Abstract
Background
The diagnosis of gambiense human African trypanosomiasis (gHAT) typically involves 2 steps: a serological screen, followed by the detection of living trypanosome parasites in the blood or lymph node aspirate. Live parasites can, however, remain undetected in some seropositive individuals, who, we hypothesize, are infected with Trypanosoma brucei gambiense parasites in their extravascular dermis.
Methods
To test this hypothesis, we conducted a prospective observational cohort study in the gHAT focus of Forecariah, Republic of Guinea. Of the 5417 subjects serologically screened for gHAT, 66 were enrolled into our study and underwent a dermatological examination. At enrollment, 11 seronegative, 8 unconfirmed seropositive, and 18 confirmed seropositive individuals had blood samples and skin biopsies taken and examined for trypanosomes by molecular and immunohistological methods.
Results
In seropositive individuals, dermatological symptoms were significantly more frequent, relative to seronegative controls. T.b. gambiense parasites were present in the blood of all confirmed cases (n = 18) but not in unconfirmed seropositive individuals (n = 8). However, T. brucei parasites were detected in the extravascular dermis of all unconfirmed seropositive individuals and all confirmed cases. Skin biopsies of all treated cases and most seropositive untreated individuals progressively became negative for trypanosomes 6 and 20 months later.
Conclusions
Our results highlight the skin as a potential reservoir for African trypanosomes, with implications for our understanding of this disease’s epidemiology in the context of its planned elimination and underlining the skin as a novel target for gHAT diagnostics.
Keywords: skin, reservoir, human African trypanosomiasis, Trypanosoma brucei gambiense
Live trypanosomes can remain undetected in the blood of individuals seropositive for sleeping sickness. Here, we show that they could be infected with parasites in their extravascular dermis, highlighting the skin as a potential reservoir for trypanosomes.
The number of new cases of gambiense human African trypanosomiasis (gHAT; or sleeping sickness) has never been so low in the known epidemiological history of the disease, with only ~1500 new cases reported in 2017 [1, 2], and the World Health Organization (WHO) has targeted gHAT elimination by 2030 [3]. This objective has been encouraged by the success of active surveillance efforts that relies on a 2-step diagnosis: an initial serological screen, followed by microscope observation of blood, lymph, or cerebrospinal fluid (CSF) to detect extracellular trypanosomes and to confirm the serological diagnosis. However, some seropositive individuals remain without a confirmed parasitological diagnosis for years and have been recently described as being latent cases, raising the question as to whether reservoirs of live parasites persist in these individuals [4].
Trypanosoma brucei s. l. parasites are found in the extravascular compartment of various tissues of their mammalian hosts, including the skin, albeit mostly under experimental conditions in animal models rather than during the natural progression of the disease [5]. Recent studies have revealed that substantial quantities of trypanosomes persist within the extravascular dermis following experimental infection in mice with T.b. gambiense or T. b. brucei. These parasites can be transmitted to the tsetse vector, even in the absence of detectable parasites in the host’s blood [6]. This study also reported a retrospective screening of archived skin biopsies from a gHAT endemic region, which revealed the presence of some extravascular skin-dwelling trypanosomes [6]. However, the species of these parasites was not identified and no clinical records were available for the screened samples.
These observations raise the question as to whether T.b. gambiense might be found in the skin of confirmed gHAT cases, as well as in unconfirmed seropositive individuals, in regions of active disease transmission. To address this question, we performed a prospective observational study in the Forecariah District in the Republic of Guinea, which is one of the most active gHAT foci in Western Africa.
METHODS
More details for materials and methods are provided in the Supplementary Data.
Ethical Approval
All investigations were conducted in accordance with the Declaration of Helsinki and with the approval of the National Ethical Committee of the Republic of Guinea (Study Diag-Cut-THA 032/CNERS/17 and amendment 038/CNERS/19).
Study Enrollment, Screening, and Case Definitions
From May 2017 to February 2019, a total of 5417 individuals were screened by the HAT National Control Programme using the card agglutination test for trypanosomiasis, first on whole-blood (CATTwb), then on plasma (CATTp) for validation, in 43 villages in the active gHAT focus of the Forecariah District, Republic of Guinea. All subjects were classified as seronegative, unconfirmed seropositive, or confirmed seropositive according to the diagnostic process presented in Table 1. All parasitologically confirmed cases were diagnosed and treated by the HAT National Control Programme according to WHO recommendations and as described previously [7]. All confirmed cases (CATTp ≥1/4 with parasitological confirmation) and all unconfirmed seropositive individuals (CATTp ≥1/4 without parasitological confirmation) were proposed for study enrollment. In total, 40 seronegative controls (39 CATTwb-negative and 1 CATTwb-positive CATTp <1/4) were randomly selected from the population of 5417, of whom the first 29 individuals, enrolled in 2017, were only included in the epidemiological and clinical analysis, and the last 11 individuals, enrolled in 2019, were subjected to the entire protocol. Children under 16 years of age and pregnant women were excluded from the study. Each participant was informed about the study’s objectives and provided written informed consent.
Table 1.
Diagnostic Process, Number of Subjects, and Results
| Groups | Diagnostic Process | |||||||
|---|---|---|---|---|---|---|---|---|
| 1, Serological Screening | 2, Serological Validation | 3, Parasitological Confirmation | 4, Staging | No. of Subjects |
||||
| CATTwb / RDT | CATTpa | mAECT BC / LN aspirate observation | Parasites in CSF | No. WBCs in CSF | Screened | Enrolled | Followed Up | |
| Seronegative | − | ND | ND | ND | 5377 | 40 | 0 | |
| + | <1/4 | |||||||
| Seropositive | + | ≥1/4 | − | ND | 12 | 8 | 5 | |
| Confirmed | + | ≥1/4 | + | |||||
| Stage 1 | No | 0–5 | 8 | 4 | 4 | |||
| Stage 2 | Yes | >5 | 18 | 14 | 13 | |||
| ND | ND | 2 | 0 | 0 | ||||
| All | 28 | 18 | 17 | |||||
| Total | 5417 | 66 | 22 |
Abbreviations: CATTp, card agglutination test for trypanosomiasis on plasma; CATTwb, card agglutination test for trypanosomiasis on whole blood; CSF, cerebrospinal fluid; mAECT BC/LN aspirate, mini anion-exchange column technique on buffy coat/lymph node aspirate; ND, not determined; WBC, white blood cell.
aHighest plasma dilution with a positive result.
