Abstract
With the broader introduction of genomic medicine in research and clinical care, an increasing number of persons are offered genetic testing. Many factors, including genetic literacy, may impact the utilization of genetic results by patients and their families. We developed a rapid, self‐administered measure of genetic literacy, called Genetic Literacy Fast Test (GeneLiFT). We next evaluated the association of GeneLiFT scores with the comprehension of limitations of genomic medicine in participants undergoing genetic testing in the NIH‐sponsored eMERGE III study at Columbia University Irving Medical Center, New York. All participants underwent genetic screening for variants in 74 actionable genes associated with adult‐onset disorders. A diverse cohort of 724 participants completed the survey (60% women, 45% less than 40 years old, and 53% self‐reported White non‐Hispanic ancestry). The GeneLiFT was validated using known group differences based on education, health literacy, and numeracy, and with questions assessing genetic knowledge. GeneLiFT identified multiple standard genetics terms, that is, jargon, not recognized by more than 50% of participants (including actionability and pathogenicity). Low genetic literacy, identified in 210 participants (29%), was significantly associated with poor understanding of the limitations of genetic testing (p‐values < 10–9). This association was independent of education, health literacy, and numeracy levels, highlighting the importance of directly measuring genetic literacy. Low genetic literacy was also associated with low satisfaction with the informed consent process. GeneLiFT is a practical tool for rapid assessment of genetic literacy in large studies or clinical care. GeneLiFT will allow future research to efficiently assess the role of genetic literacy on the clinical impact of genetic testing.
Keywords: assessment, communication, genetic knowledge, genetic literacy, risk perception, screening, test
Short abstract

What is known about this topic
There is no current consensus on a genetic literacy test that can easily screen the population for low genetic literacy.
The ClinGen Consent and Disclosure Recommendations (CADRe) Workgroup advocated for adjusting the communication approach to patients' genetic literacy (Ormond et al., 2019).
What this paper adds to the topic
This paper describes a novel, rapid, self‐administered genetic literacy test that has been validated in a large heterogeneous group of individuals who underwent genetic screening. The test also confirms the association between low genetic literacy and lower satisfaction from the inform consent process and poor understanding of the limitations of genetic testing.
1. INTRODUCTION
For decades genetic counselors have ensured that patients' decisions to undergo genetic testing are informed and autonomous (American Society of Human Genetics Ad Hoc Committee on Genetic Counselling, 1975). The pre‐test genetic counseling session ensures comprehension and a personalized informed consent process (Committee on Genetics, 2017). Within these conversations, genetic counselors are regularly checking clients' understanding of the genetic terminology (Butow & Lobb, 2004; Roter, Erby, Larson, & Ellington, 2007, 2009). This ability to recognize terms related to genetics is defined as genetic literacy (Abrams et al., 2015; Erby et al., 2008) and is one of the pillars of comprehension. Although recognition cannot imply understanding, lack thereof indicates ignorance of the concept or at minimum of the specific term.
Discussion of the risks and benefits of the proposed genetic test, including its limitations, enhances informed decision‐making (Kaphingst et al., 2012). In other fields, it has been shown that when patients are involved in the discussion, and both understand and support the clinical plan, they are more likely to adhere (Drew et al., 2001). Different literacies have been associated with the effectiveness of clinical care. Numeracy, health, health insurance, and eHeath literacies have been independently associated with better understanding of the benefits of testing and acting on medical instructions (Dewalt et al., 2004; Levitt, 2015; Norman & Skinner, 2006; Schillinger et al., 2002; Schwartz et al., 1997). Even though education, health literacy, and numeracy were reported to correlate with genetic knowledge, they each have limitations, especially when used separately, and are not reliable surrogates of genetic comprehension, highlighting the importance of testing genetic literacy (Kaphingst et al., 2016; Lea et al., 2011; Syurina et al., 2011).
As increasing genetic testing in research and clinical care has led to a shortage of genetic counselors (Kurian et al., 2017; Rehm, 2017), the ClinGen Consent and Disclosure Recommendations (CADRe) Workgroup advocated for adjusting the communication approach to patients' genetic literacy (Ormond et al., 2019). A lower level of genetic literacy would require a longer, more traditional genetic counseling approach, while patients with higher genetic literacy can be seen by other professionals. Like other forms of literacy, higher genetic literacy is expected to enhance informed decision‐making in genetic testing. Conversely, low genetic literacy is expected to negatively impact understanding of the genetic test, leading to inappropriate follow‐up or noncompliance with recommended care, such as additional testing and preventive clinical screenings (Schleit et al., 2019). It is therefore vital to have a test that can rapidly and easily assess the level of genetic literacy. However, due to limitations of previously developed tests, there is no consensus on which instrument to use to measure genetic literacy (Abrams et al., 2015; Ostergren et al., 2015). Two genetic literacy tests have been developed. The Real‐G requires in‐person interaction (Erby et al., 2008); the other, the GLAC, includes multiple‐choice questions and takes longer to complete (Hooker et al., 2014). These formats are a barrier to large‐scale implementation of genetic literacy assessments. The evaluation of the association between genetic literacy and factors influencing the clinical impact of genetic testing is thus limited by low utilization of those tests.
We therefore developed a brief, self‐administered ‘Genetic Literacy Fast Test’ (GeneLiFT), formatted as a vocabulary recognition test. Contrary to current test formats, GeneLiFT can be easily implemented in different settings as it enables high‐throughput self‐screening for genetic literacy with instantaneous results. As it generates a list of words the testee does not recognize, GeneLiFT can help reduce the utilization of jargon in conversations and online platforms.
Utilization of medical jargon has been shown to inhibit dialogue and reduce patients' satisfaction (Pitt & Hendrickson, 2020; Roter et al., 2007), and the limitations of genomic medicine are generally poorly understood (Bernhardt et al., 2015). We therefore hypothesized that low genetic literacy would be associated with lower satisfaction with the informed consent process and poorer understanding of the limitations of genomic testing. We tested those hypotheses in a cohort of individuals who underwent preventive genetic screening as part of the Electronic Medical Records and Genomics (eMERGE) consortium Phase III project (Milo Rasouly et al., 2019). eMERGE III offered all participants' genetic screening using a targeted panel of genes with return of both positive and negative results (eMERGE Consortium, 2019). This research study therefore mimics preventive clinical genetic screening to identify people at risk of developing adult‐onset disorders with effective intervention or treatment available (Fossey et al., 2018; Halverson et al., 2020). Participants were not asked hypothetical questions but were rather asked to share their comprehension and expectations from the genetic test to which they consented.
2. METHODS
2.1. Participants
Participants were enrolled at Columbia University Irving Medical Center (CUIMC) as part of the eMERGE III consortium. One of the goals of the eMERGE III study at CUIMC was to enroll a diverse cohort of participants naïve to genetic testing (Milo Rasouly et al., 2019). Adult participants able to give written informed consent and willing to provide a blood sample were enrolled using three recruitment methods: clinics, flyers, and staff network; the last consisted of friends, family members, and colleagues of the study staff (Milo Rasouly et al., 2019).
The consent process was conducted by clinical research coordinators (CRCs) who were specifically trained for the eMERGE study by a genetic counselor and used a script (Supplemental Online File 1). During the consent process, CRCs described how genes may contribute to disease risk, and how this knowledge can potentially transform routine preventive medicine. Participants were informed that eMERGE III primarily screened for a set of actionable genes linked to highly penetrant conditions for which preventive measures and/or treatment are available, such as breast, ovarian and colon cancer, severe cardiovascular diseases, and sudden death. CRCs took special care to explain that negative results do not mean a ‘clean bill of health’. They also explained that identification of a pathogenic variant meant that, while there is a high likelihood of developing the disease, not all those carrying such variants will develop the disease. CRCs were instructed to interrupt the consent process intermittently to ask the potential participant whether he/she needed a clearer explanation and proceed only whether he/she verbalized understanding.
All samples underwent genetic screening in a Clinical Laboratory Improvement Amendments (CLIA)‐certified laboratory. At CUIMC, results were returned for 74 genes and 24 variants and placed in the electronic medical record (eMERGE Consortium, 2019). The study was approved by the Columbia University Institutional Review Board (IRB‐AAAQ9205).
