Abstract
Background
Healthcare workers are a key occupational group at risk for suicidal thoughts and behaviors (STB). We investigated the prevalence and correlates of STB among hospital workers during the first wave of the Spain COVID‐19 outbreak (March–July 2020).
Methods
Data come from the baseline assessment of a cohort of Spanish hospital workers (n = 5450), recruited from 10 hospitals just after the height of the coronavirus disease 2019 (COVID‐19) outbreak (May 5–July 23, 2020). Web‐based self‐report surveys assessed 30‐day STB, individual characteristics, and potentially modifiable contextual factors related to hospital workers' work and financial situation.
Results
Thirty‐day STB prevalence was estimated at 8.4% (4.9% passive ideation only, 3.5% active ideation with or without a plan or attempt). A total of n = 6 professionals attempted suicide in the past 30 days. In adjusted models, 30‐day STB remained significantly associated with pre‐pandemic lifetime mood (odds ratio [OR] = 2.92) and anxiety disorder (OR = 1.90). Significant modifiable factors included a perceived lack of coordination, communication, personnel, or supervision at work (population‐attributable risk proportion [PARP] = 50.5%), and financial stress (PARP = 44.1%).
Conclusions and Relevance
Thirty‐day STB among hospital workers during the first wave of the Spain COVID‐19 outbreak was high. Hospital preparedness for virus outbreaks should be increased, and strong governmental policy response is needed to increase financial security among hospital workers.
Keywords: COVID‐19 outbreak, hospital workers, suicidal thoughts and behaviors
1. INTRODUCTION
The coronavirus disesase 2019 (COVID‐19) pandemic has presented hospital workers with unprecedented challenges in terms of workload as well as health‐ and work‐related risk and stress exposures. The latter includes exposure to COVID‐19 patients and stress about getting infected or infecting loved ones, but also moral injury, that is, psychological distress resulting from actions, or the lack of them, that violate one's moral or ethical code (Litz et al., 2009). In the current context, moral injury may result from the lack of hospital preparedness for the pandemic, and may lead to traumatic experiences such as having to prioritize care, or seeing patients suffer or die from COVID‐19 (Greenberg et al., 2020). In line with these concerns, rates of depression, anxiety, and sleep problems among healthcare workers during COVID‐19 outbreaks are high (Muller et al., 2020; Pappa et al., 2020; Vindegaard & Benros, 2020), and those in direct contact with affected patients report posttraumatic stress and psychological distress (Kisely et al., 2020). These adverse mental health outcomes are well‐known risk factors for suicidal thoughts and behaviors (STB; Franklin et al., 2017). High rates of STB among healthcare professionals during virus outbreaks can therefore be expected (Gunnell et al., 2020) especially since this population segment already has increased risk for suicidal ideation (Tyssen et al., 2001) and suicide (Dutheil et al., 2019; Hawton et al., 2011) under normal working conditions. No research to date focused on STB during a virus outbreak in this key occupational group at risk (Salazar de Pablo et al., 2020). In addition, previous studies among hospital workers active during the COVID‐19 pandemic predominantly focused on healthcare workers (mostly doctors or nurses; Muller et al., 2020), while many hospital workers not involved in patient care may also be at risk for adverse mental health.
Spain was among those countries whose healthcare systems came under extreme pressure during the first wave of the COVID‐19 pandemic (March–July 2020; Arango, 2020). The Spanish government declared a state of alarm on March 14, 2020, and between the beginning of March and mid‐April, more than 2000 new cases were reported daily. The healthcare system nearly collapsed during April–May due to lack of intensive care unit beds, ventilators, and healthcare personnel (Red Nacional de Vigilancia Epidemiólogica [RENAVE], 2020). By the time the situation stabilized in early July, Spain had the eighth highest number of confirmed cases (i.e., 249,659 on 01/07/2020), and the fifth highest COVID death rate (i.e., 60.7/100,000 on 01/07/2020) in the world (Roser et al., 2020).
