Abstract
Background
Human immunodeficiency virus (HIV) infection leads to blood–brain barrier (BBB) dysfunction that does not resolve despite viral suppression on antiretroviral therapy (ART) and is associated with adverse clinical outcomes. In preclinical models, cannabis restores BBB integrity.
Methods
We studied persons with HIV (PWH) and HIV-negative (HIV−) individuals who had used cannabis recently. We assessed 2 biomarkers of BBB permeability: the cerebrospinal fluid (CSF) to serum albumin ratio (CSAR) and CSF levels of soluble urokinase plasminogen activator receptor (suPAR), a receptor for uPA, a matrix-degrading proteolytic enzyme that disrupts the BBB. A composite index of the BBB markers was created using principal components analysis. Neural injury was assessed using neurofilament light (NFL) in CSF by immunoassay.
Results
Participants were 45 PWH and 30 HIV− individuals of similar age and ethnicity. Among PWH, higher CSF suPAR levels correlated with higher CSAR values (r = 0.47, P < .001). PWH had higher (more abnormal) BBB index values than HIV− individuals (mean ± SD, 0.361 ± 1.20 vs −0.501 ± 1.11; P = .0214). HIV serostatus interacted with cannabis use frequency, such that more frequent use of cannabis was associated with lower BBB index values in PWH but not in HIV− individuals. Worse BBB index values were associated with higher NFL in CSF (r = 0.380, P = .0169).
Conclusions
Cannabis may have a beneficial impact on HIV-associated BBB injury. Since BBB disruption may permit increased entry of toxins such as microbial antigens and inflammatory mediators, with consequent CNS injury, these results support a potential therapeutic role of cannabis among PWH and may have important treatment implications for ART effectiveness and toxicity.
Keywords: HIV, blood-brain barrier, cannabis, neuroscience, cerebrospinal fluid
Among persons with human immunodeficiency virus, more frequent use of cannabis was associated with better blood–brain barrier (BBB) indices. Better BBB indices were associated with lower neurofilament light in cerebrospinal fluid, suggesting that cannabis may have a beneficial impact on HIV-associated BBB injury.
Even with effective suppression of viral replication, human immunodeficiency virus (HIV) infection leads to abnormal blood–brain barrier (BBB) function (“leaky” BBB) and neuroinflammation. A leaky BBB may permit increased entry into the central nervous system (CNS) of toxins, including cytokines and chemokines, causing neuronal injury. This may lead to adverse clinical outcomes, including neurocognitive impairment. Blood–brain barrier dysfunction is evidenced by higher cerebrospinal fluid (CSF)-to-serum albumin (CSAR) ratios and upregulation of urokinase plasminogen activator (uPA), a matrix-degrading proteolytic enzyme, and its receptor, uPAR, disrupting the basal lamina around cerebral capillaries [1, 2].
The soluble form of uPAR, suPAR, is a biomarker of monocyte activation and chronic inflammation [3], and positively correlated with HIV-associated inflammation, neurocognitive impairment, and non-AIDS events [4–6]. In CSF higher suPAR levels are found in individuals with AIDS dementia complex [7, 8].
More than 30% of persons living with HIV (PWH) use cannabis, and the prevalence is increasing with legalization across the United States [9]. In animal and in vitro models, cannabinoids stabilize the BBB and reduce neuroinflammation [10–16]. One mechanism is via stimulation of tight junction proteins such as claudin and zona occludens type 1 (ZO-1) [17–20]. This is relevant for HIV. For example, the addition of CB2R agonist in barrier-forming primary brain microvascular endothelial cells (BMVECs) from individuals with HIV infection increased transendothelial electrical resistance induced by LPS and increased the amount of tight junction proteins (occludin and claudin-5) present in membrane fractions [17]. No previous study has evaluated BBB functions in PWH in relation to cannabis use.