2.2. Measures
At time of enrollment, participants completed an intake form for demographic information, and personal and familial history of several diseases. A blood sample was collected, and participants were then asked whether they preferred to complete the pre‐result survey online or on paper, in English or in Spanish. Participants independently completed the survey after enrolling in the study. For those who chose the paper version, the survey was provided to them at time of consent together with a pre‐stamped return envelope to send it back at their earliest convenience. A link to the survey was sent via email to participants who chose the online option. Upon completion of the survey, participants received a $25 gift card. The survey included questions related to education, health literacy, health numeracy, and previous encounters with genetic counselors. Since we assessed familiarity with genetic vocabulary, we also asked participants whether they were born in the United States, as reduced familiarity with English was a potential confounder. Further details are provided below.
2.2.1. Demographics
The intake form included questions about date of birth, race, ethnicity, and address. Using the participants' electronic medical record, we retrieved information on Medicaid status. We chose not to collect participant income information as this question is often viewed as intrusive. However, in an effort to evaluate the socioeconomic status of our participants, we used geo‐income. Geo‐income was taken from the census information on median household income of neighborhoods using participants' addresses, which has been shown to be a relatively good approximation of individual household income (Brokamp et al., 2017). In the survey, participants were asked to self‐report their education level.
2.2.2. Health literacy and numeracy measures
Health literacy is defined as the degree to which individuals have the capacity to obtain, process, and understand basic health information needed to make appropriate health decisions (Health Resources and Services Administration). To detect inadequate health literacy, participants were asked ‘How confident are you filling out medical forms by yourself?’ (Chew et al., 2008).
Numeracy, the level at which people are comfortable with numbers, is associated with health risk perception. It was tested with a question regarding understanding of risk and ratios (i.e., ‘Which of the following numbers represents the biggest risk of getting a disease? 1 in 100, 1 in 1,000, or 1 in 10’. (Lipkus et al., 2001).
2.2.3. Genetic knowledge
Genetic knowledge measures comprehension of genetic principles (Erby et al., 2008; Haga et al., 2013; Hurle et al., 2013). In keeping with previously published procedures, we asked participants to self‐rate their genetic knowledge (Sweet et al., 2017). To assess participants' understanding of genetic concepts, four previously utilized items were used (Fitzgerald‐Butt et al., 2016; Kaphingst et al., 2012; Molster et al., 2009; Ostergren et al., 2015; Sweet et al., 2017). These four sentences describe different components of genetics: heritability, inheritance, genetic risk, and penetrance (Table S1). Participants were asked to indicate how correct they thought these statements were on a five‐point Likert scale ranging from definitely correct to definitely incorrect. As the level of certainty may reflect general self‐confidence, for each question, participants received 1 point if they chose the correct answers (‘definitely’ or ‘probably’) and 0 points if they answered ‘don't know’ or the incorrect answers (‘definitely’ or ‘probably’). The sum of the points given for those four items was then used to create a genetic knowledge variable. Since the concepts were relatively basic, this variable was then dichotomized, with low genetic knowledge assigned to individuals who did not know at least two of the concepts (score ≤ 2).
2.2.4. Satisfaction with the informed consent process
Participants were asked ‘How satisfied were you with the explanation of the study's goals when you were first invited to participate?’ and given four response options. We categorized those who answered ‘extremely satisfied’ and ‘quite satisfied’ as satisfied, and those who answered ‘slightly satisfied’ or ‘not satisfied at all’ as not satisfied.
2.2.5. Understanding limitations of genetic testing
The participants were asked to rate the veracity of an additional set of four previously utilized statements to assess understanding of genetic testing limitations (Bernhardt et al., 2015; Facio et al., 2011; Kaphingst et al., 2012; Sanderson, Linderman, et al., 2016). The topics covered included prevention/curability of genetic conditions, implications of a report in which no genetic variants were identified (i.e., negative result), status of current scientific knowledge, and the predictive value of genetic testing.
2.3. Development of a novel genetic literacy test
The vocabulary recognition test (Zimmerman et al., 1977), which was validated as highly associated with vocabulary knowledge (Meara & Buxton, 1987), was used as a conceptual framework for GeneLiFT. This test format has also been widely used to determine more complex cognitive functions (Craik et al., 2015; Harrington & Carey, 2009; Lemhöfer & Broersma, 2012; Rawson et al., 2010; Stanovich et al., 1995; Zhang et al., 2019). Vocabulary recognition tests require testees to mark words they recognize as real, having been told that some of the words are not real. These are rapid tests estimated to take about 1 min for 30 words and non‐words (2 min for a test comprising 60 words and non‐words) and are self‐administered, so a large number of people can be tested easily (Pellicer‐Sánchez, 2012). The METER test is a health literacy test that uses the same format, and that highly correlates with the widely used REALM oral health literacy test (Davis et al., 2006; Rawson et al., 2010), justifying our decision for selecting this design.
Content validity was established using experts in genetics (geneticists and genetic counselors) who approved a list of real words. Using a group of 227 non‐experts (friends and colleagues), we identified real words not recognized by everybody and non‐words mistaken as real by some respondents (Table S2).
GeneLiFT (Figure S1) comprises 51 words: 31 real words and 20 non‐words (Table S3). Amongst the 31 real words, seven were included in the informed consent form for the study (Supplemental Online File 2), five in the genetic literacy test Real‐G (Erby et al., 2008), eleven in the NHGRI glossary (NHGRI Glossary, 2015), four in the ‘subjective genomic knowledge test’ (Ishiyama et al., 2008), and the remaining words were used during informed consent discussions.
The scoring of the GeneLiFT was based on previous vocabulary recognition tests (Meara & Buxton, 1987; Rawson et al., 2010; Zimmerman et al., 1977). Responses identifying the 20 non‐words as real words were scored as −1. To differentiate between health literacy and genetic literacy, the real words were divided into ‘genetic‐related words’ and ‘genetic‐specific words’. Genetic‐related words were defined as words that have definitions in the genetics realm but also in other fields. Genetic‐specific words were defined as words that only have genetic‐based definitions. Of the 31 words, 16 are genetic‐related words and 15 are genetic‐specific (Table S3). The ‘genetic‐specific words’ were given a higher weight than the ‘genetic‐related words’. Correct responses for the 16 genetic‐related words were scored +1, and for the 15 genetic‐specific words as +2. Therefore, the range of GeneLiFT is −20 to +46 (Figure S2). To simplify the scoring system and provide a rapid response for potential users, we dichotomized the outcome variable into ‘low genetic literacy’ and ‘other’. As we did not want the specific cohort analyzed to bias our analysis, we arbitrarily decided that scoring 50% of the maximum number of points or less (a score of 23 or less) would be considered ‘low genetic literacy’, but replication studies should further establish the adequacy of this cutoff point.
GeneLiFT was administered at the end of the survey. To assess GeneLiFT validity, we analyzed its association with measures known to be associated with genetic knowledge and/or genetic literacy: education (Erby et al., 2008; Kaphingst et al., 2012; Molster et al., 2009; Vassy et al., 2012), health literacy (Erby et al., 2008; Kaphingst et al., 2016), and numeracy (Langer et al., 2017; Lea et al., 2011; Ostergren et al., 2015). GeneLiFT's construct validity was assessed with the known groups differences method:
For education levels, the mean GeneLiFT score was compared between participants with a high school diploma, a GED or less, and those with any level of post‐high‐school education or training (vocational, technical, academic, etc…).
For health literacy, the mean GeneLiFT score was compared between participants who answered ‘Not at all’ or ‘A little bit’ confident and those who answered ‘Extremely’, ‘Quite a bit’, or ‘Somewhat’ confident (Chew et al., 2008).
For numeracy, the mean GeneLiFT score was compared between participants who answered correctly and those who did not answer correctly (Lipkus et al., 2001).
