [9], as well as efficacious [10]. There were no discontinuations for renal adverse events in several clinical trials of TDF without the use of pharmacoenhancers (i.e. boosting agents ritonavir or cobicistat) [11, 12, 13, 14]. However, TDF has been linked to decreased GFR [15, 16] and increased risk of CKD [16, 17, 18] in cohort studies. The potential for TDF nephrotoxicity may be enhanced by the concurrent administration of a pharmacoenhancer [19, 20, 21, 22] due to elevations of tenofovir serum levels. Greater risks of developing CKD were observed with cobicistat‐boosted elvitegravir or a ritonavir‐boosted protease inhibitor (PI) than with efavirenz among PLWH on TDF [23]. In addition, combinations containing TDF with a ritonavir‐boosted PI have been associated with a greater risk of CKD compared with regimens that included neither TDF nor a pharmacoenhancer [24].
Identifying individuals who are at an increased risk of developing CKD and who might benefit from a therapeutic or preventive intervention is essential in caring for an ever‐ageing HIV population. Given long‐term exposure to ART and the previously observed association between TDF and CKD risk, it is important to understand the impact of baseline CKD risk on this relationship. In addition, the interaction between TDF exposure and pharmacoenhancer use must be better understood to provide guidance on initial ART selection, especially as some evidence suggests an association between pharmacoenhancer use and CKD outcomes, both with and without TDF. Moreover, inhibition of tubular creatinine secretion with some ARVs [25, 26, 27] further complicates clinical decision‐making as it can be difficult to distinguish this innocuous artefact of the ARVs from true nephrotoxicity.
Using data from a large, real‐world population of PLWH receiving care in the US who were ART‐naïve and free of severe kidney disease at baseline, this study sought to estimate the incidence of CKD following ART initiation and to assess the risk of CKD associated with TDF use, stratified by baseline CKD risk as assessed by the D:A:D CKD risk score. The impact of pharmacoenhancer use on the association between TDF and risk of CKD was also evaluated.
Methods
Study design and population
This study utilized prospectively captured, clinical data from the electronic health records of 94 852 PLWH in the US in the Observational Pharmaco‐Epidemiology Research & Analysis (OPERA) cohort. OPERA complies with all Health Insurance Portability and Accountability Act and Health Information Technology for Economic and Clinical Health Act requirements and received annual institutional review board (IRB) approval from Advarra IRB, including a waiver of informed consent and authorization for the use of protected health information. The study population consisted of ART‐naïve adults (≥ 18 years of age) with a baseline viral load ≥ 1000 copies/mL who were initiated on a standard ART regimen [two NRTIs and one core agent: non‐boosted integrase strand transfer inhibitor, non‐boosted nonnucleoside reverse transcriptase inhibitor (NNRTI), boosted elvitegravir, or boosted PI] between the first OPERA visit and 30 April 2018. Individuals with kidney transplant, end‐stage renal disease, dialysis, sepsis or uncontrolled diabetes (two consecutive HbA1C ≥ 6.5%) at baseline were excluded. All included PLWH had at least one pre‐ART estimated GFR (eGFR), with the last reading ≥ 60 mL/min/1.73 m2 within 12 months prior to or at ART initiation, and at least two eGFR measures after ART initiation, with > 90 days between the first and last follow‐up eGFR measures. The observation period ranged from ART initiation up to the first of the following censoring events: (1) changes in any ARV agent of interest, (2) 12 months after the last clinical contact, (3) death, or (4) study end (31 October 2018).
Study measurements
Each person’s risk of developing CKD was estimated at baseline using the D:A:D CKD risk score. This CKD risk score was derived from the Data Collection on Adverse Events of Anti‐HIV Drugs (D:A:D) dataset [28] and was validated in several European cohorts [28], and for US PLWH in the OPERA cohort [29]. The D:A:D score is calculated based on a number of characteristics associated with increased CKD risk among PLWH (Table S1) [28].
The first composite exposure of interest consisted of TDF use (yes/no) and baseline D:A:D CKD risk group (low, medium and high‐risk); the no‐TDF/low‐risk group served as the referent group in comparative analyses. The second composite exposure consisted of TDF use (yes/no) and whether the ART regimen included a pharmacoenhancer (cobicistat or ritonavir, yes/no); the no‐TDF/non‐boosted ART regimen group served as the referent group in comparative analyses.
Chronic kidney disease was defined as a confirmed decrease in eGFR to < 60 mL/min/1.73 m2, measured with two or more consecutive eGFR results, > 90 days apart. If there were multiple eGFRs < 60 mL/min/1.73 m2, the total time between the first and last must have exceeded 90 days [1]. Time to CKD was calculated based on the date of the second eGFR value < 60 mL/min/1.73 m2. The CKD‐EPI Creatinine Equation (2009) [30] was used to calculate eGFR.