We present data from the MIND/COVID project (MIND/COVID‐19, 2020), a national multiple‐cohort study of the mental health impact of the COVID‐19 pandemic in Spain. We report here on the baseline assessment of the hospital workers cohort, conducted just after the height of the virus outbreak (May 5–July 23, 2020), when demands on the Spanish public healthcare system were substantially increased. The objectives of the current report are to examine baseline prevalence of 30‐day STB and to investigate the relationship of potentially modifiable contextual factors related to hospital workers' perceived work and financial situation, with 30‐day STB.
2. METHODS
2.1. Study design, population, and sampling
The study design consists in a multicenter, prospective, observational cohort study of Spanish hospital workers. A convenience sample of 10 hospitals from four autonomous communities in Spain (i.e., the Basque Country, Castile and Leon, Catalonia, and the Community of Madrid) agreed to participate. Hospitals were selected to reflect the geographical and sociodemographic variability in Spain. All participating hospitals came from regions with high COVID‐19 caseloads.
Here we report on the baseline assessment of the cohort, which consists of de‐identified web‐based self‐report surveys (May 5–July 23, 2020), conducted soon after the first wave of the COVID‐19 outbreak in Spain. In each participating hospital, hospital representatives contacted all employed hospital workers to participate using the hospitals' administrative email distribution lists (i.e., census sampling). The invitation email included an anonymous link to access the web‐based survey platform (qualtrics.com). Informed consent was obtained from all participants at the first survey page. Two reminder emails were sent within a 2–4 weeks period after the initial invitation.
The study complies with the principles established by national and international regulations, including the Declaration of Helsinki and the Code of Ethics. The study was approved by the Research Integrity and Good Scientific Practices Committee of IMIM‐Parc de Salut Mar, Barcelona, Spain (2020/9203/I), and by all participating centers' institutional review boards (IRBs).
2.2. Measures
2.2.1. STB
A modified self‐report version of selected items from the Columbia Suicide Severity Rating Scale (C‐SSRS; Posner et al., 2011), also used in other large‐scale epidemiological studies (e.g., Nock et al., 2014), assessed suicidal thoughts and behaviors in the past 30 days, including passive suicidal ideation (“wish you were dead or would go to sleep and never wake up”), active suicidal ideation (“have thoughts of killing yourself”), suicide plans (“think about how you might kill yourself [e.g., taking pills, shooting yourself] or work out a plan of how to kill yourself”), and suicide attempt (“make a suicide attempt [i.e., purposefully hurt yourself with at least some intent to die]”).
2.2.2. Potentially modifiable contextual factors
Potentially modifiable contextual factors refer to factors that are related to hospital workers' work and financial situation, that are relevant with regard to the COVID‐19 outbreak, and that are potentially modifiable in the future.
Six work‐related factors were assessed: (1) the average weekly hours worked, categorized into 40 h/week or less, 41–50 h/week, and 51 h or more per week; (2) the perceived lack of coordination, communication, personnel, or supervision at work, using four 5‐level Likert‐type items ranging from “none of the time” to “all of the time.” Items were summed and rescaled to a 0.0–4.0 Likert scale (Cronbach α = .858); (3) the perceived frequency of lack of protective equipment, using a 5‐level Likert‐type item ranging from “none of the time” to “all of the time;” (4) the perceived efficiency of the available protective equipment, using a 4‐level Likert‐type item ranging from “sufficient” to “completely insufficient;” (5) having had to make decisions regarding prioritizing care among COVID‐19 patients (assessed among medical doctors and nurses only); and (6) having had patient(s) in care that died from COVID‐19. All items included a specific time frame, that is, “since the onset of the virus outbreak in Spain.”
Two factors related to hospital workers' financial situation were assessed: (1) having suffered a significant loss in personal or familial income due to the COVID‐19 pandemic; and (2) financial stress, using two 5‐level Likert‐type items that assessed stress regarding one's financial situation (Dohrenwend et al., 1978) and stress regarding job loss or loss of income because of COVID‐19, with response options ranging from “none” to “very severe.” Items were summed and rescaled to 0.0–4.0 Likert scale (Cronbach α = .821).
