Abstract
Purpose
To evaluate the long‐term efficacy and safety of two minimally invasive glaucoma surgery implants with a subconjunctival drainage approach: the XEN45 Gel Stent® (Xen) implant and the PRESERFLO™ MicroShunt (MicroShunt).
Methods
Retrospective comparative case series of primary open‐angle glaucoma (POAG) patients with at least 6 months of follow‐up after a MicroShunt or Xen implantation augmented with mitomycin C.
Results
Forty‐one eyes of 31 patients underwent Xen implantation, and 41 eyes of 33 patients, MicroShunt implantation. Baseline characteristics were similar, except for more combined surgeries with phacoemulsification in the Xen group (37% vs. 2%). Mean baseline IOP ± standard deviation dropped from 19.2 ± 4.4 to 13.8 ± 3.8 mmHg (n = 26) in the Xen group and from 20.1 ± 5.0 to 12.1 ± 3.5 (n = 14) in the MicroShunt group at 24 months of follow‐up (p = 0.19, t‐test). The number of IOP‐lowering medications dropped from 2.5 ± 1.4 to 0.9 ± 1.2 in the Xen group and from 2.3 ± 1.5 to 0.7 ± 1.1 in the MicroShunt group. The probability of qualified success was 73% and 79% at 24 months of follow‐up for the Xen and MicroShunt groups, respectively. Postoperative complications were usually mild and self‐limiting. The number of bleb needling and secondary glaucoma surgery procedures was similar in both groups; however, in the Xen group more additional MicroPulse® transscleral cyclophotocoagulation procedures were performed.
Conclusion
Xen Gel Stent and PreserFlo MicroShunt implantations achieved comparable results in POAG eyes in terms of IOP‐lowering and surgical success, with a similar high safety profile.
Keywords: glaucoma, minimally invasive glaucoma surgery, PRESERFLO MicroShunt, surgery, Xen gel stent
Introduction
In recent years, surgical options for the treatment of glaucoma have increased with the introduction of minimally invasive glaucoma surgery (MIGS). These surgeries aim to reduce intraocular pressure (IOP) in a safer and less traumatic manner in comparison with traditional glaucoma filtering surgery (Francis et al. 2011; Caprioli et al. 2015).
The mechanism of action differs among MIGS. The XEN® 45 Gel Stent (Allergan Inc., Dublin, Ireland) (Xen) (Lewis 2014) and the PRESERFLO™ MicroShunt (Santen, Osaka, Japan) (MicroShunt) (Pinchuk et al. 2017) drain aqueous humour into the subconjunctival space, similar to the gold standard, trabeculectomy (Cairns 1968). The Xen, usually implanted via an ab interno approach with an injector, is a 6‐mm‐long and flexible tube with a 45‐µm lumen made of cross‐linked porcine gelatine (Lewis 2014). The 8.5‐mm‐long MicroShunt is made of a stable and flexible polymer ‘SIBS’ (poly[styrene‐block‐isobutylene‐block‐styrene]), which is already used for long‐term implantation in the body in cardiac stents (Pinchuk et al. 2008). It has a lumen diameter of 70 µm and is implanted via an ab externo approach (Pinchuk et al. 2017). The design of both tubes is based on the Hagen–Poiseuille equation, limiting early postoperative hypotony (Sheybani et al. 2015). To reduce the risk of fibrosis, both procedures are augmented with intraoperative application or injection of mitomycin C (MMC).
To the best of our knowledge, there is currently no report comparing these two techniques. In this study, we compare both the effectiveness and safety of Xen versus MicroShunt implantations after a follow‐up of 2 years.
Methods
Study design
This is a retrospective study comparing the effectiveness, safety and success rate of Xen and MicroShunt implantations augmented with MMC in patients with primary open‐angle glaucoma (POAG). The study protocol adhered to the tenets of the Declaration of Helsinki and was conducted after approval by the local ethics committee. All surgeries were performed by experienced glaucoma surgeons who were proficient in both procedures under study.
Patients and assessments
We included all Xen and MicroShunt implantations consecutively performed in patients with POAG between November 2014 and June 2019 at the University Eye Clinic, Maastricht, the Netherlands. Patients were eligible for surgery if they were inadequately controlled on maximum tolerated medical therapy and/or had progression of visual field loss. Patients were excluded from the analysis if they had less than 6 months of follow‐up.
Baseline data collected from the patient history included age, sex, race, systemic and ocular history, the treated eye, glaucoma severity, IOP and IOP‐lowering medication use. Glaucoma severity was defined using the Hodapp–Parrish–Anderson (H‐P‐A) classification (Hodapp et al. 1993). Postoperative evaluations were documented on day 1, week 1 and months 1, 3, 6, 12, 18 and 24 using the acceptable time windows proposed by the World Glaucoma Association (WGA) guidelines (Shaaraway et al. 2009). At each time‐point, slit‐lamp examination, medication use, IOP assessment by Goldmann applanation tonometry, complications and additional interventions were extracted from clinical records.
