Abstract
Background and aim
Dietary strategies that contribute to reducing incidence of Helicobacter pylori infection without negative side effects are highly desirable owing to worldwide bacterial prevalence and carcinogenesis potential. The aim of this study was to determine dosage effect of daily cranberry consumption on H. pylori suppression over time in infected adults to assess the potential of this complementary management strategy in a region with high gastric cancer risk and high prevalence of H. pylori infection.
Methods
This double‐blind, randomized, placebo‐controlled trial on 522 H. pylori‐positive adults evaluated dose–response effects of proanthocyanidin‐standardized cranberry juice, cranberry powder, or their placebos on suppression of H. pylori at 2 and 8 weeks by 13C‐urea breath testing and eradication at 45 days post‐intervention.
Results
H. pylori‐negative rates in placebo, low‐proanthocyanidin, medium‐proanthocyanidin, and high‐proanthocyanidin cranberry juice groups at week 2 were 13.24%, 7.58%, 1.49%, and 13.85% and at week 8 were 7.35%, 7.58%, 4.48%, and 20.00%, respectively. Consumption of high‐proanthocyanidin juice twice daily (44 mg proanthocyanidin/240‐mL serving) for 8 weeks resulted in decreased H. pylori infection rate by 20% as compared with other dosages and placebo (P < 0.05). Percentage of H. pylori‐negative participants increased from 2 to 8 weeks in subjects who consumed 44 mg proanthocyanidin/day juice once or twice daily, showing a statistically significant positive trend over time. Encapsulated cranberry powder doses were not significantly effective at either time point. Overall trial compliance was 94.25%. Cranberry juice and powder were well‐tolerated.
Conclusions
Twice‐daily consumption of proanthocyanidin‐standardized cranberry juice may help potentiate suppression of H. pylori infection. Trial registration: ChiCTR1800017522, per WHO ICTRP.
Keywords: Cranberry, Helicobacter pylori, Randomized clinical trial, Suppression
Introduction
Over half the world's population is infected with Helicobacter pylori, which can induce peptic ulcer and increase risk of developing gastric cancer (GC). 1 , 2 , 3 Among the Asian countries, China has one of the highest incidences of GC. 4 H. pylori screening followed by conventional triple or quadruple antibiotic treatment is effective in preventing GC 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ; however, treatment failure rates of 10–30%, significant antibiotic resistance development, and other barriers to antibiotic treatment including cost, availability, and potential side effects have left infected individuals at an increased risk for future GC development. 2 , 13 , 14 Complimentary strategies with fewer negative side effects are needed that could contribute to partial suppression of H. pylori infections within populations, potentially helping to reduce overuse of antibiotics. While not alternatives to antibiotics, certain dietary interventions have shown bacterial suppression effects, reducing overall disease incidence without total pathogen eradication. 15 , 16 There is increased interest in implementing food‐based regimens that are culturally acceptable, affordable, and well tolerated and help maintain microbiome diversity, which can benefit overall health. 17
The majority of studies on dietary components and suppression of H. pylori have been in vitro, with few clinical studies on colonized individuals. 17 Cranberry (Vaccinium macrocarpon Ait.) is a fruit that has been shown clinically to suppress infections when consumed regularly. 18 , 19 A double‐blind randomized, placebo‐controlled trial (RCT) of 189 H. pylori‐positive individuals in China in 2005 found a significant 14.3% decrease in the H. pylori infection rate at both 35 and 90 days in participants who consumed two daily servings of 250 mL of cranberry juice (27%). 18 An RCT in Chile on 295 H. pylori‐positive children found suppression rates of 16.9% after 200 mL of cranberry juice daily intake over a 3‐week period. 19 H. pylori‐infected mice administered with cranberry juice (0.5 mL/mouse) had a significant 80% bacterial suppression after 24 h and a 20% eradication rate after 4 weeks. 20 Eradication sustainability was low 1 month after cranberry interventions ceased, 19 , 20 indicating that regular consumption may be necessary to maintain suppression.
Mechanisms of action of cranberry in suppression of H. pylori may be due to phenolic compounds found in the fruit, especially the complex oligomeric A‐type proanthocyanidins (PACs) that have been implicated in prevention of adhesion of Escherichia coli to bladder cells 21 and oral bacteria to tooth surfaces. 22 This bacterial anti‐adhesion effect decreases infection but is not bactericidal, therefore reducing the potential for proliferation of resistant bacterial strains. 23 In vitro studies in Israel demonstrated that a cranberry extract containing predominantly PACs prevented adhesion of H. pylori sialic acid‐specific strains to human gastric mucus and stomach cells. 24 , 25 A polyphenolic fraction of cranberry inhibited growth of two H. pylori strains in a dose‐dependent manner, causing morphological changes in the bacteria that prevented replication. 26
Cranberry products utilized in these previous studies were not standardized for PAC content, and single clinical doses were arbitrarily determined, making it difficult to recommend effective dietary intervention strategies using cranberry. Determining dose–response, frequency of dosing, and active cranberry product forms that improve H. pylori‐negative rates is needed to develop this complementary approach to increasing bacterial suppression in susceptible populations. Thus, an RCT was designed and carried out in Linqu County, Shandong Province in China, which has one of the highest GC mortality rates worldwide and high prevalence of H. pylori infection, 13 to determine the daily dosage of PAC in cranberry juice and juice‐derived powder required to suppress H. pylori at 2 and 8 weeks following once‐daily or twice‐daily consumption.
