Abstract
Background
People who inject drugs (PWID) experience barriers to accessing testing and treatment for hepatitis C virus (HCV) infection. Opioid agonist therapy (OAT) may provide an opportunity to improve access to HCV care. This systematic review assessed the association of OAT and HCV testing, treatment, and treatment outcomes among PWID.
Methods
Bibliographic databases and conference presentations were searched for studies that assessed the association between OAT and HCV testing, treatment, and treatment outcomes (direct-acting antiviral [DAA] therapy only) among PWID (in the past year). Meta-analysis was used to pool estimates.
Results
Of 9877 articles identified, 22 studies conducted in Australia, Europe, North America, and Thailand were eligible and included. Risk of bias was serious in 21 studies and moderate in 1 study. Current/recent OAT was associated with an increased odds of recent HCV antibody testing (4 studies; odds ratio (OR), 1.80; 95% confidence interval [CI], 1.36–2.39), HCV RNA testing among those who were HCV antibody–positive (2 studies; OR, 1.83; 95% CI, 1.27–2.62), and DAA treatment uptake among those who were HCV RNA–positive (7 studies; OR, 1.53; 95% CI, 1.07–2.20). There was insufficient evidence of an association between OAT and treatment completion (9 studies) or sustained virologic response following DAA therapy (9 studies).
Conclusions
OAT can increase linkage to HCV care, including uptake of HCV testing and treatment among PWID. This supports the scale-up of OAT as part of strategies to enhance HCV treatment to further HCV elimination efforts.
Keywords: HCV, PWID, IDU, care cascade, injecting drug use
Opioid agonist therapy (OAT) was associated with increased hepatitis C virus (HCV) testing and treatment among people who inject drugs but not treatment completion or sustained virologic response. This supports the scale-up of OAT as part of strategies to enhance HCV treatment to further elimination efforts.
(See the Editorial Commentary by Kattakuzhy and Rosenthal on pages e119–21.)
Globally, 6.1 million people who inject drugs (PWID) are estimated to be living with hepatitis C virus (HCV) infection [1, 2]. The development of simple, effective, direct-acting antiviral treatments (DAAs) for the treatment of HCV infection [3] has been transformative, with evidence that DAAs are having a population-level impact on liver disease burden in settings where treatment scale-up has been broad at the population level [4–7]. The World Health Organization (WHO) has set a goal to eliminate HCV infection as a global public health threat [8]. However, in many settings, HCV testing and treatment uptake remain below the WHO elimination targets, especially among PWID [8]. People who have injected drugs comprise the majority of existing infections in many countries [1, 2, 9]. Strategies to improve HCV testing and treatment outcomes for PWID, therefore, are critical for global HCV elimination efforts.
Opioid agonist therapy (OAT) improves antiretroviral therapy outcomes for human immunodeficiency virus (HIV) infection [10] and reduces the risk of HIV and HCV acquisition [11, 12]. It is hypothesized that OAT may similarly increase engagement of PWID in the HCV care cascade. Although there are studies that have evaluated the uptake of HCV testing [13–20] and treatment uptake [14, 16, 20–24] among PWID, to our knowledge, the association between OAT and HCV testing, treatment uptake, and treatment outcomes has not been systematically reviewed. Understanding the impact of OAT on the cascade of HCV care is critical to inform the implementation of successful strategies to enable progress toward global HCV elimination efforts among PWID.
In order to address this gap, we conducted a systematic review to evaluate the association between OAT and HCV testing and treatment uptake among PWID and to evaluate the association between OAT and adherence, treatment completion, and sustained virologic response (SVR) following DAA treatment among PWID.
METHODS
The study is reported in accordance with PRISMA [25], and the protocol was registered with PROSPERO (CRD42019138921).
Eligibility Criteria
We included observational (cohorts and cross-sectional studies) or experimental studies that investigated HCV testing and treatment if the study met the following criteria: population of people with recent injecting drug use (injecting in the previous 12 months, including active/ongoing/current drug use); reported a comparison of outcomes among people who had and had not received OAT with either methadone or buprenorphine (ever or currently/recently [past 6 months]); and reported 1 of the following outcomes: HCV antibody testing (ever or recently [past year]), HCV RNA testing (ever or recently [past year]), HCV treatment uptake (interferon-based and DAA), and DAA HCV treatment outcomes (adherence, completion, and SVR).
Information Sources and Search
Literature searches of 5 bibliographic databases, including Medline (PubMed), Scopus, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), and PsycINFO, were performed. Presentations at key viral hepatitis conferences were searched, including the International Liver Congress, the Liver Meeting, the Conference on Retroviruses and Opportunistic Infections, and the International Conference on Hepatitis Care in Substance Users. Reference lists of the articles included in the analysis and relevant review articles were hand-searched. Forward citation tracking was carried out using Scopus. Searches were performed in September 2018. For searches of HCV testing and treatment uptake, there was no time restriction. For searches of DAA treatment outcomes, searches were limited to studies published since January 2013 (interferon-free DAA therapies available after this date). Combinations of search terms relating to HCV, drug use, OAT, HCV testing, and treatment were used (Supplementary Materials).
Study Selection
Records identified through primary searches were screened by title and abstract after the removal of duplicates. The full text of potentially eligible records was retrieved, reviewed, and eligible studies included. In the case of multiple publications of a single study, the one with the most up-to-date data was included.
Data Collection Process and Data Items
Data extracted included study characteristics, participant characteristics, testing outcomes, treatment uptake, and treatment outcomes (Supplementary Materials, pp 4–7). Authors were contacted if supplementary data were required and updated/unpublished data were used in analyses.
Risk of Bias in Individual Studies
The risk of bias for the included studies was assessed using the Risk Of Bias In Non-randomised Studies of Interventions (ROBINS-I) tool [26]. Studies were ranked as having low, moderate, serious, or critical risk of bias across 7 domains, and the overall risk of bias was derived.
Study selection, data extraction, and risk of bias appraisal was undertaken by 2 reviewers independently (study selection: J. G. and B. H.; data extraction: A. D., L. T., T. S., and J. G.; and risk of bias appraisal: H. V. and L. T.), with discrepancies discussed with a third reviewer (study selection: L. D.; data extraction: B. H.; and risk of bias appraisal: L. D.).
Synthesis of Results
The primary outcomes of interest were recent or ever HCV antibody testing, recent or ever HCV RNA testing (among those HCV antibody–positive), HCV treatment uptake (among those HCV RNA–positive), and DAA treatment outcomes (adherence, completion, and SVR). Treatment completion was defined as completion of the full course of the prescribed treatment among those who initiated treatment. SVR was defined as unquantifiable HCV RNA at 12 or 24 weeks after the end of treatment for those who initiated treatment (intent-to-treat). The proportion of people with each outcome of interest was assessed, and odds ratios (ORs) were calculated for the association between ever having received OAT and between currently received OAT on each outcome. For HCV treatment uptake, additional analyses were performed to evaluate the association between OAT and DAA treatment. For each study, the outcome measures and corresponding standard errors and 95% confidence intervals (95% CIs) were calculated.