Ab interno Xen Gel Stent implantation
The Xen implantation was performed under sub‐Tenon’s (90%), retrobulbar or topical anaesthesia using an ab interno approach. After anaesthesia, reference points were marked in the superonasal conjunctiva, 3 mm from the limbus. Prior to the procedure, 0.1 ml of a 0.2 mg/ml MMC solution was injected in the nasal superior quadrant, under Tenon’s capsule, and massaged over the area of the anticipated insertion site. A main 1.2‐mm corneal paracentesis incision was made in the inferotemporal quadrant, and the anterior chamber (AC) was filled with an Ophthalmic viscosurgical device (ProVisc®, Alcon Laboratories Inc., Fort Worth, TX, USA) (OVD). The injector was directed through the incision across the AC towards the superonasal quadrant and placed through the sclera in the subconjunctival space with 1–2 mm left in the AC. Placement was confirmed immediately after the implantation using a goniolens. If the surgery was combined with cataract extraction, a regular phacoemulsification was performed after MMC injection. After instilment of a miotic drug (MIOSTAT™, Alcon Laboratories Inc.), the XEN stent was implanted. In all cases, the OVD was carefully removed at the end of the procedure, and tube flow checked.
Ab externo MicroShunt procedure
All MicroShunt implantations were performed under sub‐Tenon’s anaesthesia. A fornix‐based conjunctival flap was created in the superonasal or superotemporal quadrant. A deep sub‐Tenon’s pocket was formed, and wet cautery was applied to the scleral vessels in the intended area of placement. Mitomycin C (MMC) (0.2 mg/ml) was applied to the scleral surface using several in MMC‐soaked Lasik shields placed under the conjunctival/Tenon’s flap for 3 min. After the shields were removed, the area was thoroughly rinsed with balanced salt solution (BSS®, Alcon Laboratories, Inc.). The sclera was marked 3 mm from the limbus. A scleral pocket was made using a 1‐mm triangular knife, after which in this pocket a 25‐gauge needle was introduced to create a needle track into the AC. The MicroShunt was inserted into the AC through the needle track, tucking the fin of the device tightly into the pocket. After checking for flow, Tenon’s capsule and conjunctiva were sutured in a watertight manner. If the implantation was combined with cataract extraction, phacoemulsification was performed after the irrigation of MMC. In a combined procedure, the OVD was carefully removed after phacoemulsification, after which a miotic drug (MIOSTAT™, Alcon Laboratories, Inc.) was instilled and the eye pressurized with BSS, before placing the MicroShunt.
Postoperative management
Both procedures were directly followed by a prophylactic intracameral antibiotic injection [cefuroxime, (Aprokam®, Laboratoires Théa, Clermont‐Ferrand, France)] and an anti‐inflammatory subconjunctival steroid (dexamethasone) injection in the operating room. All IOP‐lowering medications were discontinued immediately after the surgery. Postoperatively, topical unpreserved antibiotic prophylaxis [ofloxacin, (Trafloxal®, Bausch & Lomb Pharma, Bridgewater, NJ, USA)] was prescribed four times daily during a period of two weeks. Topical anti‐inflammatory therapy with unpreserved steroids (dexamethasone 0.1%) started at four to six times a day and was tapered off slowly over a period of several months according to bleb formation and wound healing. Intraocular pressure (IOP) was assessed at every postoperative visit. Postoperative IOP‐lowering medication was added in case of raised IOP, and bleb needling or interventions were performed at the discretion of the treating ophthalmologist.
Bleb needling and revision were performed in the operating room. During needling, a 30‐G needle was introduced into the bleb to release adhesions of the Tenon’s capsule, followed by injection of an anti‐fibrotic agent 5‐Fluorouracil (5‐FU) (0.1 ml of 50 mg/ml) or MMC (0.1 ml of 0.2 mg/ml). Revisions were performed by opening the conjunctival wound area and removing tube adhesions. In cases with severe bleb fibrosis, a tenonectomy was performed and, if considered necessary, the procedure was augmented with the application of MMC or an Ologen® implant (Aeon Astron Europe, the Netherlands).
Outcome measurements
The primary outcome was the IOP after 12 and 24 months of follow‐up. Secondary parameters included the number of postoperative IOP‐lowering medications, adverse events, additional glaucoma interventions and surgical success rates. Surgical success was defined as a postoperative IOP ≤ 18 mmHg at 2 consecutive follow‐up visits after 3 months of follow‐up. If success was achieved without medication, additional glaucoma surgery or other glaucoma therapy, it was considered a complete success. Qualified success was obtained if target IOP was achieved without any additional glaucoma interventions, with or without IOP‐lowering medication. Bleb needling or revision was not considered a failure. The time to failure was defined as the time to reoperation or the first visit of 2 consecutive visits after 3 months in which the patient had an IOP > 18 mmHg.
Cases that required an additional glaucoma intervention were only included in the postoperative analysis up to the moment of the decision to intervene.
Statistical analysis
Data were collected from the electronic patient records, and statistical analysis was performed using SPSS Statistics version 26 (IBM Inc., Armonk, NY, USA).
Baseline characteristics and study outcomes were compared using independent‐samples Student’s t‐test for continuous variables and chi‐square test and Fisher’s exact test, as needed, for categorical variables.
An independent‐samples Student’s t‐test was used for the evaluation of IOP from baseline up to 24 months between the Xen and MicroShunt groups.
Additionally, the probability of success was assessed using Kaplan–Meier survival curves. Success probabilities between the groups were compared using log‐rank (Mantel–Cox) test. All tests in our analysis were 2‐sided, and a p‐value of 0.05 or less was considered statistically significant.
Results
Baseline characteristics
A total of 82 implantations were included in the analysis. Forty‐one eyes in 31 patients received a Xen implantation, and 41 eyes in 33 patients received a MicroShunt implantation. The main baseline clinical and demographic characteristics are presented in Table 1. No significant differences were observed in the baseline characteristics between the Xen group and the MicroShunt group. Disease severity was evenly distributed between the groups, with most patients having moderate glaucomatous damage.
