Abstract
Introduction
The lack of approved specific therapeutic agents to treat coronavirus disease (COVID‐19) associated with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has led to the rapid implementation of convalescent plasma therapy (CPT) trials in many countries, including the United Kingdom. Effective CPT is likely to require high titres of neutralising antibody (nAb) in convalescent donations. Understanding the relationship between functional neutralising antibodies and antibody levels to specific SARS‐CoV‐2 proteins in scalable assays will be crucial for the success of a large‐scale collection. We assessed whether neutralising antibody titres correlated with reactivity in a range of enzyme‐linked immunosorbent assays (ELISA) targeting the spike (S) protein, the main target for human immune response.
Methods
Blood samples were collected from 52 individuals with a previous laboratory‐confirmed SARS‐CoV‐2 infection. These were assayed for SARS‐CoV‐2 nAbs by microneutralisation and pseudo‐type assays and for antibodies by four different ELISAs. Receiver operating characteristic (ROC) analysis was used to further identify sensitivity and specificity of selected assays to identify samples containing high nAb levels.
Results
All samples contained SARS‐CoV‐2 antibodies, whereas neutralising antibody titres of greater than 1:20 were detected in 43 samples (83% of those tested) and >1:100 in 22 samples (42%). The best correlations were observed with EUROimmun immunoglobulin G (IgG) reactivity (Spearman Rho correlation coefficient 0.88; p < 0.001). Based on ROC analysis, EUROimmun would detect 60% of samples with titres of >1:100 with 100% specificity using a reactivity index of 9.1 (13/22).
Discussion
Robust associations between nAb titres and reactivity in several ELISA‐based antibody tests demonstrate their possible utility for scaled‐up production of convalescent plasma containing potentially therapeutic levels of anti‐SARS‐CoV‐2 nAbs.
Keywords: convalescent plasma, COVID‐19, neutralising antibody level, SARS‐CoV‐2, testing of plasma
1. INTRODUCTION
The emergence of a novel coronavirus as a cause of respiratory disease occasionally leading to severe acute respiratory syndrome (SARS) was first noted in the Hubei province, China in December 2019. From there, it rapidly spread to a number of countries, including Italy, Iran, Spain and France. 1 Subsequently, this virus was classified as SARS coronavirus 2 (SARS‐CoV‐2) within the genus Betacoronavirus 2 and its associated disease termed COVID‐19. Mortality due to COVID‐19 is as high as 50% for patients admitted to intensive care units. 3
The first imported cases of SARS‐CoV‐2 were identified in the United Kingdom at the end of January 2020, and local transmission within the United Kingdom became evident 1 month later. As of 1st May 2020, a total of 182 260 cases and 28 131 deaths have been reported, and the numbers are predicted to continue to rise in this first pandemic wave. Currently, there are no approved specific antivirals targeting the novel virus, and convalescent plasma therapy (CPT) has been suggested as an immediately available therapy. A systematic review and retrospective meta‐analysis, including 699 treated patients with SARS‐CoV‐1 infection or severe influenza and 568 untreated controls, demonstrated a statistically significant reduction in mortality and in the pooled odds of mortality following treatment, compared with placebo or no therapy (odds ratio 0.25; 95% CI: 0.14–0.45). 4
Convalescent plasma may be an effective treatment for COVID‐19, with success linked to levels of neutralising antibody present in plasma, which reduce viral replication and increase viral clearance. 5 , 6 Virus‐specific neutralising antibodies play a key role in viral clearance. The spike (S) protein is responsible for the SARS‐CoV‐2 attachment and entry to the target cells via the ACE‐2 receptor, and neutralising antibodies recognising the receptor‐binding domain (RBD) on the S protein have been shown to block viral entry. 7 Antibodies against other domains of S protein or possibly even against other proteins may contribute to the functional neutralisation of the virus. Neutralising antibodies are known to be detectable in patients approximately 10–15 days after the onset of SARS‐CoV‐2 infection, 8 but this antibody response continues to mature for at least 3 weeks 9 and potentially longer.
The issue of the potential toxicity of convalescent plasma via antibody‐dependent enhancement (ADE) also needs to be addressed carefully. It has been shown to occur when non‐neutralising or heterotypic antibodies facilitate viral entry into host cells and enhance viral infectivity. 10 It is likely to occur when antibody levels or specificities do not permit neutralisation. 11 For these reasons, it is important to determine neutralising antibody titres in donated plasma, as well as a practical cut‐off titre level, to evaluate not only its safety but also its effectiveness for convalescent plasma transfusion.