GeneLiFT's validity was also assessed with the concurrent measures' technique using self‐reported genetic knowledge and the dichotomized genetic knowledge variable:
For self‐reported low genetic literacy, the mean GeneLiFT score was compared between participants who answered ‘Low’ and those who answered ‘High’, ‘Average,’ or ‘Moderate’.
For genetic knowledge, the mean GeneLiFT score of participants with low genetic knowledge, as calculated by the dichotomized score, was compared to the others.
2.4. Data analysis
Data were analyzed using Rstudio software (RStudio Team, 2016). Ordinal and categorical variables are reported as percentages. Differences between groups were calculated using an independent 2‐group t‐test. Logistic regression was used to validate GeneLiFT and to test the association between genetic literacy (GeneLiFT) (predictor) and understanding of the limitations of genetic testing, as well as satisfaction with the informed consent process (outcomes). Logistic regression included adjustment for covariates. Covariates were dichotomized to empower the comparisons. The cutoffs for the dichotomizations were chosen a priori to differentiate between ‘low’ and ‘other’ for each measurement. The following covariates were analyzed based on prior literature: education (divided into 3 levels: low as described above, medium for those with some college, associate degree, or other post‐high‐school training, and high for those with a college degree and more), gender, numeracy (low vs. other), health literacy (low vs. other), dichotomized genetic knowledge (low vs. other), self‐reported genetic knowledge (low vs. other), previous encounter with a genetic counselor (yes vs. no), place of birth (United States vs. other), Medicaid health coverage (Medicaid vs. other), and recruitment modality (clinic vs. flyers or network).
3. RESULTS
3.1. Diverse participants
A total of 1,132 individuals were recruited, with 1,016 (90%) requesting a survey in English. Spanish consent forms and study material were made available for the remaining 116 participants, but due to small sample size these were not included in this report (Figure S3). The survey was completed by 724 of the 1,016 participants (71%). Participants had a mean (SD) age of 46.5 (17.3) years, ranging from 18 to 94 years (Table 1). They were of diverse racial and ethnic ancestry, with 140 (19%) self‐identified as Hispanic, 118 (16%) as Black non‐Hispanic, 70 (10%) as Asian, and 253 (35%) not born in the United States. The participants' socio‐economic background included 84 (12%) participants with a high school degree or less, 145 (20%) with low numeracy, 40 (6%) with low health literacy, 32 (4%) with Medicaid insurance, and 73 (10%) living in neighborhoods with average annual household income under $33,948 (Medicaid eligibility in New York State for a family of 4 in 2017, when the study took place). Most participants (588, 81%) had never met a geneticist or a genetic counselor.
TABLE 1.
Characteristics of the participants who completed the survey (n = 724)
| Completed the survey no. (%) | |
|---|---|
| Gender | |
| Female | 435 (60) |
| Male | 289 (40) |
| Age | |
| Less than 40 years | 323 (45) |
| 40−65 years | 260 (36) |
| 65 years+ | 141 (19) |
| Self‐reported race and ethnicity | |
| White, non‐Hispanic | 381 (53) |
| Hispanic a | 140 (19) |
| Asian, non‐Hispanic | 72 (10) |
| Black, non‐Hispanic | 118 (16) |
| Other, non‐Hispanic (including multiple races) b | 13 (2) |
| Disease status | |
| Self‐reported healthy | 332 (46) |
| Sick | 316 (44) |
| Missing | 9 (<1) |
| Health Insurance | |
| Medicaid | 32 (4) |
| Medicare | 19 (3) |
| Missing | 673 (93) |
| Recruitment method | |
| Flyers or staff network | 286 (40) |
| Clinics | 438 (60) |
| Geo‐income | |
| Less than $33,948 | 73 (10) |
| $33,948–$74,999 | 270 (37) |
| 75,000–$99,999 | 163 (23) |
| $100,000+ | 194 (27) |
| Missing | 24 (3) |
| Questionnaire format | |
| Paper | 49 (7) |
| Electronic | 675 (93) |
| Education | |
| Some school but did not graduate high school | 16 (2) |
| High school graduate or GED | 68 (9) |
| Some college, associate degree, or other post‐high school training (vocational, technical, etc.) | 139 (19) |
| College graduate | 244 (34) |
| Master's degree/some graduate degree | 186 (26) |
| Doctoral degree | 71 (10) |
| Health Literacy | |
| Low | 40 (6) |
| Other | 684 (94) |
| Numeracy | |
| Low | 145 (20) |
| Other | 575 (79) |
| Missing | 4 (1) |
| Self‐reported genetic knowledge | |
| Low | 126 (17) |
| Other | 598 (83) |
| Place of birth | |
| Born in the USA | 471 (65) |
| Not born in the USA | 253 (35) |
| Experience with genetic counseling | |
| No previous contact with genetic counseling | 588 (81) |
| Previous contact with genetic counseling | 110 (15) |
| Not sure | 26 (4) |
| Genetic literacy | |
| Low | 210 (29) |
| Other | 514 (71) |
Including 64 White, 16 Black, 4 American Indian or Alaskan Native, 2 Native Hawaiian or other Pacific Islander, and 1 Asian.
Including 2 American Indian or Alaskan Native and 1 Native Hawaiian or other Pacific Islander.
Overall, the characteristics of those who completed the survey were similar to the total sample (Table S4), but a lower proportion of individuals reporting a disease at time of enrollment completed the survey (p‐value = 2 × 10−5), and individuals enrolled through flyers or the staff network were more likely to answer the survey (p‐value = 2 × 10−4).
3.2. Genetic knowledge
Of the 724 participants, 162 (22%) did not know that ‘some people with a genetic mutation may not develop the genetic condition’, 110 (15%) did not know that ‘genetic testing may find genetic mutations that increase a person's chance of developing a genetic condition’, 104 (14%) did not know that ‘healthy parents can have a child with a genetic condition’, and 67 (9%) did not know that ‘genetic testing may find genetic variants that a person can pass on to his/her children’ (Table S1). Overall, 275 (38%) did not know the concept corresponding to at least one of those four statements, and 112 (15%) participants did not know at least two concepts (flagged as having low genetic knowledge using the dichotomized variable).
3.3. Distribution of GeneLiFT scores
GeneLiFT scores ranged from −3 to 46 (mean = 28.3, SD = 11, median = 28). The correlation between the number of genetic‐specific words and genetic‐related words recognized was statistically significant but relatively weak (r = 0.39, p‐value < 10–16, Figure S4). The GeneLiFT score reflected the proportion of genetic‐specific words recognized by participants, with all participants scoring > 40 recognizing more than 75% of the genetic‐specific words (Figure 1). Less than half of the 724 participants (244, 44.6%) recognized more than a third of the 15 genetic‐specific words. Ten genetic words were not recognized as real by more than half of the participants (Table S3).
FIGURE 1.
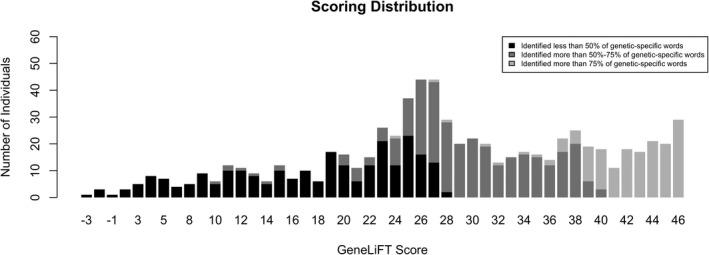
Distribution of the GeneLiFT score. Black represents those who recognized less than 50% of the genetic‐specific words, dark grey represents those who recognized 50%–75% of the genetic‐specific words, and light grey those who recognized more than 75% of the genetic‐specific words. Low genetic literacy was assigned to those with a score of 23 points or less. The mean score in this cohort was 28.3 points and the median was 28 points
3.4. GeneLiFT validity
We observed significantly lower GeneLiFT scores for persons with lower education levels, lower health literacy, and lower numeracy (all p‐value < 10–4; Figure 2) supporting its construct validity. Participants who self‐reported low genetic knowledge and those with low genetic knowledge based on the four questions had significantly lower GeneLiFT scores (both p‐value < 10–15, Figure 2) supporting concurrent validity of GeneLiFT with other measures.