Field Procedure and Sampling
Participants underwent an epidemiological interview and a clinical examination, during which dermatological symptoms including pruritus (skin itch) and dermatitis (skin inflammation) were assessed at enrollment as well as at each subsequent follow-up at 6 and 20 months after enrollment/treatment. Epidemiological and clinical parameters are detailed in the Supplementary Data. The absence of dermatitis lesions at the skin sampling site was verified and a 2-mm blood-free skin punch biopsy was sampled from the right back shoulder of all confirmed seropositive cases, all unconfirmed seropositive individuals, and for the final 11 seronegative controls. Touch preparations were obtained by gently rolling the biopsy on a clean glass slide and Giemsa staining in the field. The positivity of a given slide was defined by the detection of at least 3 trypanosomes. Biopsies were fixed for immunohistochemistry (IHC) and molecular analyses. Plasma aliquots from blood samples were also obtained for serological trypanolysis tests [8].
Immunohistochemical Detection
Skin biopsy sections were stained with hematoxylin-eosin (H&E) and Giemsa stains, and immunolabelled with the T. brucei–specific anti-ISG65 antibody that targets the Invariant Surface Glycoprotein 65 expressed at the surface of the mammalian host stages of T. brucei s.l. parasites [9] and the T. brucei–specific anti-Hsp70 antibody that recognizes the endoplasmic reticulum molecular chaperone heat-shock protein 70 homolog [10]. Slides were blindly assessed by at least 2 readers. Slides from seronegative controls were mixed with slides from seropositive cases in order to guarantee blind reading. The positivity of a given skin-section slide was defined by the detection of at least 3 trypanosomes.
Polymerase Chain Reaction Detection
DNAs were extracted from paraffin-embedded biopsies and blood samples with tissue-specific commercial kits (Qiagen, Germany). For each sample, at least 2 polymerase chain reactions (PCRs) were performed with T. brucei-specific primers targeting a DNA satellite repeated sequence (10 000 copies per cell) [11], and T. brucei gambiense surface glycoprotein (TgsGP) primers directed against the single copy TgsGP gene [12], for detecting T. brucei s. l. and T.b. gambiense DNAs, respectively.
Data Analyses
For epidemiological, clinical, and diagnostic parameters, differences between seronegative controls versus unconfirmed seropositive individuals and confirmed cases were assessed using the following 2-sided tests at 5% confidence: Fisher’s exact tests for qualitative data (Tables 2 and 3) and/or Mann-Whitney tests for quantitative data (age in Table 2). For the follow-up analyses, differences between results at enrolment versus results at 6 months and 20 months after treatment/enrollment were assessed for each group using 2-sided Fisher’s exact tests at 5% confidence (Table 4).
Table 2.
Epidemiological and Clinical Characteristics of Case Subjects
| Parameters | Groups (N = 66) | ||||||
|---|---|---|---|---|---|---|---|
|
Seronegative (n = 40) |
Seropositive (n = 8) |
P | Confirmed (n = 18) | ||||
| Stage 1 (n = 4) | Stage 2 (n = 14) | All (n = 18) | P | ||||
| Epidemiologic | |||||||
| Age (n = 66), years | 37.9 (14) | 36.6 (18) | .7647a | 31.0 (17) | 35.6 (15) | 34.6 (15) | .3502a |
| Male sex (n = 66) | 22/40 (55%) | 3/8 (38%) | .4538 | 2/4 (50%) | 5/14 (36%) | 7/18 (39%) | .3950 |
| HAT case(s) in the family since 2010 (n = 65) | 11/40 (28%) | 2/7 (29%) | >.9999 | 2/4 (50%) | 5/14 (36%) | 7/18 (39%) | .5404 |
| Occupational risk (n = 66) | 17/40 (43%) | 4/8 (50%) | .7155 | 2/4 (50%) | 5/14 (36%) | 7/18 (39%) | >.9999 |
| Clinical | |||||||
| Swollen LNs (n = 65) | 5/39 (13%) | 6/8 (75%) | .0010 | 4/4 (100%) | 13/14 (93%) | 17/18 (94%) | <.0001 |
| Any dermatological symptoms (n = 66) | 8/40 (20%) | 5/8 (63%) | .0252 | 4/4 (100%) | 13/14 (93%) | 17/18 (94%) | <.0001 |
| Dermatitis (n = 66) | 7/40 (18%) | 5/8 (63%) | .0166 | 4/4 (100%) | 11/14 (79%) | 15/18 (83%) | <.0001 |
| Pruritus (n = 66) | 3/40 (8%) | 2/8 (25%) | .1887 | 0/4 (0%) | 11/14 (79%) | 11/18 (61%) | <.0001 |
| Asthenia (n = 65) | 17/39 (44%) | 4/8 (50%) | >.9999 | 4/4 (100%) | 14/14 (100%) | 18/18 (100%) | <.0001 |
| Fever (n = 63) | 6/38 (16%) | 1/7 (14%) | >.9999 | 2/4 (50%) | 9/14 (64%) | 11/18 (61%) | .0013 |
| Weight loss (n = 61) | 6/39 (15%) | 3/8 (38%) | .1672 | 2/4 (50%) | 6/10 (60%) | 8/14 (57%) | .0046 |
| Eating disorders (n = 66) | 4/40 (10%) | 1/8 (13%) | >.9999 | 0/4 (0%) | 7/14 (50%) | 7/18 (39%) | .0250 |
| Headache (n = 65) | 23/39 (59%) | 6/8 (75%) | .6918 | 3/4 (75%) | 13/14 (93%) | 16/18 (89%) | .0322 |
| Circadian rhythm disruptions (n = 66) | 3/40 (8%) | 1/8 (13%) | .5303 | 0/4 (0%) | 5/14 (36%) | 5/18 (28%) | .0925 |
| Sexual dysfunctions (n = 65) | 4/39 (10%) | 1/8 (13%) | >.9999 | 0/4 (0%) | 5/14 (36%) | 5/18 (28%) | .1236 |
| Behavior changes (n = 63) | 4/39 (10%) | 0/7 (0%) | >.9999 | 0/4 (0%) | 3/13 (23%) | 3/17 (18%) | .6624 |
Data are presented as n/total (%) or mean (SD). For each group and each parameter, total values correspond to the numbers of subjects for which a value was available (n/total). P values were obtained by comparing one by one the parameters of each group of seropositive subjects (unconfirmed and all confirmed) with those of seronegative controls using 2-sided Fisher’s exact tests or 2-sided Mann-Whitney tests at 5% confidence.