Neutralising antibody levels can either be determined directly using native or pseudo‐type virus in cellular bioassays or be estimated by ELISA if there is an adequate correlation between neutralising antibody titre and ELISA reactivity. Neutralising antibody titre can be detected and quantified in a microneutralisation assay format in which samples are assayed for their ability to block infection of cells by SARS‐CoV‐2. Similarly, a pseudo‐type assay can be used to measure neutralising antibody levels using a virus construct containing SARS‐CoV‐2 S protein in the surface of a luciferase tagged vesicular stomatitis virus or lentivirus viral vector. 12 , 13 Both types of assays use suitably characterised target cells. Although a limitation of microneutralisation assays using live virus is the necessity to undertake work at biosafety level (BSL)‐3 laboratory, a pseudo‐type assay is more suitable for high‐throughput screening of convalescent plasma donors as it can be performed at a BSL‐2 facility.
In the current study, we have first determined the neutralising antibody levels in our convalescent plasma donors and estimated a cut‐off to be used in clinical trials. Second, we have also assessed whether there is a correlation between neutralisation antibody titres (measured either using microneutralisation or pseudo‐type assay) and ELISA reactivity using a variety of assays formats including cell lysate, in‐house assays and two commercial ELISAs. Identification of a suitable high‐throughput assay is required urgently to support scaling up convalescent plasma production and to support the comparison of data between countries.
2. MATERIALS AND METHODS
Convalescent plasma donors
We initiated the collection of convalescent plasma using the established infrastructure and standard UK donor selection guidelines during March 2020; serum and EDTA blood samples were collected from individuals with a previous laboratory‐confirmed SARS‐CoV‐2 infection at least 28 days after the resolution of their symptoms. These donor samples were submitted to Public Health England and tested initially for SARS‐CoV‐2 RNA by in‐house reverse transcription polymerase chain reaction assay, 14 as well as for SARS‐CoV‐2 antibodies using a native virus antigen ELISA and microneutralisation assays, both based on the UK prototype strain (GISAID accession number EPI/ISL/407073), and the samples were subsequently subjected to testing by pseudo‐type neutralisation assay and trimeric S ELISA. Basic donor information including age, gender and virology testing data were collected.
Ethical statement
Signed consent was obtained from each donor at the time of donation. Donors consent to the NHS blood and transplant holding information about them, including their health, attendances and donations, and using their information for the purposes explained in the donor welcome booklet and data protection leaflet, which donors are asked to read at the time of donation. This includes using data for the purposes of clinical audit to assess and improve the service and for research, specifically to improve our knowledge of the donor population.
Infected virus lysate assay
Native virus antigen ELISA was modified from a previously described MERS‐CoV assay. 15 Serial dilutions of convalescent plasma samples were added to microplates containing the bound detergent‐extracted lysates of SARS‐CoV‐2 (isolate England/02/2020)‐infected Vero E6 cells and uninfected cells. The reactivity was determined using a chemiluminescent substrate labelled secondary antibody. Virus lysates contain a mixture of viral proteins expressed in Vero E6 cells, including viral nucleocapsid and S proteins, and these proteins are presented in the same structure as the native virus infecting the host. ELISA index value was defined as the difference between infected and uninfected cell reactivity expressed relative to control calibrator serum.
Microneutralisation assay and neutralising antibody titre
SARS‐CoV‐2 (isolate England/02/2020)‐specific neutralising antibody levels were measured using a modification of the World Health Organization (WHO) influenza microneutralisation methodology. 16 Briefly, the virus was incubated with a serial dilution of convalescent plasma obtained from recovered patients, after which a suspension of VeroE6 cells was added. After 22 h, cells were fixed, and in‐cell SARS‐CoV‐2 nucleoprotein (NP) expression was determined by ELISA. The virus‐neutralising antibody titre was determined as the serum concentration that inhibited 50% of SARS‐CoV‐2 NP expression. All work was undertaken in a BSL‐3 laboratory.
2.1. Enzyme‐linked trimeric S immunosorbent assay (ELISA–Oxford)
Antibodies to the trimeric S (based on YP009724390.1) protein were detected by ELISA as previously described, using 2% skimmed milk in phosphate buffered saline as a blocking agent and alkaline phosphatase‐conjugated anti‐human IgG (A95455; Sigma) at 1:10 000 dilution. 12 Optical densities (ODs) were measured at 405 nm.