Statistical analyses
Baseline demographic and clinical characteristics at the time of ART initiation were described by TDF use and D:A:D CKD risk group. Poisson regression was employed to estimate unadjusted incidence rates (IRs) of CKD and 95% confidence intervals (CIs) within each level of the composite exposure defined by TDF use and baseline D:A:D CKD risk. Pooled logistic regression, estimated with generalized estimating equations (first‐order autoregressive correlation structure), was used to assess the association between both composite exposure groups and incident CKD. Follow‐up time was modelled flexibly using restricted cubic splines with knots at the 5th, 33rd, 67th and 95th percentiles. Both adjusted models included baseline age, sex, race/ethnicity, and calendar year of ART initiation, as well as time‐updated alcohol dependence, HIV viral load, use of nephrotoxic drugs (see Table 1 footnote), use of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, hepatitis C co‐infection, incident diabetes, and hypertension/hypertension treatment. The association between CKD risk and TDF‐D:A:D CKD risk group was further adjusted for pharmacoenhancer use; the association between CKD risk and TDF‐pharmacoenhancer use was further adjusted for baseline D:A:D CKD risk group.
Table 1.
Baseline demographic and clinical characteristics by tenofovir disoproxil fumarate intake and D:A:D chronic kidney disease risk strata
| Median (IQR) or N (%) | No TDF | TDF | ||||
|---|---|---|---|---|---|---|
|
Low‐risk (N = 2827) |
Medium‐risk (N = 481) |
High‐risk (N = 272) |
Low‐risk (N = 4743) |
Medium‐risk (N = 994) |
High‐risk (N = 485) |
|
| Age (years) | 28.9 (24.7–35.1) | 49.2 (40.6–53.3) | 52.4 (46.7–57.4) | 30.5 (25.5–38.1) | 47.7 (41.3–52.7) | 51.7 (46.4–57.0) |
| Female | 254 (9%) | 97 (20%) | 79 (29%) | 566 (12%) | 174 (18%) | 146 (30%) |
| Race/ethnicity | ||||||
| Black non‐Hispanic | 1334 (47%) | 205 (43%) | 140 (52%) | 2004 (42%) | 337 (34%) | 183 (38%) |
| Other non‐Hispanic | 623 (22%) | 154 (33%) | 84 (31%) | 1283 (27%) | 435 (44%) | 204 (42%) |
| Hispanic | 775 (27%) | 98 (20%) | 37 (14%) | 1283 (27%) | 171 (17%) | 66 (14%) |
| Missing | 95 (3%) | 24 (5%) | 11 (4%) | 173 (4%) | 51 (5%) | 32 (7%) |
| Year of ART initiation | 2017 (2016–2017) | 2016 (2015–2017) | 2016 (2014–2017) | 2013 (2011–2014) | 2012 (2010–2014) | 2012 (2010–2014) |
| Backbone | ||||||
| TAF/FTC | 1394 (49%) | 244 (51%) | 131 (48%) | NA | NA | NA |
| ABC/3TC | 1294 (46%) | 206 (43%) | 125 (46%) | NA | NA | NA |
| TDF/FTC | NA | NA | NA | 4677 (99%) | 966 (97%) | 470 (97%) |
| Other | 139 (5%) | 31 (6%) | 16 (6%) | 66 (1%) | 28 (3%) | 15 (3%) |
| Anchor agent | ||||||
| Non‐boosted NNRTI | 197 (7%) | 43 (9%) | 26 (10%) | 2107 (44%) | 422 (43%) | 217 (45%) |
| Non‐boosted INSTI | 1502 (53%) | 216 (45%) | 122 (45%) | 405 (9%) | 115 (12%) | 61 (13%) |
| Boosted PI | 249 (9%) | 66 (14%) | 51 (19%) | 963 (20%) | 250 (25%) | 135 (28%) |
| Elvitegravir/cobicistat | 879 (31%) | 156 (32%) | 73 (27%) | 1268 (27%) | 207 (21%) | 72 (15%) |
| Log10(HIV viral load) (copies/mL) | 4.7 (4.2–5.1) | 4.7 (4.1–5.2) | 4.8 (4.2–5.3) | 4.7 (4.2–5.1) | 4.8 (4.2–5.2) | 4.6 (4.1–5.0) |
| CD4 cell count (cells/µL) | 383 (236–538) | 310 (145–504) | 243 (98–431) | 344 (208–496) | 282 (139–456) | 290 (129–439) |
| eGFR (mL/min/1.73 m2) | 117.8 (106.2–128.2) | 96.0 (85.3–105.9) | 77.4 (68.9–85.6) | 115.7 (104.8–125.9) | 95.8 (85.1–106.8) | 80.5 (73.1–86.9) |
| Alcohol abuse | 51 (2%) | 16 (3%) | 6 (2%) | 109 (2%) | 42 (4%) | 10 (2%) |
| Nephrotoxic medication* | 303 (11%) | 82(17%) | 47 (17%) | 838 (18%) | 253 (25%) | 134 (28%) |
| ACE inhibitor/ARB | 69 (2%) | 68 (14%) | 57 (21%) | 159 (3%) | 119 (12%) | 93 (19%) |
| HCV co‐infection | 58 (2%) | 44 (9%) | 29 (11%) | 112 (2%) | 119 (12%) | 75 (16%) |
| HBV co‐infection | 84 (3%) | 26 (5%) | 10 (4%) | 202 (4%) | 63 (6%) | 35 (7%) |
| Diabetes | 34 (1%) | 49 (10%) | 40 (15%) | 34 (1%) | 70 (7%) | 42 (9%) |
| Hypertension | 561 (20%) | 202 (42%) | 157 (58%) | 1074 (23%) | 383 (39%) | 252 (52%) |
3TC, lamivudine; ABC, abacavir; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ART, antiretroviral therapy; D:A:D, Data Collection on Adverse Events of Anti‐HIV Drugs; eGFR, estimated glomerular filtration rate; FTC, emtricitabine; HBV, hepatitis B virus; HCV, hepatitis C virus; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NA, not applicable; NRTI, nucleos(t)ide reverse transcriptase inhibitor; NNRTI, nonNRTI; PI, protease inhibitor; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Atazanavir/ritonavir, lopinavir/ritonavir, acyclovir, cidofovir, valacyclovir, ganciclovir, valganciclovir, dipyridamole, NSAID, probenecid.