2.2.3. Individual characteristics
Twelve individual characteristics were assessed: (1) age; (2) gender; (3) marital status; (4) having children in care; (5) self‐reported lifetime mental disorders before the onset of the COVID‐19 outbreak, using Composite International Diagnostic Interview (CIDI) 3.0 adapted screener items (Kessler & Üstün, 2004), including mood (i.e., depressive and bipolar disorders), anxiety (i.e., panic, generalized anxiety, and obsessive–compulsive disorders), substance use (i.e., alcohol, illicit drugs, and prescription drugs with or without prescription), and other disorders; (6) profession, categorized into five categories: medical doctors, nurses, auxiliary nurses, other professions involved in patient care (i.e., midwives; dentists or odontologists; pharmaceutical, laboratory, or radiology technicians; psychologists, physiotherapists, social workers, patient transport) and other professions not involved in patient care (i.e., administrative and management personnel, logistic support [e.g. food, maintenance, supplies], research‐only personnel); (7) the frequency of direct exposure to COVID‐19 patients during professional activity, using one 5‐level Likert type item, ranging from “none of the time” to “all of the time;” (8) changes in assigned functions, team, or working location, categorized into having changed to a specific COVID‐19‐related work location (e.g., emergency room, COVID ward, fever clinic, intensive care unit, quarantine center, field hospital), having changed of team or assigned functions, and all others; (9) the frequency of working at home during the COVID‐19 outbreak, using a 6‐level Likert item, ranging from “never” to “always;” (10) COVID‐19 infection history, categorized into having been hospitalized for COVID‐19, having had a positive COVID‐19 test or medical diagnosis not requiring hospitalization, and all others; (11) having been in isolation of quarantine because of exposure to COVID‐19‐infected person(s); and (12) having close ones infected with COVID‐19.
2.3. Data representativeness and quality
A total of 5450 hospital workers participated (response rate = 11.8%). It is important to note that the survey view rate (i.e., the proportion of hospital workers that opened the invitation email; Eysenbach, 2004) is unknown, except for one hospital (26.4%), suggesting low survey view rates, and questioning the validity of the response rate as an indicator of data representativeness. Post‐stratification weights were used in all analyses to adjust for potential nonresponse bias, taking into account sample versus target population differences in age, gender, and profession. Differences in post‐stratifying variables between our sample and the target population were small, suggesting good data representativeness. See Table S1 and S2 for more details on response rates and poststratification. To further assess data representativeness, we compared our observed COVID‐19 infection rates stratified by Autonomous Community in Spain (range 8.4%–21.8%) with official seroprevalence results, and found that they are in very close agreement (see Table S3).
For this study, analyses were restricted to n = 5169 (94.8%) that completed all STB items. An additional n = 5 were excluded because they did identify with neither male or female gender (of those, n = 1 reported 30‐day passive ideation only; n = 2 reported a 30‐day suicide attempt). No statistical differences in gender or age were found between those that completed the STB items and those that did not (females 80.8% vs. 82.1%, χ 2(1) = 0.315, p = .574; mean age 42.9 vs. 42.1, t(5448] = −1.18, p = .240). Median % missingness per variable in the analysis sample was 1.4% (see Table S4). Missing data were handled using multivariate imputation by chained equations (van Buuren, 2012).
2.4. Statistical analysis
All analyses were conducted with SAS version 9.4, and R version 3.6.2. First, prevalence estimates of 30‐day STB were estimated, both total and stratified by individual characteristics, with associated modified Rao–Scott χ 2 tests and Fisher‐Exact tests. Second, we estimated multivariable associations between 30‐day STB and individual characteristics. Logistic regression was used for all multivariable analysis. Third, we estimated the multivariable association between 30‐day STB and each potentially modifiable contextual factor separately, adjusting for all individual characteristics. Fourth, we identified the subset of individual characteristics and potentially modifiable contextual factors that best explain STB in multivariable models, using the lasso shrinkage method (Hastie et al., 2009), optimizing the Bayesian Information Criterion. Variance was estimated using the Taylor series linearization method taking into account post‐stratification and within‐hospital clustering of data. Potential deviations from a continuous linear effect in the logit were assessed using likelihood ratio tests comparing full categorical versus continuous variable specifications. All analyses were adjusted for hospital membership and time of survey. Fifth, population‐attributable risk proportions (PARP; Krysinska & Martin, 2009), and associated bootstrap percentile confidence intervals (500 replications), were simulated based on individuals' predicted probabilities estimated by the multivariable logistic regression equations (Nock et al., 2015). PARP provide estimates of the proportions of STB that could potentially be attributed to specific predictor variables assuming a causal pathway between these predictor variables and STB.