Abbreviations: HAT, human African trypanosomiasis; LN, lymph node.
aTwo-sided Mann-Whitney test.
Table 3.
Serological, Molecular, and Histological Analysis Results From Blood and Skin Samples
| Parameters | Groups (n = 37) | ||||||
|---|---|---|---|---|---|---|---|
|
Seronegative (n = 11) |
Seropositive (n = 8) |
P | Confirmed (n = 18) | ||||
| Stage 1 (n = 4) | Stage 2 (n = 14) | All (n = 18) | P | ||||
| Trypanolysis | |||||||
| LiTat 1.3 positive (n = 36) | 0/10 (0%) | 2/8 (25%) | .1830 | 4/4 (100%) | 14/14 (100%) | 18/18 (100%) | <.0001 |
| LiTat 1.5 positive (n = 36) | 0/10 (0%) | 2/8 (25%) | .1830 | 4/4 (100%) | 12/14 (86%) | 16/18 (89%) | <.0001 |
| LiTat 1.6 positive (n = 36) | 0/10 (0%) | 2/8 (25%) | .1830 | 4/4 (100%) | 12/14 (86%) | 16/18 (89%) | <.0001 |
| Positive for all VATs (n = 36) | 0/10 (0%) | 2/8 (25%) | .1830 | 4/4 (100%) | 12/14 (86%) | 16/18 (89%) | <.0001 |
| Negative for all VATs (n = 36) | 10/10 (100%) | 6/8 (75%) | .1830 | 0/4 (0%) | 0/14 (0%) | 0/18 (0%) | <.0001 |
| PCR on blood | |||||||
| TBR positive (n = 37) | 0/11 (0%) | 0/8 (0%) | >.9999 | 4/4 (100%) | 14/14 (100%) | 18/18 (100%) | <.0001 |
| TgsGP positive (n = 37) | 0/11 (0%) | 0/8 (0%) | >.9999 | 2/4 (50%) | 10/14 (71%) | 12/18 (67%) | .0004 |
| Negative for all PCRs on blood (n = 37) | 11/11 (100%) | 8/8 (100%) | >.9999 | 0/4 (0%) | 0/14 (0%) | 0/18 (0%) | <.0001 |
| PCR on skin | |||||||
| TBR positive (n = 37) | 0/11 (0%) | 6/8 (75%) | .0010 | 1/4 (25%) | 13/14 (93%) | 14/18 (78%) | <.0001 |
| TgsGP positive (n = 37) | 0/11 (0%) | 0/8 (0%) | >.9999 | 0/4 (0%) | 0/14 (0%) | 0/18 (0%) | >.9999 |
| Negative for all PCRs on skin (n = 37) | 11/11 (100%) | 2/8 (25%) | .0010 | 3/4 (75%) | 1/14 (7%) | 4/18 (22%) | <.0001 |
| Histology | |||||||
| Dermal touchpreps (n = 22, 3 reads) | ND | 2/6 (33%) | 1/3 (33%) | 12/13 (92%) | 13/16 (81%) | ||
| H&E section (n = 36, 1 read) | 0/11 (0%) | 6/8 (75%) | .0010 | 4/4 (100%) | 8/13 (62%) | 12/17 (71%) | .0003 |
| Giemsa section (n = 37, 2 reads) | 0/11 (0%) | 4/8 (50%) | .0181 | 0/4 (0%) | 14/14 (100%) | 14/18 (78%) | <.0001 |
| IHC Hsp70 (n = 31, 1 read) | 0/11 (0%) | 1/4 (25%) | .2667 | 1/4 (25%) | 11/12 (92%) | 12/16 (75%) | .0002 |
| IHC ISG65 (n = 37, 3 reads) | 0/11 (0%) | 8/8 (100%) | <.0001 | 4/4 (100%) | 14/14 (100%) | 18/18 (100%) | <.0001 |
| Negative for all reads (n = 37) | 11/11 (100%) | 0/8 (0%) | <.0001 | 0/4 (0%) | 0/17 (0%) | 0/18 (0%) | <.0001 |
Data are presented as n/total (%). For each group and each parameter, total values correspond to the numbers of subjects for which a value was available (n/total). P values were obtained by comparing one by one the parameters of each group of seropositive subjects (unconfirmed and all confirmed) with those of seronegative controls using 2-sided Fisher’s exact tests at 5% confidence.
Abbreviations: H&E, hematoxylin-eosin; Hsp70, heat shock protein 70; IHC, immunohistochemistry; ISG65, invariant surface glycoprotein 65; PCR, polymerase chain reaction; TBR, T. brucei–specific; TgsGP, Trypanosoma gambiense-specific glycoprotein; VAT, variable antigen type.
Table 4.