Sensitivity analyses
Because some ARVs inhibit tubular creatinine secretion, leading to artificially low eGFRs, this study considered the impact of using corrected eGFRs calculated based on the median increase in serum creatinine reported in clinical trials (Table S2) [31]. To assess the magnitude of potential measurement error bias caused by inhibition of creatinine tubular secretion by certain ARVs, all models were repeated using corrected eGFRs in sensitivity analyses.
Results
Of the 21 356 ART‐naïve PLWH in the OPERA database, 58 were < 18 years of age, 3036 initiated ART with a non‐standard regimen by current guidelines, 110 had either end‐stage liver disease, dialysis, uncontrolled diabetes or sepsis at initiation, 1889 lacked a baseline eGFR, 250 had a baseline eGFR < 60 mL/min/1.73 m2, and 6241 did not have two or more follow‐up eGFRs. The remaining 9802 PLWH (10%) met criteria for inclusion in this study; 6222 initiated ART including TDF (CKD risk: 76% low, 16% medium, 8% high) and 3580 did not (CKD risk: 79% low, 13% medium, 8% high). Low‐risk individuals were the youngest (median age 29 and 31 years in TDF and no‐TDF groups, respectively), compared with all other groups (range of median ages: 49–52 years). Compared with all other groups, individuals not on TDF with low CKD risk were the most likely to be male (91% vs. range: 70–88%), had received their HIV diagnosis closer to baseline (median 1.6 months vs. range: 2.9–12.7 months), and had the highest median CD4 cell count (383 cells/µL vs. range: 243–344 cells/µL). They were also the least likely to use nephrotoxic ARVs (3% vs. range 5–12%) or nephrotoxic non‐ARVs (8% vs. range: 11–16%) (Table 1). Regardless of TDF use, individuals with low CKD risk had lower body mass index [24 kg/m2 vs. range: 25–26 kg/m2] and were less likely to have diabetes [1% vs. range: 7–10%], cardiovascular diseases [1% vs. range: 4–7%] or hepatitis C co‐infection [2% vs. range: 9–16%]) compared with those with medium or high CKD risk.
TDF use/D:A:D CKD risk score and incidence of CKD
There were 125 incident cases of CKD over 24 382 person‐years of follow‐up, for an overall IR of 5.1 events/1000 person‐years (95% CI: 4.3–6.1). The median follow‐up time among individuals not using TDF was of 19–20 months across the D:A:D CKD risk groups; the median follow‐up time among individuals using TDF was higher, at 27–29 months across risk groups (Table 2). Regimen discontinuation and switches occurred more frequently with higher baseline D:A:D risk categories among PLWH not taking TDF, but was consistently high regardless of risk group among those on TDF. Moreover, the last eGFR before discontinuation or switch tended to be lower with higher baseline CKD risk (Table 2).
Table 2.
Follow‐up characteristics by tenofovir disoproxil fumarate intake and D:A:D chronic kidney disease risk strata
| Median (IQR) or N (%) | No TDF | TDF | ||||
|---|---|---|---|---|---|---|
|
Low‐risk (N = 2827) |
Medium‐risk (N = 481) |
High‐risk (N = 272) |
Low‐risk (N = 4743) |
Medium‐risk (N = 994) |
High‐risk (N = 485) |
|
| Duration of follow‐up (months) | 19.5 (13.0–27.5) | 18.6 (12.0–27.6) | 18.7 (12.0–27.7) | 28.8 (18.3–45.1) | 28.5 (16.8–44.8) | 27.2 (16.1–45.8) |
| Regimen discontinuation* | 850 (30%) | 183 (38%) | 122 (45%) | 4091 (86%) | 907 (91%) | 434 (90%) |
| Last eGFR (mL/min/1.73 m2) | 106.2 (91.5–118.3) | 89.5 (77.2–99.6) | 75.4 (65.3–90.3) | 107.5 (93.6–119.1) | 88.9 (75.8–100.5) | 79.3 (66.3–90.2) |
| Last eGFR < 60 mL/min/1.73 m2 | ≤ 5 † | 9 (5%) | 22 (18%) | 39 (1%) | 49 (5%) | 67 (15%) |
eGFR, estimated glomerular filtration rate; IQR, interquartile range; TDF, tenofovir disoproxil fumarate.
Regimen discontinuation defined as discontinuation of any antiretroviral agent or regimen switch.
Cell count ≤ 5 deemed insufficient for reporting.