Table 1.
Baseline demographics and clinical characteristics of the study population
| Xen (n = 41) | MicroShunt (n = 41) | p‐value | |
|---|---|---|---|
| Study eye, OD, no. (%) | 21 (51%) | 21 (51 %) | 1.00 |
| Mean age ± SD, yrs | 69 ± 8 | 66 ± 9 | 0.13 |
| Gender, male, no. (%) | 20 (49%) | 21 (51%) | 0.83 |
| Race, Caucasian, no. (%) | 41 (100%) | 41 (100%) | 1.00 |
| Mean central corneal thickness ± SD, µm | 540 ± 35 | 531 ± 40 | 0.36 |
| Mean IOP ± SD, mmHg | 19.2 ± 4.4 | 20.1 ± 5.0 | 0.39 |
| Mean no. of IOP‐lowering medications ± SD | 2.5 ± 1.4 | 2.3 ± 1.5 | 0.65 |
| Number of IOP‐lowering medications, no (%) | |||
| 0 | 4 (10%) | 6 (15%) | 0.73 |
| 1 | 9 (22%) | 7 (17%) | |
| 2 | 4 (10%) | 8 (20%) | |
| 3 | 12 (29%) | 8 (20%) | |
| 4 | 11 (27%) | 11 (27%) | |
| 5 | 1 (2%) | 1 (2%) | |
| Use of oral acetazolamide, no. (%) | 7 (17%) | 5 (12%) | 0.53 |
| Glaucoma severity, no. (%) | |||
| Mild up to −6.00 dB | 14 (34%) | 11 (27%) | 0.76 |
| Moderate −6.01 dB up to −12.00 dB | 14 (34%) | 15 (37%) | |
| Advanced −12.01 dB or worse | 12 (29%) | 14 (34%) | |
| Missing | 1 (2%) | 1 (2%) | |
| History of phacoemulsification | 10 (24%) | 18 (44%) | 0.06 |
| History of laser trabeculoplasty | 15 (37%) | 19 (46%) | 0.37 |
Comparisons between treatment groups were performed using the 2‐sided Student’s t‐test for continuous variables and the chi‐square test for categorical variables.
The Xen implantation was performed in combination with cataract extraction in 15 (37%) cases versus 1 (2%) case in the MicroShunt group (p < 0.001). Mean follow‐up duration after the primary surgery was 22.4 and 18.9 months in the Xen and MicroShunt groups, respectively.
Intraocular pressure
IOP measurements for the Xen and MicroShunt groups are reported in Table 2. At baseline, mean IOP was similar between the groups (p = 0.39). Postoperatively, patients were censored from the time‐point and additional incisional glaucoma surgery was performed. Mean IOP in the Xen group dropped from 19.2 ± 4.4 mmHg at baseline to 13.3 ± 2.9 mmHg (31%) at 12 months and 13.8 ± 3.8 mmHg (28%) at 24 months of follow‐up. In patients who received a MicroShunt, mean IOP decreased from 20.1 ± 5.0 mmHg at baseline to 12.1 ± 3.5 mmHg (40%) at 12 months and 12.1 ± 3.5 mmHg (39%) at 24 months of follow‐up. We found lower IOP values in the MicroShunt group at all time‐points; however, this difference was only statistically significant at month 1 and month 3 (Fig. 1).
Table 2.
Mean IOP and medication use for patients at baseline and at time‐points during follow‐up after Xen and MicroShunt implantations
| Xen | MicroShunt | p‐value | |||
|---|---|---|---|---|---|
| Combined | Stand‐alone | All | All | ||
| Baseline | |||||
| IOP (mmHg) | 18.4 ± 3.3 | 19.6 ± 4.9 | 19.2 ± 4.4 | 20.1 ± 5.0 | 0.39 |
| no. of medications | 2.1 ± 1.5 | 2.7 ± 1.3 | 2.5 ± 1.4 | 2.3 ± 1.5 | |
| n | 15 | 26 | 41 | 41 | |
| Day 1 | |||||
| IOP (mmHg) | 11.1 ± 4.2 | 7.5 ± 3.6* | 8.8 ± 4.1 | 7.9 ± 4.0 | 0.34 |
| no. of medications | 0.1 ± 0.3 | 0.1 ± 0.4 | 0.1 ± 0.3 | 0.1 ± 0.4 | |
| n | 14 | 26 | 40 | 41 | |
| Week 1 | |||||