FIGURE 2.
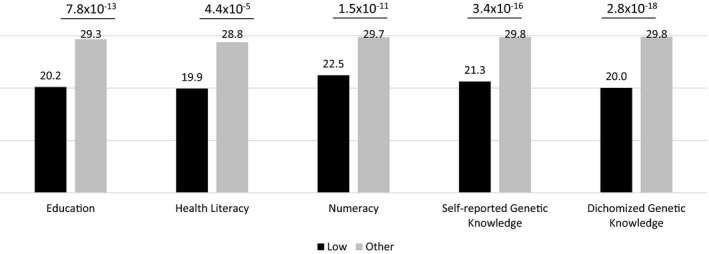
Validation of GeneLiFT. Difference between the GeneLiFT score of participants with different levels of education, health literacy, numeracy, self‐reported genetic literacy, and genetic knowledge. The mean GeneLiFT scores are reported above each bar and the differences are significant (independent t‐test, p‐values are shown on the top of the bars)
3.5. Genetic literacy
Of the 724 participants, 210 (29%) were categorized as having low genetic literacy based on their GeneLiFT score (score ≤ 23 points), with 196 (93%) recognizing fewer than 8 of 15 (≤50%) genetic‐specific words (Figure 1). The remaining 7% were penalized for endorsing non‐words. Of those with low genetic literacy, 86% did not recognize ‘actionability,’ 76% did not recognize ‘pathogenicity,’ and 97% did not recognize ‘penetrance’ as real words. Low genetic literacy was significantly associated with genetic knowledge (based on the dichotomized variable), independently of education, health literacy, numeracy (p‐value = 5.3 × 10−6, Table 2). Health literacy was not associated with genetic knowledge. After adjustments to the other covariates, education was still associated with genetic knowledge (p‐value = 0.01, Table 2).
TABLE 2.
Association of GeneLiFT scores with genetic knowledge score, controlling for education, health literacy, and numeracy
| Low genetic knowledge score | ||||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| GeneLiFT | ||||
| Low | 4.4 (2.9–6.7) | 2.8 × 10−12 | 2.9 (1.8–4.7) | 5.3 × 10−6 |
| Education a | ||||
| High school or less | 2.0 (1.1–3.6) | 0.02 | 2.2 (1.2–4.1) | 0.01 |
| College or more | 0.3 (0.2–0.5) | 2.3 × 10−6 | 0.5 (0.3–0.8) | 0.01 |
| Health literacy | ||||
| Low | 0.6 (0.3–1.3) | 0.21 | 1.4 (0.6–3.2) | 0.45 |
| Numeracy | ||||
| Low | 2.4 (1.6–3.8) | 9.1 × 10−5 | 1.5 (0.9–2.4) | 0.13 |
Abbreviations: 95% CI, 95% Confidence Interval; OR, Odds Ratio. Adjusted OR: adjusted to all other variables in the table.
Reference group: Some college, associate degree, or other post‐high school training (vocational, technical, etc…).
3.6. Association of genetic literacy with satisfaction with the informed consent process
Only 60 (4%) participants indicated that they were ‘slightly or not satisfied at all’ with the informed consent process. Low genetic literacy was associated with lower satisfaction with the informed consent process (OR, 2.6; 95% CI, 1.2–5.3; p‐value = 0.01). Additional covariates tested including age, gender, education, self‐reported genetic knowledge, recruitment method, previous encounter with a genetic counselor, and Medicaid coverage were not statistically significant.
3.7. Association of genetic literacy with comprehension of the limitations of genetic testing
The limitations of genomic medicine were poorly understood by 381 of the 724 participants (53%) based on at least one of their answers to the four related questions. Low genetic literacy was significantly associated with higher likelihood of believing that ‘all genetic conditions can be prevented or cured’ (OR, 5.6; 95% CI, 3.8–8.2; p‐value = 6 × 10−19, Table 3), that ‘if nothing is identified, it means a “clean bill of health” (no risk for genetic diseases)’ (OR, 4.4; 95% CI 3.1–6.2; p‐value = 3 × 10−16), that ‘scientists know how all variants in genes will affect a person's chance of developing a genetic condition’ (OR, 4.1; 95% CI, 2.6–6.4; p‐value = 5 × 10−10), and that ‘the exact chance of developing a genetic condition can be determined through genetic testing’ (OR, 3.4; 95% CI, 2.4–4.8; p‐value = 8 × 10−13). Low genetic literacy was associated with those misconceptions independently of low genetic knowledge (all p‐values < 10–8, Table 3), and independently of other potential confounding variables, including education, health literacy, numeracy, age, gender, Medicaid coverage, generation living in the United States, and genetic knowledge, self‐reported genetic knowledge, recruitment method, and previous encounter with a genetic counselor (all p‐values < 0.001, Tables S5–S8).
TABLE 3.
Association between genetic literacy and probability of misunderstanding the limitations of genetic testing
| Dichotomized 5‐Likert scale questions | Individuals who did not answer correctly | Genetic literacy a | |||
|---|---|---|---|---|---|
| No. (%) |
Unadjusted OR (95% CI) |
p value |
Adjusted OR (95% CI) b |
p value | |
| ‘If nothing is identified, it means a "clean bill of health" (no risk for genetic diseases)’ | 191 (26) |
4.4 (3.1–6.2) |
3.1 × 10−16 |
3.3 (2.3–4.9) |
2.9 × 10−10 |
| ‘The exact chance of developing a genetic condition can be determined through genetic testing’ | 319 (44) |
3.4 (2.4–4.8) |
7.8 × 10−13 |
2.8 (2.0–4.1) |
5.0 × 10−9 |
| ‘All genetic conditions can be prevented or cured’ | 155 (21) |
5.6 (3.8–8.2) |
6.3 × 10−19 |
4.4 (2.9–6.5) |
2.7 × 10−13 |
| ‘Scientists know how all variants in genes will affect a person's chance of developing a genetic condition’ | 96 (13) |
4.1 (2.6–6.4) |
4.6 × 10−10 |
4.2 (2.7–6.7) |
8.2 × 10−10 |
Odds Ratio 95% Confidence Interval Lower and Upper (OR 95% CI L or U). (%) Proportion from the 724 participant. The sentences are presented as provided to the participants, including underlines.
GeneLiFT‐based low genetic literacy as a predictor to give the wrong answer.
GeneLiFT‐based low genetic literacy as a predictor to give the wrong answer, adjusted to genetic knowledge. Adjusted odds ratio to additional variables can be found in Tables S4–S7.
4. DISCUSSION
We report here a novel, rapid, self‐administered genetic literacy test, GeneLiFT. This test was developed and validated on a sample of research participants who consented to undergo genetic screening with return of results. The heterogeneous cohort utilized to validate GeneLiFT, and in particular the low number of participants with prior interactions with genetic counseling, emphasizes its applicability to the general population. Importantly, we identified an association between low genetic literacy and reduced comprehension of the limitations of the genetic test to which these participants consented.
Demonstrating a high prevalence of poor genetic knowledge in participants who just underwent genetic screening, more than a third of the cohort did not understand all the genetic concepts tested, comparable to previous reports about low genetic knowledge in the population (Chapman et al., 2019). Not understanding potential genetic risk in the absence of personal or familial history of disease (i.e., ‘Healthy parents can have a child with a genetic condition’, 14%) was particularly concerning as all participants had completed informed consent for preventive genetic screening for adult‐onset genetic disorders, all of which have incomplete penetrance and/or can occur de novo. Despite explanations regarding the panel of genes tested and their variable penetrance, many participants believed in the deterministic impact of genetic mutations and did not understand that ‘some people with a genetic mutation may not develop the genetic condition’ (22%). It was also worrisome that some participants did not understand that the test may identify genetic risk (i.e., ‘Genetic testing may find genetic mutations that increase a person's chance of developing a genetic condition’, 9%). As genetic results should lead to cascade screening, it was also disquieting that the concept of heritability was not understood by all (i.e., ‘Genetic testing may find genetic variants that a person can pass on to his/her children’, 15%). To validate our novel genetic literacy test, we demonstrated that low genetic literacy as measured by GeneLiFT was significantly associated with lower comprehension of those genetic concepts. As low genetic knowledge is one of the factors impeding the ability to grasp genetic‐based risk and implement necessary behavioral changes (Haga et al., 2013), those participants are at high risk to have difficulties in understanding the implications of their genetic results. Although studies have not conclusively shown a negative psychological impact of genetic test results on patients with a genetic risk (Oliveri et al., 2018; Wade, 2019), those studies may be biased by a high level of genetic literacy amongst those asked or by previously known high‐risk for genetic disease. GeneLiFT can help analyze the association between genetic literacy and psychological impact of genetic testing.