The lowest CKD incidence was observed in the low‐risk group, both for those on TDF (IR: 0.6/1000 person‐years, 95% CI: 0.3–1.2) and not on TDF (IR: 1.7/1000 person‐years, 95% CI: 0.9–3.3). CKD incidence was elevated in the medium‐risk group for those on TDF (IR: 8.2/1000 person‐years, 95% CI: 5.5–12.4) and not on TDF (IR: 7.8/1000 person‐years, 95% CI: 3.7–16.4). The highest incidence of CKD occurred among PLWH in the high‐risk group, both for individuals on TDF (IR: 30.5/1000 person‐years, 95% CI: 22.6–41.3) and for those not on TDF (IR: 67.0/1000 person‐years, 95% CI: 48.3–92.8) (Fig. 1). In adjusted models, TDF use was not statistically significantly associated with incident CKD among PLWH in the low‐risk group [adjusted odds ratio (aOR) = 0.55, 95% CI: 0.19–1.54]. Compared with low‐risk individuals not using TDF, the risk of CKD was significantly higher in the high‐risk group, regardless of TDF use (no TDF: aOR = 19.55, 95% CI: 7.35–52.00; TDF: aOR = 12.84, 95% CI: 4.57–36.07). The risk of CKD was also higher in the medium‐risk groups than in the no‐TDF/low‐risk group, but the increase in risk was only statistically significant in the TDF/medium‐risk group (aOR = 3.96/, 95% CI: 1.38–11.39) (Table 3).
Fig 1.
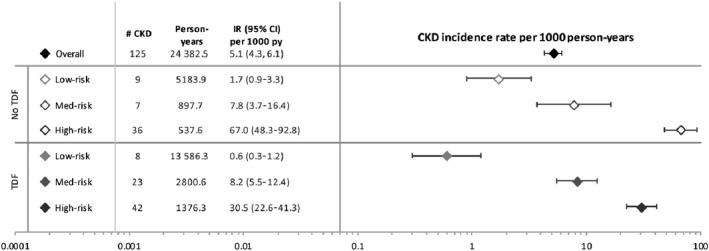
Incidence rates of chronic kidney disease by tenofovir disoproxil fumarate intake and D:A:D chronic kidney disease risk strata. CI, confidence interval; CKD, chronic kidney disease; D:A:D, Data Collection on Adverse Events of Anti‐HIV Drugs; IR, incidence rate; TDF, tenofovir disoproxil fumarate; py, person‐years.
Table 3.
Association* between tenofovir disoproxil fumarate (TDF) intake/D:A:D chronic kidney disease risk strata and incidence of chronic kidney disease †
| TDF/D:A:D Risk Group | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| No TDF | ||
| Low‐risk | 1.00 (Ref) | 1.00 (Ref) |
| Medium‐risk | 4.69 (1.70–12.96) | 2.32 (0.72–7.52) |
| High‐risk | 37.56 (17.20–82.02) | 19.55 (7.35–52.00) |
| TDF | ||
| Low‐risk | 0.42 (0.16–1.11) | 0.55 (0.19–1.54) |
| Medium‐risk | 5.37 (2.40–12.01) | 3.96 (1.38–11.39) |
| High‐risk | 18.30 (8.42–39.78) | 12.84 (4.57–36.07) |
CI, confidence interval; D:A:D, Data Collection on Adverse Events of Anti‐HIV Drugs; OR, odds ratio.
Pooled logistic regression estimated with generalized estimating equations (first‐order autoregressive correlation structure), adjusted for baseline age (restricted cubic splines), sex, race/ethnicity†, index calendar year (restricted cubic splines) and regimen, as well as time‐updated alcohol misuse, HIV viral load ≥ 50 copies/mL.
386 individuals were excluded from analysis due to missing race/ethnicity information.
In sensitivity analysis, after applying the eGFR correction to adjust for the impact of specific ARVs on tubular creatinine secretion, the overall incidence of CKD was reduced by half to a corrected IR of 2.4 events/1000 person‐years (95% CI: 1.8–3.1) (Table S3). Pooled logistic regression could not be performed after eGFR correction due to the small number of CKD events within each exposure group (range: 0–26).
TDF use/boosted ART regimen and incidence of CKD
After adjusting for important confounding factors, including baseline D:A:D CKD risk score, there was no statistically significant difference in risk of incident CKD across the four groups defined by TDF and pharmacoenhancer use (Table 4). In sensitivity analysis, after applying the eGFR correction, there was a numerically increased risk of CKD with boosted regimens with or without TDF when compared with no‐TDF/non‐boosted regimens, but the number of events without TDF was very small (n ≤ 5) and CIs were wide (Table S4).
Table 4.
Association* between tenofovir disoproxil fumarate (TDF) intake/boosted regimen and incidence of chronic kidney disease †
| TDF/boosted regimen | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| No TDF | ||
| Non‐boosted regimen | 1.00 (Ref) | 1.00 (Ref) |
| Boosted regimen | 1.52 (0.87–2.68) | 1.28 (0.70–2.33) |
| TDF | ||
| Non‐boosted regimen | 0.59 (0.35–0.97) | 0.69 (0.35–1.35) |
| Boosted regimen | 0.84 (0.50–1.39) | 1.10 (0.59–2.03) |
CI, confidence interval; OR, odds ratio.