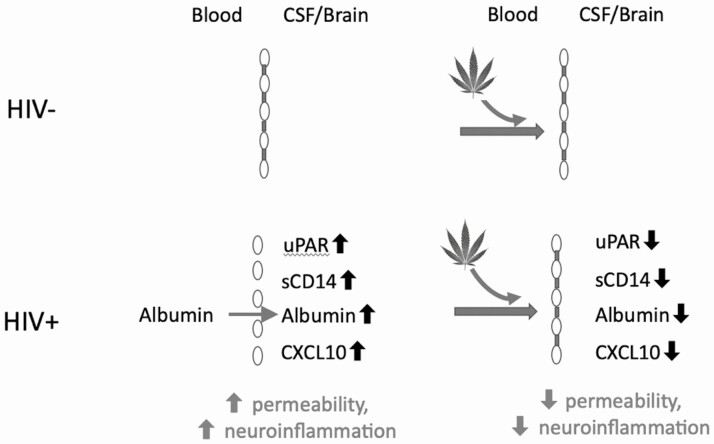
As depicted schematically in Figure 1, we hypothesized that an interaction between HIV serostatus and cannabis use such as that in PWH with a leaky BBB, cannabis would improve BBB permeability, but that this would not be the case in individuals without HIV without a leaky BBB.
Figure 1.
Proposed model of interaction between HIV and cannabis with respect to the blood–brain barrier. In the presence of elevated permeability associated with HIV, cannabis reduces permeability and neuroinflammation but has no effect when the blood–brain barrier is intact in HIV. Abbreviations: CSAR, cerebrospinal fluid-to-serum albumin ratio; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; HIV+, HIV positive; HIV−, HIV negative; suPAR, soluble urokinase plasminogen activator receptor.
METHODS
Participants
Forty-five PWH and 30 individuals without HIV who had used cannabis at least once within the past month were prospectively enrolled in a cohort study at the University of California, San Diego. Recent users were selected to reduce potential bias due to unmeasured confounding factors, since cannabis users are different from nonusers in a variety of ways. Inclusion criteria included a sufficient volume of CSF and plasma in storage at −80oC to perform assays. Exclusions included abuse or dependence on methamphetamine, alcohol, and other substances within the past 18 months; significant CNS confounding conditions such as history of AIDS-defining opportunistic infection of the CNS; traumatic brain injury resulting in permanent neurological deficits; and major, active psychiatric disorders such as schizophrenia. All participants signed informed consent documents approved by the local institutional review board.
Blood–Brain Barrier Permeability Markers
We assessed CSF levels of suPAR using immunoassay-based methods (Quantikine Human uPAR ELISA; R&D Systems), and albumin was measured in serum at each site’s clinical laboratory and in CSF at a reference laboratory using immunonephelometry. A principal components analysis was used to create a composite index of the 2 highly related BBB markers.
Additional Clinical and Laboratory Assessments
The standardized timeline follow-back substance-use interview, which has been validated for use in clinical populations [21], was used to estimate frequency of cannabis use over the past month, lifetime total quantity of cannabis use, and total lifetime days of cannabis use. Comprehensive neuromedical assessments were performed. These assessments included vital signs, neurological and physical examination, collection of medical history including antiretroviral (ARV) regimen, and collection of blood and CSF. HIV serostatus was documented by enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blot. Routine clinical assays, such as blood CD4+ T-cell count and CSF total protein, were measured in the Clinical Laboratory Improvement Amendments (CLIA)–certified laboratory at the University of California, San Diego Medical Center. HIV RNA levels were measured in CSF and plasma by real-time polymerase chain reaction with a lower quantification limit of 50 copies/mL (Abbott Diagnostics). Urine specimens were screened for THC (tetrahydrocannabinol) by a 10-panel drug screen card (Rapid Response; Biotechnostix Inc). Neurofilament light (NFL) in CSF was measured using ELISA; values below the limit of detection were assigned the lower quantitation limit.