Supporting the difference between general and genetic literacies, we observed limited correlation between the recognition of genetic‐specific words and genetic‐related words. We showed that GeneLiFT's association with genetic knowledge is independent of health literacy, numeracy and education, and also explains most of the unadjusted associations between genetic knowledge and education or numeracy. These results emphasize the importance of measuring genetic literacy as opposed to education, health literacy, or numeracy when analyzing the impact of an intervention on genetic knowledge. Thus, as recommended by the CADRe Workgroup (Ormond et al., 2019), it is crucial to account for genetic literacy level prior to genetic testing, to ensure that proper consent is obtained from participants undergoing clinical testing and participating in genomic research.
In accord with observations in other fields where utilization of jargon decreases satisfaction (Pitt & Hendrickson, 2020; Roter et al., 2007), low genetic literacy was associated with lower satisfaction with the informed consent process. The uniform format of informed consent in this study and the complexity of the information provided may have frustrated those with low genetic literacy. On the other hand, the overwhelming satisfaction with the informed consent process may be explained by completion bias, as only a subset of the participants completed the survey. Overall, these results suggest that genetic literacy is a potential confounding variable for user satisfaction. GeneLiFT can easily be incorporated in any survey on users' satisfaction when evaluating novel formats for the informed consent process. Analyzing a mixed population of individuals with low and higher genetic literacy may prevent the identification of preferred educational tools. Stratification may reveal that some tools are superior for individuals with low genetic literacy, while others are preferred by those with higher genetic literacy. Implementation of the GeneLiFT prior to online education could personalize the tool presented to the user.
As hypothesized, low genetic literacy was also associated with poorer understanding of genetic testing limitations. Misjudgment was highly associated with low genetic literacy. A large proportion of participants overestimated the capabilities of science. Disappointingly, more than a quarter of participants did not understand that a negative result would not provide them with a ‘clean bill of health’, despite the emphasis on this during the informed consent process. This underlines the importance of developing educational tools for the pre‐test consent/counseling process to ensure that all participants, patients, and customers understand the limitations of a ‘negative’ result. As previously shown in cohorts with relatively high socio‐economic backgrounds (Bernhardt et al., 2015), the limitations of genomic medicine were poorly understood even after adjusting for education level, highlighting the risk associated with socio‐economic based predictions. Surprisingly, the associations between low genetic literacy and misconceptions regarding genetic testing were not completely explained by low genetic knowledge. Our results reinforced the importance of measuring genetic literacy as an independent variable associated with understanding of genetic test limitations (Kaphingst et al., 2016; Lea et al., 2011; Syurina et al., 2011). As low genetic literacy was significantly associated with lower understanding of test limitations, GeneLiFT could help stratify cohorts to test such educational tools.
GeneLiFT also has the advantage of rapidly flagging the words the participant, client, or patient does not recognize. Those words may be part of the written information provided to the individual as part of research, clinical care, or direct‐to‐consumer genetic testing. In clinical care, asking patients to take GeneLiFT prior to a counseling session may help providers tailor the conversation to the clients. If some of those words are present in the clinical report, the provider may want to spend more time defining them or simply avoid them all together. For instance, our study revealed that only 33% of participants recognized the word ‘actionability’ as real, highlighting the relevance of a recent paper on the importance of defining the word ‘actionable’ to patients and clinicians (Gornick et al., 2019). On the other hand, some jargon may be avoided altogether, as words like ‘penetrance’ and ‘pathogenicity’ were recognized by less than 50% of participants. Such tailoring is imperative, as the utilization of medical jargon can negatively impact the session by inhibiting dialogue and reducing patients' satisfaction (Pitt & Hendrickson, 2020; Roter et al., 2007). Even though universal utilization of plain language may be the most strategic solution to address the genetic literacy challenge (Stableford & Mettger, 2007), plain language communication is not a part of standard education for health care providers and can be harder to achieve than targeted recommendations (Warde et al., 2018). Nevertheless, the written communication, including the informed consent, should strive to use plain language and carefully define terms deemed essential for patients' or participants' understanding. Future studies are needed to assess the impact of genetic literacy testing prior to genetic counseling.
Importantly, GeneLiFT has the advantage of being self‐administered and amenable to use in many contexts. Interestingly, the proportion of participants identified as having low genetic literacy was similar to the proportion reported using the REAL‐G test on another population (Erby et al., 2008). As genetic testing is increasingly offered as part of clinical care, research and through direct‐to‐consumer companies, new communication formats and new models of informed consent, including tele‐genetics and e‐consent, have been introduced (Biesecker et al., 2018; Cadigan et al., 2017; Green et al., 2004; Sanderson, Suckiel, et al., 2016; Vrečar et al., 2017). These formats have become more prevalent given the current shortage in genetic counselors (Cichon & Feldman, 2014; Stephenson, 1997). Self‐administered education tools are being developed to enhance individual understanding of genetic testing (Cadigan et al., 2017; McBride et al., 2009). As these new approaches are introduced into medicine and through direct‐to‐consumer companies, little is known about the factors impacting their efficacy (Biesecker et al., 2018; Green et al., 2004). Implementation of the GeneLiFT prior to online consent and education could personalize the tool presented to the user.
4.1. Study limitations
This study should be interpreted in the context of its limitations. First, although the sample was heterogeneous, it only included participants who consented to a genetic screening study and completed the survey. It was not designed as a representative study. The low rate of patients approached who declined participation reduces the bias associated with the consent to undergo genetic screening (Milo Rasouly et al., 2019); however, the reasons for not completing the survey could be associated with genetic literacy, as participants with low health literacy are underrepresented in this cohort. For instance, healthy individuals who actively volunteered for the study were more likely to fill out the survey, potentially because of the financial incentive or because of higher genetic literacy. Therefore, the proportion of individuals with low genetic literacy in the overall patient population may be significantly higher. The cohort also underrepresents participants on Medicaid, those under the poverty line and individuals from minorities like Native Americans, who may also experience lower genetic literacy. Secondly, the analysis of the association of genetic literacy with expectations from genetic testing was performed on the same sample used to validate GeneLiFT. Therefore, replication studies using diverse populations and in different settings are needed to assess the robustness of the findings. As the GeneLiFT threshold for low genetic literacy was set independently of the cohort, we expect to observe similar results in other cohorts; however, future studies may help identify a more sensitive and specific threshold. Utilization of GeneLiFT as a continuous co‐variate could also be tested. In this study, GeneLiFT was placed at the end of a survey that contained some of the real words tested. Exposure to those words may have inflated the numbers of real words recognized and again reduced the number of participants identified as having low genetic literacy. Replication studies should administer GeneLiFT at different time points to test the impact of exposure to those technical terms. Finally, GeneLiFT tests genetic literacy and not genetic knowledge; it should not therefore replace testing of genetic knowledge when appropriate.
4.2. Future research and practice implications
To our knowledge, this study represents the first evidence of an association between genetic literacy and expectations from genetic testing in a cohort undergoing genetic testing with return of results. As GeneLiFT is a rapid, self‐administered test that can be scored automatically in real time, it could be incorporated in different settings, including clinical care, research, and online tools. Incorporation of GeneLiFT may enable future studies to develop evidence‐based guidelines to personalize formats of education, informed consent, and return of results.