Pooled logistic regression estimated with generalized estimating equations (autoregressive correlation structure), adjusted for baseline age (restricted cubic splines), sex, race/ethnicity†, index calendar year (restricted cubic splines), and D:A:D chronic kidney disease risk score, as well as time‐updated alcohol misuse, HIV viral load ≥ 50 copies/mL, nephrotoxic medications, medications affecting proteinuria, hepatitis C co‐infection, diabetes and hypertension.
386 individuals were excluded from analysis due to missing race/ethnicity information.
Discussion
In this large cohort of PLWH in the US, incident CKD was relatively rare over more than 24 000 person‐years of follow‐up among treatment‐naïve individuals initiating ART. Among PLWH with a low D:A:D CKD risk score at baseline (77% of study population), TDF did not appear to increase the risk of CKD when compared with the no‐TDF group. The risk of CKD was, however, increased with high baseline D:A:D CKD risk scores, regardless of TDF use, compared with individuals with low baseline risk score and no TDF use. No association was identified with boosted ART regimens, although the small number of incident CKD events limited the ability to investigate multiple exposure categories.
The overall incidence of CKD in this study population of ART‐naïve PLWH was low (5.1/1000 person‐years), but consistent with rates of incident CKD in other populations of PLWH, with incidence rates ranging from 1.3 to 11.2/1000 person‐years [15, 32, 33, 34]. In a previous study in the OPERA cohort, progression to CKD among PLWH with no kidney disease at baseline was strongly associated with baseline CKD risk, as measured by the D:A:D CKD risk score [29]. In the current study, there was no increased risk of CKD with TDF use among ART‐naïve PLWH with low baseline risk of CKD, which encompassed > 70% of the population analysed. Of note, the overall median follow‐up was longer among TDF users (29 months) than among non‐TDF users (19 months). These findings may provide relevant context to the previous cohort studies that have reported an association between TDF and CKD [15, 16, 17, 18, 35, 36]. In a systematic review of 11 studies (5767 participants), there was a significantly greater decrease in Cockcroft–Gault eGFR with TDF than without TDF (mean difference = 3.92 mL/min, 95% CI: 2.13–5.70); however, there was statistical heterogeneity and evidence of publication bias. Among 10 841 PLWH from the Veterans Health Administration who initiated ART between 1997 and 2007, there was a 33% increased risk of CKD (95% CI: 18–51%) with each additional year of cumulative TDF exposure [16]. In a meta‐analysis pooling estimates from five studies, the relative risk of CKD was estimated to be 1.56 (95% CI: 0.83–2.93) with TDF vs. without TDF [37]. Among participants with baseline eGFR ≥ 90 mL/min in the D:A:D study, each additional year of cumulative TDF use was associated with an increased CKD risk (adjusted IR ratio = 1.14, 95% CI: 1.10–1.19) over 7 years of follow‐up [18]. However, these studies did not account for baseline CKD risk or for pharmacoenhancer use and included ART‐experienced PLWH who were not explicitly free of kidney disease at baseline. By contrast, there was no statistical difference in kidney function in a pooled analysis of six studies that reported a change in eGFR using the more accurate Modification of Diet in Renal Disease calculation [17]. The absence of a statistically significant association between TDF use and risk of incident CKD among PLWH with a low risk of CKD at ART initiation in the OPERA cohort suggests that TDF may still be considered as initial therapy in low‐risk individuals in settings where this would increase access to first‐line regimens. This finding is of great clinical importance because TDF is widely used, efficacious and recommended as a front‐line ARV agent, and a cost‐saving generic TDF‐emtricitabine (TDF‐FTC) has recently been introduced [38]. These findings could thus have an impact on treatment options for a large proportion of PLWH, as 77% of the study population were classified as low‐risk, a slightly higher prevalence than the D:A:D CKD risk score derivation cohort (62%) and its two validation cohorts (SMART/ESPRIT, 65%; Royal Free Hospital Clinic Cohort, 73%) [28]. This approach would, however, require the proper identification of individuals with a low baseline risk of CKD prior to ART initiation, as well as appropriate reassessment of kidney function and CKD risk during the course of treatment with TDF.