Meta-analysis was used to synthesize the outcome measure estimates. Heterogeneity across studies was assessed using the I2 statistic, with an I2 of less than 25%, 25%–75%, and more than 75% considered as low, moderate, and high heterogeneity, respectively [27]. Random-effect models were used when heterogeneity was medium or high (I2 ≥25%).
Logit transformed outcome estimates were used in all meta-analyses, while the estimates were back-transformed for reporting. A fixed continuity correction of 0.5 was applied where there was a zero cell in calculating ORs. Two-sided P values of less than .05 were deemed to be statistically significant. All analyses were done with Stata version 14.0.
RESULTS
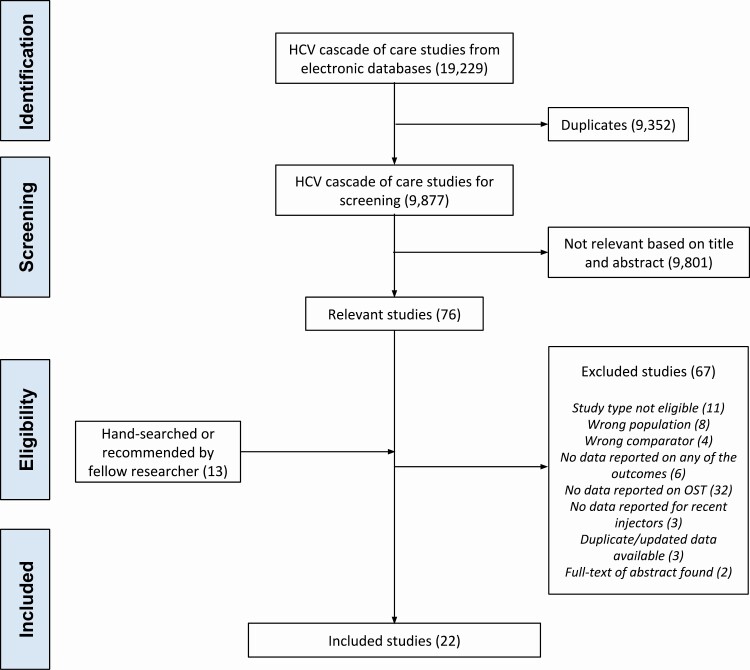
A total of 9877 records in bibliographic databases and 12 records from other sources were identified, with 22 studies included (Figure 1) [13–24, 28–37].
Figure 1.
PRISMA flow chart. Abbreviations: HCV, hepatitis C virus; OAT, opioid agonist therapy.
Study characteristics are summarized in Tables 1, 2 and Supplementary Table 1. We identified 9 published studies that measured the impact of exposure to OAT on having ever received HCV antibody testing (ever OAT, 7 studies [14, 16–19, 28]; recent OAT, 7 studies [13, 14, 16–18, 20, 28]) or recently received HCV antibody testing (ever OAT, 3 studies [18, 28]; recent OAT, 4 studies [15, 18, 20, 28]; Table 2). We identified 5 published studies that measured the impact of exposure to OAT on having ever received HCV RNA testing (ever OAT, 5 studies [14, 16, 17, 28]; recent OAT, 5 studies [14, 16, 17, 20, 28]; Table 2) or recently received HCV RNA testing among those HCV antibody–positive (ever OAT, 2 studies [28]; recent OAT, 2 studies [20, 28]; Table 2). We identified 8 published studies that measured the impact of exposure to OAT on having ever received HCV treatment among those HCV RNA detectable (ever OAT, 6 studies [14, 16, 20–22, 28]; recent OAT, 7 studies [14, 16, 20, 21, 23, 24, 28]; Table 2). We identified 9 published studies that measured the impact of exposure to recent OAT on DAA treatment completion (9 studies) and SVR (9 studies; Supplementary Table 1) (none of these studies included data on ever OAT) [29–38]. There was insufficient data on adherence to include this outcome.
Table 1.
Characteristics of the Studies Included in the Analysis
| Characteristic | Number of Studies (N = 22) (%) | Number of Study Participants |
|---|---|---|
| Study design | ||
| Observational, prospective | 7 (32) | 2016 |
| Observational, retrospective | 5 (23) | 1539 |
| Cross-sectional | 8 (36) | 14 236 |
| Clinical trial | 2 (10) | 305 |
| Study setting | ||
| Community clinic | 3 (14) | 437 |
| Tertiary care | 3 (14) | 431 |
| Needle and syringe program | 5 (23) | 10 357 |
| Mixed | 6 (27) | 3730 |
| Other/not reported | 5 (23) | 2953 |
| Number of centers | ||
| Single-center | 8 (35) | 1359 |
| Multicenter | 14 (64) | 16 549 |
| Definition of recent drug usea | ||
| During the past 1 month | 3 (14) | 1323 |
| During the past 6 months | 14 (64) | 6223 |
| During the past 12 months | 2 (9) | 301 |
| Ongoing or active drug use | 4 (18) | 10 713 |
| Definition of opioid agonist therapya | ||
| Current | 20 (91) | 8574 |
| Past 6 months | 1 (5) | 345 |
| Ever | 8 (36) | 10 867 |
aTotal equals more than 100% due to 6 studies reporting multiple groups.
Table 2.