| IOP (mmHg) | 10.6 ± 3.3 | 9.5 ± 5.7 | 9.9 ± 4.9 | 8.7 ± 4.1 | 0.21 |
| no. of medications | 0.2 ± 0.6 | 0.0 ± 0.0 | 0.1± 0.4 | 0.0 ± 0.2 | |
| n | 14 | 24 | 38 | 41 | |
| Month 1 | |||||
| IOP (mmHg) | 15.0 ± 6.2 | 12.0 ± 6.4 | 13.1 ± 6.4 | 10.3 ± 3.2 | 0.019 |
| no. of medications | 0.3 ± 0.7 | 0.2 ± 0.7 | 0.2 ± 0.7 | 0.0 ± 0.0 | |
| n | 14 | 24 | 38 | 40 | |
| Month 3 | |||||
| IOP (mmHg) | 13.8 ± 3.6 | 13.8 ± 5.3 | 13.8 ± 4.6 | 10.9 ± 2.8 | 0.002 |
| no. of medications | 0.5 ± 0.9 | 0.6 ± 1.1 | 0.5 ± 1.0 | 0.2 ± 0.5 | |
| n | 14 | 23 | 37 | 38 | |
| Month 6 | |||||
| IOP (mmHg) | 15.5 ± 5.7 | 14.0 ± 4.3 | 14.5 ± 4.8 | 12.5 ± 4.2 | 0.07 |
| no. of medications | 0.3 ± 0.6 | 0.8 ± 1.2 | 0.6 ± 1.0 | 0.2 ± 0.5 | |
| n | 13 | 22 | 35 | 34 | |
| Month 12 | |||||
| IOP (mmHg) | 14.4 ± 3.4 | 12.7 ± 2.5 | 13.3 ± 2.9 | 12.1 ± 3.5 | 0.17 |
| no. of medications | 0.5 ± 1.0 | 1.0 ± 1.2 | 0.8 ± 1.2 | 0.6 ± 1.0 | |
| n | 11 | 19 | 30 | 28 | |
| Month 18 | |||||
| IOP (mmHg) | 14.0 ± 2.9 | 12.6 ± 1.8 | 13.2 ± 2.3 | 12.5 ± 3.3 | 0.46 |
| no. of medications | 0.7 ± 1.2 | 1.5 ± 1.3 | 1.2 ± 1.3 | 0.6 ± 0.9 | |
| n | 10 | 16 | 26 | 16 | |
| Month 24 | |||||
| IOP (mmHg) | 12.5 ± 1.7 | 14.6 ± 4.6 | 13.8 ± 3.8 | 12.1 ± 3.5 | 0.19 |
| no. of medications | 0.5 ± 1.1 | 1.1 ± 1.3 | 0.9 ± 1.2 | 0.7 ± 1.1 | |
| n | 10 | 16 | 26 | 14 | |
IOP = intraocular pressure. Shown is mean ± standard deviation. Patients were censored from analysis when they received an additional intervention. Comparisons between treatment groups were performed using the 2‐sided Student t‐test. *p‐value < 0.05.
Fig. 1.
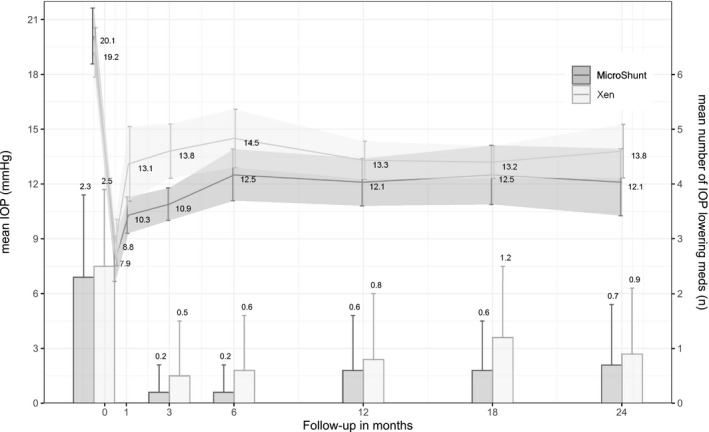
Mean intraocular pressure and medication use during follow‐up after Xen and MicroShunt implantations in patients with primary open‐angle glaucoma. Error bars represent the 95% confidence interval.
Medication
The mean number of IOP‐lowering medications dropped from 2.5 ± 1.4 and 2.3 ± 1.5 at baseline to 0.8 ± 1.2 and 0.6 ± 1.0 at 12 months and 0.9 ± 1.2 and 0.7 ± 1.1 at 24 months of follow‐up in the Xen and MicroShunt groups, respectively. At 24 months, 62% (16/26) versus 64% (9/14) of patients were free of IOP‐lowering medication in the Xen group versus the MicroShunt group, respectively (p = 0.86). Mean IOP at 24 months in the medication‐free patients was 13.4 ± 3.7 mmHg in the Xen group and 11.6 ± 4.2 mmHg in the MicroShunt group (p = 0.26). None of the patients used oral acetazolamide in either of the groups at two years of follow‐up.
Surgical success rates
Kaplan–Meier survival curves for surgical success are shown in Fig. 2. The probability of complete success was 46% and 58% at 12 months and 34% and 49% at 24 months of follow‐up with a mean time to failure (95% CI) of 14.0 (11.3–16.8) and 16.6 (13.7–19.4) months for Xen and MicroShunt implantations, respectively (p = 0.21). The probability of qualified success was 78% and 79% at 12 months and 73% and 79% at 24 months of follow‐up with a mean time to failure (95%‐CI) of 20.0 (17.8–22.2) and 20.2 (17.9–22.6) months for the Xen and MicroShunt groups, respectively (p = 0.68).
Fig. 2.