Clinical, Serological, Molecular, and Histological Follow-up Analyses at 6 and 20 Months After Enrollment
| Parameters | Seropositive | Confirmed | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | ||||||||||||||
| Enrollment | 6 Months | 20 Months | Enrollment | 6 Months | 20 Months | Enrollment | 6 Months | 20 Months | |||||||
| n/Total (%) | n/Total (%) | P | n/Total (%) | P | n/Total (%) | n/Total (%) | P | n/Total (%) | P | n/Total (%) | n/Total (%) | P | n/Total (%) | P | |
| Clinical | |||||||||||||||
| Asthenia | 3/5 (60%) | 1/5 (20%) | .5238 | 2/4 (50%) | >.9999 | 4/4 (100%) | 0/4 (0%) | .0286 | 2/3 (67%) | .4286 | 13/13 (100%) | 3/13 (23%) | .0001 | 3/9 (33%) | .0011 |
| Swollen LNs | 4/5 (80%) | 1/4 (25%) | .2063 | 3/4 (75%) | >.9999 | 4/4 (100%) | 2/3 (67%) | .4286 | 2/3 (67%) | .4286 | 12/13 (92%) | 2/11 (18%) | .0005 | 3/9 (33%) | .0066 |
| Any dermatological symptoms | 4/5 (80%) | 1/5 (20%) | .2063 | 1/4 (25%) | .2063 | 4/4 (100%) | 1/4 (25%) | .1429 | 0/3 (0%) | .0286 | 12/13 (92%) | 5/13 (38%) | .0112 | 3/9 (33%) | .0066 |
| Fever | 1/5 (20%) | 1/5 (20%) | >.9999 | 0/4 (0%) | >.9999 | 2/4 (50%) | 0/4 (0%) | .4286 | 0/3 (0%) | .4286 | 9/13 (69%) | 0/13 (0%) | .0005 | 1/9 (11%) | .0115 |
| Headache | 5/5 (100%) | 2/5 (40%) | .1667 | 3/4 (75%) | .4444 | 3/4 (75%) | 0/4 (0%) | .1429 | 0/3 (0%) | .1429 | 12/13 (92%) | 2/13 (15%) | .0002 | 4/9 (44%) | .0231 |
| Pruritus | 1/5 (20%) | 0/5 (0%) | >.9999 | 1/4 (25%) | >.9999 | 0/4 (0%) | 0/4 (0%) | >.9999 | 0/3 (0%) | >.9999 | 10/13 (77%) | 3/13 (23%) | .0169 | 2/9 (22%) | .0274 |
| Weight loss | 2/5 (40%) | 2/5 (40%) | >.9999 | 0/4 (0%) | .4444 | 2/4 (50%) | 1/4 (25%) | >.9999 | 0/3 (0%) | .4286 | 5/9 (56%) | 0/13 (0%) | .0048 | 0/9 (0%) | .0294 |
| Dermatitis | 4/5 (80%) | 1/5 (20%) | .2063 | 1/4 (25%) | .2063 | 4/4 (100%) | 1/4 (25%) | .1429 | 0/3 (0%) | .0286 | 10/13 (77%) | 5/13 (38%) | .1107 | 3/9 (33%) | .0789 |
| Eating disorders | 1/5 (20%) | 1/5 (20%) | >.9999 | 1/4 (25%) | >.9999 | 0/4 (0%) | 0/4 (0%) | >.9999 | 0/3 (0%) | >.9999 | 6/13 (46%) | 0/13 (0%) | .0149 | 1/9 (11%) | .1649 |
| Sexual dysfunctions | 1/5 (20%) | 3/5 (60%) | .5238 | 0/4 (0%) | >.9999 | 0/4 (0%) | 0/4 (0%) | >.9999 | 0/3 (0%) | >.9999 | 5/13 (38%) | 5/13 (38%) | >.9999 | 1/9 (11%) | .3330 |
| Circadian rhythm disruptions | 1/5 (20%) | 1/5 (20%) | >.9999 | 0/4 (0%) | >.9999 | 0/4 (0%) | 0/4 (0%) | >.9999 | 0/3 (0%) | >.9999 | 4/13 (31%) | 1/13 (8%) | .3217 | 1/9 (11%) | .3602 |
| Behavior changes | 0/4 (0%) | 1/5 (20%) | >.9999 | 0/4 (0%) | >.9999 | 0/4 (0%) | 0/4 (0%) | >.9999 | 0/3 (0%) | >.9999 | 2/12 (17%) | 0/13 (0%) | .2200 | 0/9 (0%) | .4857 |
| Diagnosis | |||||||||||||||
| CATTwb | 5/5 (100%) | 4/5 (80%) | >.9999 | 3/4 (75%) | .4444 | 4/4 (100%) | 4/4 (100%) | >.9999 | 2/3 (67%) | .4286 | 13/13 (100%) | 10/13 (77%) | .2200 | 4/9 (44%) | .0048 |
| CATTp | 5/5 (100%) | 1/5 (20%) | .0476 | 2/4 (50%) | .1667 | 4/4 (100%) | 0/4 (0%) | .0286 | 1/3 (33%) | .1429 | 13/13 (100%) | 3/13 (23%) | .0001 | 0/9 (0%) | <.0001 |
| Parasitology | 0/5 (0%) | 0/5 (0%) | >.9999 | 0/4 (0%) | >.9999 | 4/4 (100%) | 0/4 (0%) | .0286 | 0/3 (0%) | .0286 | 13/13 (100%) | 0/13 (0%) | <.0001 | 0/9 (0%) | <.0001 |
| Trypanolysis | |||||||||||||||
| LiTat 1.3 positive | 1/5 (20%) | 1/5 (20%) | >.9999 | 0/4 (0%) | >.9999 | 4/4 (100%) | 4/4 (100%) | >.9999 | 3/3 (100%) | >.9999 | 13/13 (100%) | 10/11 (91%) | .2200 | 5/9 (56%) | .0172 |
| LiTat 1.5 positive | 1/5 (20%) | 1/5 (20%) | >.9999 | 0/4 (0%) | >.9999 | 4/4 (100%) | 4/4 (100%) | >.9999 | 2/3 (67%) | .4286 | 11/13 (85%) | 8/11 (73%) | .6299 | 5/9 (56%) | .1778 |
| LiTat 1.6 positive | 1/5 (20%) | 1/5 (20%) | >.9999 | 0/4 (0%) | >.9999 | 4/4 (100%) | 4/4 (100%) | >.9999 | 0/3 (0%) | .0286 | 11/13 (85%) | 6/11 (54%) | .1819 | 0/9 (0%) | .0002 |
| Positive for all VATs | 1/5 (20%) | 1/5 (20%) | >.9999 | 0/4 (0%) | >.9999 | 4/4 (100%) | 4/4 (100%) | >.9999 | 0/3 (0%) | .0286 | 11/13 (85%) | 6/11 (54%) | .1819 | 0/9 (0%) | .0002 |
| Negative for all VATs | 4/5 (80%) | 4/5 (80%) | >.9999 | 4/4 (100%) | >.9999 | 0/4 (0%) | 0/4 (0%) | >.9999 | 0/3 (0%) | >.9999 | 0/13 (0%) | 1/11 (9%) | .4583 | 3/9 (33%) | .0545 |
| TBR PCR | |||||||||||||||
| Blood | 0/5 (0%) | 0/5 (0%) | >.9999 | 0/4 (0%) | >.9999 | 4/4 (100%) | 0/4 (0%) | .0286 | 0/3 (0%) | .0286 | 13/13 (100%) | 0/13 (0%) | <.0001 | 0/9 (0%) | <.0001 |