AUTHOR CONTRIBUTIONS
Hila Milo Rasouly designed the study, collected, analyzed and interpreted the data, and wrote the manuscript. Maddalena Marasa was responsible of the participants enrollment, helped with the questionnaire design and the revision of the manuscript. Nicole Cuneo helped with the development of GeneLiFT and the revision of the manuscript. Jacqueline J. Thompson, Natalia DeMaria, and David A. Fasel helped with the analysis of the data. Julia Wynn, Wendy K. Chung, and Paul Appelbaum helped with the design of the questionnaire and critically reviewed the manuscript. Debanjana Chatterjee helped with participants' enrollment, study design, and critically reviewed the manuscript. Suzanne Bakken helped with the statistical analysis, GeneLiFT validation, and critically reviewed the manuscript. Chunhua Weng and Ali G. Gharavi supervised the work, provided funding, and critically reviewed the manuscript.
Author Hila Milo Rasouly confirms that she has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All of the authors gave final approval of this version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
COMPLIANCE WITH ETHICAL STANDARDS
CONFLICT OF INTEREST
Author Hila Milo Rasouly, Author Nicole Cuneo, Author Maddalena Marasa, Author Natalia DeMaria, Author Debanjana Chatterjee, Author Jacqueline J. Thompson, Author David A. Fasel, Author Julia Wynn, Author Wendy K. Chung, Author Paul Appelbaum, Author Chunhua Weng, Author Suzanne Bakken, and Author Ali G. Gharavi declare that they have no conflict of interest.
HUMAN STUDIES AND INFORMED CONSENT
Approval to conduct this human subjects research was obtained from the Columbia University Institutional Review Board (IRB‐AAAQ9205). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants before being included in the study.
ANIMAL STUDIES
No non‐human animal studies were carried out by the authors for this article.
DATA SHARING AND DATA ACCESSIBILITY
The GeneLiFT test copyright, authored by Drs Rasouly and Gharavi, is owned by Columbia University. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Supporting information
Fig S1‐S4
Table S1‐S8
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all the participants in the study and the clinical research coordinators who enrolled them. We also thank Dr Victoria Kolupaeva for her comments on the manuscript. The research was supported by the eMERGE (grant U01HG008680), an investigator award from the Renal Research Institute and the Columbia Precision Medicine Initiative.
Rasouly HM, Cuneo N, Marasa M, et al. GeneLiFT: A novel test to facilitate rapid screening of genetic literacy in a diverse population undergoing genetic testing. J Genet Couns.2021;30:742–754. 10.1002/jgc4.1364
Contributor Information
Hila Milo Rasouly, Email: hm2673@columbia.edu.
Ali G. Gharavi, Email: ag2239@columbia.edu.
REFERENCES
- Abrams, L. R. , McBride, C. M. , Hooker, G. W. , Cappella, J. N. , & Koehly, L. M. (2015). The many facets of genetic literacy: Assessing the scalability of multiple measures for broad use in survey research. PLoS One, 10(10), e0141532. 10.1371/journal.pone.0141532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society of Human Genetics Ad Hoc Committee on Genetic Counselling (1975). Genetic counselling. American Journal of Human Genetics, 27(2), 240–242.1124768 [Google Scholar]
- Bernhardt, B. A. , Roche, M. I. , Perry, D. L. , Scollon, S. R. , Tomlinson, A. N. , & Skinner, D. (2015). Experiences with obtaining informed consent for genomic sequencing. American Journal of Medical Genetics. Part A, 167, 2635–2646. 10.1002/ajmg.a.37256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker, B. B. , Lewis, K. L. , Umstead, K. L. , Johnston, J. J. , Turbitt, E. , Fishler, K. P. , Patton, J. H. , Miller, I. M. , Heidlebaugh, A. R. , & Biesecker, L. G. (2018). Web platform vs in‐person genetic counselor for return of carrier results from exome sequencing: A randomized clinical trial. JAMA Internal Medicine, 178(3), 338–346. 10.1001/jamainternmed.2017.8049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokamp, C. , Wolfe, C. , Lingren, T. , Harley, J. , & Ryan, P. (2017). Decentralized and reproducible geocoding and characterization of community and environmental exposures for multisite studies. Journal of the American Medical Informatics Association: JAMIA, 25(3), 309–314. 10.1093/jamia/ocx128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butow, P. N. , & Lobb, E. A. (2004). Analyzing the process and content of genetic counseling in familial breast cancer consultations. Journal of Genetic Counseling, 13(5), 403–424. 10.1023/B:JOGC.0000044201.73103.4f [DOI] [PubMed] [Google Scholar]
- Cadigan, R. J. , Butterfield, R. , Rini, C. , Waltz, M. , Kuczynski, K. J. , Muessig, K. , Goddard, K. A. B. , & Henderson, G. E. (2017). Online education and e‐consent for GeneScreen, a preventive genomic screening study. Public Health Genomics, 20(4), 235–246. 10.1159/000481359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, R. , Likhanov, M. , Selita, F. , Zakharov, I. , Smith‐Woolley, E. , & Kovas, Y. (2019). New literacy challenge for the twenty‐first century: Genetic knowledge is poor even among well educated. Journal of Community Genetics, 10(1), 73–84. 10.1007/s12687-018-0363-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, L. D. , Griffin, J. M. , Partin, M. R. , Noorbaloochi, S. , Grill, J. P. , Snyder, A. , Bradley, K. A. , Nugent, S. M. , Baines, A. D. , & VanRyn, M. (2008). Validation of screening questions for limited health literacy in a large VA outpatient population. Journal of General Internal Medicine, 23(5), 561–566. 10.1007/s11606-008-0520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon, M. , & Feldman, G. L. (2014). Opportunities to improve recruitment into medical genetics residency programs: Survey results of program directors and medical genetics residents. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 16(5), 413–418. 10.1038/gim.2013.161 [DOI] [PubMed] [Google Scholar]
- Committee on Genetics (2017). Committee Opinion No. 693: Counseling about genetic testing and communication of genetic test results. Obstetrics and Gynecology, 129(4), e96–e101. 10.1097/AOG.0000000000002020 [DOI] [PubMed] [Google Scholar]
- Craik, F. I. M. , Rose, N. S. , & Gopie, N. (2015). Recognition without awareness: Encoding and retrieval factors. Journal of Experimental Psychology. Learning, Memory, and Cognition, 41(5), 1271–1281. 10.1037/xlm0000137 [DOI] [PubMed] [Google Scholar]
- Davis, T. C. , Wolf, M. S. , Arnold, C. L. , Byrd, R. S. , Long, S. W. , Springer, T. , Kennen, E. , & Bocchini, J. A. (2006). Development and validation of the Rapid Estimate of Adolescent Literacy in Medicine (REALM‐Teen): A tool to screen adolescents for below‐grade reading in health care settings. Pediatrics, 118(6), e1707–e1714. 10.1542/peds.2006-1139 [DOI] [PubMed] [Google Scholar]
- Dewalt, D. A. , Berkman, N. D. , Sheridan, S. , Lohr, K. N. , & Pignone, M. P. (2004). Literacy and health outcomes: A systematic review of the literature. Journal of General Internal Medicine, 19(12), 1228–1239. 10.1111/j.1525-1497.2004.40153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew, P. , Chatwin, J. , & Collins, S. (2001). Conversation analysis: A method for research into interactions between patients and health‐care professionals. Health Expectations, 4(1), 58–70. 10.1046/j.1369-6513.2001.00125.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- eMERGE Consortium (2019). Harmonizing clinical sequencing and interpretation for the eMERGE III network. American Journal of Human Genetics, 105(3), 588–605. 10.1016/j.ajhg.2019.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erby, L. H. , Roter, D. , Larson, S. , & Cho, J. (2008). The rapid estimate of adult literacy in genetics (REAL‐G): A means to assess literacy deficits in the context of genetics. American Journal of Medical Genetics. Part A, 146A(2), 174–181. 10.1002/ajmg.a.32068 [DOI] [PubMed] [Google Scholar]