Statistical Analysis
Demographics, medical history, and HIV disease characteristics were summarized using means and standard deviations, medians and interquartile ranges (IQRs), or counts and percentages as appropriate. Demographic data and cannabis use over the past month were compared between PWH and individuals without HIV using independent-samples t test for continuous variables and Fisher’s exact test for categorical variables. Log10 transformations were applied to biomarker measures to improve symmetry and normality of distributions. Correlations among biomarkers were assessed using Pearson’s r. A principal components analysis was used to create a composite index of the 2 highly related BBB markers explaining 75.5% of the variance. Separate multivariable regressions were used to assess the interaction effects between HIV serostatus and cannabis use (ie, cannabis days used over the past month, lifetime days of cannabis use, and lifetime total quantity of cannabis use) on the BBB index and the impact of potential covariates. Additive effects were tested when the interaction effect was not significant. Demographics (ie, age and ethnicity) were included in multivariable models and retained as covariates if the P value was less than 0.2 in backward model selection. Finally, the association of CSF NFL and BBB index was evaluated using linear regression. The HIV serostatus by BBB index interaction was then included in the model to test the effect of HIV serostatus on the relationship between NFL and BBB index. The results were considered statistically significant at the 5% ɑ level. JMP Pro version 14 (SAS Institute Inc.) was used for all analyses.
RESULTS
Participant Characteristics
Participants were 45 PWH and 30 individuals without HIV. Table 1 shows demographic and clinical characteristics, including cannabis use frequency. Age and ethnicity were comparable between the 2 groups. The proportion of women among PWH was significantly higher than among individuals without HIV (16.7% vs 0%). Nadir and current CD4 were 350 (IQR, 213, 519) and 702 (479, 1057), respectively. Of PWH, 60.9% were virally suppressed. Persons living with HIV had higher CSF albumin levels than those without HIV (mean ± SD: 17.4 ± 8.09 vs 12.2 ± 6.39; P = .0285). Among PWH, CSF albumin (mg/dL) was above the upper limit of normal, 25 mg/dL in 6 PWH (14.0%). Persons living with HIV had higher CSF total protein than those without HIV (39.2 ± 13.6 vs 28.8 ± 9.38; P = .0285). Persons living with HIV had worse CSAR values (0.534 ± 0.208 vs 0.387 ± 0.233; P = .0353) and uPAR values (2.91 ± 0.182 vs 2.80 ± 0.199; P = .0626) than those without HIV.
Table 1.
Demographic and Clinical Characteristics
| PWH (n = 25) | Individuals Without HIV (n = 18) | P | |
|---|---|---|---|
| Age (mean ± SD), years | 41.1 ± 12.7 | 37.7 ± 14.1 | .26 |
| Female, n (%) | 5 (16.7) | 0 (0.0) | .0046 |
| Non-Hispanic white, n (%) | 24 (53.3) | 10 (33.3) | .083 |
| Nadir CD4+ cells/μL, median [IQR] | 370 [288, 453] | … | |
| Current CD4+/μL, median [IQR] | 660 [554, 767] | … | |
| Virally suppressed, n (%) | 23 (57.5) | … | |
| On ART, n (%) | 29 (67.4) | … | |
| Days cannabis used in past month, median [IQR] | 9 [2, 30] | 9.5 [1.8, 30] | .97 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; PWH, persons living with HIV.
Principal Components Analysis
Among all participants, log CSAR and log CSF suPAR were highly correlated (r = 0.509, P = .0005). The principal components analysis constructed a composite index of the 2 highly related BBB markers that explained 75.5% of the variance; higher values indicated a leakier BBB. Thus, the principal components analysis permitted the creation of a reduced set of variables that was easier to analyze and interpret. Both CSAR and uPAR were highly related to the BBB index (for CSAR, r = 0.869, P < .0001; for uPAR, r = 0.869, P < .0001).