Methods
Study design
The study intervention phase was an RCT, designed to assess the dose–response effects at 2 and 8 weeks of daily consumption of either cranberry juice or juice‐based powder standardized by PAC dosage or matching placebos on suppression of H. pylori. Additional testing was done to determine if bacterial suppression was sustained 45 days after completion of the intervention phase. The study was conducted in Linqu County, between August 2018 and April 2019, by researchers from Peking University Cancer Hospital & Institute (PUCHI), China. The study was approved by the institutional review board of PUCHI and was registered as ChiCTR1800017522 in accordance with World Health Organization International Clinical Trials Registry Platform requirements prior to execution of the study. Standard triple therapy with proton pump inhibitors and antibiotics was provided to subjects who remained H. pylori positive at the conclusion of the trial. Written informed consent was obtained from all subjects.
Study population
The 13C‐urea breath tests (13C‐UBT) conducted between August and December 2018 identified 1017 eligible subjects from 21 communities in Linqu County. A total of 525 H. pylori‐positive adults, aged 18–60 years, met the recruitment criteria and were invited to participate in the trial. Given the 5% initial eradication for the placebo and 20% for active, the smallest sample size of 67 subjects in each group was required to reach the power (1 − β) of 0.80 with the significant level (α) at 0.05.
Informed consent for participation was obtained from each subject; it was explicitly explained by trained interviewers that H. pylori‐positive residents would be assigned into a high‐dose, low‐dose, or placebo group; they would be free to terminate enrollment and leave the study at any time; and they had a right to receive any medical care. A structured questionnaire was used at baseline requesting information on demographic characteristics, family history of cancer, personal history of major diseases, and lifestyle factors. Eligible subjects were allowed to taste cranberry juice to determine palatability and asked if they are able to swallow capsules. As an incentive to participate, subjects were informed that they would receive standard triple therapy antibiotic treatments at the conclusion of the study if they were H. pylori positive following the trial.
Residents with any medical history of peptic ulcer or reported upper abdominal symptoms including pain, burning sensation, acidic taste in the mouth, and unpleasant regurgitation of stomach contents were excluded as suspected cases of ulcerous disease. In addition, subjects with the following conditions were also excluded from the trial: serious medical conditions, undergoing active cancer treatment, currently or previously on antibiotic therapy for H. pylori infection, history of congestive heart failure, respiratory failure, stroke, seizures, pregnancy, and mental or psychiatric illness.
Participants were provided with a written information sheet to report any potential adverse events during the study. In addition, participants would be withdrawn from the study within 24 h of any episodes of ulcer, perforation, upper gastrointestinal bleeding or any moderate or severe adverse events.
Investigational products
Cranberry juice concentrate (50° Brix), pH 2.69, titratable acidity 10.1 (wt/wt) as citric, derived from USA‐sourced berries and press extracted to enhance and preserve PAC/polyphenol levels, was formulated into two standardized investigational product beverages. One formulation contained 17.5% cranberry juice (2.282% cranberry concentrate, 5.91% beet sugar, and 91.81% water), and the second formulation contained 35% cranberry juice (4.563% cranberry concentrate, 11.81% beet sugar, and 83.62% water). Juices were hot filled into 240‐mL polyethylene terephthalate bottles to pasteurize contents and produce shelf‐stability at room temperatures. Placebo beverage that did not contain active cranberry constituents but was cranberry flavored was provided by Ocean Spray Cranberries Inc., Middleboro, MA, USA. The standardized cranberry powder derived from cranberry juice extract was mixed with excipients (mannitol [Pearlitol 160C], silica colloidal anhydrous, and magnesium stearate) at a ratio of 77.7% cranberry powder to 22.3% excipients, and 280 mg was packaged in each red opaque gelatin capsule. The equivalent amount of placebo powder consisting of excipient ingredients was packaged in identical capsules. Phenolic component analyses of the finished investigational products were performed by Complete Phytochemical Solutions, LLC, Cambridge, WI, USA; and results are presented in Table S1.
Randomization and blinding
A total of 525 H. pylori‐positive subjects from 21 communities (divided into two blocks) were initially enrolled; three refused to receive the intervention and were excluded before randomization. Consequently, a total of 522 subjects were allocated into the juice arm or encapsulated powder arm at random by block and then were assigned to seven intervention groups by individual randomization via computer (Fig. 1). Briefly, 294 subjects living in nine communities were assigned into one of four intervention groups (placebo, low‐PAC, medium‐PAC, or high‐PAC dose) of the juice arm (73 or 74 subjects for each group), and 228 subjects from 12 communities were assigned into one of three intervention groups (placebo, medium‐PAC, or high‐PAC dose) of the encapsulated powder arm (76 subjects for each group). Within the two arms of the trial (juice and encapsulated powder), dose–response effects on H. pylori suppression were evaluated based on PAC dose.
FIGURE 1.