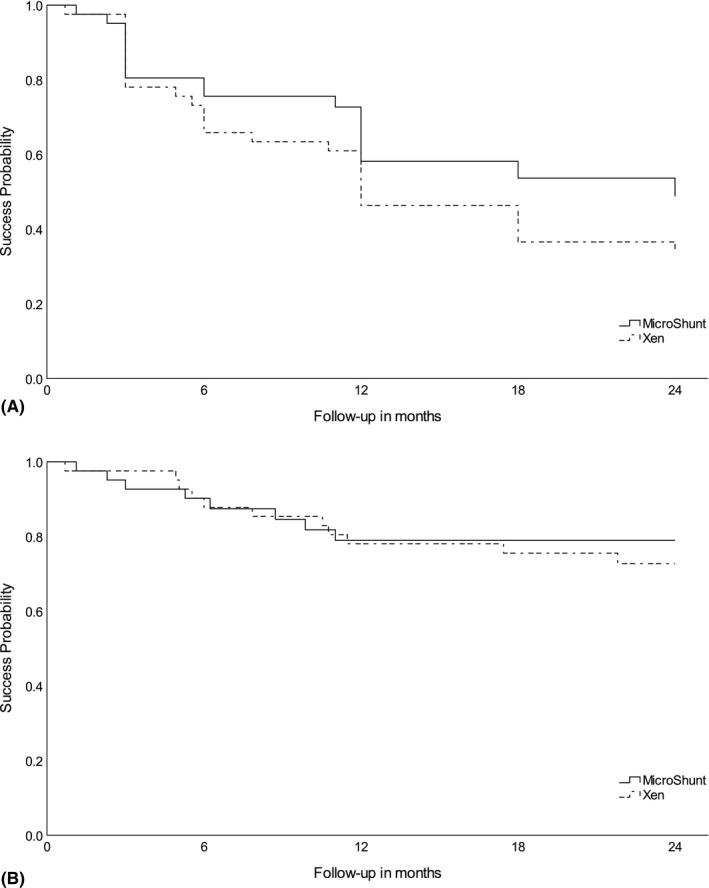
Kaplan–Meier survival curve showing the probability of success after Xen and MicroShunt implantations. (A) Complete success is defined as an IOP ≤ 18 mmHg without medication or additional glaucoma interventions. (B) Qualified success is defined as an IOP ≤ 18 mmHg without additional glaucoma interventions, with or without medication.
Postoperative interventions
Table 3 lists the postoperative interventions. Eight eyes (20%) of patients in the Xen group and 2 (5%) eyes in patients in the MicroShunt group underwent bleb needling (p = 0.09). In both groups, two eyes (5%) underwent bleb revision (p = 1.00). The median (range) follow‐up time between the surgery and the first revision or needling was 2.3 (0.8–6.0) months in the Xen group and 3.0 (2.1–5.1) months in the MicroShunt group. Another postoperative intervention that was performed was a MicroPulse® transscleral cyclophotocoagulation (IRIDEX Corporation, Mountain View, CA, USA) (MP‐TSCPC) procedure in 8 (20%) cases of the Xen group and in 1 case in the MicroShunt group (p = 0.029). One eye in each group underwent a phacoemulsification combined with a trabecular micro‐bypass stent procedure (iStent, Glaukos, San Clemente, CA, USA) (phaco‐iStent). Three (7%) cases after Xen implantation and 6 (15%) cases after MicroShunt implantation failed and underwent additional glaucoma filtration surgery (p = 0.48). The median (range) follow‐up time between the surgery and the additional glaucoma filtration surgery was 4.9 (0.7–6.0) months in the Xen group versus 7.0 (1.1–14.7) months in the MicroShunt group. In both the Xen and MicroShunt groups, one case required operative adjustment of the stent’s placement.
Table 3.
Summary of interventions after Xen and MicroShunt implantations
| Xen (n = 41) | MicroShunt (n = 41) | p‐value | |
|---|---|---|---|
| Postoperative bleb management | 10 (24%) | 4 (10%) | 0.08 a |
| Bleb revision | 2 (5%) | 2 (5%) | 1.00 b |
| Bleb needling | 8 (20%) | 2 (5%) | 0.09 b |
| Other laser/surgery | |||
| Phacoemulsification§ | 6 (38%) | 4 (18%) | 0.27 b |
| MP‐TSCPC | 8 (20%) | 1 (2%) | 0.029 b |
| Trabecular micro‐bypass stent | 1 (2%) | 1 (2%) | 1.00 b |
| Glaucoma filtration surgery | 3 (7%) | 6 (15%) | 0.48 b |
| Glaucoma filtration device | 2 (5%) | 4 (10%) | 0.68 b |
| Trabeculectomy | 1 (2%) | 2 (5%) | 0.99 b |
Data are presented in no. (%).
MP‐TSCPC = MicroPulse transscleral cyclophotocoagulation.
Chi‐square test.
Fisher’s exact test. § % is corrected for phakic eyes only.
Postoperative complications
Regarding the safety profile, a comparison of postoperative complications is shown in Table 4.
Table 4.
Summary of reported adverse events after Xen and MicroShunt implantations during follow‐up
| Xen (n = 41) | MicroShunt (n = 41) | |
|---|---|---|
| Early postoperative complications | ||
| Hypotony ≤ 5 mmHg at anytime | 10 (24%) | 16 (39%) |
| Hypotony requiring reformation of AC | 2 (5%) | 1 (2%) |
| Early (micro)hyphema | 9 (22%) | 8 (20%) |
| Choroidal detachment | 1 (2%) | 1 (2%) |
| Late postoperative complications | ||
| Hypotony | 3 (8%) | 0 |
| Ptosis | 0 | 1 (2%) |
| Curling of stent | 6 (15%) | 0 |
| Tube occlusion | 0 | 1 (2%) |
| Migration of stent | 1 (2%) | 0 |
Data are presented in no. (%) Late postoperative complications are considered > 1 month after the surgery.