2.2. Pseudoparticle neutralisation test
A lentivirus‐based SARS‐CoV‐2 pseudovirus particle was constructed displaying the full S protein on the surface of pseudoparticle as previously described (accession number: YP009724390.1). 12 Neutralising antibody titres were measured by the reduction in luciferase gene expression after 72 h incubation of HEK 293T ACE2‐transfected cells at 37°C. The 50% inhibitory dilution (IC50) was defined as the plasma dilution at which the relative light units (RLUs) were reduced by 50% compared with the virus control wells after subtraction of the background RLUs in the groups with cells only.
2.3. Commercial assays, EUROimmun (IgG) and Fortress (total antibodies)
EUROimmun assay is based on the S1 protein and Fortress assay on the RBD of S protein. These assays were performed according to the manufacturer's recommendation (EUROimmun, PerkinElmer, London, UK and Fortress Diagnostics, Belfast, Northern Ireland).
2.4. Statistics
Associations between test assays were compared using Pearson correlation coefficients and the non‐parametric Spearman's rank correlation. p‐Values were derived using Student's t test for correlations and Pearson correlation coefficient under the null hypothesis that the correlation was zero. The sensitivity and specificity were calculated to assess the performance of the different assays in classifying the level of neutralising antibody titres obtained by microneutralisation assay using live SARS‐CoV‐2 virus. Exact binomial confidence intervals were used to derive confidence intervals.
3. RESULTS
The initial assessment included samples from 52 recovered patients who would qualify as donors of convalescent plasma for clinical trials. They were all males (to avoid the need for additional human leukocyte antigen and human neutrophil antigen antibody testing that was not available at the required scale at the time of the study) and at least 28 days from the recovery after laboratory‐confirmed SARS‐CoV‐2 infection. They were sampled during the first 2 weeks of April, implying that their illness began at the beginning of March. Therefore, they would all have been hospitalised as a part of the containment strategy. However, no data on the severity of their infection are currently available. EDTA and serum samples were obtained from each individual, and a whole‐blood donation was collected from 10. All samples were submitted to Public Health England Colindale, and available samples were distributed from there to the University of Oxford and Public Health England Porton Down for further testing. All samples tested negative for SARS‐CoV‐2 RNA. Assay specificity (particularly the rate of false reactives) has not been included in this analysis.
Neutralising antibodies were detected by microneutralisation assay in 43 of 52 tested samples using a cut‐off titre 1:20; the highest detectable titre was 1:4096 (Figure 1). In other assays, SARS‐CoV‐2 antibodies were detected in most samples tested by pseudo‐type assay (47/51), lysate ELISA (47/50) and EUROimmun (47/50) and in all samples by trimeric S ELISA (51/51) and Fortress total antibody ELISA (50/50). Based on these initial observations, all assays demonstrated good sensitivity for detecting antibodies in the study subjects 28 days after their recovery. For most assays, quantitative measures of serological reactivity (IC50 in the pseudo‐type assay, ODs or signal to cut‐off ratios (S/CO)) suggested a trend with neutralising antibody titres based on the live virus microneutralisation assay (Figure 1).
FIGURE 1.
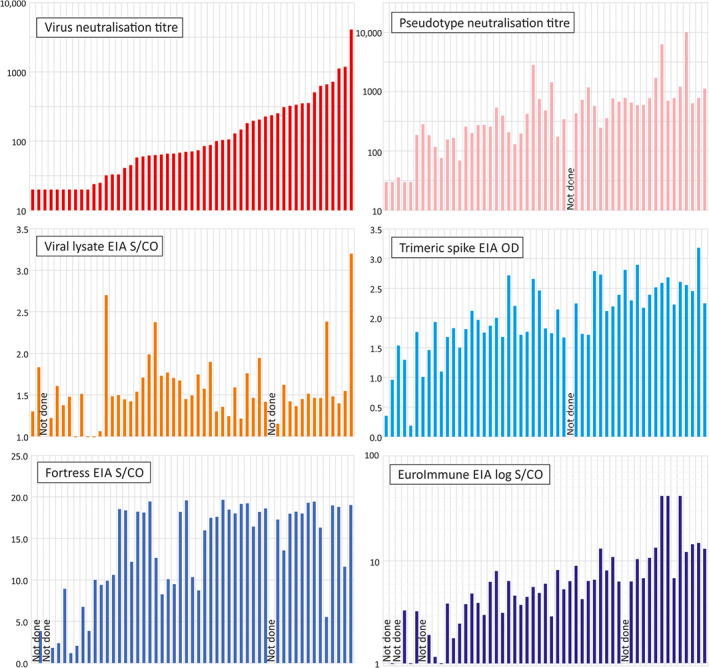
Comparison of neutralising antibody titres with reactivity in other assays. Comparison of neutralising antibody titres of the 52 test samples in the virus neutralisation assay with those of the pseudo‐type assay and reactivities in enzyme immunoassay (EIAs). In all graphs, samples were ordered by virus‐neutralising antibody titres. The following assay cut‐off values were used: 0.049 for trimeric spike EIA, 1.0 for Fortress EIA and 1.1 for EUROimmun [Color figure can be viewed at wileyonlinelibrary.com]
We have further assessed the correlation between neutralising antibody titre and serological reactivities in different ELISA platforms (Figure 2) where Pearson correlation coefficients and the non‐parametric Spearman's Rank correlation tests were performed. The Pearson correlation tests were used for a linear association between variables (using log‐transformed values for the neutralisation, pseudo‐type and EUROimmun assays; R2 values), whereas Spearman's coefficient determined correlations in ranking irrespective of magnitude. A further comprehensive pairwise comparison between all assays is provided in Figure S1.