Characteristics of Included Studies and Reported Outcomes for Hepatitis C Virus (HCV) Antibody Testing, HCV RNA Testing, and HCV Treatment Uptake
| HCV Antibody Testing | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCV Antibody Testing Ever | Recent HCV Antibody Testing | |||||||||||||||||
| First Author, Year (Country) | Study Design | Definition of Recent Injecting Drug Use | Total n | Age Mean or Median, Y | Male (%) | Used Opioids Ever (%) | OAT Ever (%) |
OAT Recently (%) | HCV Antibody Testing Ever (%) | HCV Antibody Testing Recently (%) | No OAT Ever | OAT Ever | No Recent OAT | OAT Recently | No OAT Ever | OAT Ever | No Recent OAT | OAT Recently |
| Bajis, 2019 (Australia) [13] | Observational cohort | Previous 6 months | 605 | 42 | 67 | NA | NA | 65 | 72 | NA | NA | NA | 137/210 (65%) |
297/395 (75%) |
NA | NA | NA | NA |
| Butler, 2015 (Australia) [14] | Cross-sectional | Previous 6 months | 854 | 40 | 64 | 98 | 74 | 44 | 94 | NA | 201/233 (90%) | 601/630 (95%) | 441/477 (92%) | 362/377 (96%) | NA | NA | NA | NA |
| Butler, 2019 (Australia) [16] | Cross-sectional | Previous 6 months | 887 | 43 | 67 | 97 | 64 | 38 | 92 | NA | 272/311 (87%) |
540/571 (95%) |
493/546 (90%) |
323/341 (95%) |
NA | NA | NA | NA |
| Day, 2008 (Australia) [15] | Cross-sectional | Previous 6 months | 197 | 36 | 64 | NA | NA | 68 | NA | 71 | NA | NA | NA | NA | NA | NA | 37/63 (59%) |
103/134 (77%) |
| Gibbs, 2019 (Australia) [28] | Cross-sectional | Previous 6 months | 905 | 43* | 66 | 95 | 66 | 38 | 88 | 57 | 252/308 (82%) |
541/594 (91%) |
482/564 (85%) |
314/341 (92%) |
151/308 (49%) |
364/594 (61%) |
297/564 (53%) |
220/341 (65%) |
| Iakunchykova, 2018 (Ukraine) [17] | Cross-sectional | Ongoing/active | 1002 | 36* | 76 | 100 | 52 | 30 | 83 | NA | 215/481 (45%) |
352/521 (68%) |
215/481 (45%) |
215/300 (72%) |
NA | NA | NA | NA |
| Roux, 2016 (France) [18] | Intervention | Ongoing/active | 202 | 30 | 77 | 98 | 87 | 71 | 82 | 48 | 16/57 (28%) |
149/176 (85%) |
27/57 (47%) |
121/143 (85%) |
7/57 (12%) |
90/176 (51%) |
16/57 (28%) |
73/143 (51%) |
| Ti, 2013 (Thailand) [19] | Cross-sectional | Previous 6 months | 427 | 38 | 81 | NA | 76 | NA | 33 | NA | 13/104 (13%) |
128/323 (40%/) |
NA | NA | NA | NA | NA | NA |
| Valerio, 2019 (Australia) [20] | Observational cohort | Previous 6 months | 1147 | 43 | 65 | 96 | 82 | 67 | 85 | 51 | 139/205 (68%) | 837/942 (89%) | 284/373 (76%) | 692/774 (89%) | 84/205 (41%) | 500/942 (53%) | 159/373 (43%) | 500/774 (65%) |
| HCV RNA Testing | ||||||||||||||||||
| HCV RNA Testing Ever | Recent HCV RNA Testing | |||||||||||||||||
| First Author, Year (Country) | Study Design | Definition of Recent Injecting Drug Use | Total n | Age Mean or Median, Year | Male (%) | Used Opioids Ever (%) | OAT Ever (%) |
OATRecently (%) | HCV RNA Testing Ever (%) | HCV RNA Testing Recently (%) | No OAT Ever | OAT Ever | No Recent OAT | OATRecently | No OAT Ever | OAT Ever | No Recent OAT | OATRecently |
| Butler, 2015 (Australia) [14] | Cross-sectional | Previous 6 months | 547 | 41 | 62 | 99 | 82 | 49 | 59 | NA | 48/96 (50%) |
275/451 (61%) |
155/277 (56%) |
168/270 (62%) |
NA | NA | NA | NA |
| Butler, 2019 (Australia) [16] | Cross-sectional | Previous 6 months | 481 | 44 | 67 | 98 | 77 | 47 | 89 | NA | 97/113 (86%) |
332/368 (90%) |
225/257 (88%) |
203/224 (91%) |
NA | NA | NA | NA |
| Gibbs, 2019 (Australia) [28] | Cross-sectional | Previous 6 months | 796 | 43* | 66 | 95 | 68 | 39 | 68 | 45 | 132/252 (52%) |
405/541 (75%) |
301/482 (62%) |
238/314 (76%) |
84/252 (33%) |
272/541 (50%) |
199/482 (41%) |
158/314 (50%) |
| Iakunchykova, 2018 (Ukraine) [17] | Cross-sectional | Ongoing/active | 1002 | 37* | 76 | 100 | 52 | 30 | 35 | NA | 126/481 (26%) |
220/521 (42%) |
126/481 (26%) |
145/300 (48%) |
NA | NA | NA | NA |
| Valerio, 2019 (Australia) [20] | Observational cohort | Previous 6 months | 796 | 45 | 66 | 99 | 89 | 75 | 77 | 45 | 55/86 (64%) | 559/710 (79%) | 144/202 (71%) | 470/594 (79%) | 32/86 (37%) | 329/710 (46%) | 79/202 (39%) | 282/594 (47%) |
| HCV Treatment Uptake | ||||||||||||||||||
| HCV Treatment Uptake | ||||||||||||||||||
| First Author, Year (Country) | Study Design | Definition of Recent Injecting Drug Use | Total Number of Participants | Age Mean or Median, Year | Male (%) | Used Opioids Ever (%) |
OAT Ever
(%) |
OATRecently (%) | HCV Treatment Ever (%) | No OAT Ever | OAT Ever | No Recent OAT | OATRecently | |||||
| Butler, 2015 (Australia) [14] | Cross-sectional | Previous 6 months | 179 | 41 | 61 | 98 | 88 | 57 | 20 | 6/21 (29%) |
29/158 (18%) |
13/77 (17%) |
18/102 (18%) |
|||||
| Butler, 2019 (Australia) [16] | Cross-sectional | Previous 6 months | 289 | 43 | 72 | 99 | 77 | 45 | 32 | 15/68 (22%) |
77/223 (35%) |
37/159 (23%) |
55/130 (42%) |
|||||
| Gibbs, 2019 (Australia) [28] | Cross-sectional | Previous 6 months | 334 | 44* | 71 | 98 | 81 | 50 | 72 | 35/62 (56%) |
204/271 (75%) |
108/168 (64%) |
131/166 (79%) |
|||||
| Iversen, 2014 (Australia) [21] | Cross-sectional | Ongoing/active | 9478 | 35 | 64 | NA | 81 | 50 | 6 | 128/1767 (7%) | 468/7683 (6%) |
128/1767 (7%) |
283/4743 (6%) |
|||||
| Iverson, 2019 (Australia) [22] | Cross-sectional | Previous 1 month | 486 | NA | 66 | NA | 76 | NA | 41 | 32/117 (27%) |
165/369 (45%) |
NA | NA | |||||
| Makarenko, 2019 (Canada) [23] | Observational cohort | Previous 6 months | 308 | 42 | 85 | NA | NA | 33 | 26 | NA | NA | 46/206 (22%) |
34/102 (33%) |
|||||
| Socías, 2019 (Canada) [24] | Observational cohort | Previous 6 months | 611 | 47 | 60 | NA | NA | 56 | 13 | NA | NA | 25/266 (9%) |
53/345 (15%) |
|||||
| Valerio, 2019 (Australia) [20] | Observational cohort | Previous 6 months | 620 | 44 | 70 | 98 | 89 | 74 | 64 | 29/69 (42%) | 364/551 (66%) | 82/159 (52%) | 311/461 (67%) | |||||
*Asterisk indicates median age. Abbreviations: HCV, hepatitis C virus; OAT, opioid agonist therapy.