Early self‐limiting hyphema was commonly reported in both groups. To evaluate hypotony, we used the numeric definition of an IOP ≤ 5 mmHg after surgery suggested by the World Glaucoma Association guidelines (Shaaraway et al. 2009). A single measurement of hypotony at day 1 and/or week 1 was seen in 10 (24%) of the patients in the Xen group and 16 (39%) of the patients in the MicroShunt group (p = 0.15). By month 1, hypotony had resolved in all cases in the MicroShunt group; however, it persisted in three cases of the Xen group. Due to hypotony, two patients in the Xen group and one patient in the MicroShunt group needed reformation of the AC in the first postoperative week. In both groups, one case of (nonkissing) choroidal detachment was observed, requiring reformation of the AC after the Xen implantation and resolving spontaneously for the case in the MicroShunt group.
In the Xen group, 6 (15%) cases of curling of the stent were described. In one Xen patient, the stent migrated into the direction of the AC, requiring repositioning in the operating room. No cases of device exposure or migration were seen in the MicroShunt group. In one case, the iris occluded the lumen of the tube and repositioning was performed in the operating room.
Discussion
In this study, we compared the outcome on efficacy and safety of two minimally invasive glaucoma implants for subconjunctival drainage (the Xen and MicroShunt implants, augmented with MMC), in patients with POAG.
Both procedures were found to be effective in lowering IOP and reducing the number of postoperative IOP‐lowering medications. Our results suggest that Xen and MicroShunt implants may have similar IOP‐lowering potential and surgical effectiveness in POAG patients. Overall, 73% of eyes with Xen implantation and 79% of the MicroShunt implantations showed qualified success (i.e. an IOP value ≤ 18 mmHg without additional glaucoma intervention) after 24 months of follow‐up. In both groups, mean IOP dropped to the low teens at 12 months and 24 months of follow‐up. The mean number of IOP‐lowering medications was also reduced in both groups, with more than half of the patients completely off medications after 24 months.
We found no differences in the number of bleb needling and additional glaucoma filtration surgery rates. However, MP‐TSCPC was performed less often in the MicroShunt group.
We were challenged by how to interpret the additional MP‐TSCPC and phaco‐iStent procedures performed during follow‐up. These new procedures are less invasive in comparison with conventional incisional surgeries but could have a significant moderating effect on outcomes. Performing a sensitivity analysis showed no differences in outcomes in our cohort. However, as the literature suggests a significant IOP reduction up to 30–40% after both MP‐TSCPC and phaco‐iStent procedures we decided to include cases in the analysis only up to these interventions (Neuhann 2015; Davids et al. 2018; Macher et al. 2018; de Crom et al. 2020).
Previously published prospective and retrospective studies showed that Xen implantation in patients with open‐angle glaucoma is a safe and effective method with a mean IOP in the mid‐teens after one or two years of follow‐up (Fea et al. 2017; Mansouri et al. 2018a; Tan et al. 2018; Widder et al., 2018; Gillmann et al. 2019; Heidinger et al. 2019; Kalina et al. 2019; Reitsamer et al., 2019; Smith et al. 2019). In a large prospective study of 202 Xen implantations in POAG patients, Reitsamer et al., (2019) showed an overall mean IOP of 14.9 ± 4.5 and 15.2 ± 4.2 mmHg at 12 and 24 months postoperatively. Outcomes in our current study are at least as favourable as in this prior study.
Currently, limited evidence is available on the MicroShunt, including and restricted to industry‐initiated trials. A pioneering study showed a mean IOP of 11.9 ± 3.7, two years after MicroShunt implantation augmented with 0.4 mg/ml MMC (Batlle et al. 2016). However, preliminary results from an international multicenter prospective trial presented at the World Glaucoma Congress showed a mean IOP of 14.8 ± 5.0 at two years of follow‐up (García‐Feijoó et al. 2019). In this latter study, 0.2 mg/ml MMC was used. Our present results show a more favourable outcome with this lower concentration of MMC. More evidence is needed on the desired concentration of MMC.
Hypotony was quite common in both groups within the first postoperative month. However, the incidence of hypotony‐related complications was low. In a cohort following 300 trabeculectomy patients, numerical hypotony of ≤ 5 mmHg was seen in 47% at any time during follow‐up and persistent hypotony in two consecutive visits after 1 month in 11% of patients (Abbas et al. 2018). Our cohort shows lower rates of hypotony with both minimally invasive glaucoma implants in comparison with this study on trabeculectomy.
Even though the two implants have a similar drainage approach, they differ in a few aspects. Firstly, the Xen implant is implanted via an ab interno approach, without opening the conjunctiva, whereas the MicroShunt is implanted via an ab externo approach, quite similar to trabeculectomy. Secondly, the two implants use a different method of administration and amount of MMC. It is known that both the concentration and duration of MMC exposure have an effect on the surgical success of glaucoma filtration surgery (Al Habash et al. 2015). Thirdly, the material and design of the two implants differ, which may possibly have different effects on biocompatibility, foreign‐body reaction and migration after implantation. Widder et al. (2019) recently published a case report of stent degradation after Xen implantation. The long‐term effect of these materials in glaucoma patients is yet to be established.