Relationship of the Blood–Brain Barrier Index to Human Immunodeficiency Virus Serostatus and Clinical Characteristics
Persons living with HIV had higher (more abnormal) BBB index values than individuals without HIV (0.361 ± 1.20 vs −0.501 ± 1.11; P = .0214). Persons living with HIV had higher log10 CSAR than individuals without HIV (3.79 ± 1.74 vs 2.78 ± 1.44; P = .0490). Log10 suPAR levels were marginally higher in PWH than in those without HIV (2.91 ± 0.179 vs 2.80 ± 0.199; P = .0641). Within the HIV-positive group, worse BBB index values were marginally associated with lower nadir CD4 counts (r = −0.379, P = .0615), but not with current CD4 (r = −0.00377, P = .986). Virally suppressed PWH had nonsignificantly lower BBB index values than unsuppressed PWH (0.177 ± 1.313 vs 0.801 ± 0.971; P = .236). Females had nonsignificantly better BBB index values than males (−0.329 ± 1.02 vs 0.0254 ± 0.821; P = .48). Within the whole sample, greater BBB permeability was significantly associated with older age and non-Hispanic white ethnicity (r = 0.3917, P = .0094; 0.426 ± 0 610 vs −0.252 ± 0.844; P = .0077).
Interaction Between Human Immunodeficiency Virus and Cannabis Use Frequency
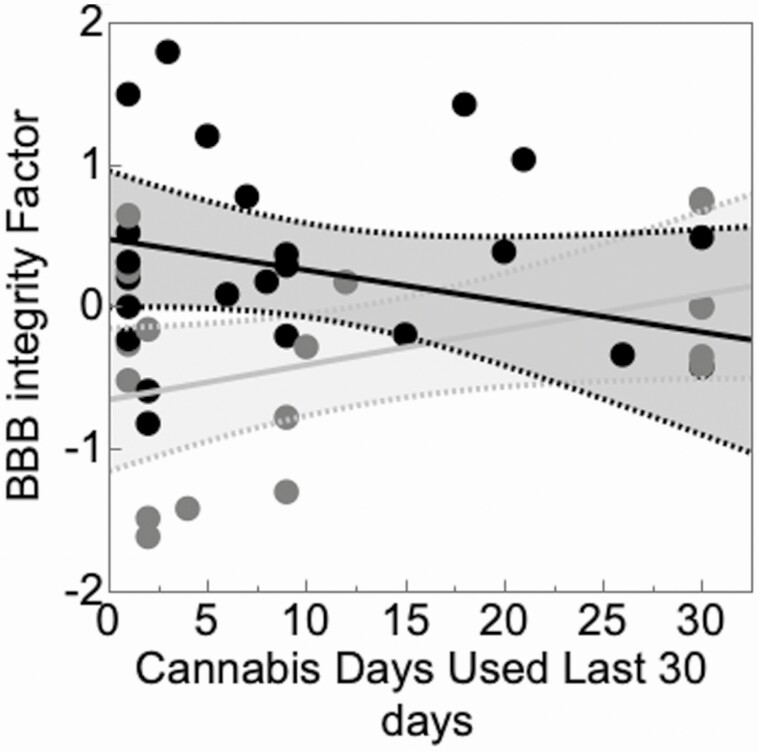
In a multivariable model, BBB index values were significantly better in those with more frequent cannabis use over the past month for PWH but not for those without HIV (interaction P = .0294; HIV status P = .0148; for HIV+, r = −0.273; 95% confidence interval [CI], −.603, .137; P = .187; for HIV−, r = 0.422; 95% CI, −.0556, .743; P = .0809) (Figure 2). The associations of BBB index with estimated total lifetime days of cannabis use in PWH and individuals without HIV were then assessed using a multivariable model including HIV status, lifetime days of cannabis use, and their interaction. The main effects of HIV status and cannabis lifetime days were significant, while the interaction was not (P = .427). Additive effects were tested and indicated that HIV infection was associated with worse BBB index values (P = .00142), while lifetime days of cannabis use was associated with better BBB index values (P = .00581). Also, in a multivariable model, estimated lifetime total quantity of cannabis use was associated with better BBB index values (P = .0642) and HIV was associated with worse BBB index values; the interaction was not significant (P = .288). In addition, BBB index values were not associated with use of other drugs, including methamphetamine (total quantity, cumulative density, duration; all P > .10) and alcohol (total days, total quantity, cumulative density; all P > .10).