Several studies have suggested that pharmacoenhancers may increase the risk of CKD. A meta‐analysis of five clinical trials compared TDF with tenofovir alafenamide (TAF), a prodrug of tenofovir that is absorbed more quickly than TDF. In this meta‐analysis, the risk for discontinuation due to renal adverse events was 1% lower with boosted TAF than with boosted TDF (P = 0.002), although no difference was observed when comparing unboosted TAF with unboosted TDF [39]. Clinical trials and observational studies have shown an increase in CKD risk with regimens including TDF and boosted PIs (usually lopinavir or atazanavir) compared with regimens including TDF and NNRTIs [19, 20, 21, 22]. In the Veterans Health Administration clinical and administrative datasets, PLWH taking efavirenz with TDF and FTC were significantly less likely to develop CKD than those taking elvitegravir/cobicistat and TDF [adjusted hazard ratio (aHR) = 0.75, 95% CI: 0.59–0.95) or a ritonavir‐boosted PI (aHR = 0.62, 95% CI: 0.53–0.72) [23]. In the Centers for AIDS Research Network of Integrated Clinical Systems cohort, PLWH taking TDF and a ritonavir‐boosted PI were more than three times more likely to develop stage 3–4 CKD compared with those taking neither TDF nor a pharmacoenhancer; the inclusion of either TDF or ritonavir‐boosted PI alone resulted in comparatively small and non‐significant increases in risk when compared with regimens that included neither [24]. In the D:A:D cohort, TDF, ritonavir‐boosted atazanavir and ritonavir‐boosted lopinavir use were all identified as independent predictors of CKD [40], although cumulative exposure to ritonavir‐boosted darunavir was not associated with an increased incidence of CKD [41]. By contrast, this study did not find an association between boosted regimens and incident CKD among ART‐naïve individuals initiating ART in the OPERA cohort. In a sensitivity analysis adjusting for the expected impact of agents that interfere with tubular creatinine secretion, a numerically higher risk of CKD with pharmacoenhancers was observed; however, the number of events was very small and CIs were wide. As the small number of incident CKD events limited the ability to fully evaluate these questions, further research is needed to better understand the impact of pharmacoenhancers and TDF on incident CKD, taking into account baseline CKD risk.
This study has several strengths. The OPERA cohort represents PLWH in care at both small, rural clinics and large, urban health centres from 84 clinical sites in 17 states and one US territory; the 94 852 PLWH in OPERA at the time of this study represent approximately 8% of PLWH in care in the US [42]. The use of electronic health records in this large sample of PLWH reflects real‐world clinical practice and provided access to all clinical interactions, including laboratory results and provider notes. The large study population of 9802 ART‐naïve PLWH allowed the study of CKD, a rare outcome. Confirmation of CKD events over a minimum of 3 months was required in this study, as per the KDIGO guidelines [1], to avoid misclassification of acute kidney impairment or transient low eGFRs as CKD. The CKD‐EPI creatinine equation was selected to calculate the eGFR because it is less biased and more accurate than other equations among PLWH on ART [43, 44, 45]. In sensitivity analyses, an attempt was made to correct the value of eGFR for the use of certain ARVs known to inhibit tubular creatine secretion, based on the median increase in serum creatinine reported in clinical trials for each ARV. While an approximation, this approach allowed us to illustrate the potential magnitude of bias that could have been introduced into this and previous studies by the inclusion of artificially low eGFRs. Overall, results were robust to the eGFR correction sensitivity analyses despite wider confidence intervals. Finally, models were adjusted for time‐varying confounders, including development of comorbidities associated with CKD and introduction of nephrotoxic medications.
This study is not without limitations. Despite a large sample size of 9802 ART‐naïve PLWH without kidney disease at baseline, incident CKD was rare, resulting in limited power to detect differences between exposure groups defined by ART regimen and baseline CKD risk. The overall small number of events, which was further reduced by half after eGFR correction, prevented statistical modelling to evaluate the additional role of anchor ARV class on incident CKD. Moreover, censoring at ART modification may have led to an underestimation of CKD events, which, in turn could have led to bias if more PLWH were switched off TDF before meeting the definition for CKD. TDF exposure was not measured cumulatively, although some studies have suggested that TDF toxicity increases with longer exposure. This may explain in part the smaller impact of TDF and pharmacoenhancers observed in OPERA compared with other studies. Proteinuria was not measured in this study, which may have introduced information bias in the identification of kidney disease. Despite controlling for several important baseline and time‐varying confounders, residual confounding probably remains. Confounding by indication is likely to have arisen after the first reports of TDF nephrotoxicity. Control for such confounding by indication may have been incomplete, despite the flexible adjustment for calendar year at ART initiation. Additionally, there was no adjustment for duration of CKD risk factors such as nephrotoxic medication use. Channelling bias could not be ruled out, although individuals with lower eGFR did not appear to be preferentially channelled away from TDF‐containing regimens. Finally, it is important to note that variability in the frequency of testing for CKD in clinical practice potentially contributed to classification bias, as sicker individuals, those with higher risk of CKD, or those under the care of a more zealous physician may get tested more often.
Our findings indicate that incident CKD was uncommon among ART‐naïve PLWH in this large US‐based clinical population. In individuals with a low baseline D:A:D CKD risk score, the largest group of ART‐naïve PLWH in this study, TDF‐containing regimens did not appear to increase the risk of incident CKD, suggesting that it may remain a viable treatment option in appropriate settings.
Author contributions
LB and JF share the responsibility for the design of this study. LB conducted all the analyses. RH, LB, JF, AB, GP, CW, MW and GF contributed to the interpretation of results. All authors critically reviewed and approved the manuscript and participated sufficiently in the work to take public responsibility for its content.
Supporting information
Table S1 D:A:D chronic kidney disease risk score determination.
Table S2 eGFR correction factors.
Table S3 Chronic kidney disease incidence rates by TDF/D:A:D risk group, with eGFR correction.
Table S4 Association between TDF/boosted regimen and incident chronic kidney disease, with eGFR correction.