3. RESULTS
Prevalence of 30‐day STB was estimated at 8.4% (Table 1). Of those, more than half (4.9% in total) were passive suicidal ideation only. The others (3.5%) consisted of active suicidal ideation without plan or attempt (0.8%) and with plan or attempt (2.7%). A total of n = 6 reported a 30‐day suicide attempt. Thirty‐day STB was substantially elevated among those with pre‐existing mood and anxiety disorders (any STB range 13.5%–22.3%; plan or attempt range 4.8%–9.6%) and among those with a hospitalization for COVID‐19 (any STB = 12.6%; plan or attempt = 8.1%). STB was also elevated among those aged 18–29, non‐married respondents, those without children in care, those that had frequent exposure to COVID‐19 patients, those that changed to specific COVID‐19‐related work locations, and those that never worked at home. Any STB ranged from 6.2% to 12.0% across hospitals (Table S1).
Table 1.
Thirty‐day prevalence of suicidal thoughts and behaviors (STB), total, and stratified by individual characteristics (n = 5164)
| Any STB (n = 395) | Passive ideation only (n = 243) | Active ideation without plan or attempt (n = 41) | Active ideation with plan or attempt (n = 111) | |||
|---|---|---|---|---|---|---|
| n | % (SE) | % (SE) | % (SE) | % (SE) | % (SE) | |
| Total | 8.4 (0.8) | 4.9 (0.5) | 0.8 (0.2) | 2.7 (0.3) | ||
| Age | ||||||
| 50 years or more | 1730 | 37.0 (2.1) | 7.6 (0.6) | 4.7 (0.5) | 0.1 (0.0) | 2.9 (0.5) |
| 30–49 years | 2553 | 47.7 (1.1) | 7.5 (1.1) | 4.3 (0.6) | 1.0 (0.3) | 2.2 (0.3) |
| 18–29 years | 881 | 15.3 (1.6) | 12.7 (1.8) | 7.2 (1.3) | 2.1 (0.6) | 3.3 (0.6) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(2) = 12.59, p = .002* | R–S χ 2(2) = 8.63, p = .013* | F‐E p < .001* | R–S χ 2(2) = 2.66, p = .265 | ||
| Gender | ||||||
| Male | 992 | 22.7 (1.3) | 8.5 (1.0) | 3.1 (0.5) | 1.3 (0.5) | 4.1 (0.5) |
| Female | 4172 | 77.3 (1.3) | 8.3 (0.8) | 5.4 (0.6) | 0.7 (0.1) | 2.2 (0.3) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(1) = 0.11, p = .738 | R–S χ 2(1) = 10.73, p = .001* | F‐E p = .425 | R–S χ 2(1) = 10.82, p = .001* | ||
| Marital status | ||||||
| Single, divorced, or legally separated, or widowed | 2753 | 52.1 (2.0) | 10.7 (1.2) | 6.1 (1.0) | 1.3 (0.3) | 3.3 (0.4) |
| Married | 2411 | 47.9 (2.0) | 5.8 (0.3) | 3.6 (0.3) | 0.3 (0.1) | 1.9 (0.5) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(1) = 21.46, p < .001* | R–S χ 2(1) = 4.71, p = .030* | F‐E p < .001* | R–S χ 2(1) = 4.25, p = .039* | ||
| Having children in care | ||||||
| Children in care | 1974 | 38.7 (0.8) | 5.8 (0.7) | 3.5 (0.3) | 0.3 (0.1) | 2.0 (0.5) |
| No children in care | 3190 | 61.3 (0.8) | 10.0 (1.0) | 5.8 (0.7) | 1.1 (0.2) | 3.1 (0.4) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(1) = 15.79, p < .001* | R–S χ 2(1) = 10.20, p = .001* | F‐E p = .002* | R–S χ 2(1) = 2.57, p = .109 | ||
