Abstract
Bergamot has been traditionally used for the relief of diseases related to oxidative stress. Our aim was to investigate the effect of bergamot phytosome on visceral adipose tissue (VAT) and on metabolic profile, in overweight and obese subjects with mild hypercholesterolemia. A total of 64 participants were randomized into two groups for 12 weeks: a supplemented group (33 individuals, BMI 27 ± 3 kg/m2 receiving 500 mg of bergamot phytosome, two daily tablets) and placebo group (31 subjects, BMI 28 ± 3 kg/m2, two daily tablets). As to the within differences, the parameters of VAT, total and LDL‐cholesterol were significantly decreased in the bergamot phytosome group, but not in the placebo group. As to between‐group differences, a statistically significant interaction between time and group, that is, the change in score over time differs between the two groups was observed 30 days after supplementation for VAT (p‐value = .005), total cholesterol (p‐value <.0002), and LDL (p = .004) in respect to placebo. The other parameters (glucose, insulin, Homeostasis Model Assessment, high‐density lipoprotein cholesterol, triglycerides, fat free mass, fat mass) were not significant. In conclusion, this clinical study gives evidence that bergamot phytosome provides beneficial effects, such as decrease of VAT and modulation of metabolic alterations, after just 30 days of supplementation, resulting a very promising protection of cardiovascular health.
Keywords: bergamot phytosome, cardiovascular health, dietary supplement, hypercholesterolemia, obesity, visceral adipose tissue
1. INTRODUCTION
Control of lipid levels is one of the most effective strategies for cardiovascular disease (CVD) prevention. Epidemiologic data have demonstrated the crucial role of dyslipidemia, especially hypercholesterolemia, in the development of CVD (Verschuren et al., 1995).
Available evidence confirms that reducing plasma low‐density lipoprotein cholesterol (LDL‐C) reduces CVD risk, and it is generally accepted that the reduction of LDL‐C levels is of prime importance in the prevention of CVD; most current guidelines on the management of dyslipidemia or the prevention of CVD identify LDL‐C as a primary target (Anderson et al., 2013; Catapano et al., 2016; Perk et al., 2012). Many clinical trials have clearly demonstrated that cholesterol lowering, primarily with statins, reduces major vascular events and mortality (Baigent et al., 2010; Catapano et al., 2011).
Another critical factor that predisposes individuals to CVD is increased visceral adipose tissue (VAT; Van Gaal, Mertens, & De Block, 2006).
It is becoming clear that the distribution of body fat is more important in this regard than simply the amount of fat. For instance, android rather than gynoid distribution of fat is more often associated with the metabolic syndrome, diabetes, and CVD (Despres et al., 1989; Janssen, Katzmarzyk, & Ross, 2004; Krotkiewski, Bjorntorp, Sjostrom, & Smith, 1983; Kuk et al., 2006; Lapidus et al., 1984; Larsson, Svardsudd, & Welin, 1984). Therefore, anthropometric measurements, such as the waist size, and the evaluation of VAT by DXA, have been shown in epidemiological studies to predict adverse outcomes (Hayashi et al., 2007; Janssen et al., 2004; Poirier & Després, 2003).
A number of supplements for dyslipidemia are available in the market with beneficial effects on plasma lipids, although their impact on cardiovascular risk remains largely unknown (Mannarino, Ministrini, & Pirro, 2014).
Several studies have demonstrated multiple health‐related properties of the Citrus flavonoids on cardiovascular health protection (Benavente‐García & Castillo, 2008).
It is generally believed that bioactive phytochemicals can play an important therapeutic role in attenuating oxidative damage induced by atherogenic dyslipidemia and inflammation (Mangge, 2014). Bergamot is an endemic plant of the Calabrian region in southern Italy with a unique profile of flavonoids and flavonoid glycosides present in its juice and albedo, such as neoeriocitrin, neohesperidin, naringin, rutin, neodesmin, rhoifolin, and poncirin (Gliozzi, Walker, & Mollace, 2014). Last year, a detailed phytochemical investigation on the polyphenolic fraction was completed to fully characterize the bouquet of this distinctive source of phytonutrients (Formisano et al., 2019).
Recent studies on the juice from the endocarp of bergamot have shown that it has several bioactivities such as antioxidant, antiinflammatory, neuroprotective, as well as hypolipidemic and hypoglycemic properties (Currò et al., 2016; Miceli et al., 2007; Mollace et al., 2008; Risitano et al., 2014; Trovato et al., 2010; Tundis et al., 2012). Bergamot phytosome is an innovative, food‐grade formulation of polyphenols from bergamot (Citrus bergamia Risso et Poiteau, exclusively from plantations in Calabria) standardized to contain 11–19% of total flavonones by HPLC. The recommended dose is 500 mg twice a day. The phytosome delivery system optimizes the biological absorption of polyphenols, normally characterized by low solubility. A recent study in humans shows that bergamot phytosome is effective in modulating total cholesterol (TC), high‐density lipoprotein (HDL), LDL, triglycerides (TGs), and glucose levels, through antioxidant, hypoglycemic and hypolipidemic action (Mollace et al., 2011). These characteristics make it particularly suitable for its use against metabolic syndrome and for any condition that might impair the maintenance of good cardiovascular health.
We investigated whether bergamot phytosome could be used for metabolic management in overweight and obese class I subjects (BMI between 25 and 35 kg/m2) with newly diagnosed mild hypercholesterolemia (5.4–7.0 mmoL/L).
The primary end point of the study is the evaluation of VAT, by Dxa Corescan, and the secondary end‐points are the lipid and glycemic parameters.
2. MATERIALS AND METHODS
2.1. Experimental design
The study was a 12‐week randomized double‐blind placebo‐controlled trial. Participants were randomized to either the bergamot phytosome or placebo arm. Randomization was performed by a block randomization schedule provided by an external randomization service. One researcher (HP) screened and enrolled participants, and the random allocation sequence was implemented via sequentially numbered but otherwise identical sealed tablet containers allocated to participants in the order of enrolment. The randomization code was provided in sealed envelopes only to be broken at the end of the clinical trial or in the case of serious adverse events (AEs).
2.2. Population
The subjects were recruited from the Dietetic and Metabolic Unit of the “Santa Margherita” Institute, University of Pavia, Italy.
The inclusion criteria of the subjects were: age between 18 and 60 years, mild hypercholesterolemia (5.4–7.0 mmoL/L), body mass index (BMI) ranging from 25 to 35 kg/m2, no history of CVD, not taking any medication likely to affect lipid metabolism (such as statins) or to affect appetite and body fat storage (such as corticosteroids, antihistamines, antidepressants), free of overt liver, renal and thyroid diseases and sedentary. The exclusion criteria were smoked or drank more than two standard alcoholic beverages/day (20 g of alcohol/day), not taking dietary supplements which affects appetite, lipogenesis, or hyperlipemia. The experimental protocol was approved by the Ethics Committee of the University of Pavia (ethical code Number: 9222/14122018) and all volunteers gave their written informed consent.
2.3. Dietary supplement
Bergamot Phytosome (Vazguard, Indena SpA) is a food‐grade innovative lecithin formulation of the bergamot enriched polyphenols fraction (BPF provided by Herbal and Antioxidant Derivatives Srl, Bianco, Italy). Briefly, Citrus bergamia Risso & Poiteau fruits were collected from plants located in Calabria, Italy. These were the specific source for the preparation of the BPF extract then formulated in Phytosome. BPF was prepared as previously described (Mollace et al., 2011). Dietary phospholipids (sunflower lecithin) were formulated with 40% in weight standardized BPF extract as described previously by Mollace et al. (2019), in order to enhance oral bioavailability of bergamot main flavonoids. Tablets containing 500 mg of bergamot phytosome (standardized to contain 11–19% of total flavanones) and placebo were provided by Indena SpA (Milan, Italy). Tablets with no active ingredient were used as placebo and were identical to bergamot phytosome ones, in terms of size, shape, color, odor, and taste. The other no active food‐grade components of the bergamot phytosome and placebo film‐coated tablets were the following: Microcrystalline cellulose E460, calcium phosphate E341 polyvinylpolypirrolidone E1202, sodium croscarmellose E468, silicon dioxide E551, talc E553b, magnesium stearate E470b, hydroxypropylmethylcellulose E464‐based film‐coating. Before release, the film‐coated tablets were tested for appearance, average mass, uniformity of mass, HPLC‐content of total bergamot flavanones, disintegration time and microbiological quality. All procedures have been performed according to Food Supplement European Regulation.