Acknowledgements
This research would not be possible without the generosity of PLWH and their OPERA caregivers. Additionally, we are grateful to the following individuals: Robin Beckerman (SAS programming), Jeff Briney (QA), Bernie Stooks (Database Arch & Mgmt), Judy Johnson (Med Terminology Classification), Rodney Mood (Site Support), & Rachel Palmieri Weber (Manuscript Preparation).
Conflicts of interest: RH has received a research grant from Gilead, speaker honoraria and advisory boards from ViiV Healthcare, BMS, Merck, Gilead Sciences and Janssen, and advisory board participation of Epividian. LB, JF and GF are employed by Epividian, Inc.; Epividian has had research funded by ViiV Healthcare, Merck & Co., Inc., Janssen Pharmaceutica and Gilead Sciences. AB and GP are employed by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. CW is a consultant for Epividian, Inc. MW has participated in post‐conference advisory boards for the Conference on Retroviruses and Opportunistic Infections (CROI) and International AIDS Conference (IAC) and also serves as a principal investigator on ViiV Healthcare clinical trials but does not receive personal compensation for this work, which goes directly to the AIDS Healthcare Foundation.
References
- 1. KDIGO Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013; 3: 5–14. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . National Chronic Kidney Disease Fact Sheet, 2017. Atlanta, GA, Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 3. Bash LD, Coresh J, Kottgen A et al. Defining incident chronic kidney disease in the research setting: the ARIC Study. Am J Epidemiol 2009; 170: 414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . Chronic Kidney Disease Surveillance System‐United States. Available at: http://www.cdc.gov/ckd. Accessed 8 January 2020.
- 5. Campos P, Ortiz A, Soto K. HIV and kidney diseases: 35 years of history and consequences. Clin Kidney J 2016; 9: 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mallipattu S, Salem F, Wyatt C. The changing epidemiology of HIV‐related chronic kidney disease in the era of antiretroviral therapy. Kidney Int 2014; 86: 259–265. [DOI] [PubMed] [Google Scholar]
- 7. Bertoldi A, De Crignis E, Miserocchi A et al. HIV and kidney: a dangerous liaison. New Microbiol 2017; 40: 1–10. [PubMed] [Google Scholar]
- 8. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV Department of Health and Human Services 2019. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 18 December 2019.
- 9. Nelson MR, Katlama C, Montaner JS et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. Aids 2007; 21: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 10. Hemkens LG, Ewald H, Santini‐Oliveira M et al. Comparative effectiveness of tenofovir in treatment‐naive HIV‐infected patients: systematic review and meta‐analysis. HIV Clin Trials 2015; 16: 178–189. [DOI] [PubMed] [Google Scholar]
- 11. Gallant JE, DeJesus E, Arribas JR et al. Lamivudine, and Efavirenz for HIV. N Engl J Med 2006; 354: 251–260. [DOI] [PubMed] [Google Scholar]
- 12. Gallant JE, Staszewski S, Pozniak AL et al. Efficacy and safety of tenofovir df vs stavudine in combination therapy in antiretroviral‐naive patients: a 3‐year randomized trial. JAMA 2004; 292: 191–201. [DOI] [PubMed] [Google Scholar]
- 13. Cohen CJ, Molina JM, Cahn P et al. Efficacy and safety of rilpivirine (TMC278) versus efavirenz at 48 weeks in treatment‐naive HIV‐1‐infected patients: pooled results from the phase 3 double‐blind randomized ECHO and THRIVE Trials. J Acquir Immune Defic Syndr 2012; 60: 33–42. [DOI] [PubMed] [Google Scholar]
- 14. Post FA, Moyle GJ, Stellbrink HJ et al. Randomized comparison of renal effects, efficacy, and safety with once‐daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral‐naive, HIV‐1‐infected adults: 48‐week results from the ASSERT study. J Acquir Immune Defic Syndr 2010; 55: 49–57. [DOI] [PubMed] [Google Scholar]
- 15. Mocroft A, Kirk O, Reiss P, De Wit S, Sedlacek D, Beniowski M. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV‐positive patients. AIDS 2010; 24: 1667–1678. [DOI] [PubMed] [Google Scholar]
- 16. Scherzer R, Estrella M, Li Y et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 2012; 26: 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooper R, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta‐analysis: renal safety of tenofovir disoproxil fumarate in HIV‐infected patients. Clin Infect Dis 2010; 51: 496–505. [DOI] [PubMed] [Google Scholar]
- 18. Mocroft A, Lundgren J, Ross M et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV‐positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV 2016; 3: e23–e32. [DOI] [PubMed] [Google Scholar]
- 19. Albini L, Cesana BM, Motta D et al. A randomized, pilot trial to evaluate glomerular filtration rate by creatinine or cystatin C in naive HIV‐infected patients after tenofovir/emtricitabine in combination with atazanavir/ritonavir or efavirenz. J Acquir Immune Defic Syndr 2012; 59: 18–30. [DOI] [PubMed] [Google Scholar]
- 20. Goicoechea M, Liu S, Best B et al. Greater tenofovir‐associated renal function decline with protease inhibitor‐based versus nonnucleoside reverse‐transcriptase inhibitor‐based therapy. J Infect Dis 2008; 197: 102–108. [DOI] [PubMed] [Google Scholar]
- 21. Young J, Schafer J, Fux CA et al. Renal function in patients with HIV starting therapy with tenofovir and either efavirenz, lopinavir or atazanavir. AIDS 2012; 26: 567–575. [DOI] [PubMed] [Google Scholar]