- Facio, F. M. , Brooks, S. , Loewenstein, J. , Green, S. , Biesecker, L. G. , & Biesecker, B. B. (2011). Motivators for participation in a whole‐genome sequencing study: Implications for translational genomics research. European Journal of Human Genetics: EJHG, 19(12), 1213–1217. 10.1038/ejhg.2011.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald‐Butt, S. M. , Bodine, A. , Fry, K. M. , Ash, J. , Zaidi, A. N. , Garg, V. , Gerhardt, C. A. , & McBride, K. L. (2016). Measuring genetic knowledge: A brief survey instrument for adolescents and adults. Clinical Genetics, 89(2), 235–243. 10.1111/cge.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossey, R. , Kochan, D. , Winkler, E. , Pacyna, J. , Olson, J. , Thibodeau, S. , Connolly, J. , Harr, M. , Behr, M. , Prows, C. , Cobb, B. , Myers, M. , Leslie, N. , Namjou‐Khales, B. , Milo Rasouly, H. , Wynn, J. , Fedotov, A. , Chung, W. , Gharavi, A. , … Kullo, I. (2018). Ethical considerations related to return of results from genomic medicine projects: The eMERGE network (Phase III) experience. Journal of Personalized Medicine, 8(1), 2. 10.3390/jpm8010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornick, M. C. , Ryan, K. A. , Scherer, A. M. , Scott Roberts, J. , De Vries, R. G. , & Uhlmann, W. R. (2019). Interpretations of the term “Actionable” when discussing genetic test results: What you mean is not what i heard. Journal of Genetic Counseling, 28(2), 334–342. 10.1007/s10897-018-0289-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. J. , Peterson, S. K. , Baker, M. W. , Harper, G. R. , Friedman, L. C. , Rubinstein, W. S. , & Mauger, D. T. (2004). Effect of a computer‐based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: A randomized controlled trial. JAMA, 292(4), 442–452. 10.1001/jama.292.4.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga, S. B. , Barry, W. T. , Mills, R. , Ginsburg, G. S. , Svetkey, L. , Sullivan, J. , & Willard, H. F. (2013). Public knowledge of and attitudes toward genetics and genetic testing. Genetic Testing and Molecular Biomarkers, 17(4), 327–335. 10.1089/gtmb.2012.0350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson, C. M. E. , Bland, S. T. , Leppig, K. A. , Marasa, M. , Myers, M. , Rasouly, H. M. , Wynn, J. , & Clayton, E. W. (2020). Ethical conflicts in translational genetic research: Lessons learned from the eMERGE‐III experience. Genetics in Medicine, 22(10), 1667–1672. 10.1038/s41436-020-0863-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington, M. , & Carey, M. (2009). The on‐line Yes/No test as a placement tool. System, 37(4), 614–626. 10.1016/j.system.2009.09.006 [DOI] [Google Scholar]
- Health Resources and Services Administration . (n.d.). Retrieved from HRSA website: https://www.hrsa.gov/about/organization/bureaus/ohe/healthliteracy/index.html#:~:text=Health%20literacy%
- Hooker, G. W. , Peay, H. , Erby, L. , Bayless, T. , Biesecker, B. B. , & Roter, D. L. (2014). Genetic literacy and patient perceptions of IBD testing utility and disease control: A randomized vignette study of genetic testing. Inflammatory Bowel Diseases, 20(5), 901–908. 10.1097/MIB.0000000000000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurle, B. , Citrin, T. , Jenkins, J. F. , Kaphingst, K. A. , Lamb, N. , Roseman, J. E. , & Bonham, V. L. (2013). What does it mean to be genomically literate?: National Human Genome Research Institute Meeting Report. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 15(8), 658–663. 10.1038/gim.2013.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama, I. , Nagai, A. , Muto, K. , Tamakoshi, A. , Kokado, M. , Mimura, K. , Tanzawa, T. , & Yamagata, Z. (2008). Relationship between public attitudes toward genomic studies related to medicine and their level of genomic literacy in Japan. American Journal of Medical Genetics. Part A, 146A(13), 1696–1706. 10.1002/ajmg.a.32322 [DOI] [PubMed] [Google Scholar]
- Kaphingst, K. A. , Blanchard, M. , Milam, L. , Pokharel, M. , Elrick, A. , & Goodman, M. S. (2016). Relationships between health literacy and genomics‐related knowledge, self‐efficacy, perceived importance, and communication in a medically underserved population. Journal of Health Communication, 21(Suppl. 1), 58–68. 10.1080/10810730.2016.1144661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphingst, K. A. , Facio, F. M. , Cheng, M.‐R. , Brooks, S. , Eidem, H. , Linn, A. , Biesecker, B. B. , & Biesecker, L. G. (2012). Effects of informed consent for individual genome sequencing on relevant knowledge. Clinical Genetics, 82(5), 408–415. 10.1111/j.1399-0004.2012.01909.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian, A. W. , Griffith, K. A. , Hamilton, A. S. , Ward, K. C. , Morrow, M. , Katz, S. J. , & Jagsi, R. (2017). Genetic testing and counseling among patients with newly diagnosed breast cancer. JAMA, 317(5), 531–534. 10.1001/jama.2016.16918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer, M. M. , Roche, M. I. , Brewer, N. T. , Berg, J. S. , Khan, C. M. , Leos, C. , Moore, E. , Brown, M. , & Rini, C. (2017). Development and validation of a genomic knowledge scale to advance informed decision making research in genomic sequencing. MDM Policy & Practice, 2(1), 238146831769258. 10.1177/2381468317692582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea, D. H. , Kaphingst, K. A. , Bowen, D. , Lipkus, I. , & Hadley, D. W. (2011). Communicating genetic and genomic information: Health literacy and numeracy considerations. Public Health Genomics, 14(4–5), 279–289. 10.1159/000294191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemhöfer, K. , & Broersma, M. (2012). Introducing LexTALE: A quick and valid lexical test for advanced learners of English. Behavior Research Methods, 44(2), 325–343. 10.3758/s13428-011-0146-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt, L. (2015). Why health insurance literacy matters. JAMA, 313(6), 555–556. 10.1001/jama.2014.17419 [DOI] [PubMed] [Google Scholar]
- Lipkus, I. M. , Samsa, G. , & Rimer, B. K. (2001). General performance on a numeracy scale among highly educated samples. Medical Decision Making: An International Journal of the Society for Medical Decision Making, 21(1), 37–44. 10.1177/0272989X0102100105 [DOI] [PubMed] [Google Scholar]
- McBride, C. M. , Alford, S. H. , Reid, R. J. , Larson, E. B. , Baxevanis, A. D. , & Brody, L. C. (2009). Characteristics of users of online personalized genomic risk assessments: Implications for physician‐patient interactions. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 11(8), 582–587. 10.1097/GIM.0b013e3181b22c3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meara, P. , & Buxton, B. (1987). An alternative to multiple choice vocabulary tests. Language Testing, 4(2), 142–154. 10.1177/026553228700400202 [DOI] [Google Scholar]
- Milo Rasouly, H. , Wynn, J. , Marasa, M. , Reingold, R. , Chatterjee, D. , Kapoor, S. , Piva, S. , Kil, B. H. , Mu, X. , Alvarez, M. , Nestor, J. , Mehl, K. , Revah‐Politi, A. , Lippa, N. , Ernst, M. E. , Bier, L. , Espinal, A. , Haser, B. , Sinha, A. , … Chung, W. K. (2019). Evaluation of the cost and effectiveness of diverse recruitment methods for a genetic screening study. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 21, 2371–2380. 10.1038/s41436-019-0497-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molster, C. , Charles, T. , Samanek, A. , & O'Leary, P. (2009). Australian study on public knowledge of human genetics and health. Public Health Genomics, 12(2), 84–91. 10.1159/000164684 [DOI] [PubMed] [Google Scholar]
- NHGRI glossary (2015, August 1). Retrieved from https://www.genome.gov/glossary/