FIGURE 2.
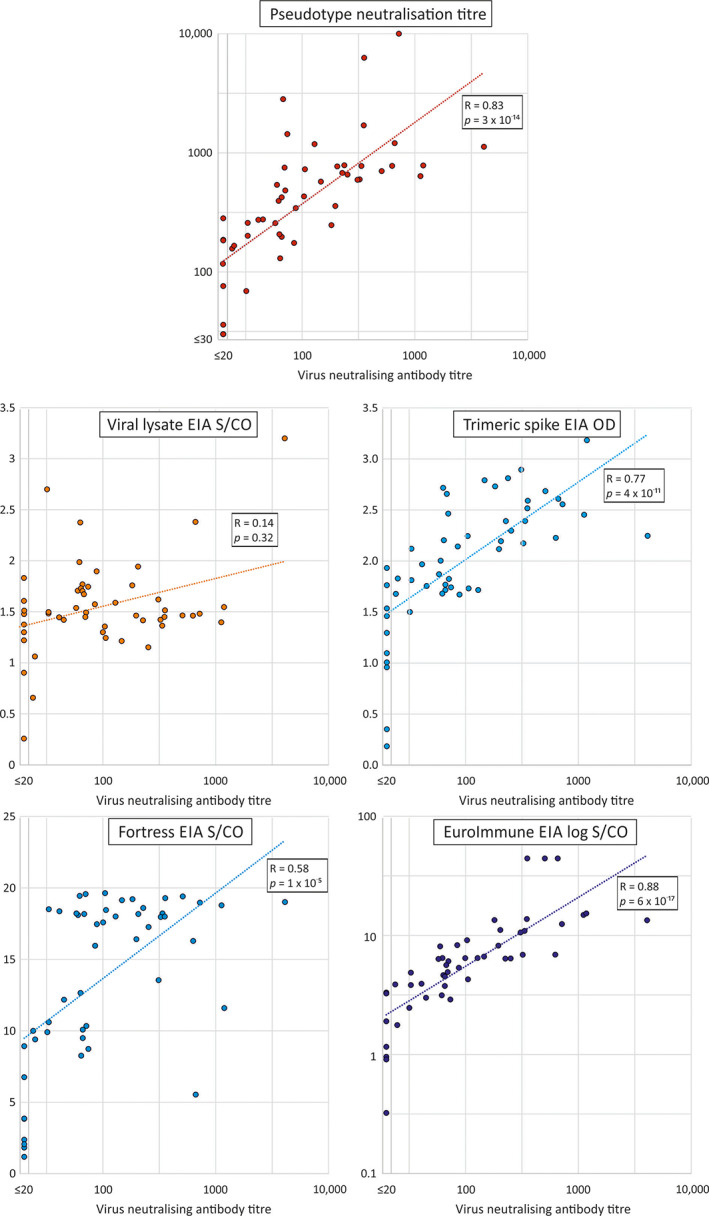
Correlations between neutralising and pseudo‐type antibody titres and reactivities in EIAs. Scatter plots of neutralising antibody titres of test samples in the virus neutralisation assay with those of the pseudo‐type assay and reactivities in EIAs. A line of best fit was estimated by linear regression using log‐transformed values for the virus and pseudo‐type neutralising antibody assays and the EUROimmun EIA. Correlation coefficients and (two‐tailed) p values were calculated by Spearman non‐parametric test [Color figure can be viewed at wileyonlinelibrary.com]
The strongest correlation was observed between neutralising antibody titres and reactivity in the EUROimmun IgG ELISA (Spearman's rank correlation: 0.88; p < 0.0001, n = 48). Correlations were also observed between neutralising antibody titres with IC50 values in the pseudo‐type assay (Spearman's rank correlation: 0.82; p < 0.0001, n = 51) and trimeric S ELISA (Spearman's rank correlation: 0.76; p < 0.0001, n = 51).