Subjects were randomly allocated to one of two groups, using a block randomization code to ensure balanced groups with 1:1 ratio: dietary supplement:
Active intervention: Bergamot Phytosome.
Placebo.
Supplementation regimen was two daily tablets, one at breakfast and one at dinner, for 12 continuous weeks. The daily dose of bergamot phytosome was based on the pharmacokinetics profile and the efficacy human study recently reported by Mollace et al. (2019).
Regarding blinding, the active intervention and placebo were given in identical containers devoid of any labeling by the principal investigator, who was not involved in any of the assessments.
Compliance to the supplementation regimen was defined as the number of tablets actually taken by each subject, divided by the number of tablets that should have been taken over the course of the study. AEs were based on spontaneous reporting by subjects as well as open‐ended inquiries by members of the research staff. Safety was assessed by laboratory tests performed at baseline and end of treatment (EoT) detailed below, and by recording volunteered AEs.
2.4. Lipid and glycaemic parameters
The lipid and glycemic parameters were assessed at the start of the study, after 30 days, 60 days, and 90 days as EoT. In order to avoid venipuncture distress, blood samples were obtained through an indwelling catheter inserted in an antecubital vein. Subjects were instructed to fast for 12 hours before obtaining the blood sample. Blood samples were immediately centrifuged and stored at −80°C. Fasting blood glucose, TC, LDL‐C, HDL cholesterol (HDL‐C), and TG levels were measured by automatic biochemical analyzer (Hitachi 747, Tokyo, Japan) with kits by Roche Diagnostics Ltd (CV: 3–5%). The serum insulin was evaluated by a double antibody RIA (Kabi Pharmacia Diagnostics AB, Uppsala, Sweden) and expressed as pmol/L. The intra‐ and inter‐assay coefficients of variation were below 6% and the low detection limit was 10.7 pmol/L. The subjects refrained from any form of physical exercise for 48 hours before the study. Female subjects were tested during the early follicular phase of their menstrual cycles (days 3–10). Insulin resistance was evaluated using the Homeostasis Model Assessment (Haffner, Kennedy, Gonzalez, Stern, & Miettinen, 1996). Finally, for the assessment of safety, routine blood biochemistry parameters (blood count, serum protein electrophoresis, creatinine, liver, and thyroid function) were evaluated at the start and at the end of intervention.
2.5. Anthropometric measurements
Nutritional status was assessed using anthropometric measurements at the start of the study, after 30, 60, and 90 days as EoT. Body weight and height were measured and the BMI was calculated (kg/m2; Frisancho, 1984). Anthropometric parameters were always collected by the same investigator.
2.6. Dietary counseling and physical activity
Subjects were trained to follow a regimen that maintained a prudent balance of macronutrients based on WHO criteria (World Health Organization, 1985): 28% of energy from fat (cholesterol <200 mg), 57% of energy from carbohydrates (10% from simple carbohydrates), with 20–25 g of bran and 15% of energy from protein. A registered dietician performed initial dietary counseling. A three‐day weighed‐food record of two weekdays and one weekend day was performed during the first and the last week of the study. Dietary records were analyzed using a food‐nutrient database (Rational Diet, Milan, Italy).
Regarding physical activity, according to the patient inclusion criteria, only sedentary patients were recruited and participants were asked to maintain their habitual activity.
2.7. Body composition
Body composition (FFM, fat mass, and gynoid and android fat distribution) was measured by dual‐energy X‐ray absorptiometry (DXA) with the use of a Lunar Prodigy DXA (GE Medical Systems). The in vivo CVs were 0.89 and 0.48% for whole body fat (fat mass) and FFM, respectively.
VAT volume was estimated using a constant correction factor (0.94 g/cm3). The software automatically places a quadrilateral box, which represents the android region, outlined by the iliac crest and with a superior height equivalent to 20% of the distance from the top of the iliac crest to the base of the skull (Mohammad et al., 2017).
2.8. Statistical analysis
Sample size was calculated using parameters as from in (Parker et al., 2019): for the primary outcome a loss of body fat of 0.0 kg “between groups” (−0.2 kg intervention vs. –0.00 kg placebo) with standard deviation on supplementation variables of ±0.4 kg for 12 weeks of treatment. Considering two balanced groups with 1:1 allocation (n1 = n2), an alpha significance level set at 0.05, an output rate (dropout) of 10 and 80% of power in detecting differences between the groups, a sample size of 60 subjects in total (30 subjects per arm; Parker et al., 2019) were calculated.
Data were investigated using an intention to treat analysis. Differences between groups at baseline were investigated in each continuous variable using t tests for independent data. To evaluate statistically significant changes over time for primary and secondary endpoints we fitted a linear mixed model for longitudinal data with time, group and the interaction time*group as fixed effect, including a random effect in the form of time|subject and an autocorrelation term (corAR1) in order to take into account for intra‐subject correlation and temporal effect for sampling/measuring (Pinheiro & Bates, 2006; Verbeke & Molenberghs, 2000). All models were adjusted for age, sex, and BMI. Normality of residuals was assessed graphically and with Shapiro–Wilk test. When the pattern of change of the variable's mean over time in the longitudinal study was not simply linear, to accommodate for non‐linear trends we fitted a piecewise mixed‐effects model, thus modeling segmented change over time (Fitzmaurice, Laird, & Ware, 2011). In fact, in some instances, a more evident change in the mean could occur in the first observation period, that is, 30 days after supplementation, while the long‐term, that is, from 30 to 60 days and even more from 60 to 90 days after supplementation, could be relatively minimal or could follow a different trend. In these cases, we proceeded by dividing the time axis into two segments and fitting a mixed model as specified above for each segment, thus obtaining separate slope coefficients that represent the actual slopes in the respective time period. Additionally, for these variables, we also performed a t test to compare the difference in the mean delta (pre‐T1 and post‐T4 measurement difference) between the two groups.
Pearson's pairwise partial correlations adjusted for sex, age, and BMI (z), between the primary endpoint (x) and each selected secondary endpoint (y), r(x,y|z), in the two groups at baseline and at the different time points were computed.
Descriptive statistics are reported as mean ± standard deviation (SD). All analysis was performed on R 3.5.1 software (R Core Team, 2017).
3. RESULTS
A total of 64 participants were recruited, randomized, and analyzed for primary and secondary endpoints. Thirty‐three subjects were assigned to supplemented group (18 female and 15 male), while 31 were assigned to placebo group (18 female and 13 male).
Baseline characteristics of participants separately for supplementation and placebo groups are shown in Table 1. No statistically significant differences were observed between the two groups at baseline.
TABLE 1.