| Skin | 3/5 (60%) | 0/5 (0%) | .1667 | 0/4 (0%) | .1667 | 1/4 (25%) | 0/4 (0%) | >.9999 | 0/3 (0%) | >.9999 | 12/13 (92%) | 0/13 (0%) | <.0001 | 0/9 (0%) | <.0001 |
| Histology | |||||||||||||||
| Dermal touchpreps | 1/3 (33%) | 0/2 (0%) | >.9999 | ND | 1/3 (33%) | 1/4 (25%) | >.9999 | ND | 11/12 (92%) | 2/13 (15%) | .0002 | ND | |||
| IHC Hsp70 | 1/4 (25%) | ND | 0/4 (0%) | >.9999 | 1/4 (25%) | ND | 0/3 (0%) | >.9999 | 11/12 (92%) | ND | 0/9 (0%) | <.0001 | |||
| IHC ISG65 | 5/5 (100%) | 1/5 (20%) | .0476 | 0/4 (0%) | .0079 | 4/4 (100%) | 0/4 (0%) | .0286 | 0/3 (0%) | .0286 | 13/13 (100%) | 5/13 (38%) | .0016 | 0/9 (0%) | <.0001 |
| Negative for all reads | 0/5 (0%) | 2/5 (40%) | .4444 | 4/4 (100%) | .0079 | 0/4 (0%) | 2/4 (50%) | .4286 | 2/3 (67%) | .1429 | 0/13 (0%) | 1/13 (8%) | >.9999 | 9/9 (100%) | <.0001 |
Total values correspond to the numbers of subjects for which a value was available (n/total). For each group of subjects, P values were obtained by comparing one by one the parameters recorded at 6 months and 20 months after treatment/enrollment with those obtained at enrollment, using 2-sided Fisher’s exact tests at 5% confidence.
Abbreviations: CATTp, card agglutination test for trypanosomiasis on plasma; CATTwb, card agglutination test for trypanosomiasis on whole blood; Hsp70, heat shock protein 70; IHC, immunohistochemistry; ISG65, invariant surface glycoprotein 65; LN, lymph node; ND, not determined; PCR, polymerase chain reaction; TBR, T. brucei–specific; VAT, variable antigen type.
RESULTS
Epidemiological and Clinical Results
Results of the initial screening of 5417 individuals are shown in Table 1. Out of 5377 seronegative subjects (CATTwb-negative or CATTp <1/4), 40 were enrolled as seronegative controls, of whom 11 provided skin biopsies. A total of 40 seropositive individuals (CATTwb-positive and CATTp ≥1/4) were identified during the survey, of whom 12 tested negative upon parasitological examination (0.22%) and 28 were confirmed as HAT cases (0.52%). Eight nonconfirmed seropositive individuals and 18 confirmed HAT cases had no exclusion criteria and accepted to be enrolled in the study.
As shown in Table 2, the occurrence of dermatitis was significantly more frequent in confirmed HAT cases (15/18, 83%; P < .0001) and nonconfirmed seropositive individuals (5/8, 63%; P = .0166) as compared with seronegative controls (7/40, 18%). Pruritus was the most frequent dermatological sign in patients with confirmed HAT (11/18, 61%), as compared with seronegative controls (3/40, 8%). Among the various observed clinical manifestations of localized dermatitis, we unambiguously identified typical cases of intertrigo (in 4/18 confirmed cases vs 2/40 seronegative controls), pityriasis (in 3/18 vs 2/40), scabies (in 3/18 vs 1/40), dermatophytosis (in 3/18 vs 1/40), molluscum (in 3/18 vs 1/40), and ulceration (in 3/18 vs 1/40). The main clinical manifestations in nonconfirmed seropositive individuals were eczema (3/8), intertrigo (2/8), and pityriasis (1/8). Apart from general pruritus and intertrigo, all dermatological signs were observed in upper regions of the body, especially on the thorax and arms.
Biological Results
Plasma from all confirmed and unconfirmed seropositive cases, and from 11 of 40 seronegative controls, was assessed using the trypanolysis test, which detects complement-mediated immune responses activated by T.b. gambiense–specific antigens. All confirmed cases were positive for the LiTat 1.3 antigen, and 89% (16/18) of these cases were positive for both the LiTat 1.5 and 1.6 antigens (Table 3). Only 25% (2/8) of the unconfirmed seropositive individuals were positive for all antigens, while the others remained negative for all 3 variants, as seronegative controls.
A skin-punch biopsy was sampled from all enrolled confirmed and unconfirmed seropositive cases and from 11 of 40 seronegative controls. Dermal touch preparations were then generated in the field and full-length trypanosomes were observed on slides from 81% (13/16) of the confirmed cases and from 33% (2/6) of the unconfirmed seropositive individuals (Table 3, Supplementary Figure 1). One of the unconfirmed seropositive individuals who tested positive in this dermal test also tested positive in the trypanolysis test.