We selected a neutralising antibody titre of 1:100 as a likely therapeutic threshold for plasma donation selection (see discussion) and determined the best corresponding cut‐off value in the EUROimmun ELISA by ROC analysis (Table 1; Figure 3). A total of 22 of 48 samples with a EUROimmun result had a neutralising antibody titre higher than or equal to 1:100 and hence contributed to the sensitivity calculations. Similarly, the remaining 26 samples with neutralising antibody titre below 1:100 contributed to the specificity calculations. Five potential cut‐off values in the EUROimmun ELISA (S/CO values between 6.37 and 10) were investigated for sensitivity and specificity; a value of 9.1 correctly identified 65% of donations (14/22) above the 1:100 neutralising antibody threshold, whereas all donations below this neutralising antibody threshold were identified correctly using this value (26/26). In contrast, the pseudo‐type assay was unable to identify 50% or more donations >1:100 without false identification.
TABLE 1.
Threshold values for optimal sensitivity and specificity of EUROimmun and pseudo‐type neutralisation assays by ROC analysis
| Cut‐off value | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| EUROimmun S/CO | ||
| 6.37 | 0.95 (0.76, 1.00) | 0.89 (0.98, 0.77) |
| 6.64 | 0.76 (0.53, 0.92) | 0.93 (1.00, 0.83) |
| 8.19 | 0.68 (0.48, 0.83) | 0.96 (0.99, 0.85) |
| 9.1 a | 0.65 (0.45, 0.81) | 1.00 (1.00, 0.92) |
| 10 | 0.52 (0.30, 0.74) | 1.00 (1.00, 1.00) |
| Pseudo‐type neut. titre | ||
| 573 | 0.86 (0.64, 0.97) | 0.90 (0.98, 0.73) |
| 770 | 0.48 (0.26, 0.70) | 0.93 (0.99, 0.78) |
Note: These calculations are based on 48 samples, from which 22 had neutralising antibody levels of or over 1:100, and the remaining 26 were below 1:100.
Optimal value selected for donation selection shown in bold.
FIGURE 3.
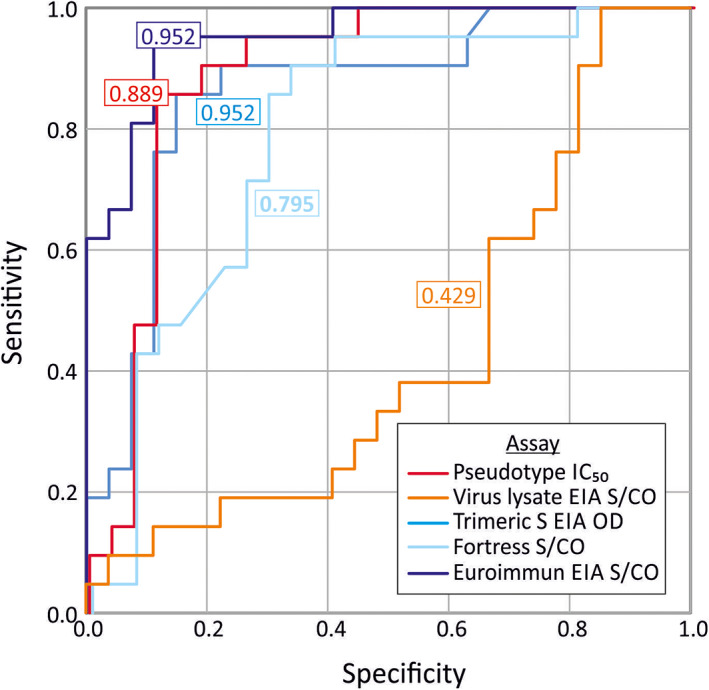
ROC analysis of serology assays predicting virus‐neutralising antibody titres of ≥1/100. OC curves for the pseudo‐type, virus lysate and three EIAs to correctly identify samples with neutralising antibody titres of 1:100 and over in the virus neutralisation assay. A total of 48 samples were included in these calculations (22 with neutralising antibody levels of or over 1:100 and the remaining 26 below 1:100). Areas under the curve for each assay are shown in colour‐coded boxes [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Here, we have described the first evaluation of the relationship between neutralising antibody titres and measures of antibodies to SARS‐CoV‐2 proteins in a variety of assays. These data can guide the selection of units of convalescent plasma for clinical use and for randomised clinical trials.