Description of Studies
Tables 1 and 2 and Supplementary Table 1 summarize the characteristics of the included studies undertaken in Australia (n = 10), Canada (n = 4), France (n = 1), Georgia (n = 1), Italy (n = 1), Thailand (n = 1), Ukraine (n = 1), and the United States (n = 2). Twenty studies were observational (12 cohort studies and 8 cross-sectional studies), 1 study was a clinical trial, and 1 study was an interventional trial (Table 1). Definition of recent injecting drug use, proportion ever receiving OAT (52%–88%), and proportion recently/currently receiving OAT (25%–73%) varied across studies.
Risk of Bias
Risk of bias was serious in 21 studies and moderate in 1 study (Supplementary Materials). The domains that were most often associated with serious risk of bias included bias due to confounding and bias in the selection of participants. For all other risk of bias domains, most studies were rated as being at low risk of bias. It was not appropriate to conduct sensitivity analyses (eg, excluding studies at serious/critical risk of bias) because all but 1 study met this criteria.
Impact of OAT on HCV Antibody Testing
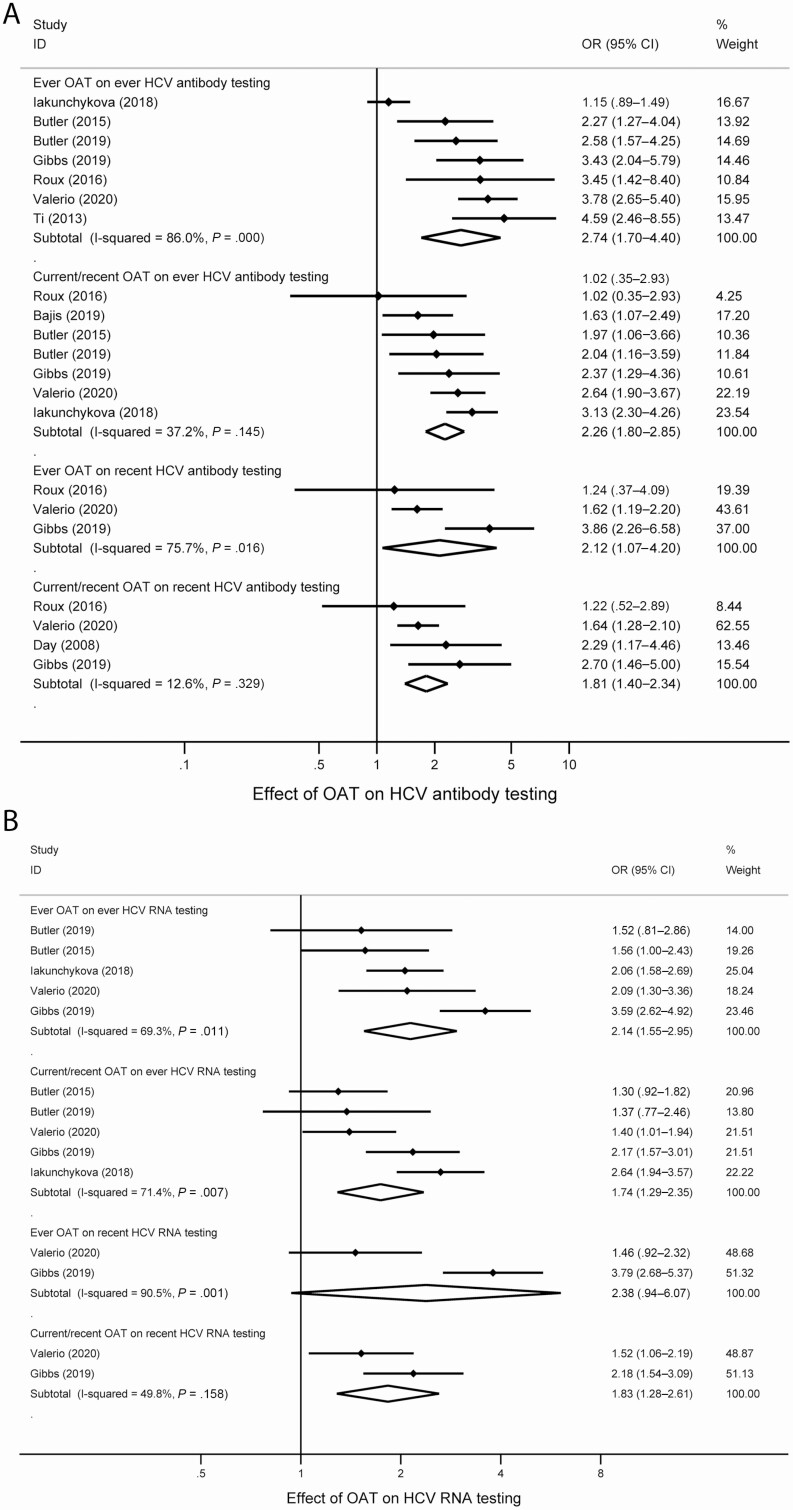
Across 8 studies, the proportion of people who ever received HCV antibody testing was between 33% and 94% (Table 2). Studies were pooled measuring the impact of ever having received OAT (7 studies) and recently/currently receiving OAT (7 studies) on having ever received HCV antibody testing (Figure 2). Random-effect meta-analysis of estimates demonstrated that having ever received OAT was associated with an increased odds of having ever received HCV antibody testing (OR, 2.74; 95% CI, 1.70–4.40; I2 = 86.0%). Recent exposure to OAT was associated with an increased odds of having ever received HCV antibody testing (OR, 2.26; 95% CI, 1.80–2.85; I2 = 37.2%).
Figure 2.
Forest plots examining the association between and (A) HCV antibody and (B) HCV RNA testing. Abbreviations: CI, confidence interval; HCV, hepatitis C virus; OAT, opioid agonist therapy; OR, odds ratio.
The proportion who recently received HCV antibody testing was between 48% and 71% (Table 2). We also pooled data from studies that measured the impact of ever having received OAT (3 studies) and recently/currently receiving OAT (4 studies) on having recently received HCV antibody testing (Figure 2). Having ever received OAT was associated with an increased odds of recent HCV antibody testing (OR, 2.12; 95% CI, 1.07–4.20; I2 = 75.7%). Recent exposure to OAT was associated with an increased odds of recent HCV antibody testing (OR, 1.81; 95% CI, 1.40–2.34; I2 = 12.6%).
Impact of OAT on HCV RNA Testing
The proportion of people who had ever received HCV RNA testing among those who were HCV antibody–positive was between 35% and 89% (Table 2). Studies were pooled measuring the impact of ever having received OAT (5 studies) and recently/currently receiving OAT (5 studies) on having ever received HCV RNA testing (Figure 2). Having ever received OAT was associated with an increased odds of having ever received HCV RNA testing (OR, 2.14; 95% CI, 1.55–2.95; I2 = 69.3%). Recent OAT exposure was associated with an increased odds of having ever received HCV RNA testing (OR, 1.74; 95% CI, 1.29–2.35; I2 = 71.4%).