Figure 2.
BBB index values were better in those with more frequent cannabis use over the past month for PWH (black) but not for those without HIV (gray). Abbreviations: BBB, blood–brain barrier; HIV, human immunodeficiency virus; PWH, persons living with HIV.
Confounding Evaluation
To adjust for potential confounders, we included in multivariable models demographic factors that were significantly correlated with BBB index values (age, ethnicity). Gender and ethnicity were nonsignificant in the multivariable model (P = .886 and .227). In backward model selection, gender was included as a covariate in the final model, and ethnicity was removed.
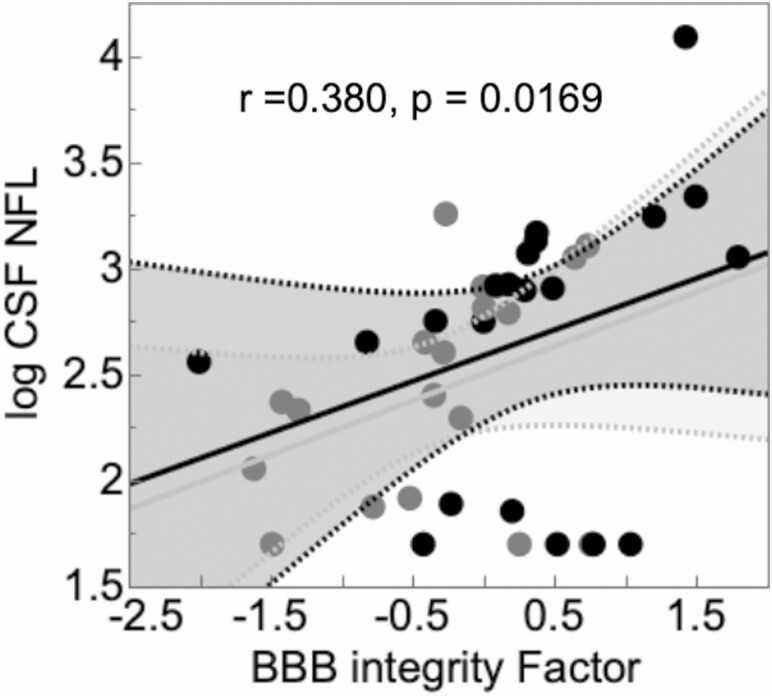
Worse BBB indices were associated with greater axonal injury as reflected in CSF NFL levels (Figure 3). This relationship was not modified by HIV serostatus and the interaction between HIV and BBB index was not significant. Persons living with HIV had nonsignificantly higher levels of NFL than the group without HIV (log10, 2.66 ± 0.665 vs 2.42 ± 0.512; P = .206).
Figure 3.
Worse BBB indices were associated with greater axonal injury as reflected in CSF NFL levels. Black, PWH; gray, individuals without HIV. Abbreviations: BBB, blood–brain barrier; CSAR, cerebrospinal fluid-to-serum albumin ratio; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; NFL, neurofilament light; PWH, persons living with HIV; uPAR, urokinase plasminogen activator receptor.
Discussion
This is the first report consistent with improved BBB function with cannabis use in PWH. As expected, PWH had more abnormal BBB function than individuals without HIV. Multiple parameters of increased cannabis use (frequency of recent use, estimated total lifetime quantity, and estimated total lifetime days of use) were associated with improved BBB function in PWH but not in persons without HIV. Other drugs of abuse did not affect BBB function. Higher levels of NFL, a marker of axonal injury, were seen with poor BBB function.