Baseline (T1) characteristics in the supplemented and placebo groups
| Supplement (n = 33) mean (SD) | Placebo (n = 31) mean (SD) | p Value a | |
|---|---|---|---|
| Age (years) | 59.03 (8.06) | 57.29 (8.13) | .39 |
| BMI (kg/m2) | 27.86 (3.35) | 28.58 (3.00) | .362 |
| Primary endpoint | |||
| VAT (g) | 1,042.55 (629.56) | 1,095.32 (610.56) | .735 |
| Secondary endpoints | |||
| Total cholesterol (mg/dl) | 245.09 (21.32) | 237.29 (18.97) | .123 |
| LDL (mg/dl) | 156.30 (28.70) | 161.19 (25.60) | .474 |
| HDL (mg/dl) | 61.12 (18.46) | 60.77 (19.22) | .942 |
| VLDL (mg/dl) | 23.85 (9.38) | 23.68 (9.29) | .940 |
| Triglycerides (mg/dl) | 119.27 (46.91) | 118.39 (46.43) | .940 |
| Glycemia (mg/dl) | 87.12 (8.02) | 87.06 (7.05) | .977 |
| Insulin (mcU/ml) | 9.56 (5.74) | 10.64 (5.79) | .456 |
| HOMA | 2.10 (1.33) | 2.34 (1.39) | .475 |
| APOB (mg/dl) | 133.21 (23.54) | 133.77 (18.55) | .916 |
| APOA (mg/dl) | 165.58 (33.81) | 164.68 (42.31) | .926 |
| AST (IU/l) | 20.88 (6.04) | 19.93 (5.85) | .530 |
| ALT(IU/l) | 21.61 (9.42) | 21.81 (7.94) | .927 |
| GGT (U/l) | 26.58 (28.73) | 19.77 (6.80) | .195 |
| Creatinine (mg/dl) | 0.80 (0.14) | 0.82 (0.11) | .597 |
| Waist circumference (cm) | 91.23 (13.08) | 96.40 (17.15) | .182 |
| Weight (kg) | 69.85 (12.53) | 76.23 (17.45) | .104 |
| Fat mass (g) | 25,237.94 (8,104.81) | 30,656.03 (13,614.78) | .06 |
| Lean mass (g) | 42,324.30 (7,710.22) | 43,299.29 (7,520.21) | .610 |
| ApoB/ApoA | 0.83 (0.21) | 0.86 (0.25) | .611 |
| Total cholesterol/HDL | 4.32 (1.19) | 4.25 (1.29) | .811 |
| ApoA/HDL | 2.79 (0.34) | 2.78 (0.35) | .905 |
| LDL/HDL | 2.72 (0.77) | 2.88 (0.94) | .465 |
Abbreviations: ALT, alanine aminotransferase; APOA‐I, Apolipoprotein A‐I; APOB, apolipoprotein B; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; VAT, visceral adipose tissue; VLDL, very low‐density lipoprotein.
p Value for between group comparisons.
Figure 1 displays the mean of the variables over time separately in the two groups. In some instances, more evident change in the mean, both in terms of decreasing and increasing, occurs in the first observation period, that is, 30 days, and then remains constant in the subsequent observation period, that is, 60 and 90 days after supplementation.
FIGURE 1.
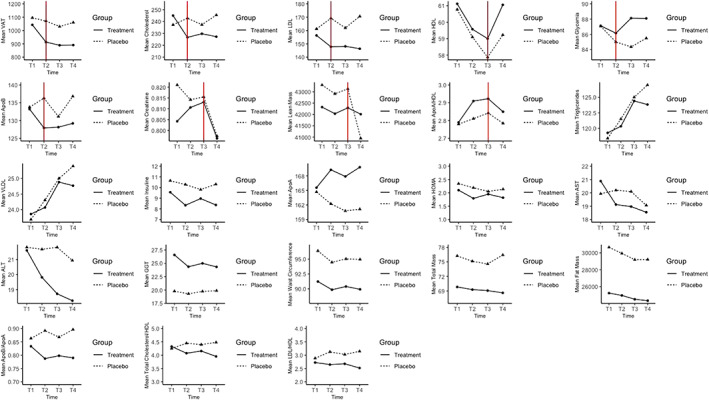
Mean of the primary and secondary outcomes over time separately in supplement and placebo groups. Red vertical line, where present, represents the break point, that is, knot, decided by data‐driven graphical representations [Colour figure can be viewed at wileyonlinelibrary.com]
This tendency, as shown in Figure 1, can be seen for VAT in both groups while for TC, LDL, and ApoB in the supplement group only. With regard to HDL, we observe in both groups a decrease of its mean over time until T3 (i.e., 60 days) and a subsequent increase of its levels at T4 (90 days). Glycemia showed a moderate decrease from T1 to T2 (i.e., 30 days) in both groups followed by a subsequent moderate increase of its levels in the subsequent observation period. Creatinine mean over time until T3 (60 days) follows a different trend for the two groups, a small decrease in placebo group and a small increase in supplement group, but at 60 days, we assist to a decrease in both groups. As to the lean mass in both groups, in the first 60 days, we do not observe any decreasing or increasing trend, but after 60 days, we observe a rapid decrease that is more pronounced in placebo group. Lastly, for ApoA/HLD ratio, there is an increasing trend for both groups until T3 (60 days) followed by a decrease at T4 (90 days). To evaluate statistically significant changes over time within and between the two groups for these variables in which the knot is set, we created an additional time spline variable based on time and knot in order to fit the piecewise mixed effects model. Results of the within‐group and between‐group differences, using piecewise mixed effect linear regression, are reported, respectively, in Tables 2 and 3 both for the primary and the secondary endpoints, together with the descriptive statistics in terms of mean ± SD for each endpoint at each time point.
TABLE 2.
Within group pre–post supplementation difference for primary and secondary endpoints using piecewise mixed effect linear regression. At each time point, mean ± SD are reported
| Group | Mean (SD) T1 | Mean (SD) T2 | Mean (SD) T3 | Mean (SD) T4 | Effect | Estimate | SE | p Value* | |
|---|---|---|---|---|---|---|---|---|---|
| Primary endpoint | |||||||||
| VAT (g) | Supplement | 1,042.55 (629.56) | 913.12 (635.59) | 889.36 (623.74) | 890.88 (630.87) |
time T1‐T2 time T2‐T4 |
−130.08 −11.12 |
23.67 16.21 |
<.0001 .49 |
| Placebo | 1,095.32 (610.56) | 1,070.77 (653.26) | 1,029.81 (643.92) | 1,060.03 (626.53) |
time T1‐T2 time T2‐T4 |
−29.75 −5.37 |
26.93 15.92 |
.27 .74 |
|
| Secondary endpoints | |||||||||
| Total cholesterol (mg/dl) | Supplement | 245.09 (21.32) | 226.73 (18.18) | 229.79 (21.65) | 227.30 (22.79) |
time T1‐T2 time T2‐T4 |
−18.06 0.29 |
2.38 1.59 |
<.0001 .86 |
| Placebo | 237.29 (18.97) | 242.87 (25.77) | 237.39 (23.96) | 245.52 (22.60) |
time T1‐T2 time T2‐T4 |
2.07 1.32 |
4.90 1.68 |
.67 .43 |
|
| LDL (mg/dl) | Supplement | 156.30 (28.70) | 147.79 (26.41) | 148.15 (27.58) | 146.36 (30.99) |
time T1‐T2 time T2‐T4 |
−8.16 −0.71 |
2.35 1.62 |
.0008 .66 |
| Placebo | 161.19 (25.60) | 169.19 (31.01) | 161.94 (28.55) | 170.61 (28.32) |
time T1‐T2 time T2‐T4 |
3.66 0.71 |
3.73 1.60 |
.33 .66 |
|
| HDL (mg/dl) | Supplement | 61.12 (18.46) | 59.58 (16.44) | 59.00 (17.10) | 61.06 (16.49) |
time T1‐T3 time T3‐T4 |
−2.06 0.74 |
0.95 0.45 |
.03 .10 |