The proportion who had recently received HCV RNA testing was 44% in 1 study and 45% in the other study (Table 2). We pooled data from studies that measured the impact of ever having received OAT (2 studies) and having recently/currently receiving OAT (2 studies) on having recently received HCV RNA testing (Figure 2). Having ever received OAT was not associated with an increased odds of having recently received HCV RNA testing (OR, 2.38; 95% CI, .94–6.07; I2 = 90.5%). Having recently received OAT was associated with an increased odds of having received HCV RNA testing (OR, 1.83; 95% CI, 1.28–2.61; I2 = 49.8%).
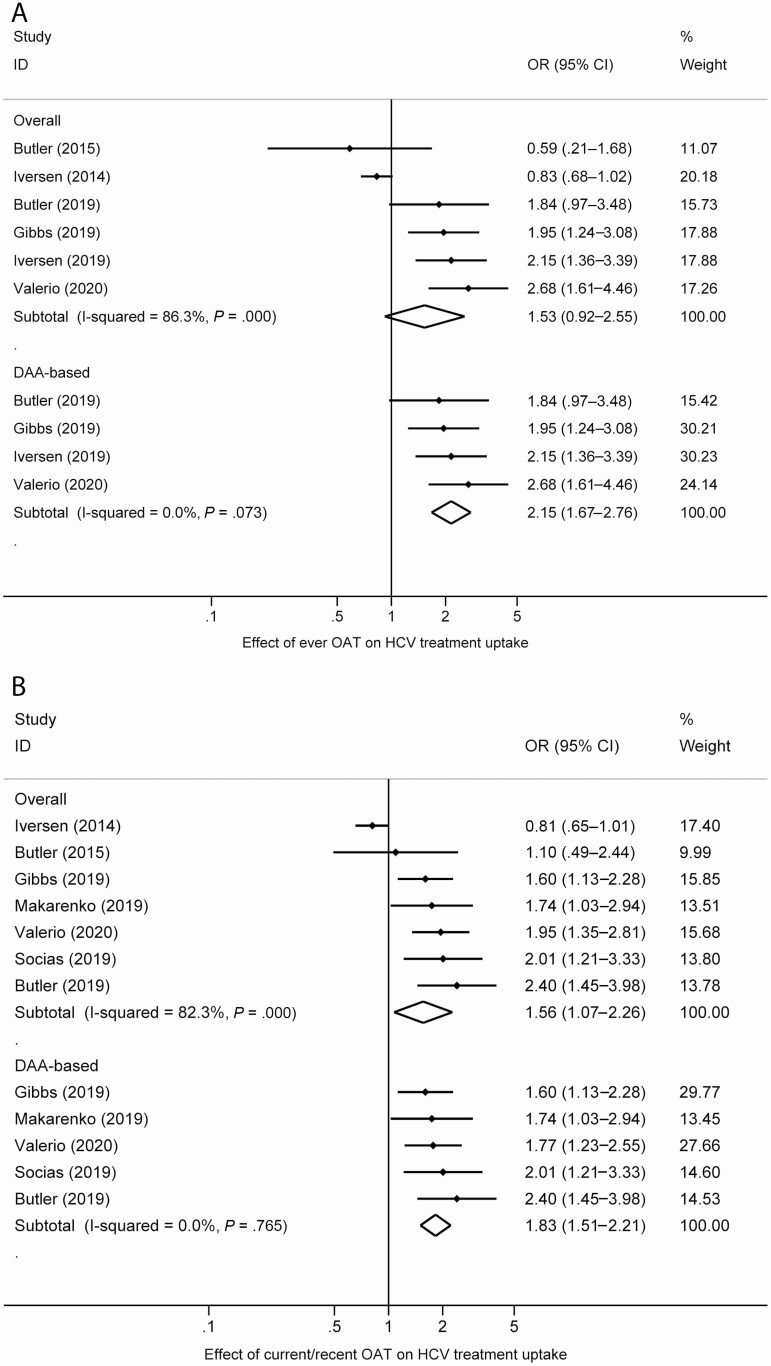
Impact of OAT on HCV Treatment Uptake
The proportion of people who had ever received HCV treatment among those who were HCV RNA detectable was between 6% and 72% (Table 2). Data from studies that measured the impact of ever having received OAT (6 studies; DAA: 4 studies) and recently/currently receiving OAT (7 studies; DAA: 5 studies) on having ever received HCV treatment were pooled (Figure 3). The association of having ever received OAT and having ever received HCV treatment was not statistically significant (OR, 1.53; 95% CI, .92–2.55; I2 = 86.3%). Recent OAT exposure was associated with an increased odds of having ever received HCV treatment (OR, 1.56; 95% CI, 1.07–2.26; I2 = 82.3%). The intervention association strengthened and heterogeneity decreased when only studies in the DAA era were considered (6 studies; OR, 1.83; 95% CI, 1.51–2.21; I2 = 0.0%). Having ever received OAT was associated with an increased odds of having ever received DAA HCV treatment (4 studies; OR, 2.15; 95% CI, 1.67–2.76; I2 = 0.0%).
Figure 3.
Forest plots examining the association between (A) ever OAT and (B) current/recent OAT and HCV treatment uptake. Abbreviations: CI, confidence interval; HCV, hepatitis C virus; OAT, opioid agonist therapy; OR, odds ratio.