Alterations of the BBB occur in the earliest stages of infection and persist throughout the infection [22, 23]. Several mechanisms may contribute to impaired BBB function in HIV. Inflammation in HIV has been shown to reduce BBB integrity [24, 25]. Additionally, antiretroviral drugs can contribute to BBB damage. For example, PWH exposed to rilpivirine showed endothelial cell dysfunction [26]. Exposure of endothelial cells to efavirenz can severely impact BBB integrity by decreasing levels of tight junction proteins claudins-1/5, occludin, ZO-1, and JAM-1. This results in an increase in permeability, a phenomenon that has been shown both in vivo and in vitro [27–29].
Cannabis may improve BBB function by several mechanisms. One, discussed above, is upregulation of claudin and occludin by cannabis, which has been demonstrated in animal models and in human brain tissue [17–20] and should be examined in future research. Additionally, reduced inflammation may be one mechanism by which cannabis restores BBB function. Numerous studies have demonstrated anti-inflammatory effects of cannabis [30, 31]. Both in vitro studies and animal models show that CB2R agonists are anti-inflammatory and may be part of the general neuroprotective action of the endocannabinoid system (ECS) by decreasing glial reactivity [32]. In one study, heavy cannabis users had fewer interleukin-23 and phorbol 12-myristate-13-acetate induced tumor necrosis factor-α (TNF-α)–producing antigen-presenting cells in blood [33]. In another, cannabis users had lower TNF- α levels than nonusers [34].
The function of the BBB, in part, is to protect the brain parenchyma from circulating toxins. In the context of HIV, potential toxins include ARV medications. The BBB plays a pivotal role in the maintenance of the CNS. It controls the inflow of nutrients, while at the same time actively pumps out metabolic byproducts and toxic compounds [35–37]. Antiretroviral drugs can be neurotoxic by off-target inhibition of mitochondrial polymerase γ, the enzyme responsible for normal mitochondrial DNA and other mechanisms [38–41]. This is important because disruption of the BBB may enhance entry of neurotoxic antiretrovirals into the CNS. Our finding that BBB dysfunction was associated with higher levels of axonal injury, as indexed by NFL, suggests that BBB dysfunction may indeed have clinical consequences.
Limitations of this study include the small sample size. We did not measure ARV or toxin concentrations in CSF to determine if increased levels correlated with BBB breakdown. We did not measure levels of exogenous cannabinoids or endocannabinoids. Cerebrospinal fluid NFL levels were numerically, but not statistically significantly, higher in PWH than in those without HIV, differing from some previous reports. However, CSF NFL levels can be elevated in a variety of conditions other than HIV, some of which may have been present in this cohort [42–44].
More frequent cannabis use may have a beneficial impact on HIV-associated BBB injury. Since BBB can lead to HIV-associated CNS injury, cannabinoids may have therapeutic benefits in the CNS
Notes
Financial support. This work was supported by National Institutes of Health (contract number N01 MH22005) (Translational Methamphetamine Research Center; principal investigator, I. Grant).