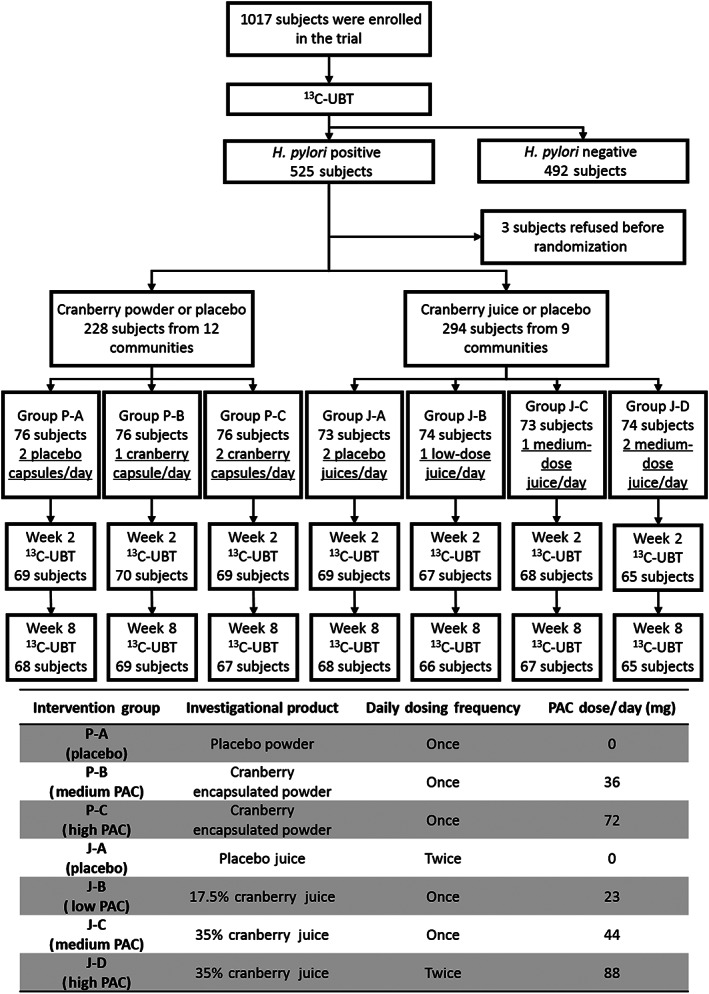
Participant flow diagram with intervention dosing scheme.
Seven codes indicating different types and dosages of the intervention products were generated by an independent statistician and were sealed in an envelope until the end of the trial. Both the participants and the investigators were masked to treatment assignment to preserve double‐blinding.
Interventions
The H. pylori‐positive subjects received an 8‐week supply of cranberry juice, encapsulated powder or matching placebos at the initiation of the trial (week 0), instructions for use of products, and subject food records and treatment diaries to record daily product use, dietary intake, changes in concomitant therapies, and any adverse events and symptoms throughout the study. To maintain double‐blinding, coded intervention products were packaged individually for each participant in eight boxes, each containing a 1‐week supply, labeled with the identity code of each participant, and delivered by research staff members. For the juice arm, PAC‐standardized, shelf‐stable bottled cranberry beverage (240‐mL serving) or a matching placebo were provided and either refrigerated or stored by participants at room temperature (out of the sun) for the duration of the trial. For the encapsulated powder arm, PAC‐standardized cranberry juice‐based powder (280‐mg servings in capsules) or a matching placebo were provided and stored at room temperature. Specifically, subjects in each intervention group received investigational products containing standardized PAC dosages and dosing instructions according to the scheme illustrated in Figure 1.
Over the 8‐week intervention period, subjects in the low‐PAC group (group J‐B) consumed one bottle (240 mL) of cranberry juice daily containing 23 mg PAC/bottle, while subjects in the medium‐PAC group (group J‐C) consumed one 240‐mL bottle containing 44 mg PAC. Subjects in the high‐PAC group (group J‐D) consumed two 240‐mL bottles of juice (44 mg PAC/bottle) daily, every morning and evening. Subjects in the encapsulated powder arm swallowed one (group P‐B) or two (group P‐C) 280‐mg capsule(s) of cranberry powder, which contained 36 mg PAC/capsule or two placebo capsules (group P‐A) daily in the morning over the 8‐week intervention period.
To measure compliance, staff members of PUCHI counted empty product containers and discussed any adverse events at weeks 2 and 8 of the intervention. A subject was considered to have poor compliance with the intervention and subsequently excluded from the per‐protocol analysis in the following situations: (i) missed more than 5% of the investigational product doses over the entire intervention period, or (ii) continually missed three doses of the investigational product, or (iii) missed the staff interview at week 2 and/or week 8.
Measurement of Helicobacter pylori infection
All subjects were given a 13C‐UBT at baseline to determine initial infection status and again at weeks 2 and 8 to test effectiveness of the investigational products. Subjects who tested negative at 8 weeks were retested 45 days later, after the investigational products stopped being administered, to determine H. pylori eradiation rate. Details of the 13C‐UBT were described in previous publications. 13
Statistical analyses
The primary outcome variable was the H. pylori‐negative rate in the cranberry and placebo groups after 2 and 8 weeks, which was analyzed by both intention‐to‐treat (ITT) and per‐protocol strategies. Statistical differences were determined using a two‐tailed χ 2 test or Fisher's exact conditional test at α = 0.05. Odds ratios and corresponding 95% confidence intervals were calculated by multivariable unconditional logistic regression to evaluate the potential factors (age, gender, tobacco smoking, alcohol consumption, or baseline body mass index [BMI]) influencing H. pylori‐negative rates at week 8. Statistical analyses and randomization were conducted by Statistical Analysis System software (version 9.2; SAS Institute, Cary, NC).
Results
The mean age of the 522 subjects was 47.24 ± 11.53 years, and 232 subjects were male (44.40%). No statistical difference was found in age, gender, tobacco smoking, alcohol consumption, or baseline BMI between intervention groups either in the juice arm or the encapsulated powder arm, ensured by the randomization of the trial participants (Table 1).
TABLE 1.