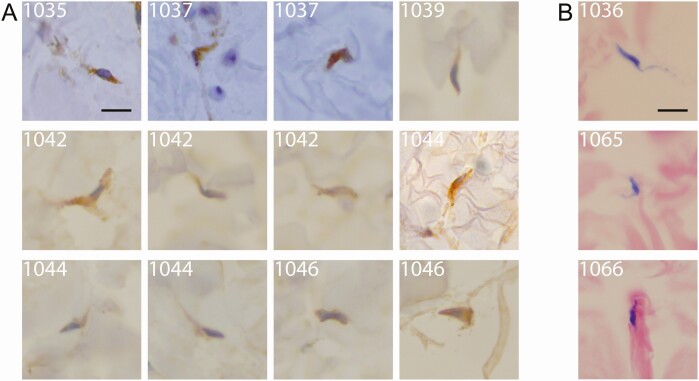
The skin biopsy samples were processed for IHC analyses in the laboratory. Skin samples obtained from the seronegative controls (11/11) did not test positive for trypanosomes (Table 3). By contrast, all unconfirmed seropositive individuals (8/8) and all confirmed cases (18/18) were found to be positive at least following staining by a T. brucei–specific anti-ISG65 antibody (Figure 1, Supplementary Figure 2, Table 3). In addition, all samples from nonconfirmed seropositive individuals and confirmed cases were also found to be positive following either unspecific Giemsa staining and/or unspecific H&E staining and/or labelling with a T. brucei–specific anti-Hsp70 antibody (Figure 1, Supplementary Figure 2, Table 3). In positive skin sections, T. brucei parasites were evenly distributed in the reticular dermis, and were occasionally associated with edema. No other parasites were detected in any of the skin samples.
Figure 1.
Extravascular trypanosomes in the dermal matrix of human skin biopsies. For each enrolled study subject, paraffin-embedded skin biopsy sections were stained either with a specific anti-ISG65 antibody (brown) (A) or with Giemsa (purple) (B) and screened at 100× magnification. Representative trypanosome sections from confirmed stage 1 (subject 1044) and stage 2 cases (subjects 1035, 1036, 1037, 1039, and 1042), as well as from unconfirmed seropositive individuals (subjects 1046, 1065, and 1066), are shown. The scale bars represent 10 μm. More images of extravascular Trypanosoma brucei parasites in human skin biopsies are available in Supplementary Figure 2.
To confirm the identity of these skin-dwelling parasites, T. brucei–specific PCR (TBR-PCR) assays were performed on total DNA extracted from fresh blood and from paraffin-embedded skin samples. Both blood and skin DNA samples from the seronegative controls (11/11) were found to be negative by the TBR-PCR assays. By contrast, 100% of blood (18/18) and 78% of skin samples (14/18) from confirmed cases tested positively in the TBR-PCR assays. Parasite DNA was only detected in the skin of unconfirmed seropositive individuals (6/8, 75%) but not in their blood (0/8) (Table 3). T.b. gambiense–specific TgsGP-PCR assays were performed on the same DNA samples and were positive for only 67% (12/18) of the blood samples of confirmed cases (Table 3). We reasoned that the use of fresh skin biopsies would be more appropriate than paraffin-embedded skin samples for TgsGP-PCR due to the low sensitivity of this method targeting a single-copy gene. To test this hypothesis, we obtained fresh skin samples from an outgroup of 9 additional confirmed cases, who were identified in 2018 in the same district by using the same study protocol (Supplementary Table 1). The fresh skin samples from 89% (8/9) of these confirmed cases were found to be positive to TBR-PCR, and 33% (3/9) were also found to be positive to TgsGP-PCR (Supplementary Table 1).
Follow-up Results
The same panels of analyses were repeated at 6 and 20 months after study enrollment of the unconfirmed seropositive individuals or after treatment of the confirmed cases (Table 4). In total, 17 of 18 and 12 of 18 confirmed cases were followed up at 6 months and 20 months after treatment, respectively, with 12 of 18 confirmed cases followed up twice, 5 of 18 followed up once, and 1 lost to follow-up (Table 4). Most of the clinical symptoms associated with the stage 2 cases at enrollment, including dermatological signs, significantly decreased in frequency during the first 6 months after treatment. Although all parasitological observations and PCR results became negative within 6 months after treatment in all confirmed cases (17/17), trypanosomes were still detected by histological methods in up to 38% of them (5/13 by IHC anti-ISG65). Twenty months after treatment, all CATTp and histological tests became negative (12/12), with 2 of 3 confirmed cases remaining positive to the trypanolysis test (Table 4).
In total, 5 of 8 and 4 of 8 unconfirmed seropositive individuals were followed up at 6 months and 20 months after enrollment, respectively, with 4 of 8 unconfirmed seropositive individuals followed up twice, 1 of 8 followed-up once, and 3 of 8 lost to follow-up (1 death, 1 pregnancy, and 1 resignation) (Table 4). In 80% (4/5) of the unconfirmed seropositive individuals who were monitored after enrollment, dermatological signs progressively disappeared (Table 4). The 4 unconfirmed seropositive individuals whose trypanolysis test was negative at enrollment became negative to CATTp, TBR-PCR on skin, and IHC anti-ISG65 in the same period. In contrast, the only trypanolysis-positive individual who could be monitored at 6 months maintained a serological reactivity to CATTp. No parasite DNA was detected by TBR-PCR in either blood or skin, but the skin biopsy remained positive by IHC-ISG65. Although this individual was lost to follow-up at the 20-month time point, he was diagnosed as a stage 1 case (CATTp 1/8, mAECT-BC+, CSF−, and WBC 4 : 4 white blood cells counted in the LCR) during an active surveillance campaign that was led in November 2019 (ie, after the end of this study) and was treated accordingly.
DISCUSSION
Here, we set out to investigate whether T.b. gambiense parasites might be found in the skin of confirmed gHAT cases, as well as in unconfirmed seropositive individuals, in regions of active disease transmission. Although this study is somewhat limited to a restricted population and to the detection methods used, 100% of the confirmed cases and unconfirmed seropositive subjects were found to carry extravascular trypanosomes in their skin.
Dermatological Signs in gambiense Human African Trypanosomiasis
Our results indicate that dermatological symptoms might be an important aspect of gHAT’s clinical presentation. The few reports that exist on this topic in the literature describe a wide array of skin pathologies associated with sleeping sickness, including pruritus, chancre, rashes, and localized edemas [13, 14]. However, detailed dermatological profiles of HAT cases have mostly been derived from light-skinned travelers with imported HAT [14]. Whereas chancres and rashes remain anecdotal, pruritus was the most commonly observed dermatological sign in endemic cases (in up to 57% of stage 2 cases) [14]. Here, we observed a higher occurrence of pruritus and dermatitis in unconfirmed seropositive individuals and in confirmed cases relative to seronegative controls (Table 2). The observed dermatitis profiles included some conditions the etiologies of which might not be directly related to a trypanosome infection. However, it could be hypothesized that the immune status of the infected host skin is somehow altered by the presence of trypanosomes in a way that promotes the outcome of dermatitis caused by other pathogens and/or increases skin sensitivity.