Our initial observation of convalescent plasma donors sampled at least 28 days after recovery from a laboratory‐confirmed SARS‐CoV‐2 infection showed that all of them demonstrated serological evidence of past SARS‐CoV‐2 infection in one or more assays, whereas the neutralising antibody levels detected by microneutralisation assay varied from low (1:20) to high (1:4096; Figure 1). Furthermore, approximately 43% of donor samples showed neutralising antibody titres greater than 1:100. These neutralising antibody titres obtained by the microneutralisation assay correlated with values obtained by pseudovirus assay; a titre of 1:100 corresponded to 1:300 calculated based on luminescence reading. Although the pseudo‐type assay can be automated and does not require working with the live virus in a biosafety level 3 laboratory, it is still time‐consuming compared to the ELISA‐based assay and requires the use of live cells and BSL‐2 facilities that are often lacking from blood donation screening and reference laboratories.
In a previous study, most convalescent plasma donors with previous COVID‐19 infection showed high neutralising antibody titres of at least 1:160 determined by the plaque reduction neutralisation test (PRNT; 39/40). For CPT, only donations with antibody titres above 1:640 were used. 5 In a separate study, donations with a neutralising antibody titre equal or higher than 1:80 based on the microneutralisation test were used successfully. 6 It is important to note that antibody titres obtained by different assays may not be comparable; based on previous data on SARS‐CoV‐2, neutralising antibody titres obtained by PRNT were approximately four‐fold higher than those obtained by a cytopathic effect (CPE)‐based microneutralisation assay. 17 CPE refers to structural changes in host cell, caused by virus invasions. Further comparative work is required to determine how the neutralising antibody level obtained by our microneutralisation assays compares with the PRNT titres and also with assays performed outside the United Kingdom. The future availability of WHO international standards will facilitate such comparisons; this is anticipated to be available in December 2020.
A minimum neutralising antibody titre in convalescent plasma needs to be determined before plasma is supplied for clinical trials. This needs to be balanced with the difficulty of collecting a required number of such components and providing a sufficient dose of antibodies to potentially be effective. For the planned trial, the use of plasma with a too‐low cut‐off may prevent or prolong a clear demonstration of efficacy; conversely, a too high cut‐off may prevent a sufficient supply of plasma to fulfil trial needs. The chosen neutralising antibody level, 1:100, was selected as a pragmatic cut‐off that enables an estimated 40% of collected plasma to be used. The actual dose of neutralising antibody given to patients also depends on the number of units given, and giving two units from different donors may substantially increase the mean dose to more than 1:300. Although considered potentially effective, how this level obtained by the microneutralisation assay compares with PRNT titres used in previous studies requires further work. This cut‐off will be reviewed after a larger number of samples have been analysed to see if supply is meeting demand.
In order to support the scaling up the convalescent plasma production, it is important to identify a suitable high‐throughput ELISA assay that can be used to estimate the neutralising antibody levels in convalescent plasma samples and thus could determine which donations are offered for clinical use. Serological reactivity in both the EUROimmun SARS‐CoV‐2 IgG ELISA and the trimeric S SARS‐CoV‐2 ELISA showed a strong correlation with neutralising antibodies obtained either by microneutralisation test or by pseudo‐type assay. Although the EUROimmun assay has been shown to lack sensitivity for samples collected from patients with recent infection, 18 we have shown that it could be used to identify donations containing high levels of neutralising antibodies with a good level of specificity. By selecting an S/CO cut‐off value of 9.1, the assay would only identify units if the neutralising antibody titre was 1:100 or higher. This is consistent with a previous finding where plasma with high titres of neutralising SARS‐CoV‐2 antibodies also showed higher titres of RBD, S domain 1 or 2 and specific binding antibodies. 8 Trimeric S ELISA falls within the RBD domain located in the S domain 1, whereas EUROimmun targets S domain 1. However, it is important to note that this is based on testing a preselected cohort of individuals at least 28 days after recovery from a previous laboratory‐confirmed SARS‐CoV‐2 infection. The evaluation should be repeated if these criteria are changed or if the screening of native blood donor populations without a prior history of SARS‐CoV‐2 infection is considered.
As only a small number of samples from preselected convalescent plasma donors have been tested so far, which is a limitation of this study, we propose that several assay formats should be employed in a larger group of donors to validate these findings before the scaling up can be finalised. For practical and economic reasons, we decided to extend neutralising antibody testing up to 300 samples and then finalise analysis. Nevertheless, the results provide guidance for the many convalescent plasma programmes in progress around the world.