Selected characteristics of subjects in each group by intervention product arms
| Characteristics | Juice arm | Encapsulated powder arm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group J‐A (n = 73) | Group J‐B (n = 74) | Group J‐C (n = 73) | Group J‐D (n = 74) | P | Group P‐A (n = 76) | Group P‐B (n = 76) | Group P‐C (n = 76) | P | |
| Age, mean ± SD | 48.07 ± 9.31 | 45.76 ± 11.43 | 47.42 ± 11.91 | 47.64 ± 10.63 | 0.590 † | 46.26 ± 11.88 | 45.8 ± 12.40 | 49.72 ± 12.61 | 0.102 † |
| Sex, n (%) | 0.482 ‡ | 0.983 ‡ | |||||||
| Male | 33 (45.21) | 26 (35.14) | 26 (35.62) | 32 (43.24) | 38 (50.00) | 39 (51.32) | 38 (50.00) | ||
| Female | 40 (54.79) | 48 (64.86) | 47 (64.38) | 42 (56.76) | 38 (50.00) | 37 (48.68) | 38 (50.00) | ||
| Smoking, n (%) | 0.666 ‡ | 0.921 ‡ | |||||||
| No | 56 (76.71) | 59 (79.73) | 59 (80.82) | 54 (72.97) | 52 (68.42) | 52 (68.42) | 54 (71.05) | ||
| Yes | 17 (23.29) | 15 (20.27) | 14 (19.18) | 20 (27.03) | 24 (31.68) | 24 (31.68) | 22 (28.95) | ||
| Drinking, n (%) | 0.223 | 0.286 ‡ | |||||||
| No | 49 (67.12) | 54 (72.97) | 57 (78.08) | 60 (81.08) | 42 (55.26) | 49 (64.47) | 51 (67.11) | ||
| Yes | 24 (32.88) | 20 (27.03) | 16 (21.92) | 14 (18.92) | 34 (44.74) | 27 (35.53) | 25 (32.89) | ||
| BMI, mean ± SD | 24.54 ± 2.74 | 23.53 ± 3.57 | 24.32 ± 2.88 | 24.06 ± 2.29 | 0.179 † | 24.65 ± 3.03 | 23.70 ± .56 | 24.32 ± 2.75 | 0.161 † |
One‐way anova.
Chi‐square test.
J‐A, placebo juice; J‐B, low‐proanthocyanidin (PAC) juice; J‐C, medium‐PAC juice; J‐D, high‐PAC juice; P‐A, placebo encapsulated powder; P‐B, medium‐PAC encapsulated powder; P‐C, high‐PAC encapsulated powder.
Following the 8‐week intervention, 492 out of 522 subjects completed the treatment (compliance 94.25%), of which 477 (compliance 96.95%) received the second 13C‐UBT (week 2) and 470 (compliance 95.53%) received 13C‐UBT both at week 2 and week 8 (Fig. 1). No severe side effects or life‐threatening events were observed among all subjects within the intervention period.
Effect of cranberry juice on Helicobacter pylori suppression at weeks 2 and 8
Of the 294 subjects receiving cranberry juice intervention or placebo, 13C‐UBT data were successfully collected on 266 subjects at both weeks 2 and 8. ITT analysis including all 294 subjects revealed H. pylori‐negative rates at week 2 of 13.70% (10/73), 6.67% (5/74), 1.37% (1/73), and 12.16% (9/74) in J‐A (placebo), J‐B (low‐PAC), J‐C (medium‐PAC), and J‐D (high‐PAC) groups, respectively, with statistically significant difference across four groups (P = 0.028). Further analysis found the H. pylori‐negative rate in high‐PAC group was significantly higher than that of the medium‐PAC group (P = 0.009). No statistical difference was found between high‐PAC with placebo or low‐PAC groups. Per‐protocol analysis including the 266 subjects with 13C‐UBT data at both weeks 2 and 8 found that H. pylori‐negative rates at week 2 were 13.24% (9/68), 7.58% (5/66), 1.49% (1/67), and 13.85% (9/65) in placebo, low‐PAC, medium‐PAC, and high‐PAC groups, respectively, with statistically significant difference across four groups (P = 0.041). The rate in high‐PAC group was significantly higher than in medium‐PAC group (P = 0.007); however, no statistical difference was observed between high‐PAC group and placebo.
For the long‐term effect after 8 weeks, ITT analysis found the H. pylori‐negative rates were 6.85% (5/73), 6.67% (5/74), 4.11% (3/73), and 17.57% (13/74) in placebo, low‐PAC, medium‐PAC, and high‐PAC groups, respectively, with a P value of 0.012. Further inter‐group analysis found the H. pylori‐negative rate was significantly higher in high‐PAC group than in the other three groups (P < 0.05). In pre‐protocol analysis, H. pylori‐negative rates were 7.35%, 7.58%, 4.48%, and 20.00%, respectively (P = 0.012). The rate was significantly higher in high‐PAC group than in the other three groups (P < 0.05) (Table 2).
TABLE 2.
Effect of cranberry juice on Helicobacter pylori suppression at weeks 2 and 8
| H. pylori status | Intention‐to‐treat analysis | Per‐protocol analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group J‐A (n = 73) | Group J‐B (n = 74) | Group J‐C (n = 73) | Group J‐D (n = 74) | P † | Group J‐A (n = 68) | Group J‐B (n = 66) | Group J‐C (n = 67) | Group J‐D (n = 65) | P † | |
| Week 2 | 0.028 | 0.041 | ||||||||
| Negative | 10 (13.70) | 5 (6.76) | 1 (1.37) | 9 (12.16) | 9 (13.24) | 5 (7.58) | 1 (1.49) | 9 (13.85) | ||
| Positive | 63 (86.30) | 69 (93.24) | 72 (98.63) | 65 (87.84) | 59 (86.76) | 61 (92.42) | 66 (98.51) | 56 (86.15) | ||
| Week 8 | 0.021 | 0.012 | ||||||||
| Negative | 5 (6.85) | 5 (6.76) | 3 (4.11) | 13 (17.57) | 5 (7.35) | 5 (7.58) | 3 (4.48) | 13 (20.00) | ||
| Positive | 68 (93.15) | 69 (93.24) | 70 (95.89) | 61 (82.43) | 63 (92.65) | 61 (92.42) | 64 (95.52) | 52 (80.00) | ||
Chi‐square test.