Trypanosome Detection
The direct detection of trypanosomes in the human skin is not well documented in the literature [13]. As there is no gold-standard approach for that purpose, we implemented 7 distinct molecular and immunohistological methods in parallel, yet with their own specific strengths and weaknesses. Here, dermal touch preparations were generated in the field in suboptimal ambient conditions (31°C at 75% humidity, on average), which could explain the unusual morphology of some trypanosomes, which were probably altered by osmotic shock while drying. Then, only a limited portion of each parasite is visible in the 2.5-μm skin sections because entire trypanosomes do not necessarily lie in the section plan. For the same reason, the parasite nucleus, kinetoplast, and flagellum are rarely all visible in the same given cell section. However, the specificities of the anti-ISG65 and anti-Hsp70 antibodies enable to unambiguously detect most T. brucei parasites within the extracellular dermal matrix, and this is confirmed by TBR-PCR assays. Considering that T.b. brucei are noninfectious to humans and killed within a couple of hours by human serum, the dermal parasites detected here, at least in confirmed cases, are likely to be T.b. gambiense parasites, as confirmed by the positivity of some direct TgsGP-PCR assays performed on fresh skin samples from an outgroup. However, further genetic studies would be necessary to rule out the hypothesis of infections with a peculiar T.b. brucei strain.
The detection of skin-dwelling parasites at enrollment in most of the 2-mm skin-punch biopsies sampled from seropositive individuals indicates that skin-dwelling parasites might be present over a considerable proportion of the skin surface. However, the precise dynamics of parasite load and distribution in the extravascular dermal compartment over the course of an infection remain unknown. According to historic (reviewed in [5]) and more recent [6] studies in experimental animal models, skin-dwelling parasites could theoretically be detected in almost the entire skin surface, yet with a variable distribution and at variable local densities.
A Dermal Reservoir of Trypanosomes in Nonconfirmed Seropositive Individuals
One possible explanation for the persistence of disease foci in certain regions is the presence of animal reservoirs [15]. Another possibility, as increasing evidence suggests, is that traditionally used diagnostic approaches do not detect some T.b. gambiense infections among seropositive cases [15]. Indeed, bloodstream parasite numbers in T.b. gambiense infections can periodically fluctuate to less than 100 trypanosomes/mL, falling below the detection limit of the most sensitive methods currently in use [16]. Another study estimated that 20–30% of gHAT cases are missed in active case detection by standard parasitological techniques and are left untreated [17]. These infected individuals might ultimately progress to clinical disease or remain almost asymptomatic until undergoing a possible self-cure [15].
Here, routine molecular analyses confirmed the presence of T. brucei parasites in the skin of unconfirmed seropositive individuals, including those testing negative to the LiTat 1.3 trypanolysis test known to be highly specific of T.b. gambiense. As previously observed in the same transmission focus [7], trypanolysis-negative individuals rapidly became negative to CATTp and this was associated with the disappearance of detectable dermal trypanosomes. In such subjects, dermal infections could be transient and too short to allow T.b. gambiense to invade the bloodstream and express the LiTat 1.3 antigen. Alternatively, these infections could possibly be caused by other trypanosome species cross-reacting with the CATT. More sensitive and extensive molecular analyses will be required to solve this question.
Only 2 unconfirmed seropositive individuals were positive to the LiTat 1.3 trypanolysis test in this study. One died before the first follow-up and the other was lost to follow-up after 6 months. Nevertheless, it is noteworthy that this last individual, who was still positive to histological tests at 6 months, was eventually diagnosed as a stage 1 case during a medical survey almost 2.5 years after enrollment. Systematic characterization and follow-up of dermal trypanosomes in unconfirmed seropositive individuals testing positive to the LiTat 1.3 trypanolysis test would be required to better address the role of these individuals in the transmission of T.b. gambiense.
Transmission and Epidemiological Contribution of Dermal Trypanosomes
Mathematical modeling recently predicted that, in the absence of any animal reservoirs, these unconfirmed seropositive individuals could contribute to disease transmission by maintaining an overlooked reservoir of skin-dwelling parasites [18]. The infected skin of seropositive unconfirmed individuals could provide a population of parasites that are readily accessible to the tsetse fly. Indeed, this mode of transmission has been demonstrated in experimental animal models, in which skin-dwelling trypanosomes were efficiently transmitted to the tsetse vector, even in the absence of detectable parasitemia [6, 19, 20]. However, the presence of the stumpy parasite forms that are assumed to be most adapted for development in tsetse flies was not investigated here. This is an important question for future studies to address in order to estimate the actual infectivity potential of human skin–dwelling parasites. Our reported observations should also be confirmed in a larger number of unconfirmed seropositive individuals (including Rapid Diagnostic Test (RDT)-positive subjects), and the study scaled up to include other endemic transmission foci in Africa, in order to confidently determine the actual prevalence of dermal trypanosomes.
Our results raise questions about the strategies used to diagnose this disease, which currently focus on detecting parasites in the blood and lymph. If the human skin is indeed a reservoir for trypanosomes, it could represent a novel target for diagnostics, and it could (1) allow more carriers to be treated, (2) help to determine a more accurate estimate of the true prevalence of the disease, and (3) help identify as yet undetected reservoirs in both human and animal populations. The development of less invasive and field-adapted diagnostic methods to detect extravascular dermal trypanosomes, such as the serological detection of skin-related biomarkers or the identification of specific biophysical profiles by skin scanning, would benefit these goals. The current WHO recommendation, based on risk–benefit analyses, is to not treat unconfirmed seropositive individuals without knowing if they have an active infection [1]. Importantly, we observed that the routinely administered trypanocide treatments (pentamidine for stage 1 and Nifurtimox-Eflornithine Combination Therapy (NECT) for stage 2 cases) efficiently targeted both bloodstream and dermal trypanosomes in all of the patients followed up over 20 months. With the promise of new, cheaper, less toxic, and easier to administer drugs on the horizon, the policy of treating unconfirmed seropositive individuals could possibly be reconsidered. Indeed, the new drug Acoziborole (SCYX-7158, Anacor Pharmaceuticals), which requires a single oral administration, could be the next revolutionary treatment against gHAT. As gHAT approaches its elimination targets, we propose from our findings that the current algorithms, used to identify and manage disease cases, could be adapted to include the detection of skin-dwelling parasites, which likely represent a previously unaccounted for anatomical reservoir.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Mariame C., A. M. S., and N.-R. K. S. conducted the clinical study in the field and commented on the manuscript. H. I., I. S., C. T., C. C., A. Cooper, A. Crouzols, O. C., E. C. A., and J.-M. B. performed sample analyses and commented on the manuscript. Mamadou C. and V. J. performed logistical aspects, analyzed part of the data, and commented on the manuscript. A. M., B. B., and B. R. designed the study, organized logistical aspects, analyzed the data, and wrote the manuscript as co-last authors.