Neutralising antibody levels are partly dependent on the timing of collection relative to the recovery from infection. Seroconversion following SARS‐CoV‐2 infection has been observed between 8 and 21 days after the onset of symptoms, 9 , 19 , 20 , 21 and higher levels of antibodies have been determined in plasma collected at least 14 days after the symptom resolution. 5 It is likely that the antibody maturation continues for longer as demonstrated for other viruses, and hence, the collection point of 28 days after recovery has been chosen here. This maximises the chances of collecting the most clinically effective donations. However, it is still unclear how long neutralising antibody levels are maintained, and hence, repeat testing will be performed at every donation.
Higher neutralising SARS‐CoV‐2 antibody levels have been associated with older age and a worse clinical outcome, 8 , 21 although good neutralising antibody levels have also been measured in individual patients with milder infections. 22 , 23 The monitoring of neutralising antibody levels in different patient groups (including females not included in this study) and over time is required and will inform future screening strategies.
In conclusion, here, we have demonstrated a correlation between the neutralising antibody level and antibody reactivity measured by ELISA, which will allow scaling up of the convalescent plasma production. However, continuous monitoring of assay performance, antibody decay and adaptation of selection strategies will be required in order to deliver the best clinical outcomes for patients receiving neutralising SARS‐CoV‐2 antibodies through CPT.
CONFLICT OF INTEREST
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
Heli Harvala: designed the study; coordinated the testing and collected and analysed the data, as well as wrote the first draft of manuscript; reviewed and accepted the final manuscript. Matthew L. Robb: drafted the statistical analysis; reviewed and accepted the final manuscript. Nick Watkins: critically reviewed the manuscript; supported the logistics of this study via the convalescent plasma group; reviewed and accepted the final manuscript. Samreen Ijaz: responsible for sample aliquoting and logistics between different sites; reviewed and accepted the final manuscript. Steven Dicks: responsible for sample aliquoting and logistics between different sites; reviewed and accepted the final manuscript. Monika Patel: performed testing with live virus neutralisation and lysate assays in PHE Colindale; reviewed and accepted the final manuscript. Piyada Supasa: responsible for developing spike‐ELISA in Oxford and performing testing of samples with that assay; reviewed and accepted the final manuscript. Wanwisa Dejnirattisai: responsible for developing spike‐ELISA in Oxford and performing testing of samples with that assay; reviewed and accepted the final manuscript. Chang Liu: responsible for developing spike‐ELISA in Oxford and performing testing of samples with that assay; reviewed and accepted the final manuscript. Juthathip Mongkolsapaya: responsible for developing spike‐ELISA in Oxford and performing testing of samples with that assay; reviewed and accepted the final manuscript. Abbie Brown: organised and performed EUROimmun testing at PHE Porton Down; reviewed and accepted the final manuscript. Daniel Bailey: organised and performed EUROimmun testing at PHE Porton Down; reviewed and accepted the final manuscript. Richard Vipond: organised and performed EUROimmun testing at PHE Porton Down; reviewed and accepted the final manuscript. Nicholas Grayson: organised and performed pseudo‐type neutralisation testing of these samples; reviewed and accepted the final manuscript. Nigel Temperton: organised and performed pseudo‐type neutralisation testing of these samples; reviewed and accepted the final manuscript. Sunetra Gupta: organised and performed pseudo‐type neutralisation testing of these samples; reviewed and accepted the final manuscript. Rutger J. Ploeg: reviewed and accepted the final manuscript. Jai Bolton: organised and performed pseudo‐type neutralisation testing of these samples; reviewed and accepted the final manuscript. Alex Fyfe: organised and performed pseudo‐type neutralisation testing of these samples; reviewed and accepted the final manuscript. Robin Gopal: critically reviewed the manuscript; performed testing with live virus neutralisation and lysate assays in PHE Colindale; reviewed and accepted the final manuscript. Peter Simmonds: critically reviewed the manuscript; drafted the statistical analysis; reviewed and accepted the final manuscript. Gavin Screaton: responsible for developing spike‐ELISA in Oxford and performing testing of samples with that assay; reviewed and accepted the final manuscript. Craig Thompson: organised and performed pseudo‐type neutralisation testing of these samples; reviewed and accepted the final manuscript. Tim Brooks: organised and performed EUROimmun testing at PHE Porton Down; reviewed and accepted the final manuscript. Maria Zambon: designed the study; critically reviewed the manuscript; performed testing with live virus neutralisation and lysate assays in PHE Colindale; reviewed and accepted the final manuscript. Gail Miflin: critically reviewed the manuscript; supported the logistics of this study via the convalescent plasma group; reviewed and accepted the final manuscript. David J. Roberts: designed the study; critically reviewed the manuscript; reviewed and accepted the final manuscript.