- Norman, C. D. , & Skinner, H. A. (2006). eHealth literacy: Essential skills for consumer health in a networked world. Journal of Medical Internet Research, 8(2), e9. 10.2196/jmir.8.2.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri, S. , Ferrari, F. , Manfrinati, A. , & Pravettoni, G. (2018). A systematic review of the psychological implications of genetic testing: A Comparative analysis among cardiovascular, neurodegenerative and cancer diseases. Frontiers in Genetics, 9, 624. 10.3389/fgene.2018.00624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormond, K. E. , Hallquist, M. L. G. , Buchanan, A. H. , Dondanville, D. , Cho, M. K. , Smith, M. , Roche, M. , Brothers, K. B. , Coughlin, C. R. , Hercher, L. , Hudgins, L. , Jamal, S. , Levy, H. P. , Raskin, M. , Stosic, M. , Uhlmann, W. , Wain, K. E. , Currey, E. , & Faucett, W. A. (2019). Developing a conceptual, reproducible, rubric‐based approach to consent and result disclosure for genetic testing by clinicians with minimal genetics background. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 21(3), 727–735. 10.1038/s41436-018-0093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergren, J. E. , Gornick, M. C. , Carere, D. A. , Kalia, S. S. , Uhlmann, W. R. , Ruffin, M. T. , Mountain, J. L. , Green, R. C. , Roberts, J. S. ; PGen Study Group (2015). How well do customers of direct‐to‐consumer personal genomic testing services comprehend genetic test results? Findings from the impact of personal genomics study. Public Health Genomics, 18(4), 216–224. 10.1159/000431250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer‐Sánchez, A. , & Schmitt, N. (2012). Scoring Yes–No vocabulary tests: Reaction time vs. nonword approaches. Language Testing, 29(4), 489–509. 10.1177/0265532212438053 [DOI] [Google Scholar]
- Pitt, M. B. , & Hendrickson, M. A. (2020). Eradicating Jargon‐Oblivion—A proposed classification system of medical Jargon. Journal of General Internal Medicine, 35(6), 1861–1864. 10.1007/s11606-019-05526-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson, K. A. , Gunstad, J. , Hughes, J. , Spitznagel, M. B. , Potter, V. , Waechter, D. , & Rosneck, J. (2010). The METER: A brief, self‐administered measure of health literacy. Journal of General Internal Medicine, 25(1), 67–71. 10.1007/s11606-009-1158-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm, H. L. (2017). Evolving health care through personal genomics. Nature Reviews. Genetics, 18(4), 259–267. 10.1038/nrg.2016.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roter, D. L. , Erby, L. H. , Larson, S. , & Ellington, L. (2007). Assessing oral literacy demand in genetic counseling dialogue: Preliminary test of a conceptual framework. Social Science & Medicine, 65(7), 1442–1457. 10.1016/j.socscimed.2007.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roter, D. L. , Erby, L. , Larson, S. , & Ellington, L. (2009). Oral literacy demand of prenatal genetic counseling dialogue: Predictors of learning. Patient Education and Counseling, 75(3), 392–397. 10.1016/j.pec.2009.01.005 [DOI] [PubMed] [Google Scholar]
- RStudio Team (2016). RStudio: Integrated development for R. RStudio Team. Retrieved from http://www.rstudio.com/ [Google Scholar]
- Sanderson, S. C. , Linderman, M. D. , Suckiel, S. A. , Diaz, G. A. , Zinberg, R. E. , Ferryman, K. , Wasserstein, M. , Kasarskis, A. , & Schadt, E. E. (2016). Motivations, concerns and preferences of personal genome sequencing research participants: Baseline findings from the HealthSeq project. European Journal of Human Genetics: EJHG, 24(1), 14–20. 10.1038/ejhg.2015.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson, S. C. , Suckiel, S. A. , Zweig, M. , Bottinger, E. P. , Jabs, E. W. , & Richardson, L. D. (2016). Development and preliminary evaluation of an online educational video about whole‐genome sequencing for research participants, patients, and the general public. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 18(5), 501–512. 10.1038/gim.2015.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillinger, D. , Grumbach, K. , Piette, J. , Wang, F. , Osmond, D. , Daher, C. , & Bindman, A. B. (2002). Association of health literacy with diabetes outcomes. JAMA, 288(4), 475–482. 10.1001/jama.288.4.475 [DOI] [PubMed] [Google Scholar]
- Schleit, J. , Naylor, L. V. , & Hisama, F. M. (2019). First, do no harm: Direct‐to‐consumer genetic testing. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 21(2), 510–511. 10.1038/s41436-018-0071-z [DOI] [PubMed] [Google Scholar]
- Schwartz, L. M. , Woloshin, S. , Black, W. C. , & Welch, H. G. (1997). The role of numeracy in understanding the benefit of screening mammography. Annals of Internal Medicine, 127(11), 966–972. 10.7326/0003-4819-127-11-199712010-00003 [DOI] [PubMed] [Google Scholar]
- Stableford, S. , & Mettger, W. (2007). Plain language: A strategic response to the health literacy challenge. Journal of Public Health Policy, 28(1), 71–93. 10.1057/palgrave.jphp.3200102 [DOI] [PubMed] [Google Scholar]
- Stanovich, K. E. , West, R. F. , & Harrison, M. R. (1995). Knowledge growth and maintenance across the life span: The role of print exposure. Developmental Psychology, 31(5), 811–826. 10.1037/0012-1649.31.5.811 [DOI] [Google Scholar]
- Stephenson, J. (1997). As discoveries unfold, a new urgency to bring genetic literacy to physicians. JAMA, 278(15), 1225–1226. 10.1001/jama.1997.03550150029014 [DOI] [PubMed] [Google Scholar]
- Sweet, K. , Sturm, A. C. , Schmidlen, T. , McElroy, J. , Scheinfeldt, L. , Manickam, K. , Gordon, E. S. , Hovick, S. , Scott Roberts, J. , Toland, A. E. , & Christman, M. (2017). Outcomes of a randomized controlled trial of genomic counseling for patients receiving personalized and actionable complex disease reports. Journal of Genetic Counseling, 26(5), 980–998. 10.1007/s10897-017-0073-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syurina, E. V. , Brankovic, I. , Probst‐Hensch, N. , & Brand, A. (2011). Genome‐based health literacy: A new challenge for public health genomics. Public Health Genomics, 14(4–5), 201–210. 10.1159/000324238 [DOI] [PubMed] [Google Scholar]
- Vassy, J. L. , O'Brien, K. E. , Waxler, J. L. , Park, E. R. , Delahanty, L. M. , Florez, J. C. , Meigs, J. B. , & Grant, R. W. (2012). Impact of literacy and numeracy on motivation for behavior change after diabetes genetic risk testing. Medical Decision Making: An International Journal of the Society for Medical Decision Making, 32(4), 606–615. 10.1177/0272989X11431608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrečar, I. , Hristovski, D. , & Peterlin, B. (2017). Telegenetics: An update on availability and use of telemedicine in clinical genetics service. Journal of Medical Systems, 41(2), 21. 10.1007/s10916-016-0666-3 [DOI] [PubMed] [Google Scholar]
- Wade, C. H. (2019). What Is the psychosocial impact of providing genetic and genomic health information to individuals? An Overview of Systematic Reviews. Hastings Center Report, 49, S88–S96. 10.1002/hast.1021 [DOI] [PubMed] [Google Scholar]
- Warde, F. , Papadakos, J. , Papadakos, T. , Rodin, D. , Salhia, M. , & Giuliani, M. (2018). Plain language communication as a priority competency for medical professionals in a globalized world. Canadian Medical Education Journal, 9(2), e52–e59. 10.36834/cmej.36848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Liu, J. , & Ai, H. (2019). Pseudowords and guessing in the Yes/No format vocabulary test. Language Testing, 37(1), 6–30. 10.1177/0265532219862265 [DOI] [Google Scholar]
- Zimmerman, J. , Broder, P. K. , Shaughnessy, J. J. , & Underwood, B. J. (1977). A recognition test of vocabulary using signal‐detection measures, and some correlates of word and nonword recognition. Intelligence, 1(1), 5–31. 10.1016/0160-2896(77)90025-3 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S4
Table S1‐S8
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
The GeneLiFT test copyright, authored by Drs Rasouly and Gharavi, is owned by Columbia University. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.