Acknowledgments. The authors thank M. Carrington (Cambridge, United Kingdom) and J. D. Bangs (Buffalo, NY) for providing antibodies. They are grateful to Y. Madec (Paris, France) for his help in statistical analysis. They warmly thank the team of the Programme National de Lutte contre la Trypanosomiase Humaine Africaine of Conakry, as well as all our collaborators of the Forecariah District Health Department. They thank Dominique N’Diaye (Paris, France) for his critical reading and Jane Alfred (Catalyst Editorial, UK) for her editorial work on the manuscript.
Disclaimer. None of the funding sources has a direct scientific or editorial role in the present study.
Financial support. This work was supported by the Institut Pasteur, the Wellcome Trust (grant number 209511/Z/17/Z); the Institut de Recherche pour le Développement, the French Government Investissement d’Avenir Programme—Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases” (ANR-10-LABX-62-IBEID); and the French National Agency for Scientific Research (projects ANR-14-CE14-0019-01 EnTrypa and ANR-18-CE15-0012 TrypaDerm).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Büscher P, Cecchi G, Jamonneau V, Priotto G. Human African trypanosomiasis. Lancet 2017; 390:2397–409. [DOI] [PubMed] [Google Scholar]
- 2. Lindner AK, Lejon V, Chappuis F, et al. New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: substantial changes for clinical practice. Lancet Infect Dis 2020; 20:e38–46. [DOI] [PubMed] [Google Scholar]
- 3. Franco JR, Cecchi G, Priotto G, et al. Monitoring the elimination of human African trypanosomiasis: update to 2016. PLoS Negl Trop Dis 2018; 12:e0006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berthier D, Brenière SF, Bras-Gonçalves R, et al. Tolerance to trypanosomatids: a threat, or a key for disease elimination? Trends Parasitol 2016; 32:157–68. [DOI] [PubMed] [Google Scholar]
- 5. Goodwin LG. The pathology of African trypanosomiasis. Trans R Soc Trop Med Hyg 1970; 64:797–817. [DOI] [PubMed] [Google Scholar]
- 6. Capewell P, Cren-Travaille C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. eLife 2016; 5:e17716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ilboudo H, Jamonneau V, Camara M, et al. Diversity of response to Trypanosoma brucei gambiense infections in the Forecariah mangrove focus (Guinea): perspectives for a better control of sleeping sickness. Microbes Infect 2011; 13:943–52. [DOI] [PubMed] [Google Scholar]
- 8. Van Meirvenne N, Magnus E, Buscher P. Evaluation of variant specific trypanolysis tests for serodiagnosis of human infections with Trypanosoma brucei gambiense. Acta Trop 1995; 60:189–99. [DOI] [PubMed] [Google Scholar]
- 9. Ziegelbauer K, Overath P. Organization of two invariant surface glycoproteins in the surface coat of Trypanosoma brucei. Infect Immun 1993; 61:4540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDowell MA, Ransom DM, Bangs JD. Glycosylphosphatidylinositol-dependent secretory transport in Trypanosoma brucei. Biochem J 1998; 335 (Pt 3):681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cunningham LJ, Lingley JK, Haines LR, Ndung’u JM, Torr SJ, Adams ER. Illuminating the prevalence of Trypanosoma brucei s.l. in glossina using LAMP as a tool for xenomonitoring. PLoS Negl Trop Dis 2016; 10:e0004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radwanska M, Claes F, Magez S, et al. Novel primer sequences for polymerase chain reaction-based detection of Trypanosoma brucei gambiense. Am J Trop Med Hyg 2002; 67:289–95. [DOI] [PubMed] [Google Scholar]
- 13. McGovern TW, Williams W, Fitzpatrick JE, Cetron MS, Hepburn BC, Gentry RH. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol 1995; 131:1178–82. [PubMed] [Google Scholar]
- 14. Blum JA, Neumayr AL, Hatz CF. Human African trypanosomiasis in endemic populations and travellers. Eur J Clin Microbiol Infect Dis 2012; 31:905–13. [DOI] [PubMed] [Google Scholar]
- 15. Buscher P, Bart JM, Boelaert M, et al. ; Informal Expert Group on Gambiense HATR . Do cryptic reservoirs threaten gambiense-sleeping sickness elimination? Trends Parasitol 2018; 34:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Büscher P, Deborggraeve S. How can molecular diagnostics contribute to the elimination of human African trypanosomiasis? Expert Rev Mol Diagn 2015; 15:607–15. [DOI] [PubMed] [Google Scholar]
- 17. Robays J, Bilengue MM, Van der Stuyft P, Boelaert M. The effectiveness of active population screening and treatment for sleeping sickness control in the Democratic Republic of Congo. Trop Med Int Health 2004; 9:542–50. [DOI] [PubMed] [Google Scholar]
- 18. Capewell P, Atkins K, Weir W, et al. Resolving the apparent transmission paradox of African sleeping sickness. PLoS Biol 2019; 17:e3000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caljon G, Van Reet N, De Trez C, Vermeersch M, Pérez-Morga D, Van Den Abbeele J. The dermis as a delivery site of Trypanosoma brucei for tsetse flies. PLoS Pathog 2016; 12:e1005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wombou Toukam CM, Solano P, Bengaly Z, Jamonneau V, Bucheton B. Experimental evaluation of xenodiagnosis to detect trypanosomes at low parasitaemia levels in infected hosts. Parasite 2011; 18:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.