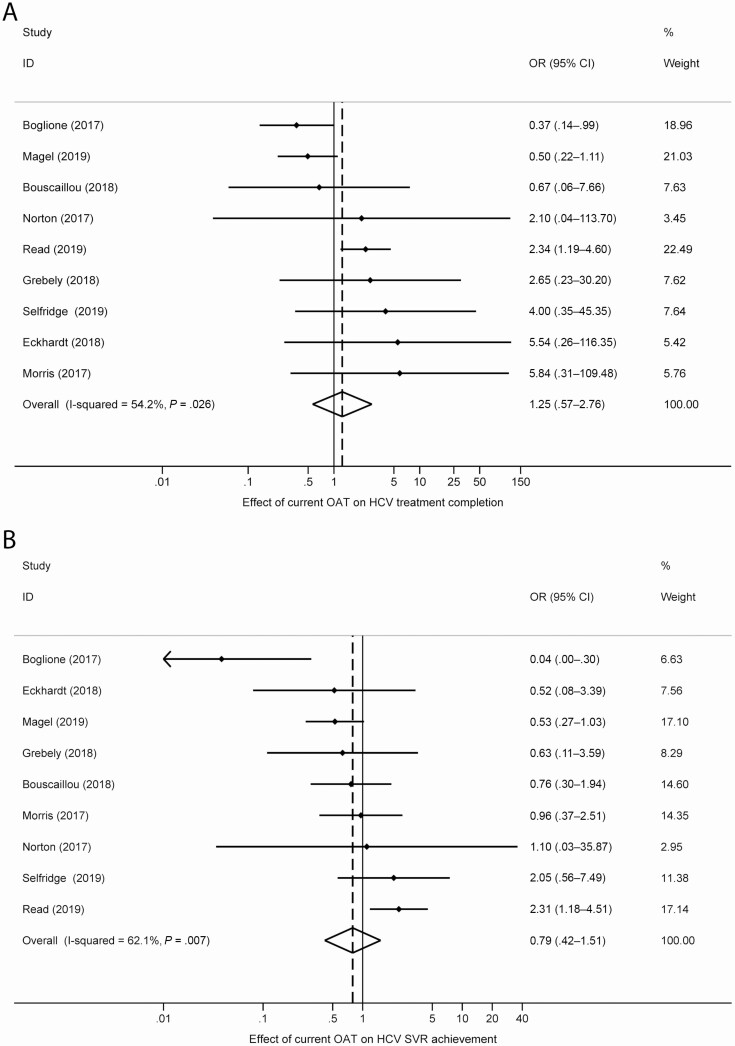
Impact of OAT on HCV Treatment Completion and SVR
The proportion of people who had completed HCV treatment among those who initiated HCV treatment was between 65% and 100% and the proportion who had achieved SVR was between 64% and 94% (Supplementary Table 1). We pooled data from studies that measured the impact of recently/currently receiving OAT on having completed HCV treatment (9 studies) or having achieved an SVR (9 studies; Figure 4). There was no impact of having recently received OAT on treatment completion (OR, 1.25; 95% CI, .57–2.76; I2 = 54.2%) or SVR (OR, 0.79; 95% CI, .42–1.51; I2 = 62.1%).
Figure 4.
Forest plots examining the association between current OAT and (A) treatment completion and (B) SVR. Abbreviations: CI, confidence interval; HCV, hepatitis C virus; OAT, opioid agonist therapy; OR, odds ratio; SVR, sustained virologic response.
DISCUSSION
We found evidence of an association between recent OAT exposure and ever receiving OAT on HCV testing and treatment uptake among PWID. Recent OAT was not associated with DAA treatment completion or SVR. These data have important implications for clinical management and health policy, supporting the integration of services for the treatment of opioid dependence and HCV care among PWID.
OAT was associated with improvements in HCV testing and treatment uptake, consistent with literature demonstrating that OAT reduces harms across multiple health outcomes for people who are opioid dependent [39]. OAT improves engagement in HIV treatment, adherence, and virologic suppression [10]. OAT is also associated with reductions in injecting risk behavior [40], risk of HIV and HCV infections [11, 12], criminal activity [41], and all-cause [42] and overdose [42] mortality. It is unsurprising that current OAT was not associated with DAA treatment completion or SVR, given the high proportion of PWID who complete and respond to DAA therapy [43].
The mechanism behind the association between OAT and improvements in HCV testing and treatment is likely multifactorial, relating to the interplay between system-, provider-, social-, and patient-level factors. Most people receiving OAT attend drug treatment clinics or community health centers that provide services other than OAT, including other medical care (including HCV), mental health services, and vocational and other assistance. People receiving OAT often have regular contact with health services with persistent cues for engagement and education [44], offering increased opportunities for engaging in HCV education, testing and treatment, particularly when services are integrated and on-site [45].
Qualitative interviews with people receiving and providing services in drug treatment clinics have highlighted key facilitators for engagement in HCV care [44, 46–51]. In drug treatment settings, engagement in HCV care is facilitated by existing relationships of trust between people receiving OAT and their healthcare providers [46–50], with HCV care providing opportunities to strengthen therapeutic relationships [51]. People using drug treatment services report that the provision of HCV testing and treatment on-site allows more immediate and accessible care [49]. This eliminates the need for often problematic and unsuccessful referral from OAT to off-site hospital-based models of HCV care [49], which may be associated with negative, stigmatizing, or discriminatory experiences [44, 47]. People receiving OAT also highlight that drug treatment clinics offer the potential for greater familiarity [47, 48, 51], flexibility [47], and convenience through on-site care (including reduced travel time and costs) [44, 47–51].
Integration of OAT and HCV treatment has been shown to be highly acceptable to both clients and staff [52]. In a study of people with ongoing injecting drug use and opioid dependence offered HCV and buprenorphine treatment, 79% (53 of 67) not receiving OAT at baseline subsequently initiated buprenorphine during HCV therapy, with reductions in injecting risk observed among those receiving OAT [53]. Integration of OAT and HCV services can occur in a range of settings where people are already accessing health services (eg, drug treatment clinics, HIV clinics, harm reduction services) in combination with different interventions (eg, financial incentives, telemedicine, peer-based support) [13, 45]. No one size will fit all, with models of care requiring person-centric approaches [54]. However, key barriers to HCV treatment among PWID must be addressed, including stigma, housing, criminalization, and healthcare systems [55].
Major strengths of this study include synthesizing estimates for the association of OAT with components of the HCV cascade of care among PWID and the supplementary data included through contacting authors. Key limitations of the evidence include the small number of studies and that the majority of studies were from 1 country (Australia). Most studies were at serious risk of bias due to the potential for confounding and biases in the selection of participants into the studies. The control of confounders was limited and inconsistent across the studies. As such, unadjusted ORs had to be pooled and there were insufficient studies to perform a meta-regression to explore sources of heterogeneity. The majority of studies identified were cross-sectional, and the effect of residual confounding on OAT and components of the HCV cascade of care cannot be ruled out. People who accessed OAT may also have been more likely to have characteristics that may have led to increased HCV testing and treatment uptake. Since most studies were cross-sectional, it is possible that OAT use may not have preceded the outcome. This temporality of the association between the exposure (OAT) and outcome (HCV testing and treatment) is a limitation. We cannot, therefore, assume that OAT use commenced before, rather than after, HCV testing or treatment. The impact of OAT on HCV testing and treatment uptake at a population level will also be determined by the proportion of PWID within that population with opioid dependence. Although the majority of studies had a high proportion of participants with a history of opioid use, not all participants may have been opioid dependent and/or required OAT. This misclassification bias may have overestimated the observed association between OAT and HCV outcomes.
In conclusion, this study demonstrated that recent OAT was associated with improvements in HCV testing and treatment uptake, supporting the integration of HCV services in drug treatment settings. This study also provides important information to inform mathematical modeling of interventions to enhance HCV care among PWID. Further work is needed to understand strategies to optimize HCV testing and treatment within drug treatment settings and improve the overall health of people who use drugs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. J. G., L. T., L. D., S. L., M. H., P. V., and B. H. conceived the scope of the review, which was critically revised by all coauthors. Screening, review, data extraction, and verification was done by J. G., L. T., L. D., A. D-D., T. S., H.V., and B. H. Data analysis was done by L. T. and T. S., which was reviewed by J. G. J. G., L. D., and B. H. drafted the first draft of the manuscript. All authors made substantial contributions to the critical review, editing, and revision of the manuscript. All authors approved the final version of the manuscript.