| Placebo | 60.77 (19.22) | 59.10 (17.72) | 57.87 (17.64) | 59.23 (17.77) |
time T1‐T3 time T3‐T4 |
−2.41 0.06 |
1.16 0.66 |
.04 .92 |
|
| Glycemia (mg/dl) | Supplement | 87.12 (8.02) | 86.15 (11.97) | 88.12 (19.77) | 88.09 (20.90) |
time T1‐T2 time T2‐T4 |
−0.81 0.97 |
1.71 1.21 |
.64 .42 |
| Placebo | 87.06 (7.05) | 85.00 (7.74) | 84.35 (7.41) | 85.48 (7.81) |
time T1‐T2 time T2‐T4 |
−2.33 0.24 |
1.19 0.63 |
.05 .70 |
|
| ApoB (mg/dl) | Supplement | 133.21 (23.54) | 127.94 (22.50) | 128.15 (20.71) | 129.21 (24.20) |
time T1‐T2 time T2‐T4 |
−5.39 0.64 |
2.60 1.85 |
.04 .73 |
| Placebo | 133.77 (18.55) | 136.29 (21.75) | 131.13 (19.94) | 136.81 (21.99) |
time T1‐T2 time T2‐T4 |
−0.45 0.26 |
2.44 1.27 |
.85 .84 |
|
| Creatinine (mg/dl) | Supplement | 0.80 (0.14) | 0.81 (0.14) | 0.81 (0.13) | 0.80 (0.15) |
time T1‐T3 time T3‐T4 |
0.01 −0.007 |
0.01 0.007 |
.45 .30 |
| Placebo | 0.82 (0.11) | 0.81 (0.13) | 0.82 (0.14) | 0.80 (0.12) |
time T1‐T3 time T3‐T4 |
−0.003 −0.008 |
0.01 0.007 |
.83 .22 |
|
| Lean mass (g) | Supplement | 42,324.30 (7,710.22) | 42,032.73 (7,673.63) | 42,291.18 (7,754.14) | 42,011.15 (7,548.28) |
time T1‐T3 time T3‐T4 |
−218.11 −10.79 |
193.97 105.11 |
.26 .92 |
| Placebo | 43,299.29 (7,520.21) | 42,911.74 (7,167.12) | 43,129.55 (7,356.75) | 40,945.29 (12,307.01) |
time T1‐T3 time T3‐T4 |
717.57 −983.23 |
760.04 770.27 |
.35 .20 |
|
| ApoA/HDL | Supplement | 2.79 (0.34) | 2.91 (0.37) | 2.92 (0.40) | 2.85 (0.40) |
time T1‐T3 time T3‐T4 |
0.12 −0.03 |
0.04 0.02 |
.001 .23 |
| Placebo | 2.78 (0.35) | 2.81 (0.31) | 2.84 (0.33) | 2.78 (0.28) |
time T1‐T3 time T3‐T4 |
0.05 −0.01 |
0.04 0.02 |
.14 .49 |
|
Note: The last four columns of the table report the estimate of the effect, its SE and the p‐value of the null hypothesis of a no effect. time x‐y refers to the x–y days interval as set by the breaking point or knot. All models were adjusted for age and sex. Significance level (two‐sided p‐value) of the null hypothesis of a no effect for the within group pre–post supplementation difference was obtained using piecewise linear model to accommodate for nonlinear trend. All models were adjusted for age, sex, and BMI.
TABLE 3.
Between‐group pre–post supplementation difference for primary and secondary endpoints using piecewise mixed effect linear regression. The estimate of the effect, its SE and the p‐value of the null hypothesis of a no effect are reported
| Effect | Estimate | SE | p Value* | |
|---|---|---|---|---|
| Primary endpoint | ||||
| VAT (g) | time T1‐T2 *group | −51.49 | 17.99 | .005 |
| time T2‐T4 *group | −2.87 | 11.25 | .80 | |
| Secondary endpoints | ||||
| Total cholesterol (mg/dl) | time T1‐T2 *group | −10.02 | 2.67 | .0002 |
| time T2‐T4 *group | −0.52 | 1.14 | .65 | |
| LDL (mg/dl) | time T1‐T2 *group | −6.34 | 2.16 | .004 |
| time T2‐T4 *group | −0.71 | 1.13 | .53 | |
| HDL (mg/dl) | time T1‐T2 *group | 0.06 | 0.75 | .94 |
| time T3‐T4 *group | 0.34 | 0.39 | .38 | |
| Glycemia (mg/dl) | time T1‐T2 *group | 0.72 | 1.06 | .50 |
| time T2‐T4 *group | 0.36 | 0.70 | .60 | |
| ApoB (mg/dl) | time T1‐T2 *group | −2.92 | 1.76 | .10 |
| time T2‐T4 *group | 0.19 | 1.13 | .87 | |
| Creatinine (mg/dl) | time T1‐T2 *group | 0.006 | 0.009 | .49 |
| time T3‐T4 *group | 0.0006 | 0.005 | .89 | |
| Lean mass (g) | time T1‐T2 *group | −349.66 | 367.30 | .34 |
| time T3‐T4 *group | 486.22 | 376.69 | .20 | |
| ApoA/HDL | time T1‐T2 *group | 0.04 | 0.02 | .09 |
| time T3‐T4 *group | −0.008 | 0.01 | .59 |
Note: time x‐y refers to the x–y days interval as set by the breaking point or knot. Significance level (two‐sided p‐value) of the null hypothesis of a no effect for the between group pre–post supplementation difference was obtained using piecewise linear model to accommodate for nonlinear trend. All models were adjusted for age, sex, and BMI.
With regard to the within‐group differences, the results showed a statistically significant decrease after 30 days of VAT (p < .0001), of TC (p < .0001), LDL (p = .0008), and ApoB (p = .04) in the supplemented group. We observe a decrease in HDL after 60 days both in supplement (p = .03) and in placebo group (p = .04) and a statistically significant increase in ApoA/HLD after 60 days in the supplement group only (p = .001). As to the between‐group differences, a statistically significant interaction between time and group, meaning that the change in score over time is different for each group, was observed 30 days after supplementation for VAT (p‐value = .005), TC (p‐value <.0002), and LDL (p = .004).
Results of the t test performed to compare the difference in the mean delta (difference between baseline and the end of observation period) between the two groups, showed a general statistically significant effect of supplementation for VAT (p = .02), TC (p < .0001), and LDL (p = .0002). For the remaining variables, no statistically significant results were observed.
The remaining secondary endpoints were analyzed by fitting a linear mixed effect model, and results are reported in Tables 4 and 5. As to the within‐group differences, the supplement group shows a statistically significant decrease over time of AST (p = .02), ALT (p = .04), weight (p = .001), fat mass (p = .002), TC/HDL (p = .004); while, albeit of a low magnitude, a significant decrease in supplement (p = .02) and a significant increase in placebo group (p = .04) were observed for LDL/HLD. As to the between‐group differences, a significant interaction between time and group, meaning that the change in score over time is different for each group, was observed for ApoA (p‐value = .04), ApoB/ApoA (p‐value = .03), TC /HDL (p = .006), and LDL/HDL(p = .001).
TABLE 4.