Supporting information
Figure S1. Supporting information.
ACKNOWLEDGMENTS
We thank all the donors who have kindly donated convalescent plasma. We are grateful for everybody within the NHS Blood and Transplant who have participated in the convalescent plasma programme and at the operations level for donor outreach, collection, transporting, processing and storing convalescent plasma. We also thank the staff members of the Virus Reference Division at Public Health England Colindale. Public Health England is acknowledged their financial support towards this work. Prof Sunetra Gupta has been funded via European Research Council “UNIFLUVAC” (grant number 812816) and MRC CiC 6. This work was funded by the Department of Health and Social Care (DHSC)/UKRI/NIHR COVID‐19 Rapid Response Grant (COV19‐RECPLA). [Correction added on 13 March 2021, after first online publication: The funding details in the Acknowledgments section has been updated in this version.]
Harvala H, Robb ML, Watkins N, et al. Convalescent plasma therapy for the treatment of patients with COVID‐19: Assessment of methods available for antibody detection and their correlation with neutralising antibody levels. Transfusion Medicine. 2021;31:167–175. 10.1111/tme.12746
Funding information European Research Council, Grant/Award Number: 812816; Public Health England; Department of Health and Social Care (DHSC)/UKRI/NIHR COVID‐19 Rapid Response Grant (COV19‐RECPLA)
REFERENCES
- 1. Novel coronavirus 2019 . https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed July 1, 2020.
- 2. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5(4):536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Docherty AB, Harrison EM, Green CA, et al. Features of 16,749 hospitalised UKpatients with COVID‐19 using the ISARIC WHO clinical characterisation protocol. medRxiv 2020: 2020.04.23.20076042.
- 4. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211(1):80‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490‐9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323:1582‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID‐19. Nat Rev Immunol. 2020;20:339‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS‐CoV‐2 in a COVID‐19 recovered patient cohort and their implications. medRxiv 2020: 2020.03.30.20047365.
- 9. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26:845‐848. [DOI] [PubMed] [Google Scholar]
- 10. Wan Y, Shang J, Sun S, et al. Molecular mechanism for antibody‐dependent enhancement of coronavirus entry. J Virol. 2020;94(5):e02015‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ricke DO, Malone RW. Medical countermeasures analysis of 2019‐nCoV and vaccine risks for antibody‐dependent enhancement (ADE). Preprints 2020; 2020030138.
- 12. Thompson C, Grayson N, Paton R, et al. Neutralising antibodies to SARS coronavirus 2 in Scottish blood donors ‐ a pilot study of the value of serology to determine population exposure. medRxiv 2020: 2020.04.13.20060467.
- 13. Nie J, Li Q, Wu J, et al. Establishment and validation of a pseudovirus neutralization assay for SARS‐CoV‐2. Emerg Microbes Infect. 2020;9(1):680‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):2000045. 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agnihothram S, Gopal R, Yount BL Jr, et al. Evaluation of serologic and antigenic relationships between middle eastern respiratory syndrome coronavirus and other coronaviruses to develop vaccine platforms for the rapid response to emerging coronaviruses. J Infect Dis. 2014;209(7):995‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The WHO Global Influenza Surveillance and Response System (GISRS) Serological diagnosis of influenza by microneutralisation assay. https://www.who.int/influenza/gisrs_laboratory/2010_12_06_serological_diagnosis_of_influenza_by_microneutralization_assay.pdf. Accessed July 1, 2020.
- 17. Perera RA, Mok CK, Tsang OT, et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Euro Surveill. 2020;25(16):2000421. 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lassaunière R, Frische A, Harboe ZB, et al. Evaluation of nine commercial SARS‐CoV‐2 immunoassays. medRxiv 2020: 2020.04.09.20056325.
- 19. Okba NMA, Muller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2‐specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26(7):1478‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis. 2020;71:778‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;71(16):2027‐2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465‐469. [DOI] [PubMed] [Google Scholar]
- 23. Haveri A, Smura T, Kuivanen S, et al. Serological and molecular findings during SARS‐CoV‐2 infection: the first case study in Finland. Euro Surveill. 2020;25(11):2000266. 10.2807/1560-7917.ES.2020.25.11.2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Supporting information.