J‐A, placebo juice; J‐B, low‐proanthocyanidin (PAC) juice; J‐C, medium‐PAC juice; J‐D, high‐PAC juice.
Effect of cranberry powder on Helicobacter pylori suppression at weeks 2 and 8
Of the 228 subjects receiving cranberry powder intervention or placebo, 13C‐UBT data were successfully collected on 204 subjects at both weeks 2 and 8. In ITT analysis including all 228 participants, subjects without second or third 13C‐UBT data were considered H. pylori‐positive. H. pylori‐negative rates at week 2 were 10.53% (8/76), 10.53% (8/76), and 9.21% (7/76) in groups P‐A (placebo), P‐B (medium‐PAC), and P‐C (high‐PAC), respectively, which did not show statistically significant difference across comparison groups (P = 0.953). Per‐protocol analysis of the 204 subjects with 13C‐UBT data at both weeks 2 and 8 found suppression rates at week 2 were 11.67% (8/68), 11.59% (8/69), and 10.45% (7/67), respectively, without statistical significance (P = 0.966).
At week 8, suppression rates were 11.84% (9/76), 6.58% (5/76), and 7.89% (6/76) in placebo, medium‐PAC, and high‐PAC powder groups, respectively, yet no statistical differences were observed (P = 0.490) by ITT analysis. In pre‐protocol analysis, suppression rates were 13.24% (9/68), 7.25% (5/69), and 8.96% (6/67), respectively, without statistical differences (P = 0.479) (Table 3).
TABLE 3.
Effect of cranberry encapsulated powder on Helicobacter pylori suppression at weeks 2 and 8
| H. pylori status | Intention‐to‐treat analysis | Per‐protocol analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Group P‐A (n = 76) | Group P‐B (n = 76) | Group P‐C (n = 76) | P † | Group P‐A (n = 68) | Group P‐B (n = 69) | Group P‐C (n = 67) | P † | |
| Week 2 | 0.953 | 0.966 | ||||||
| Negative | 8 (10.53) | 8 (10.53) | 7 (9.21) | 8 (11.76) | 8 (11.59) | 7 (10.45) | ||
| Positive | 68 (89.47) | 68 (89.47) | 69 (90.79) | 60 (88.24) | 61 (88.41) | 60 (89.55) | ||
| Week 8 | 0.490 | 0.479 | ||||||
| Negative | 9 (11.84) | 5 (6.58) | 6 (7.89) | 9 (13.24) | 5 (7.25) | 6 (8.96) | ||
| Positive | 67 (88.16) | 71 (93.42) | 70 (92.11) | 59 (86.76) | 64 (92.75) | 61 (91.04) | ||
Chi‐square test.
P‐A, placebo encapsulated powder; P‐B, medium‐proanthocyanidin (PAC) encapsulated powder; P‐C, high‐PAC encapsulated powder.
Temporal trends of Helicobacter pylori suppression rates in each active intervention group
We further investigated the temporal trends of H. pylori suppression rates over the entire intervention period in each active group in the per‐protocol datasets. Subjects in the cranberry juice arm receiving the dose of 44 mg/day PAC (group J‐C) exhibited an increasing trend of the H. pylori‐negative rate from 1.49% to 4.48% from week 2 to week 8 with statistical significance (P for trend = 0.045). Similarly, the H. pylori‐negative rate significantly increased from 13.85% at week 2 to 20.00% at week 8 for high‐PAC juice group (J‐D) receiving 44 mg PAC/bottle twice daily (P for trend = 0.001). No statistical temporal trend was found in subjects who received the low‐dose intervention (group J‐B) over time (P for trend = 0.334) (Fig. 2a).
FIGURE 2.
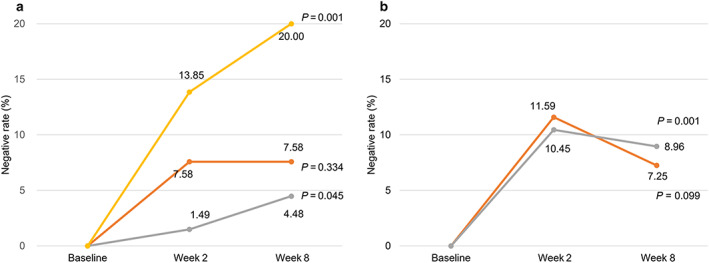
Temporal trends of Helicobacter pylori suppression rates in each active intervention group in the per‐protocol datasets. (a) Juice arm.  , J‐B;
, J‐B;  , J‐C;
, J‐C;  , J‐D. (b) Powder arm. ;
, J‐D. (b) Powder arm. ;  , P‐B;
, P‐B;  , P‐C. *Temporal trends were determined by Fisher's exact test. [Color figure can be viewed at wileyonlinelibrary.com]
, P‐C. *Temporal trends were determined by Fisher's exact test. [Color figure can be viewed at wileyonlinelibrary.com]
For the cranberry powder arm, subjects in both the medium‐PAC group (P‐B) and the high‐PAC group (P‐C) showed decreased trends of H. pylori‐negative rates at week 8 compared with week 2. The H. pylori‐negative rate in group P‐C decreased from 10.45% at week 2 to 8.96% at week 8 with statistical significance (P for trend = 0.001) (Fig. 2b).