Within‐group pre–post supplementation difference for secondary endpoints obtained using linear mixed‐effect model. At each time point, mean ± SD are reported
| Group | Mean (SD) T1 | Mean (SD) T2 | Mean (SD) T3 | Mean (SD) T4 | Estimate | SE | p Value | |
|---|---|---|---|---|---|---|---|---|
| Secondary endpoints | ||||||||
| VLDL (mg/dl) | Supplement | 23.85 (9.38) | 24.07 (9.15) | 24.88 (9.48) | 24.77 (10.42) | 0.35 | 0.40 | .39 |
| Placebo | 23.68 (9.29) | 24.30 (9.43) | 25.01 (9.27) | 25.40 (9.41) | 0.58 | 0.48 | .23 | |
| Triglycerides (mg/dl) | Supplement | 119.27 (46.91) | 120.33 (45.74) | 124.42 (47.38) | 123.85 (52.12) | 1.74 | 2.01 | .39 |
| Placebo | 118.39 (46.43) | 121.52 (47.15) | 125.03 (46.36) | 127.00 (47.07) | 2.91 | 2.43 | .23 | |
| Insulin (mcU/ml) | Supplement | 9.56 (5.74) | 8.33 (4.54) | 8.96 (4.90) | 8.36 (3.85) | −0.27 | 0.23 | .25 |
| Placebo | 10.64 (5.79) | 10.28 (5.93) | 9.81 (4.62) | 10.30 (4.77) | −0.15 | 0.31 | .63 | |
| HOMA | Supplement | 2.10 (1.33) | 1.79 (1.00) | 1.95 (1.12) | 1.82 (0.95) | −0.07 | 0.06 | .23 |
| Placebo | 2.34 (1.39) | 2.19 (1.30) | 2.05 (1) | 2.14 (1.12) | −0.07 | 0.07 | .30 | |
| ApoA (mg/dl) | Supplement | 165.58 (33.81) | 169.24 (34.92) | 167.88 (35.03) | 169.79 (34.40) | 1.07 | 0.70 | .13 |
| Placebo | 164.68 (42.31) | 162.23 (37.49) | 160.77 (37.11) | 161.13 (35.49) | −1.21 | 0.97 | .21 | |
| AST (IU/l) | Supplement | 20.88 (6.04) | 19.12 (4.86) | 18.97 (4.81) | 18.55 (4.87) | −0.71 | 0.29 | .02 |
| Placebo | 19.93 (5.85) | 20.19 (5.15) | 20.10 (5.63) | 19.06 (4.75) | −0.40 | 0.30 | .19 | |
| ALT (IU/l) | Supplement | 21.61 (9.42) | 19.82 (7.23) | 18.73 (5.35) | 18.27 (4.92) | −1.11 | 0.54 | .04 |
| Placebo | 21.81 (7.94) | 21.68 (7.25) | 21.81 (10.21) | 20.94 (6.53) | −0.28 | 0.55 | .61 | |
| GGT (U/l) | Supplement | 26.58 (28.73) | 24.36 (19.64) | 25.00 (19.23) | 24.33 (19.58) | −0.61 | 0.63 | .33 |
| Placebo | 19.77 (6.80) | 19.29 (6.91) | 19.74 (7.29) | 19.87 (6.97) | 0.06 | 0.29 | .85 | |
| Waist circumference (cm) | Supplement | 91.23 (13.08) | 89.85 (12.44) | 90.39 (12.27) | 89.92 (12.01) | −0.28 | 0.26 | .28 |
| Placebo | 96.40 (17.15) | 94.47 (14.92) | 95.05 (14.68) | 94.97 (13.89) | −0.32 | 0.30 | .29 | |
| Weight (kg) | Supplement | 69.85 (12.53) | 69.28 (12.12) | 69.08 (12.09) | 68.61 (11.85) | −0.39 | 0.12 | .001 |
| Placebo | 76.23 (17.45) | 75.13 (15.97) | 74.57 (15.05) | 74.53 (14.87) | −0.62 | 0.33 | .06 | |
| Fat mass (g) | Supplement | 25,237.94 (8,104.81) | 24,961.64 (8,133.41) | 24,509.27 (7,841.96) | 24,342.64 (7,932.58) | −302.47 | 96.41 | .002 |
| Placebo | 30,656.03 (13,614.78) | 29,937.03 (12,642.36) | 29,192.35 (11,630.92) | 29,210.29 (11,245.65) | −482.01 | 268.68 | .08 | |
| ApoB/ApoA | Supplement | 0.83 (0.21) | 0.79 (0.22) | 0.80 (0.21) | 0.79 (0.22) | −0.01 | 0.01 | .06 |
| Placebo | 0.86 (0.25) | 0.89 (0.28) | 0.87 (0.27) | 0.90 (0.28) | 0.01 | 0.01 | .28 | |
| Total cholesterol/HDL | Supplement | 4.32 (1.19) | 4.07 (1.13) | 4.15 (1.04) | 3.95 (0.99) | −0.10 | 0.03 | .004 |
| Placebo | 4.25 (1.29) | 4.45 (1.34) | 4.40 (1.15) | 4.48 (1.32) | 0.06 | 0.04 | .19 | |
| LDL/HDL | Supplement | 2.72 (0.77) | 2.65 (0.84) | 2.68 (0.79) | 2.52 (0.72) | −0.07 | 0.03 | .02 |
| Placebo | 2.88 (0.94) | 3.12 (1.13) | 3.03 (0.98) | 3.14 (1.13) | 0.06 | 0.03 | .04 | |
Note: P values < 0.05 are in bold. The last three columns of the table report the estimate of the effect, its SE, and the p‐value of the null hypothesis of a no effect. Significance level (two‐sided p‐value) of the null hypothesis of a no effect for the within group pre–post supplementation difference was obtained using linear mixed model for longitudinal data, with time, group and the interaction time*group as fixed effect, and time|subject as random effect. All models were adjusted for age, sex, and BMI.
TABLE 5.
Between‐group pre–post supplementation difference for secondary endpoints obtained using linear mixed effect model. The estimate effect at the end of observation period, its SE and the p‐value of the null hypothesis of β = 0 are reported
| Estimate (β) time*group | SE | p Value | |
|---|---|---|---|
| Secondary endpoints | |||
| VLDL (g) | −0.12 | 0.31 | .70 |
| Triglycerides (mg/dl) | −0.60 | 1.56 | .70 |
| Insulin (mcU/ml) | −0.06 | 0.19 | .75 |
| HOMA | 0.001 | 0.05 | .99 |
| ApoA (mg/dl) | 1.18 | 0.57 | .04 |
| AST (IU/l) | −0.17 | 0.21 | .41 |
| ALT (IU/l) | −0.42 | 0.37 | .27 |
| gGT (U/l) | −0.34 | 0.34 | .32 |
| Waist circumference (cm) | 0.02 | 0.20 | .94 |
| Weight (kg) | 0.10 | 0.17 | .54 |
| Fat mass (g) | 91.80 | 139.41 | .51 |
| ApoB/ApoA | −0.01 | 0.005 | .03 |
| Total cholesterol/HDL | −0.08 | 0.03 | .006 |
| LDL/HDL | −0.06 | 0.02 | .001 |
Note: P values < 0.05 are in bold. Significance level (two‐sided p‐value) of the null hypothesis of a no effect for the between group pre–post supplementation difference was obtained using linear mixed model for longitudinal data with time as fixed effect, and time|subject as random effect. All models were adjusted for age, sex, and BMI.
Table 6 reports the value of Pearson's pairwise partial correlation coefficient (r) and its p‐value between VAT and the selected secondary endpoints separately for the two groups at the different time points. Results show a significant positive correlation between VAT and TC in placebo (r = .44, p = .02) at T2, VAT, and TGs in supplement group (r = .54, p = .022) at T1, VAT and glycemia in supplement group (r = .45, p = .01) at T2 and a significant negative correlation between VAT and ApoA in placebo group at T1 (r = −.41, p = .03) and at T4 (r = −.45, p = .02).
TABLE 6.
Pairwise partial correlation, adjusted for age, sex, and BMI, between primary endpoint (VAT) and each selected secondary endpoint at different time points during the study
| T1 | T2 | T3 | T4 | |||||
|---|---|---|---|---|---|---|---|---|
| r | p Value | R | p Value | r | p Value | r | p Value | |
| Cholesterol (mg/dl) | ||||||||
| Supplement | .08 | .66 | .24 | .20 | .22 | .24 | .11 | .6 |
| Placebo | .01 | .96 | .44 | .02 | .31 | .11 | .37 | .05 |
| Triglycerides (mg/dl) | ||||||||
| Supplement | .54 | .002 | .30 | .11 | .29 | .12 | .36 | .05 |
| Placebo | .14 | .46 | .16 | .42 | .31 | .10 | .24 | .22 |
| Glycemia (mg/dl) | ||||||||
| Supplement | .32 | .09 | .45 | .01 | .30 | .10 | .05 | .77 |
| Placebo | .10 | .59 | .29 | .13 | .004 | .98 | −.008 | .97 |
| Insulin (mcU/ml) | ||||||||
| Supplement | .18 | .35 | .11 | .56 | .12 | .53 | .15 | .44 |
| Placebo | .23 | .24 | .20 | .32 | .17 | .38 | .25 | .20 |
| HOMA | ||||||||
| Supplement | .20 | .28 | .22 | .23 | .19 | .31 | .13 | .47 |
| Placebo | .21 | .29 | .25 | .20 | .15 | .45 | .27 | .17 |
| ApoA (mg/dl) |
.11 |
−.22 |
.25 |
|||||
| Supplement | .23 | .22 | .33 | .08 | −.2 | |||
| Placebo | .41 | .03 | .30 | .12 | 9 −.37 | .05 |
−.45 |
.02 |
| ApoB (mg/dl) | ||||||||
| Supplement | .36 | .06 | .26 | .17 | .51 | .004 | .48 | .007 |
| Placebo | .12 | .53 | .48 | .009 | .41 | .03 | .34 | .07 |
| Total | ||||||||
| cholesterol/HDL | ||||||||
| Supplement | .46 | .01 | .58 | .0007 | .59 | .0005 | .48 | .007 |
| Placebo | .40 | .03 | .46 | .01 | .47 | .01 | .52 | .004 |
As to the correlation between VAT and ApoB, a significant positive correlation was observed in supplement group at T3 (r = .51, p = .004) and at T4 (r = .48, p = .007) and in placebo group at T2 (r = .48, p = .009), and T3 (r = .41, p = .03). As to the correlation between VAT and TC /HDL, a statistically significant positive correlation was observed in supplement group at T1 (r = .46, p = .01), at T2 (r = .58, p = .0007), at T3 (r = .59, p = .0005), and at T4 (r = .48, p = .007) and in placebo group at T1 (r = .40, p = .03), at T2 (r = .46, p = .01), at T3 (r = .47, p = .01), and at T4(r = .52, p = .004).