Acknowledgments. The authors thank the following individuals who responded to requests for additional data: Sahar Bajis (the Kirby Institute, University of New South Wales [UNSW] Sydney, Sydney, Australia); Olena Iakunchykova (State University of New York at Albany); Frederick Altice (Yale University School of Medicine, New Haven, Connecticut); Perrine Roux and Salim Mezaache (Inserm, Paris, France); Carolyn Day (University of Sydney, Australia); Jennifer Iversen (the Kirby Institute, UNSW Sydney, Australia); Julie Bouscaillou, Tamar Kikvidze, and Niklas Luhmann (Médecins du Monde, Paris, France); Kelli Wuerth and Brian Conway (Vancouver Infectious Diseases Centre, Canada); Lucio Boglione and Antonio Davolio (Unit of Infectious Diseases, University of Turin, Italy); Benjamin Eckhardt (New York University Medical Center, New York); Alain Litwin, Brianna Norton, Matthew Akiyama, and Linda Agyemang (Albert Einstein College of Medicine and Montefiore Medical Center, New York, New York); Chris Fraser and Marion Selfridge (Cool Aid Community Health Centre, Victoria, Canada); Evan Cunningham (the Kirby Institute, UNSW Sydney, Australia); and Leith Morris (School of Public Health, University of Queensland, Australia).
Disclaimer. The views expressed in this publication do not necessarily represent the position of the Australian Government.
Financial support. The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The Australian National Drug and Alcohol Research Centre is supported by funding from the Australian Government Department of Health under the Drug and Alcohol Program. The Australian National Drug and Alcohol Research Centre, UNSW Sydney, provided some funding toward the costs of this systematic review. J. G. is supported by an Australian National Health and Medical Research Council (NHMRC) Investigator Grant. L. D. is supported by an NHMRC Senior Principal Research Fellowship. L. D. and S. L. are supported by a National Institute of Health National Institute on Drug Abuse grant (R01DA1104470). M. H. and P. V. acknowledge support from the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Evaluation of Interventions. P. V. acknowledges support from the HPRU in sexually transmitted infections and blood borne virus and National Institute for Drug Abuse (grant R01 DA037773–01A1). G. D. is supported by an NHMRC Practitioner Fellowship. B. H. is supported by an Australian NHMRC Early Career Fellowship.
Potential conflicts of interest. J. G. reports grants and personal fees from AbbVie, Gilead Sciences, Merck, and Cepheid and grants from Hologic and Indivior outside the submitted work. L. D. reports grants from Indivior and Seqirus outside the submitted work. S. L. reports grants from Indivior outside the submitted work. M. H. reports personal fees from AbbVie, Gilead Sciences, and Merck outside the submitted work. P. R. reports speaker fees and institutional research funding from Gilead Sciences and speaker fees from MSD and AbbVie. G. D. reports grants from Gilead, AbbVie, Merck, and Bristol-Myers Squibb; personal fees from Gilead, AbbVie, and Merck; and nonfinancial support from Gilead, AbbVie, and Merck outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5:e1192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grebely J, Larney S, Peacock A, et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction 2019; 114:150–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017; 166:637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alavi M, Law MG, Valerio H, et al. Declining hepatitis C virus-related liver disease burden in the direct-acting antiviral therapy era in New South Wales, Australia. J Hepatol 2019; 71:281–8. [DOI] [PubMed] [Google Scholar]
- 5. Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology 2017; 65:804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belli LS, Berenguer M, Cortesi PA, et al. ; European Liver and Intestine Association . Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: a European study. J Hepatol 2016; 65:524–31. [DOI] [PubMed] [Google Scholar]
- 7. Kim D, Li AA, Gadiparthi C, et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology 2018; 155:1154–63 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Geneva, Switzerland: WHO. [Google Scholar]
- 9. Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 2013; 10:553–62. [DOI] [PubMed] [Google Scholar]
- 10. Low AJ, Mburu G, Welton NJ, et al. Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta-analysis. Clin Infect Dis 2016; 63:1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012; 345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction 2018; 113:545–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bajis S, Grebely J, Hajarizadeh B, et al. ; LiveRLife Study Group . Hepatitis C virus testing, liver disease assessment and treatment uptake among people who inject drugs pre- and post-universal access to direct-acting antiviral treatment in Australia: the LiveRLife study. J Viral Hepat 2020; 27:281–93. [DOI] [PubMed] [Google Scholar]
- 14. Butler K, Day C, Dietze P, Bruno R, Alati R, Burns L. The potential reach of opioid substitution settings to deliver HCV care to people who inject drugs in Australia. J Subst Abuse Treat 2015; 58:90–4. [DOI] [PubMed] [Google Scholar]
- 15. Day CA, White B, Thein HH, et al. Experience of hepatitis C testing among injecting drug users in Sydney, Australia. AIDS Care 2008; 20:116–23. [DOI] [PubMed] [Google Scholar]
- 16. Butler K, Larney S, Day CA, Burns L. Uptake of direct acting antiviral therapies for the treatment of hepatitis C virus among people who inject drugs in a universal health-care system. Drug Alcohol Rev 2019; 38:264–9. [DOI] [PubMed] [Google Scholar]
- 17. Iakunchykova O, Meteliuk A, Zelenev A, Mazhnaya A, Tracy M, Altice FL. Hepatitis C virus status awareness and test results confirmation among people who inject drugs in Ukraine. Int J Drug Policy 2018; 57:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roux P, Castro DR, Ndiaye K, et al. Increased uptake of HCV testing through a community-based educational intervention in difficult-to-reach people who inject drugs: results from the ANRS-AERLI study. PLoS One 2016; 11:e0157062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ti L, Kaplan K, Hayashi K, Suwannawong P, Wood E, Kerr T. Low rates of hepatitis C testing among people who inject drugs in Thailand: implications for peer-based interventions. J Public Health (Oxf) 2013; 35:578–84. [DOI] [PubMed] [Google Scholar]
- 20. Valerio H, Alavi M, Silk D, et al. Uptake of testing, linkage to care, and treatment for hepatitis C infection among people who inject drugs in Australia: the ETHOS Engage study. J Hepatol 2019; 70:e42. [Google Scholar]
- 21. Iversen J, Grebely J, Topp L, Wand H, Dore G, Maher L. Uptake of hepatitis C treatment among people who inject drugs attending needle and syringe programs in Australia, 1999–2011. J Viral Hepatitis 2014; 21:198–207. [DOI] [PubMed] [Google Scholar]
- 22. Iversen J, Dore GJ, Catlett B, Cunningham P, Grebely J, Maher L. Association between rapid utilisation of direct hepatitis C antivirals and decline in the prevalence of viremia among people who inject drugs in Australia. J Hepatol 2019; 70:33–9. [DOI] [PubMed] [Google Scholar]
- 23. Makarenko J, Artenie AA, Hoj S, et al. Transitioning from interferon-based to direct antiviral treatment options: a potential shift in barriers and facilitators of treatment initiation among people who use drugs? Int J Drug Policy 2019; 72:69–76. [DOI] [PubMed] [Google Scholar]
- 24. Socías ME, Ti L, Wood E, et al. Disparities in uptake of direct-acting antiviral therapy for hepatitis C among people who inject drugs in a Canadian setting. Liver Int 2019; 39:1400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 26. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gibbs D, Larney S, Grebely J, et al. Hepatitis C virus cascade of care among people who inject drugs: a cross-sectional study of characteristics associated with HCV testing and treatment in Australia. In: 2019 NDARC Annual Research Symposium. Sydney, Australia, 2019.