Factors associated with Helicobacter pylori suppression at week 8
We further evaluated the factors influencing the suppression of H. pylori at week 8 in the per‐protocol subjects from both the juice and encapsulated powder arms. Multivariable unconditional logistic regression adjusted for age and intervention group found that in the cranberry juice arm, female gender, tobacco smoking, alcohol consumption, and BMI were associated with failure of H. pylori suppression; however, owing to the limited number of H. pylori‐negative subjects, the regression model was not robust, and only the female gender gained statistical significance with an odds ratio of 4.54 (95% confidence interval: 1.51–13.65). For the encapsulated powder arm, no statistical association of these characteristics was found with the suppression rate of H. pylori (Table 4).
TABLE 4.
Multivariable logistic regression analysis for factors associated with Helicobacter pylori suppression at week 8 in per‐protocol subjects
| Variables | H. pylori status in juice arm at week 8 | H. pylori status in encapsulated powder arm at week 8 | ||||
|---|---|---|---|---|---|---|
| Negative/positive | OR (95% CI) † | P † | Negative/positive | OR (95% CI) † | P † | |
| Sex | ||||||
| Male | 14/90 | 1.00 | 10/95 | 1.00 | ||
| Female | 12/150 | 4.54 (1.51–13.65) | 0.007 | 10/89 | 0.53 (0.12–2.34) | 0.400 |
| Smoking | ||||||
| No | 21/185 | 1.00 | 13/127 | 1.00 | ||
| Yes | 5/55 | 3.41 (0.97–11.80) | 0.052 | 7/57 | 0.83 (0.21–3.40) | 0.800 |
| Drinking | ||||||
| No | 20/178 | 1.00 | 10/113 | 1.00 | ||
| Yes | 6/62 | 1.84 (0.57–5.92) | 0.305 | 10/71 | 0.45 (0.12–1.66) | 0.232 |
| BMI | 26/240 | 1.03 (0.89–1.19) | 0.701 | 20/184 | 1.09 (0.95–1.26) | 0.235 |
Unconditional logistic regression adjusted for age and intervention group, with sex, smoking, drinking, and BMI as initial variables entered into the model, where age and BMI were treated as continuous variables.
BMI, body mass index; CI, confidence interval; OR, odds ratio.
Post‐intervention Helicobacter pylori infection status
H. pylori status was tested 45 days after the intervention phase utilizing 13C‐UBT to determine eradication rates of subjects in the seven intervention groups that remained H. pylori negative at both weeks 2 and 8. A total of 23 subjects were sustainably H. pylori negative during the intervention period, including 12 from the juice arm and 11 from the encapsulated powder arm. Testing revealed that 75% (6/8) of the subjects who received active treatments in the juice arm and 66.7% (4/6) in the encapsulated powder arm remained H. pylori negative, whereas 50% (2/4) and 60% (3/5) remained negative in the placebo juice and powder groups, respectively. Owing to limited sample sizes, statistical analyses were not performed (see Table S2).
Discussion
This RCT determined details on efficacious cranberry product specifications, PAC doses, and frequency of consumption, which resulted in the highest statistically significant H. pylori suppression rate of 20% when compared with that of previous trials that used single doses of non‐standardized cranberry juices. Improvement in bacterial suppression rate in this trial may be due to a higher PAC dosage in the cranberry juice (44 mg/240‐mL serving), greater dosing frequency (twice daily for 8 weeks) potentially resulting in greater and more continuous contact time of the PACs in the gastric lumen, and improved bacterial anti‐adhesion effects in the gastric epithelium. H. pylori suppression rates were not significantly higher after 2 weeks on cranberry juice; however, the percentage of H. pylori‐negative participants increased from 2 to 8 weeks in a significant positive trend when juice doses containing at least 44 mg PAC/day and above were consumed, suggesting that daily dosing for longer than 8 weeks could result in even higher suppression rates in future studies.
The ineffectiveness of the cranberry powder in this trial may be due to sub‐efficacious dosages and dissolution issues. The powder was derived directly from the juice and contained similar components, but the maximum daily dose of PAC administered was less than the effective juice dose (72 mg PAC/day for the powder vs 88 mg PAC/day for the juice). Twice‐daily administration may be needed with at least 44 mg PAC per serving. It was not possible to determine if the encapsulated powder fully dissolved in the stomach or in the intestines, confounding its overall bioactive potential. Slow dissolution may have been overcome by dissolving the powder in liquid prior to administration to improve coating of the stomach cavity and interaction with H. pylori. Cranberry powder has some advantages over juice in implementing population‐based preventative practices, such as storage, portability, and product stability, making it potentially more feasible and acceptable. Future studies could incorporate methods that would fully solubilize juice‐based cranberry powder, potentially offering an additional means of consuming cranberry for H. pylori suppression.
The status of H. pylori infection measured 45 days after the intervention phase indicated that a higher percentage of participants in both the active juice and encapsulated powder arms remained H. pylori‐free than those in both placebo arms. Even though statistical analyses could not be performed owing to limited sample sizes, these preliminary results suggest that daily cranberry consumption at the proper doses for at least 8 weeks might provide a measure of sustained suppression even if cranberry consumption is terminated. Dietary cranberry PACs are known to induce sustained changes in gut microbiota composition and functionality, 27 , 28 potentially creating less favorable conditions for H. pylori survival even when cranberry is no longer being administered. And research indicates that some resident microbes may inhibit H. pylori growth, 29 but further research is necessary to determine if growth of these inhibitory bacterial species is favored by cranberry consumption. There were subjects who remained H. pylori negative in the placebo group at the 45‐day time point, suggesting the possibility that some other unaccounted dietary component with H. pylori inhibitory activity may have been consumed. Studies considering more potential influencing factors and with longer follow‐up period after stopping the cranberry usage are warranted.