Regarding safety, no significant differences were observed between the two groups (supplement and placebo) in vital signs, physical examination results, or laboratory tests performed. No relevant AEs were recorded and compliance was very good. Therefore, this study provides evidence that bergamot phytosome is safe and well tolerated even for prolonged supplementation periods.
4. DISCUSSION
To our knowledge, this clinical investigation is the first study in literature that provides early evidence that after a 12 weeks of bergamot phytosome supplementation, in a group of overweight and obese class I subjects with mild hypercholesterolemia, there are a statistically significant changes over time for VAT and for the evaluated secondary metabolic endpoints. This pattern emerged by fitting a linear mixed model for longitudinal data and a piecewise mixed‐effects model when the pattern of variation with time was not simply linear.
Our study provides the evidence that 12‐week bergamot phytosome supplementation may positively affect VAT and the metabolic derangement that characterizes obese subjects especially in the first observation period, that is, more evident change occurs in the first 30 days and then remains stable. This is a remarkable result considering that VAT is associated with metabolic syndrome and CVD and also is an independent risk factor of all‐cause mortality (Kivimäki et al., 2017).
Management of VAT is a pivotal result because VAT can now be considered as an endocrine organ orchestrating crucial interactions with vital organs and tissues such as the brain, the liver, the skeletal muscle, the heart and blood vessels themselves (Fang, Berg, Cheng, & Shen, 2018).
Moreover, another interesting result concerns lipid profile. In fact, as regard to the between‐group differences, a significant interaction between time and group, that is, the change in score over time differs between the two groups, was observed after supplementation for TC (p‐value <.0002), and LDL cholesterol (p = .004) more evident change occurs in the first 30 days after supplementation, that is, in the first observation period, and then remains stable. Thus, these parameters of lipid alteration were significantly decreased in the bergamot phytosome group, but not in placebo group. These results are in agreement with previous studies that have shown that bergamot phytosome was effective in modulating lipid profile, (Mollace et al., 2011) or in combination with other nutraceuticals (Cicero, Fogacci, Bove, Giovannini, & Borghi, 2019).
Bergamot (Citrus bergamia Risso et Poiteau) is used to flavor Earl Grey, one of the most popular teas in the world, and is an endemic plant of the Calabria region of Southern Italy. Among bergamot extracts on the market, Vazguard (Bergamot Phytosome) was developed as a more bioavailable form of a specific bergamot enriched polyphenolic fractions, namely BPF. The traditional use of bergamot is currently being rediscovered in the cardiovascular field due to its unique mix of polyphenolic content. Indeed, the choice of this specific extract was mainly due to its unique and peculiar phytochemical profile of flavonoids and flavonoid glycosides recently detailed by Formisano. The mechanisms by which bergamot phytosome reduces VAT, total, and LDL cholesterol is probably linked to the natural and unique bouquet of the polyphenols fraction (Formisano et al., 2019). As revealed in this recent study, the deep phytochemical characterization of bergamot polyphenols obtained combining different analytical techniques resulted in the identification of almost 40 components, of which about 30 belong to flavanone/flavone/flavonol derivatives (Formisano et al., 2019). More specifically, bergamot phytosome resulted in the identification of derivatives mainly composed of flavanone neohesperidosides naringin, neoeriocitrin, neohesperidin, brutieridin, and melitidin, which account for at least 45% the bergamot extract. In an articulate and complex scenario, this unique composition shows bergamot phytosome is endowed with a special multi‐target mechanism of action. Among all the cellular pathways possibly involved, AMP kinase (AMPK) activation appears as the central target. Considered as a sensor for glucose and lipid metabolism working to ensure maintenance of ATP levels AMPK, once activated, promotes the increase of glucose uptake in muscle cells, enabling ATP production via glycolysis; moreover AMPK induces catabolic reaction and inhibits anabolic processes involving ATP consumption such as cholesterol (via inhibition of 3‐hydroxy‐3methy‐glutaryl‐coenzyme A [HMG‐CoA] reductase), fatty acids (via inhibition of acetyl‐CoA carboxylase [ACC]) and protein synthesis. Both in vitro and human studies indicate that bergamot extract directly stimulates AMPK activity leading to increase AMPK levels (Janda, Lascala, Martino, Ragusa, & PharmaNutrition, 2016); this can reasonably explain the double hypolipidemic and hypoglycemic activity demonstrated for bergamot phytosome (Mollace et al., 2019). According to literature data, flavanones such as naringin and naringenin seem to be directly involved in up‐regulation of the AMPK pathway and, consequently, in lipid and glucose metabolism (Zygmunt, Faubert, MacNeil, & Tsiani, 2010).
A so‐called statin‐like effect has been also potentially mentioned for bergamot phytosome due to the content of flavanones such as brutieridin and melitidin characterized by a hydroxyl mevalonate moiety similar to HMG‐CoA and potential ability to bind and further inhibit the enzyme HMG‐CoA reductase. However, no in vitro and in vivo studies exist to support this mechanism of action. Investigations done using computational analysis actually demonstrated as brutieridin and melitidin only partially bind with the enzyme site (Leopoldini, Malaj, Toscano, Sindona, & Russo, 2010). The lack of a significant statin‐like side effect is also clinically demonstrated by the absence of any side effect reported during human studies involving bergamot phytosome, as also confirmed in our investigation. Another potential target of bergamot flavonoids can be cyclic nucleotide phosphodiesterases (PDEs), enzymes catalyzing hydrolysis of cAMP, improving its amount within cells, and directly involved in regulation of cell metabolism and lipolysis. Flavonoids contained in bergamot extracts such as neoeriocitrin, apigenin, and genistein are known to be PDEs inhibitors, thus modulating cell pathways such as improved rate of TGs hydrolysis (lipolysis) and reduction of lipogenic effect of insulin in adipocytes and liver (Janda et al., 2016).
In view of all this, clinical efficacy of bergamot phytosome scientifically demonstrated in human studies cannot be ascribed to a single mechanism of action, but instead to the cooperative effects of its natural phytocomplex rich in polyphenols, whose poor oral bioavailability has been successfully overcome thanks to the application of an innovative lecithin‐based delivery system.
Regarding safety, this study demonstrated that bergamot phytosome is safe and well tolerated even for prolonged supplementation periods, with good compliance and no relevant adverse effects recorded. This is a fundamental step for the completion of the profile of a botanical ingredient for dietary supplements. The nutraceutical market is a sector that includes a large number of very different products. As a matter of fact, often these products are lacking of clear evidences to support their use, ranging from their manufacturing quality to the rigorous studies on effectiveness and safety (Williamson, Liu, & Izzo, 2020). Firstly, given the very complex nature of botanical extract ingredients, the quality and reproducibility of their composition present a constant challenge to manufacturers. In this scenario, bergamot phytosome, guarantees bioavailable flavonoids throughout an optimize process that deeply touches all stages of the production. The raw material is sourced only from a very small and specific part of the southern Italian Calabria region, and is extensively characterized from the phytochemical point of view demonstrated to retain the same, unique phytochemical profile of the natural bergamot juice (Formisano et al., 2019). The application of Phytosome delivery system technology allows an optimized absorption of polyphenols, which are normally characterized by poor solubility in both water and organic solvents (Mollace et al., 2019). Noteworthy, the bioavailability of bergamot active components, safety, and tolerability, together with the hypoglycemic and hypolipemic effectiveness of bergamot phytosome has been recently proven in a double blind, randomized, placebo‐controlled human study (Mollace et al., 2019).