The current study has several limitations, mainly relating to its retrospective nature. Therefore, results should be interpreted in the light of this design. Outcomes are collected via routine clinical practice and not via a reporting protocol. Nevertheless, collecting data from clinical routine gives a realistic representation of current clinical practice. Because all patients were Caucasians, this study cannot offer conclusions on effectiveness and safety for other ethnic populations. In addition, although the surgeons were all experienced glaucoma surgeons, a learning curve may have influenced the results, as these are both new techniques. The effect of needling and revision had to be studied, and in several cases, this was not attempted and we directly opted for further incisional glaucoma surgery for safety reasons. We are aware that analysis of both eyes of the same patient has the potential to lead to (selection) bias. However, a post hoc sub‐analysis of the mean IOP during follow‐up including first eyes only showed similar results in both groups. We have chosen to include both eyes as the use of only one eye per individual would lead to loss of information, without changing the results of our analyses.
The rate of patients lost to follow‐up was considerable. This can be explained by the retrospective study design. Five patients returned to their referring ophthalmologist after a successful treatment.
Patients who received secondary glaucoma interventions were only included in the analysis up to the moment of the decision to intervene. Although this may bias the results in favour of the MIGS procedures, we cannot present the results in a different way, as the IOP after an additional intervention no longer reflects the outcome of the initial Xen or MicroShunt procedure.
A treatment was selected on the basis of individual patient characteristics and was not randomly assigned. A Xen implantation was more often performed in combination with cataract extraction. The effect of a combined surgery on the efficacy of the filtration surgery also remains unclear. Studies comparing the efficacy of Xen implantation with and without cataract extraction found similar outcomes in both groups (Karimi et al. 2018; Mansouri et al. 2018b; Marcos Parra et al., 2019). It may therefore be assumed that the combined procedure had no relevant influence on the results of the present comparison study. Performing a sensitivity analysis comparing Xen and MicroShunt implantations as a stand‐alone procedure versus a combined procedure showed no statistical difference except for a significant lower IOP at day 1 postoperative in favour of the stand‐alone procedure (Table 2). Further evidence on the outcome of combination surgery with the MicroShunt has to be awaited. Finally, the number of cases in our analysis might have been too low to detect statistical differences between the two implants. A larger cohort and longer follow‐up will give further insights into the pros and cons of these two new devices.
In conclusion, both the XEN45® Gel Stent and the PRESERFLO™ MicroShunt demonstrated safe and effective lowering of IOP and the need for IOP‐lowering medications, with similar success rates after 2 years. Our cohort showed a higher number of MP‐TSCPC procedures in the Xen group. Selecting a minimally invasive device should take into consideration IOP target, the patient’s compliance with IOP‐lowering medications, optimal bleb management and the surgeon’s personal preferences. Further prospective head‐to‐head trials and cost‐effectiveness studies are essential to determine the place of these new devices for the surgical treatment of glaucoma.
The authors have no proprietary or commercial interest in any materials discussed in this manuscript. Lotte M.J Scheres, Stefani Kujovic‐Aleksov, Wishal D. Ramdas, Lianne C.G. Roelofs and Tos T.J.M. Berendschot: No conflicts of interest to disclose.
Ronald M.P.C. de Crom is a consultant for Allergan and Théa Pharma and receives lecture fees from IRIDEX and Novartis.
Carroll A.B. Webers is a consultant for Alcon, Novartis, Santen and Théa Pharma.
Henny J.M. Beckers is a consultant for Alcon, Allergan, Glaukos and Santen and received grant research support from InnFocus, a Santen company, Alcon and Glaukos.
Both Allergan Inc. and Santen Inc. had no role in the conduct, design or analysis of this study.
References
- Abbas A, Agrawal P & King AJ (2018): Exploring literature‐based definitions of hypotony following glaucoma filtration surgery and the impact on clinical outcomes. Acta Ophthalmol 96: e285–e289. [DOI] [PubMed] [Google Scholar]
- Al Habash A, Aljasim LA, Owaidhah O & Edward DP (2015): A review of the efficacy of mitomycin C in glaucoma filtration surgery. Clin Ophthalmol (Auckland N.Z.) 9: 1945–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle JF, Fantes F, Riss I et al. (2016): Three‐year follow‐up of a novel aqueous humor MicroShunt. J Glaucoma 25: e58–65. [DOI] [PubMed] [Google Scholar]
- Cairns JE (1968): Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol 66: 673–679. [PubMed] [Google Scholar]
- Caprioli J, Kim JH, Friedman DS et al. (2015): Special commentary: Supporting innovation for safe and effective minimally invasive glaucoma surgery: Summary of a Joint Meeting of the American Glaucoma Society and the Food and Drug Administration, Washington, DC, February 26, 2014. Ophthalmology 122: 1795–1801. [DOI] [PubMed] [Google Scholar]