The data determined in this trial on cranberry products and dosing will be useful for designing future studies to improve outcomes when cranberry is administered with antibiotics for H. pylori eradication. In previous studies, cranberry juice (250 mL), administered in conjunction with antibiotic triple therapies, significantly improved H. pylori eradication rates in Israeli women than did triple therapy alone. 30 Preventing adhesion to the gastric mucosa may leave non‐adherent bacteria more susceptible to the antibiotics. In another study, higher concentrations of antibiotics were needed to kill adherent bacteria. 31 In an RCT of 200 participants in Iran, H. pylori eradication rates measured 6 weeks after the intervention were significantly higher when 500 mg of cranberry powder combined with antibiotic triple therapy taken for 2 weeks was compared with triple therapy alone. 32 In situations where antibiotic treatment of H. pylori is warranted but resistance to antibiotics is reducing H. pylori eradication rates, concurrent administration of cranberry might help improve treatment outcomes.
In conclusion, this study provides additional justification for the utilization of cranberry juice as part of a complementary dietary approach to H. pylori management. Compliance was high, suggesting that participants liked the taste, and they consumed the juice regularly without side effects or other health risks. Cranberry PACs do not appreciably degrade under the highly acidic conditions in the stomach, 33 allowing them to maintain bioactivity. And, polyphenols in cranberry also decrease viability of GC cell lines, 34 inhibit GC in vivo, 35 and reduce esophageal adenocarcinoma, 36 offering further potential benefits. Results of the current study suggest that regular consumption of cranberry juice, when administered at the correct dosage and frequency, has potential to partially suppress H. pylori, especially in China where endemic infection and GC rates are high. 13 A suppression rate of 20% within a population as demonstrated in this trial could contribute to reducing overall prevalence of H. pylori by reducing inoculum levels.
Supporting information
Table S1. Values of proanthocyanidin, total phenolics, anthocyanins and flavonols in cranberry and placebo investigational finished products.
Table S2. H. pylori infection status 45 days after the intervention in subjects sustainedly negative at both weeks 2 and 8.
Li, Z.‐X. , Ma, J.‐L. , Guo, Y. , Liu, W.‐D. , Li, M. , Zhang, L.‐F. , Zhang, Y. , Zhou, T. , Zhang, J.‐Y. , Gao, H.‐E. , Guo, X.‐Y. , Ye, D.‐M. , Li, W.‐Q. , You, W.‐C. , and Pan, K.‐F. (2021) Suppression of Helicobacter pylori infection by daily cranberry intake: A double‐blind, randomized, placebo‐controlled trial. Journal of Gastroenterology and Hepatology, 36: 927–935. 10.1111/jgh.15212.
Declaration of conflict of interest: None.
Author contribution: Z‐X. L. and K‐F. P. made the study design. Z‐X. L. and K‐F. P. drafted the manuscript. Z‐X. L., Y. G., and K‐F. P. performed the statistical analyses and interpretation. Y. G. carried out the randomization. All the rest of the authors have contributed to data acquisition and critical review of the manuscript. All authors approved the final version of the article, including the authorship list.
Financial support: This study was supported by a grant from the Cranberry Marketing Committee (CMC) sanctioned by the USDA‐AMS.
Guarantor of the article: Zhe‐Xuan Li and Kai‐Feng Pan.
References
- 1. Suerbaum S, Michetti P. Helicobacter pylori infection. New Engl. J. Med. 2002; 347: 1175–1186. [DOI] [PubMed] [Google Scholar]
- 2. Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006; 19: 449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uemura N, Okamoto S, Yamamoto S et al. Helicobacter pylori infection and the development of gastric cancer. New Engl. J. Med. 2001; 345: 784–789. [DOI] [PubMed] [Google Scholar]
- 4. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA‐Cancer J. Clin. 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 5. Ohata H, Kitauchi S, Yoshimura N et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int. J. Cancer 2004; 109: 138–143. [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Blot WJ, You WC et al. Helicobacter pylori antibodies in relation to precancerous gastric lesions in a high‐risk Chinese population. Cancer Epidemiol. Biomarkers Prev. 1996; 5: 627–630. [PubMed] [Google Scholar]
- 7. You WC, Li JY, Blot WJ et al. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer. Int. J. Cancer 1999; 83: 615–619. [DOI] [PubMed] [Google Scholar]
- 8. You WC, Zhang L, Gail MH et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J. Natl. Cancer Inst. 2000; 92: 1607–1612. [DOI] [PubMed] [Google Scholar]
- 9. You WC, Brown LM, Zhang L et al. Randomized double‐blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J. Natl. Cancer Inst. 2006; 98: 974–983. [DOI] [PubMed] [Google Scholar]
- 10. Wong BC, Lam SK, Wong WM et al. Helicobacter pylori eradication to prevent gastric cancer in a high‐risk region of China: a randomized controlled trial. JAMA 2004; 291: 187–194. [DOI] [PubMed] [Google Scholar]
- 11. Fukase K, Kato M, Kikuchi S et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open‐label, randomised controlled trial. Lancet 2008; 372: 392–397. [DOI] [PubMed] [Google Scholar]