The results of the present trial confirm and enlarge the human biological activity of bergamot phytosome showing that the use of a well‐characterized and standardized botanical ingredient is pivotal to obtain consistent clinical data for a correct use.
The limitations of current study are the population studied, which is overweight and obese class I subjects with mild hypercholesterolemia. Hence, the results of this randomized, double blind, placebo‐controlled trial may lack of generalization to the general population. Therefore, future studies with a larger sample size in general population should be conducted.
5. CONCLUSIONS
In conclusion, this clinical study gives strong evidence that bergamot phytosome provides beneficial effects such as decrease of VAT and modulation of metabolic alterations after just 30 days of supplementation and remain stable afterwards, thus resulting in a very promising agent for protection of cardiovascular health.
CONFLICT OF INTEREST
Antonella Riva, Giovanna Petrangolini, and Pietro Allegrini are Indena employees.
Rondanelli M, Peroni G, Riva A, et al. Bergamot phytosome improved visceral fat and plasma lipid profiles in overweight and obese class I subject with mild hypercholesterolemia: A randomized placebo controlled trial. Phytotherapy Research. 2021;35:2045–2056. 10.1002/ptr.6950
REFERENCES
- Anderson, T. J. , Grégoire, J. , Hegele, R. A. , Couture, P. , Mancini, G. B. J. , McPherson, R. , … Ur, E. (2013). 2012 update of the Canadian cardiovascular society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Canadian Journal of Cardiology, 29(2), 151–167. 10.1016/j.cjca.2012.11.032 [DOI] [PubMed] [Google Scholar]
- Baigent, C. , Blackwell, L. , Emberson, J. , Holland, L. E. , Reith, C. , Bhala, N. , … Sourjina, T. (2010). Efficacy and safety of more intensive lowering of LDL cholesterol: A meta‐analysis of data from 170 000 participants in 26 randomised trials. The Lancet, 376(9753), 1670–1681. 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente‐García, O. , & Castillo, J. (2008). Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti‐inflammatory activity. Journal of Agricultural and Food Chemistry, 56(15), 6185–6205. 10.1021/jf8006568 [DOI] [PubMed] [Google Scholar]
- Catapano, A. L. , Graham, I. , De Backer, G. , Wiklund, O. , Chapman, M. J. , Drexel, H. , … Zamorano, J. L. (2016). 2016 ESC/EAS guidelines for the Management of Dyslipidaemias: The task force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis, 253, 281–344. 10.1016/j.atherosclerosis.2016.08.018 [DOI] [PubMed] [Google Scholar]
- Catapano, A. L. , Reiner, Ž. , De Backer, G. , Graham, I. , Taskinen, M. R. , Wiklund, O. , … Wood, D. (2011). ESC/EAS guidelines for the management of dyslipidaemias. The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European atherosclerosis society (EAS). Atherosclerosis, 217(1), 3–46. 10.1016/j.atherosclerosis.2011.06.028 [DOI] [PubMed] [Google Scholar]
- Cicero, A. F. G. , Fogacci, F. , Bove, M. , Giovannini, M. , & Borghi, C. (2019). Three‐arm, placebo‐controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phytotherapy Research, 33(8), 2094–2101. 10.1002/ptr.6402 [DOI] [PubMed] [Google Scholar]
- Currò M., Risitano R., Ferlazzo N., Cirmi S., Gangemi C., Caccamo D., Ientile R., Navarra M. (2016). Citrus bergamia Juice Extract Attenuates β‐Amyloid‐Induced Pro‐Inflammatory Activation of THP‐1 Cells Through MAPK and AP‐1 Pathways. Scientific Reports, 6(1). 10.1038/srep20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres, J. P. , Moorjani, S. , Ferland, M. , Tremblay, A. , Lupien, P. J. , Nadeau, A. , … Bouchard, C. (1989). Adipose tissue distribution and plasma lipoprotein levels in obese women. Importance of intra‐abdominal fat. Arteriosclerosis, 9(2), 203–210. 10.1161/01.atv.9.2.203 [DOI] [PubMed] [Google Scholar]
- Fang, H. , Berg, E. , Cheng, X. , & Shen, W. (2018). How to best assess abdominal obesity. Current Opinion in Clinical Nutrition & Metabolic Care, 21(5), 360–365 Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6299450/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice, G. M. , Laird, N. M. , & Ware, J. H. (2011). Applied longitudinal analysis, Hoboken, NJ: Wiley. [Google Scholar]
- Formisano, C. , Rigano, D. , Lopatriello, A. , Sirignano, C. , Ramaschi, G. , Arnoldi, L. , … Taglialatela‐Scafati, O. (2019). Detailed phytochemical characterization of bergamot polyphenolic fraction (BPF) by UPLC‐DAD‐MS and LC‐NMR. Journal of Agricultural and Food Chemistry, 67(11), 3159–3167. 10.1021/acs.jafc.8b06591 [DOI] [PubMed] [Google Scholar]
- Frisancho, A. R. (1984). New standards of weight and body composition by frame size and height for assessment of nutritional status of adults and the elderly. The American Journal of Clinical Nutrition, 40(4), 808–819. 10.1093/ajcn/40.4.808 [DOI] [PubMed] [Google Scholar]
- Gliozzi, M. , Walker, R. , & Mollace, V. (2014). Bergamot polyphenols: Pleiotropic players in the treatment of metabolic syndrome. Journal of Obesity & Metabolic Syndrome. 3(2), 143. 10.4172/2167-0943.1000143 [DOI] [Google Scholar]
- Haffner, S. M. , Kennedy, E. , Gonzalez, C. , Stern, M. P. , & Miettinen, H. (1996). A prospective analysis of the HOMA model: The Mexico City diabetes study. Diabetes Care, 19(10), 1138–1141. 10.2337/diacare.19.10.1138 [DOI] [PubMed] [Google Scholar]
- Hayashi, T. , Boyko, E. J. , McNeely, M. J. , Leonetti, D. L. , Kahn, S. E. , & Fujimoto, W. Y. (2007). Minimum waist and visceral fat values for identifying Japanese Americans at risk for the metabolic syndrome. Diabetes Care, 30(1), 120–127. 10.2337/dc06-0739 [DOI] [PubMed] [Google Scholar]
- Janda, E. , Lascala, A. , Martino, C. , Ragusa, S. , & PharmaNutrition, S. N. (2016). Molecular mechanisms of lipid‐ and glucose‐lowering activities of bergamot flavonoids. Pharma Nutrition, 4, S8–S18 Retrieved from https://www.sciencedirect.com/science/article/pii/S2213434415300220 [Google Scholar]
- Janssen, I. , Katzmarzyk, P. T. , & Ross, R. (2004). Waist circumference and not body mass index explains obesity‐related health risk. American Journal of Clinical Nutrition, 79(3), 379–384. 10.1093/ajcn/79.3.379 [DOI] [PubMed] [Google Scholar]
- Kivimäki, M. , Kuosma, E. , Ferrie, J. E. , Luukkonen, R. , Nyberg, S. T. , Alfredsson, L. , … Jokela, M. (2017). Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual‐level data for 120 813 adults from 16 cohort studies from the USA and Europe. The Lancet Public Health, 2(6), e277–e285. 10.1016/S2468-2667(17)30074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotkiewski, M. , Bjorntorp, P. , Sjostrom, L. , & Smith, U. (1983). Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. Journal of Clinical Investigation, 72(3), 1150–1162. 10.1172/JCI111040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk, J. L. , Katzmarzyk, P. T. , Nichaman, M. Z. , Church, T. S. , Blair, S. N. , & Ross, R. (2006). Visceral fat is an independent predictor of all‐cause mortality in men. Obesity, 14(2), 336–341. 10.1038/oby.2006.43 [DOI] [PubMed] [Google Scholar]