- 29. Boglione L, Mornese Pinna S, De Nicolò A, et al. Treatment with direct-acting antiviral agents of hepatitis C virus infection in injecting drug users: a prospective study. J Viral Hepat 2017; 24:850–7. [DOI] [PubMed] [Google Scholar]
- 30. Bouscaillou J, Kikvidze T, Butsashvili M, et al. Direct acting antiviral-based treatment of hepatitis C virus infection among people who inject drugs in Georgia: a prospective cohort study. Int J Drug Policy 2018; 62:104–11. [DOI] [PubMed] [Google Scholar]
- 31. Eckhardt BJ, Scherer M, Winkelstein E, Marks K, Edlin BR. Hepatitis C treatment outcomes for people who inject drugs treated in an accessible care program located at a syringe service program. Open Forum Infect Dis 2018; 5:ofy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grebely J, Dalgard O, Conway B, et al. ; SIMPLIFY Study Group . Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol 2018; 3:153–61. [DOI] [PubMed] [Google Scholar]
- 33. Conway B, Raycraft T, Alimohammadi A, Bhutani Y, Kiani G, Hakobyan S. Efficacy of all-oral HCV therapy in people who inject drugs (PWID). Hepatology 2016; 64:990A-A.26705089 [Google Scholar]
- 34. Morris L, Smirnov A, Kvassay A, et al. Initial outcomes of integrated community-based hepatitis C treatment for people who inject drugs: findings from the Queensland Injectors’ Health Network. Int J Drug Policy 2017; 47:216–20. [DOI] [PubMed] [Google Scholar]
- 35. Norton BL, Fleming J, Bachhuber MA, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy 2017; 47:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Read P, Lothian R, Chronister K, et al. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int J Drug Policy 2019; 47:209–15. [DOI] [PubMed] [Google Scholar]
- 37. Selfridge M, Cunningham EB, Milne R, et al. Direct-acting antiviral treatment for hepatitis C, reinfection and mortality among people attending an inner-city community health centre in Victoria, Canada. Int J Drug Policy 2019; 72:106–13. [DOI] [PubMed] [Google Scholar]
- 38. Magel T, Holeksa J, Thiam A, Chu L, Yung R, Truong D, Conway B. HCV treatment outcomes among current and remore people to inject drugs (PWID): real life data. International Liver Conference (Abstract THU-126), Vienna, Austria, 10–14 April 2019. [Google Scholar]
- 39. Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet 2019; 394:1560–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev 2011:CD004145. [DOI] [PubMed] [Google Scholar]
- 41. Maglione MA, Raaen L, Chen C, et al. Effects of medication assisted treatment (MAT) for opioid use disorder on functional outcomes: a systematic review. J Subst Abuse Treat 2018; 89:28–51. [DOI] [PubMed] [Google Scholar]
- 42. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017; 357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2018; 3:754–67. [DOI] [PubMed] [Google Scholar]
- 44. Treloar C, Rance J, Grebely J, Dore GJ. Client and staff experiences of a co-located service for hepatitis C care in opioid substitution treatment settings in New South Wales, Australia. Drug Alcohol Depend 2013; 133:529–34. [DOI] [PubMed] [Google Scholar]
- 45. Socías ME, Karamouzian M, Parent S, Barletta J, Bird K, Ti L. Integrated models of care for people who inject drugs and live with hepatitis C virus: a systematic review. Int J Drug Policy 2019; 72:146–59. [DOI] [PubMed] [Google Scholar]
- 46. Swan D, Long J, Carr O, et al. Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration. AIDS Patient Care STDS 2010; 24:753–62. [DOI] [PubMed] [Google Scholar]
- 47. Harris M, Rhodes T, Martin A. Taming systems to create enabling environments for HCV treatment: negotiating trust in the drug and alcohol setting. Soc Sci Med 2013; 83:19–26. [DOI] [PubMed] [Google Scholar]
- 48. Treloar C, Newland J, Rance J, Hopwood M. Uptake and delivery of hepatitis C treatment in opiate substitution treatment: perceptions of clients and health professionals. J Viral Hepat 2010; 17:839–44. [DOI] [PubMed] [Google Scholar]
- 49. Treloar C, Rance J, Dore GJ, Grebely J; ETHOS Study Group . Barriers and facilitators for assessment and treatment of hepatitis C virus infection in the opioid substitution treatment setting: insights from the ETHOS study. J Viral Hepat 2014; 21:560–7. [DOI] [PubMed] [Google Scholar]
- 50. Treloar C, Rance J; ETHOS Study Group . How to build trustworthy hepatitis C services in an opioid treatment clinic? A qualitative study of clients and health workers in a co-located setting. Int J Drug Policy 2014; 25:865–70. [DOI] [PubMed] [Google Scholar]
- 51. Rance J, Treloar C; ETHOS Study Group . ‘Not just methadone Tracy’: transformations in service-user identity following the introduction of hepatitis C treatment into Australian opiate substitution settings. Addiction 2014; 109:452–9. [DOI] [PubMed] [Google Scholar]
- 52. Chronister KJ, Lothian R, Gilliver R, Kearley J, Read P. Feasibility and acceptability of adherence support for direct acting antiviral therapy for hepatitis C in a low-threshold primary health-care opioid agonist treatment program. Drug Alcohol Rev 2019; 38:185–9. [DOI] [PubMed] [Google Scholar]
- 53. Rosenthal ES, Silk R, Mathur P, et al. Concurrent initiation of hepatitis C and opioid use disorder treatment in people who inject drugs. Clin Infect Dis 2020; ciaa105. doi: 10.1093/cid/ciaa105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harris M, Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J 2013; 10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Savic M, Best D, Manning V, Lubman DI. Strategies to facilitate integrated care for people with alcohol and other drug problems: a systematic review. Subst Abuse Treat Prev Policy 2017; 12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.