- de Crom R, Slangen C, Kujovic‐Aleksov S, Webers CAB, Berendschot T & Beckers HJM (2020): Micropulse Transscleral Cyclophotocoagulation in patients with glaucoma: 1‐ and 2‐year treatment outcomes. J Glaucoma 29: 794–798. [DOI] [PubMed] [Google Scholar]
- Davids A‐M, Pahlitzsch M, Boeker A et al. (2018): iStent inject as a reasonable alternative procedure following failed trabeculectomy? Eur J Ophthalmol 28: 735–740. [DOI] [PubMed] [Google Scholar]
- Fea AM, Spinetta R, Cannizzo PML, Consolandi G, Lavia C, Aragno V, Germinetti F & Rolle T (2017): Evaluation of bleb morphology and reduction in IOP and glaucoma medication following implantation of a novel gel stent. J Ophthalmol 2017: 9364910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis BA, Singh K, Lin SC, Hodapp E, Jampel HD, Samples JR & Smith SD (2011): Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology 118: 1466–1480. [DOI] [PubMed] [Google Scholar]
- García‐Feijoó J, Batlle JF & Riss I (2019): A 2‐year pooled analysis of the MicroShunt in patients with primary open‐angle glaucoma (POAG): 0.2 versus 0.4 mg/mL mitomycin C (MMC) outcomes. Poster presented at World Glaucoma Congress (WGC), Melbourne, Australia. [Google Scholar]
- Gillmann K, Bravetti GE, Mermoud A, Rao HL & Mansouri K (2019): XEN gel stent in pseudoexfoliative glaucoma: 2‐year results of a prospective evaluation. J Glaucoma 28: 676–684. [DOI] [PubMed] [Google Scholar]
- Heidinger A, Schwab C, Lindner E, Riedl R & Mossbock G (2019): A retrospective study of 199 Xen45 stent implantations from 2014 to 2016. J Glaucoma 28: 75–79. [DOI] [PubMed] [Google Scholar]
- Hodapp E, Parrish RK & Anderson DR (1993): Clinical decisions in glaucoma. St. Louis: Mosby Incorporated; 52–61. [Google Scholar]
- Kalina AG, Kalina PH & Brown MM (2019): XEN((R)) Gel stent in medically refractory open‐angle glaucoma: results and observations after one year of use in the United States. Ophthalmology and therapy [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi A, Lindfield D, Turnbull A et al. (2018): A multi‐centre interventional case series of 259 ab‐interno Xen gel implants for glaucoma, with and without combined cataract surgery. Eye 33: 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RA (2014): Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg 40: 1301–1306. [DOI] [PubMed] [Google Scholar]
- Macher T, Häberle H, Wächter J, Thannhäuser C, Aurich H & Pham DT (2018): Trabecular microbypass stents as minimally invasive approach after conventional glaucoma filtration surgery. J Cataract Refract Surg 44: 50–55. [DOI] [PubMed] [Google Scholar]
- Mansouri K, Gillmann K, Rao HL, Guidotti J & Mermoud A (2018): Prospective evaluation of XEN gel implant in eyes with pseudoexfoliative glaucoma. J Glaucoma 27: 869–873. [DOI] [PubMed] [Google Scholar]
- Mansouri K, Guidotti J, Rao HL, Ouabas A, D'Alessandro E, Roy S & Mermoud A (2018): Prospective evaluation of standalone XEN gel implant and combined phacoemulsification‐XEN gel implant surgery: 1‐year results. J Glaucoma 27: 140–147. [DOI] [PubMed] [Google Scholar]
- Marcos Parra MT, Salinas Lopez JA, Lopez Grau NS, Ceausescu AM & Perez Santonja JJ. (2019): XEN implant device versus trabeculectomy, either alone or in combination with phacoemulsification, in open‐angle glaucoma patients. Graefe's Arch Clin Exp Ophthalmol 257: 1741–1750. [DOI] [PubMed] [Google Scholar]
- Neuhann TH (2015): Trabecular micro‐bypass stent implantation during small‐incision cataract surgery for open‐angle glaucoma or ocular hypertension: long‐term results. J Cataract Refract Surg 41: 2664–2671. [DOI] [PubMed] [Google Scholar]
- Pinchuk L, Wilson GJ, Barry JJ, Schoephoerster RT, Parel JM & Kennedy JP (2008): Medical applications of poly(styrene‐block‐isobutylene‐block‐styrene) ("SIBS"). Biomaterials 29: 448–460. [DOI] [PubMed] [Google Scholar]
- Pinchuk L, Riss I, Batlle JF et al. (2017): The development of a micro‐shunt made from poly(styrene‐block‐isobutylene‐block‐styrene) to treat glaucoma. J Biomed Mater Res B Appl Biomater 105: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsamer H, Sng C, Vera V, Lenzhofer M, Barton K & Stalmans I. (2019): Two‐year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open‐angle glaucoma. Graefe's Arch Clin Exp Ophthalmol 257: 983–996. [DOI] [PubMed] [Google Scholar]
- Shaaraway T, Sherood M & Grehn F (2009): WGA Guidelines on design and reporting of glaucoma surgical trials. Amsterdam, The Netherlands: Kugler Publications. [Google Scholar]
- Sheybani A, Reitsamer H & Ahmed II (2015): Fluid dynamics of a novel micro‐fistula implant for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci 56: 4789–4795. [DOI] [PubMed] [Google Scholar]
- Smith M, Charles R, Abdel‐Hay A et al. (2019): 1‐year outcomes of the Xen45 glaucoma implant. Eye (Lond) 33: 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SZ, Walkden A & Au L (2018): One‐year result of XEN45 implant for glaucoma: efficacy, safety, and postoperative management. Eye (Lond) 32: 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widder RA, Dietlein TS, Dinslage S, Kuhnrich P, Rennings C & Rossler G. (2018): The XEN45 Gel Stent as a minimally invasive procedure in glaucoma surgery: success rates, risk profile, and rates of re‐surgery after 261 surgeries. Graefe's Arch Clin Exp Ophthalmol 256: 765–771. [DOI] [PubMed] [Google Scholar]
- Widder RA, Kühnrich P, Hild M, Rennings C, Szumniak A, Rössler GF (2019): Intraocular Degradation of XEN45 Gel Stent 3 Years After its Implantation. Journal of Glaucoma 28: e171–e173. [DOI] [PubMed] [Google Scholar]