- Lapidus, L. , Bengtsson, C. , Larsson, B. , Pennert, K. , Rybo, E. , & Sjöström, L. (1984). Distribution of adipose tissue and risk of cardiovascular disease and death: A 12 year follow up of participants in the population study of women in Gothenburg, Sweden. British Medical Journal, 289(6454), 1257–1261. 10.1136/bmj.289.6454.1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, B. , Svardsudd, K. , & Welin, L. (1984). Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. British Medical Journal, 288(6428), 1401–1404. 10.1136/bmj.288.6428.1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldini, M. , Malaj, N. , Toscano, M. , Sindona, G. , & Russo, N. (2010). On the inhibitor effects of bergamot juice flavonoids binding to the 3‐Hydroxy‐3‐methylglutaryl‐CoA reductase (HMGR) enzyme. Journal of Agricultural and Food Chemistry, 58(19), 10768–10773. 10.1021/jf102576j [DOI] [PubMed] [Google Scholar]
- Mangge, H. (2014). Antioxidants, inflammation and cardiovascular disease. World Journal of Cardiology, 6(6), 462–477. 10.4330/wjc.v6.i6.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannarino, M. R. , Ministrini, S. , & Pirro, M. (2014). Nutraceuticals for the treatment of hypercholesterolemia. European Journal of Internal Medicine, 25(7), 592–599 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/?term=mannarino+2014+supplement+dyslipidemia [DOI] [PubMed] [Google Scholar]
- Miceli, N. , Mondello, M. R. , Monforte, M. T. , Sdrafkakis, V. , Dugo, P. , Crupi, M. L. , … Trovato, A. (2007). Hypolipidemic effects of Citrus bergamia Risso et Poiteau juice in rats fed a hypercholesterolemic diet. Journal of Agricultural and Food Chemistry, 55(26), 10671–10677. 10.1021/jf071772i [DOI] [PubMed] [Google Scholar]
- Mohammad, A. , De Lucia Rolfe, E. , Sleigh, A. , Kivisild, T. , Behbehani, K. , Wareham, N. J. , … Mohammad, T. (2017). Validity of visceral adiposity estimates from DXA against MRI in Kuwaiti men and women. Nutrition and Diabetes, 7(1), e238. 10.1038/nutd.2016.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollace, V. , Ragusa, S. , Sacco, I. , Muscoli, C. , Sculco, F. , Visalli, V. , … Romeo, F. (2008). The protective effect of bergamot oil extract on lecitine‐like oxyLDL receptor‐1 expression in balloon injury‐related neointima formation. Journal of Cardiovascular Pharmacology and Therapeutics, 13(2), 120–129. 10.1177/1074248407313821 [DOI] [PubMed] [Google Scholar]
- Mollace, V. , Sacco, I. , Janda, E. , Malara, C. , Ventrice, D. , Colica, C. , … Romeo, F. (2011). Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia, 82(3), 309–316. 10.1016/j.fitote.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Mollace, V. , Scicchitano, M. , Paone, S. , Casale, F. , Calandruccio, C. , Gliozzi, M. , … Bombardelli, E. (2019). Hypoglycemic and hypolipemic effects of a new lecithin formulation of bergamot polyphenolic fraction: A double blind, randomized, placebo‐controlled study. Endocrine, Metabolic & Immune Disorders Drug Targets, 19(2), 136–143. 10.2174/1871530319666181203151513 [DOI] [PubMed] [Google Scholar]
- Parker, H. M. , Cohn, J. S. , O'connor, H. T. , Garg, M. L. , Caterson, I. D. , George, J. , & Johnson, N. A. (2019). Effect of fish oil supplementation on hepatic and visceral fat in overweight men: A randomized controlled trial. Nutrients, 11(2), 475. 10.3390/nu11020475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perk, J. , De Backer, G. , Gohlke, H. , Graham, I. , Reiner, Ž. , Verschuren, W. M. M. , … Zannad, F. (2012). European guidelines on cardiovascular disease prevention in clinical practice (version 2012): The fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). International Journal of Behavioral Medicine, 19(4), 403–488. 10.1007/s12529-012-9242-5 [DOI] [PubMed] [Google Scholar]
- Pinheiro, J. , & Bates, D. (2006). Mixed‐effects models in S and S‐PLUS, New York: Springer Science & Business Media. [Google Scholar]
- Poirier, P. , & Després, J. P. (2003). Waist circumference, visceral obesity, and cardiovascular risk. Journal of Cardiopulmonary Rehabilitation, 23(3), 161–169. 10.1097/00008483-200305000-00001 [DOI] [PubMed] [Google Scholar]
- R Core Team . (2017). R: A language and environment for statistical computing. Retrieved from https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing
- Risitano, R. , Currò, M. , Cirmi, S. , Ferlazzo, N. , Campiglia, P. , Caccamo, D. , … Navarra, M. (2014). Flavonoid fraction of bergamot juice reduces LPS‐induced inflammatory response through SIRT1‐mediated NF‐κB inhibition in THP‐1 monocytes. PLoS One, 9(9), e107431. 10.1371/journal.pone.0107431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovato, A. , Taviano, M. F. , Pergolizzi, S. , Campolo, L. , De Pasquale, R. , & Miceli, N. (2010). Citrus bergamia Risso & Poiteau juice protects against renal injury of diet‐induced hypercholesterolemia in rats. Phytotherapy Research: PTR, 24(4), 514–519. 10.1002/ptr.2971 [DOI] [PubMed] [Google Scholar]
- Tundis, R. , Loizzo, M. R. , Bonesi, M. , Menichini, F. , Mastellone, V. , Colica, C. , & Menichini, F. (2012). Comparative study on the antioxidant capacity and cholinesterase inhibitory activity of Citrus aurantifolia Swingle, C. aurantium L., and C. bergamia Risso and Poit. Peel essential oils. Journal of Food Science, 77(1), H40–H46. 10.1111/j.1750-3841.2011.02511.x [DOI] [PubMed] [Google Scholar]
- Van Gaal, L. F. , Mertens, I. L. , & De Block, C. E. (2006). Mechanisms linking obesity with cardiovascular disease. Nature, 444(7121), 875–880. 10.1038/nature05487 [DOI] [PubMed] [Google Scholar]
- Verbeke, G. , & Molenberghs, G. (2000). A model for longitudinal data. Linear Mixed Models for Longitudinal Data, 19–29. 10.1007/978-0-387-22775-7_3 [DOI] [Google Scholar]
- Verschuren, W. M. M. , Jacobs, D. R. , Bloemberg, B. P. M. , Kromhout, D. , Menotti, A. , Aravanis, C. , … Toshima, H. (1995). Serum total cholesterol and long‐term coronary heart disease mortality in different cultures: Twenty‐five‐year follow‐up of the seven countries study. Journal of the American Medical Association, 274(2), 131–136. 10.1001/jama.274.2.131 [DOI] [PubMed] [Google Scholar]
- Williamson, E. M. , Liu, X. , & Izzo, A. A. (2020). Trends in use, pharmacology, and clinical applications of emerging herbal nutraceuticals. British Journal of Pharmacology, 177(6), 1227–1240. 10.1111/bph.14943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Heatlh Organization (1985). Energy and protein requirements, 9, 200. Geneva: Joint FAO/WHO/UNU Expert Consultation. [Google Scholar]
- Zygmunt, K. , Faubert, B. , MacNeil, J. , & Tsiani, E. (2010). Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochemical and Biophysical Research Communications, 398(2), 178–183. 10.1016/j.bbrc.2010.06.048 [DOI] [PubMed] [Google Scholar]