- 22. Cuzin L, Pugliese P, Allavena C et al. Antiretroviral therapy as a risk factor for chronic kidney disease: results from traditional regression modeling and causal approach in a large observational study. PLoS One 2017; 12: e0187517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. LaFleur J, Bress AP, Esker S et al. Brief report: Tenofovir‐associated nephrotoxicity among a US National Historical Cohort of HIV‐Infected Veterans: Risk Modification by Concomitant Antiretrovirals. J Acquir Immune Defic Syndr 2018; 77: 325–330. [DOI] [PubMed] [Google Scholar]
- 24. Kalayjian RC, Lau B, Mechekano RN et al. Risk factors for chronic kidney disease in a large cohort of HIV‐1 infected individuals initiating antiretroviral therapy in routine care. AIDS 2012; 26: 1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. German P, Liu HC, Szwarcberg J et al. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. JAIDS 2012; 61: 32–40. [DOI] [PubMed] [Google Scholar]
- 26. Chu X, Bleasby K, Chan GH, Nunes I, Evers R. The complexities of interpreting reversible elevated serum creatinine levels in drug development: does a correlation with inhibition of renal transporters exist? Drug Metab Dispos 2016; 44: 1498–1509. [DOI] [PubMed] [Google Scholar]
- 27. Milburn J, Jones R, Levy JB. Renal effects of novel antiretroviral drugs. Nephrol Dial Transplant 2016; 32: 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mocroft A, Lundgren J, Ross M et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A: D study. PLoS Medicine 2015; 12: e1001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mills A, Schulman K, Fusco J et al. Validation of the Data Collection on Adverse Events of Anti‐HIV Drugs (D:A:D) chronic kidney disease risk score in HIV‐infected patients in the USA. HIV Med 2020; 21: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brunet L, Wyatt C, Hsu R, Mounzer K, Fusco J, Fusco G. Assessing bias introduced in estimated glomerular filtration rate (eGFR) by the inhibition of creatinine tubular secretion from common antiretrovirals. Antivir Ther 2020, in press. doi: 10.3851/IMP3373 [DOI] [PubMed] [Google Scholar]
- 32. Ryom L, Mocroft A, Kirk O et al. Association between antiretroviral exposure and renal impairment among HIV‐positive persons with normal baseline renal function: the D:A: D study. J Infect Dis 2013; 207: 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ganesan A, Krantz E, Huppler Hullsiek K et al. Determinants of incident chronic kidney disease and progression in a cohort of HIV‐infected persons with unrestricted access to health care. HIV Med 2013; 14: 65–76. [DOI] [PubMed] [Google Scholar]
- 34. Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end‐stage renal disease, in HIV‐infected individuals: a tale of two races. J Infect Dis 2008; 197: 1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gallant JE, Parish MA, Keruly JC, Moore RD. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse‐transcriptase inhibitor treatment. Clin Infect Dis 2005; 40: 1194–1198. [DOI] [PubMed] [Google Scholar]
- 36. Horberg M, Tang B, Towner W et al. Impact of tenofovir on renal function in HIV‐infected, antiretroviral‐naive patients. J Acquir Immune Defic Syndr 2010; 53: 62–69. [DOI] [PubMed] [Google Scholar]
- 37. Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of renal disease among people living with HIV: a systematic review and meta‐analysis. BMC Public Health 2012; 12: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in HIV‐1‐Infected Adults and Adolescents Department of Health and Human Services 2019. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 10 July 2019.
- 39. Hill A, Hughes SL, Gotham D, Pozniak AL. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety? J Virus Eradicat 2018; 4: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ryom L, Kirk O, Lundgren JD et al. Advanced chronic kidney disease, end‐stage renal disease and renal death among HIV‐positive individuals in Europe. HIV Med 2013; 14: 503–508. [DOI] [PubMed] [Google Scholar]
- 41. Ryom L, Dilling Lundgren J, Reiss P et al. Use of contemporary protease inhibitors and risk of incident chronic kidney disease in persons with human immunodeficiency virus: the data collection on adverse events of anti‐HIV drugs (D:A:D) study. J Infect Dis 2019; 220: 1629–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Centers for Disease Control and Prevention . Estimated HIV Incidence and Prevalence in the United States, 2010–2016. 2019 February 2019. Report No.: 1.
- 43. Inker LA, Wyatt C, Creamer R et al. Performance of creatinine and cystatin C GFR estimating equations in an HIV‐positive population on antiretrovirals. J Acquir Immune Defic Syndr 2012; 61: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swanepoel CR, Atta MG, D'Agati VD et al. Kidney disease in the setting of HIV infection: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2018; 93: 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lucas GM, Ross MJ, Stock PG et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 59: e96–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 D:A:D chronic kidney disease risk score determination.
Table S2 eGFR correction factors.
Table S3 Chronic kidney disease incidence rates by TDF/D:A:D risk group, with eGFR correction.
Table S4 Association between TDF/boosted regimen and incident chronic kidney disease, with eGFR correction.