Potential conflicts of interest. M. H. reports grants from Gilead, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Marcos-Contreras OA, Martinez de Lizarrondo S, Bardou I, et al. . Hyperfibrinolysis increases blood-brain barrier permeability by a plasmin- and bradykinin-dependent mechanism. Blood 2016; 128:2423–34. [DOI] [PubMed] [Google Scholar]
- 2. Adair JC, Baldwin N, Kornfeld M, Rosenberg GA. Radiation-induced blood-brain barrier damage in astrocytoma: relation to elevated gelatinase B and urokinase. J Neurooncol 1999; 44:283–9. [DOI] [PubMed] [Google Scholar]
- 3. Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers 2009; 27:157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gianella S, Letendre SL, Iudicello J, et al. . Plasma (1 → 3)-β-D-glucan and suPAR levels correlate with neurocognitive performance in people living with HIV on antiretroviral therapy: a CHARTER analysis. J Neurovirol 2019; 25:837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoenigl M, Moser CB, Funderburg N, et al. ; Adult Clinical Trials Group NWCS 411 Study Team . Soluble urokinase plasminogen activator receptor is predictive of non-AIDS events during antiretroviral therapy-mediated viral suppression. Clin Infect Dis 2019; 69:676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rasmussen LJ, Knudsen A, Katzenstein TL, et al. . Soluble urokinase plasminogen activator receptor (suPAR) is a novel, independent predictive marker of myocardial infarction in HIV-1-infected patients: a nested case-control study. HIV Med 2016; 17:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cinque P, Nebuloni M, Santovito ML, et al. . The urokinase receptor is overexpressed in the AIDS dementia complex and other neurological manifestations. Ann Neurol 2004; 55:687–94. [DOI] [PubMed] [Google Scholar]
- 8. Sidenius N, Nebuloni M, Sala S, et al. . Expression of the urokinase plasminogen activator and its receptor in HIV-1-associated central nervous system disease. J Neuroimmunol 2004; 157:133–9. [DOI] [PubMed] [Google Scholar]
- 9. Pacek LR, Towe SL, Hobkirk AL, Nash D, Goodwin RD. Frequency of cannabis use and medical cannabis use among persons living with HIV in the United States: findings from a nationally representative sample. AIDS Educ Prev 2018; 30:169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li L, Yun D, Zhang Y, et al. . A cannabinoid receptor 2 agonist reduces blood-brain barrier damage via induction of MKP-1 after intracerebral hemorrhage in rats. Brain Res 2018; 1697:113–23. [DOI] [PubMed] [Google Scholar]
- 11. Hind WH, England TJ, O’Sullivan SE. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br J Pharmacol 2016; 173:815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amenta PS, Jallo JI, Tuma RF, Elliott MB. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J Neurosci Res 2012; 90:2293–305. [DOI] [PubMed] [Google Scholar]
- 13. Fishbein-Kaminietsky M, Gafni M, Sarne Y. Ultralow doses of cannabinoid drugs protect the mouse brain from inflammation-induced cognitive damage. J Neurosci Res 2014; 92:1669–77. [DOI] [PubMed] [Google Scholar]
- 14. French JA, Koepp M, Naegelin Y, et al. . Clinical studies and anti-inflammatory mechanisms of treatments. Epilepsia 2017; 58(Suppl 3):69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fagherazzi EV, Garcia VA, Maurmann N, et al. . Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology (Berl) 2012; 219:1133–40. [DOI] [PubMed] [Google Scholar]
- 16. Mori MA, Meyer E, Soares LM, Milani H, Guimarães FS, de Oliveira RMW. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog Neuropsychopharmacol Biol Psychiatry 2017; 75:94–105. [DOI] [PubMed] [Google Scholar]
- 17. Ramirez SH, Haskó J, Skuba A, et al. . Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci 2012; 32:4004–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang MC, Zhang HZ, Wang Z, You FL, Wang YF. The molecular mechanism and effect of cannabinoid-2 receptor agonist on the blood-spinal cord barrier permeability induced by ischemia-reperfusion injury. Brain Res 2016; 1636:81–92. [DOI] [PubMed] [Google Scholar]
- 19. Feng YJ, Li YY, Lin XH, Li K, Cao MH. Anti-inflammatory effect of cannabinoid agonist WIN55, 212 on mouse experimental colitis is related to inhibition of p38MAPK. World J Gastroenterol 2016; 22:9515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alhamoruni A, Lee AC, Wright KL, Larvin M, O’Sullivan SE. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther 2010; 335:92–102. [DOI] [PubMed] [Google Scholar]