- 12. Ley C, Mohar A, Guarner J et al. Helicobacter pylori eradication and gastric preneoplastic conditions: a randomized, double‐blind, placebo‐controlled trial. Cancer Epidemiol. Biomarkers Prev. 2004; 13: 4–10. [DOI] [PubMed] [Google Scholar]
- 13. Pan KF, Zhang L, Gerhard M et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut 2016; 65: 9–18. [DOI] [PubMed] [Google Scholar]
- 14. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59: 1143–1153. [DOI] [PubMed] [Google Scholar]
- 15. Blaser MJ. Not all Helicobacter pylori strains are created equal: should all be eliminated? Lancet 1997; 349: 1020–1022. [DOI] [PubMed] [Google Scholar]
- 16. Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. J. Clin. Invest. 2001; 107: 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fahey JW, Stephenson KK, Wallace AJ. Dietary amelioration of Helicobacter infection. Nutr. Res. 2015; 35: 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang L, Ma J, Pan K et al. Efficacy of cranberry juice on Helicobacter pylori infection: a double‐blind, randomized placebo‐controlled trial. Helicobacter 2005; 10: 139–145. [DOI] [PubMed] [Google Scholar]
- 19. Gotteland M, Andrews M, Toledo M et al. Modulation of Helicobacter pylori colonization with cranberry juice and Lactobacillus johnsonii La1 in children. Nutrition 2008; 24: 421–426. [DOI] [PubMed] [Google Scholar]
- 20. Xiao SD, Shi T. Is cranberry juice effective in the treatment and prevention of Helicobacter pylori infection of mice? Chin. J. Dig. Dis. 2003; 4: 136–139. [Google Scholar]
- 21. Howell AB, Botto H, Combescure C et al. Dosage effect on uropathogenic Escherichia coli anti‐adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect. Dis. 2010; 10: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reed JD, Howell AB. Botanical medicine from bench to bedside. In: Cooper R, Kronenberg F, eds. Biological Activity of Cranberry Proanthocyanidins: Effects on Oxidation, Microbial Adhesion, Inflammation, and Health. New York: Mary Ann Liebert, Inc., 2009; 190–201. [Google Scholar]
- 23. Ofek I, Hasty DL, Sharon N. Anti‐adhesion therapy of bacterial diseases: prospects and problems. FEMS Immunol. Med. Microbiol. 2003; 38: 181–191. [DOI] [PubMed] [Google Scholar]
- 24. Burger O, Ofek I, Tabak M et al. A high molecular mass constituent of cranberry juice inhibits Helicobacter pylori adhesion to human gastric mucus. FEMS Immunol. Med. Microbiol. 2000; 29: 295–301. [DOI] [PubMed] [Google Scholar]
- 25. Shmuely H, Burger O, Neeman I et al. Susceptibility of Helicobacter pylori isolates to the antiadhesion activity of a high‐molecular‐weight constituent of cranberry. Diagn. Microbiol. Infect. Dis. 2004; 50: 231–235. [DOI] [PubMed] [Google Scholar]
- 26. Chatterjee A, Yasmin T, Bagchi D, Stohs SJ. Inhibition of Helicobacter pylori in vitro by various berry extracts, with enhanced susceptibility to clarithromycin. Mol. Cell. Biochem. 2004; 265: 19–26. [DOI] [PubMed] [Google Scholar]
- 27. Rodríguez‐Morató J, Matthan NR, Liu J et al. Cranberries attenuate animal‐based diet‐induced changes in microbiota composition and functionality: a randomized crossover controlled feeding trial. J. Nutr. Biochem. 2018; 62: 76–86. [DOI] [PubMed] [Google Scholar]
- 28. Anhê FF, Roy D, Pilon G et al. A polyphenol‐rich cranberry extract protects from diet‐induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015; 64: 872–883. [DOI] [PubMed] [Google Scholar]
- 29. Haley KP, Gaddy JA. Nutrition and Helicobacter pylori: host diet and nutritional immunity influence bacterial virulence and disease outcome. Gastroenterol. Res. Pract. 2016; 2016: 3019362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shmuely H, Yahav J, Samra Z et al. Effect of cranberry juice on eradication of Helicobacter pylori in patients treated with antibiotics and a proton pump inhibitor. Mol. Nutr. Food Res. 2007; 51: 746–751. [DOI] [PubMed] [Google Scholar]
- 31. Megraud F, Trimoulet P, Lamouliatte H et al. Bactericidal effect of amoxicillin on Helicobacter pylori in an in vitro model using epithelial cells. Antimicrob. Agents Chemother. 1991; 35: 869–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seyyedmajidi M, Ahmadi A, Hajiebrahimi S et al. Addition of cranberry to proton pump inhibitor‐based triple therapy for eradication. J. Res. Pharm. Pract. 2016; 5: 248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rios LY, Bennett RN, Lazarus SA et al. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002; 76: 1106–1110. [DOI] [PubMed] [Google Scholar]
- 34. Boivin D, Blanchette M, Barrette S et al. Inhibition of cancer cell proliferation and suppression of TNF‐induced activation of NFkappaB by edible berry juice. Anticancer Res 2007; 27: 937–948. [PubMed] [Google Scholar]
- 35. Weh KM, Clarke J, Kresty LA. Cranberries and cancer: an update of preclinical studies evaluating the cancer inhibitory potential of cranberry and cranberry derived constituents. Antioxidants (Basel) 2016; 5: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kresty LA, Clarke J, Ezell K et al. MicroRNA alterations in Barrett's esophagus, esophageal adenocarcinoma, and esophageal adenocarcinoma cell lines following cranberry extract treatment: insights for chemoprevention. J. Carcinog. 2011; 10: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Values of proanthocyanidin, total phenolics, anthocyanins and flavonols in cranberry and placebo investigational finished products.
Table S2. H. pylori infection status 45 days after the intervention in subjects sustainedly negative at both weeks 2 and 8.