- 21. Hjorthøj CR, Hjorthøj AR, Nordentoft M. Validity of timeline follow-back for self-reported use of cannabis and other illicit substances—systematic review and meta-analysis. Addict Behav 2012; 37:225–33. [DOI] [PubMed] [Google Scholar]
- 22. Peluso MJ, Meyerhoff DJ, Price RW, et al. . Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis 2013; 207:1703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wright PW, Vaida FF, Fernández RJ, et al. . Cerebral white matter integrity during primary HIV infection. AIDS 2015; 29:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anesten B, Yilmaz A, Hagberg L, et al. . Blood-brain barrier integrity, intrathecal immunoactivation, and neuronal injury in HIV. Neurol Neuroimmunol Neuroinflamm 2016; 3:e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Atluri VS, Hidalgo M, Samikkannu T, et al. . Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front Cell Neurosci 2015; 9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Echeverría P, Gómez-Mora E, Roura S, et al. . Variable endothelial cell function restoration after initiation of two antiretroviral regimens in HIV-infected individuals. J Antimicrob Chemother 2017; 72:2049–54. [DOI] [PubMed] [Google Scholar]
- 27. Bertrand L, Dygert L, Toborek M. Antiretroviral treatment with efavirenz disrupts the blood-brain barrier integrity and increases stroke severity. Sci Rep 2016; 6:39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bertrand L, Toborek M. Dysregulation of endoplasmic reticulum stress and autophagic responses by the antiretroviral drug efavirenz. Mol Pharmacol 2015; 88:304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faltz M, Bergin H, Pilavachi E, Grimwade G, Mabley JG. Effect of the anti-retroviral drugs efavirenz, tenofovir and emtricitabine on endothelial cell function: role of PARP. Cardiovasc Toxicol 2017; 17:393–404. [DOI] [PubMed] [Google Scholar]
- 30. Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol 2005; 5:400–11. [DOI] [PubMed] [Google Scholar]
- 31. Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem 2005; 95:437–45. [DOI] [PubMed] [Google Scholar]
- 32. Martín-Moreno AM, Reigada D, Ramírez BG, et al. . Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol Pharmacol 2011; 79:964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manuzak JA, Gott TM, Kirkwood JS, et al. . Heavy cannabis use associated with reduction in activated and inflammatory immune cell frequencies in antiretroviral therapy-treated human immunodeficiency virus-infected individuals. Clin Infect Dis 2018; 66:1872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keen L 2nd, Turner AD. Differential effects of self-reported lifetime marijuana use on interleukin-1 alpha and tumor necrosis factor in African American adults. J Behav Med 2015; 38:527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cornford EM, Hyman S. Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRx 2005; 2:27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hawkins RA, O’Kane RL, Simpson IA, Viña JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr 2006; 136:218S–26S. [DOI] [PubMed] [Google Scholar]
- 37. Qosa H, Miller DS, Pasinelli P, Trotti D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res 2015; 1628:298–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther 2000; 22:685–708. [DOI] [PubMed] [Google Scholar]
- 39. Kakuda TN, Brundage RC, Anderson PL, Fletcher CV. Nucleoside reverse transcriptase inhibitor-induced mitochondrial toxicity as an etiology for lipodystrophy. AIDS 1999; 13:2311–2. [DOI] [PubMed] [Google Scholar]
- 40. Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol 2012; 18:388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schweinsburg BC, Taylor MJ, Alhassoon OM, et al. ; HNRC Group . Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol 2005; 11:356–64. [DOI] [PubMed] [Google Scholar]
- 42. Gisslén M, Price RW, Andreasson U, et al. . Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 2016; 3:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res 2003; 987:25–31. [DOI] [PubMed] [Google Scholar]
- 44. Constantinescu R, Holmberg B, Rosengren L, Corneliusson O, Johnels B, Zetterberg H. Light subunit of neurofilament triplet protein in the cerebrospinal fluid after subthalamic nucleus stimulation for Parkinson’s disease. Acta Neurol Scand 2011; 124:206–10. [DOI] [PubMed] [Google Scholar]