| Lifetime mental disorders before onset COVID‐19 outbreak | ||||||
| Lifetime mood disorder | 568 | 12.1 (0.7) | 22.3 (1.8) | 9.9 (1.9) | 2.8 (0.6) | 9.6 (1.3) |
| No lifetime mood disorder | 4596 | 87.9 (0.7) | 6.4 (0.6) | 4.2 (0.3) | 0.6 (0.1) | 1.7 (0.2) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(1) = 71.74, p < .001* | R–S χ 2(1) = 9.67, p = .002* | R–S χ 2(1) = 15.41, p < .001* | R–S χ 2(1) = 29.57, p < .001* | ||
| Lifetime anxiety disorder | 1893 | 37.9 (1.1) | 13.5 (1.3) | 7.2 (0.8) | 1.6 (0.4) | 4.8 (0.5) |
| No lifetime anxiety disorder | 3271 | 62.1 (1.1) | 5.2 (0.6) | 3.5 (0.4) | 0.4 (0.1) | 1.4 (0.3) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(1) = 50.69, p < .001* | R–S χ 2(1) = 31.63, p < .001* | R–S χ 2(1) = 9.38, p = .002* | R–S χ 2(1) = 25.02, p < .001* | ||
| Lifetime substance use disorder | 67 | 1.4 (0.2) | 9.6 (3.8) | 1.5 (1.4) | 0.0 (0.0) | 8.1 (3.0) |
| No lifetime substance use disorder | 5097 | 98.6 (0.2) | 8.3 (0.8) | 4.9 (0.5) | 0.8 (0.2) | 2.6 (0.3) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | F‐E p = .353 | F‐E p = .375 | – | F‐E p = .003* | ||
| Other lifetime mental disorder | 135 | 2.4 (0.3) | 15.1 (3.4) | 5.2 (2.2) | 0.5 (0.5) | 9.5 (3.1) |
| No other lifetime mental disorder | 5029 | 97.6 (0.3) | 8.2 (0.8) | 4.9 (0.5) | 0.8 (0.2) | 2.5 (0.3) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(1) = 3.22, p = .073 | R–S χ 2(1) = 0.02, p = .899 | F‐E p = 1.000 | R–S χ 2(1) = 4.22, p = .040* | ||
| Number of lifetime mental disorders | ||||||
| Two or more | 454 | 9.9 (0.8) | 25.1 (1.6) | 10.8 (2.3) | 3.2 (0.7) | 11.2 (2.1) |
| Exactly one | 1710 | 33.1 (1.1) | 9.2 (0.9) | 5.5 (0.6) | 0.9 (0.2) | 2.8 (0.4) |
| Zero | 3000 | 57.0 (1.2) | 4.9 (0.6) | 3.5 (0.4) | 0.4 (0.1) | 1.1 (0.3) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(2) = 118.39, p < .001* | R–S χ 2(2) = 16.09, p < .001* | R–S χ 2 (2) = 23.76, p < .001* | R–S χ 2(2) = 32.86, p < .001* | ||
| Profession | ||||||
| Medical doctor | 1372 | 20.8 (0.4) | 7.2 (1.7) | 4.0 (1.0) | 1.0 (0.4) | 2.2 (0.5) |
| Nurse | 1635 | 30.6 (0.6) | 8.3 (1.0) | 5.2 (0.5) | 0.8 (0.2) | 2.2 (0.5) |
| Auxiliary nurse | 681 | 18.8 (1.6) | 10.9 (1.8) | 5.9 (1.3) | 1.5 (0.6) | 3.5 (0.7) |
| Other profession involved in patient care | 568 | 12.8 (0.7) | 7.3 (0.9) | 3.6 (0.8) | 0.4 (0.3) | 3.3 (0.7) |
| Other profession not involved in patient care | 908 | 17.0 (1.6) | 7.9 (1.2) | 5.2 (1.0) | 0.2 (0.1) | 2.5 (0.6) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(4) = 5.48, p = .241 | R–S χ 2(4) = 4.16, p = .385 | F‐E p = .083 | R–S χ 2(4) = 4.20, p = .380 | ||
| Frequency of direct exposure to COVID‐19 patients | ||||||
| All of the time | 1478 | 29.3 (2.6) | 10.4 (0.7) | 6.6 (0.6) | 1.1 (0.3) | 2.6 (0.5) |
| Most of the time | 918 | 18.1 (1.0) | 9.8 (2.0) | 5.6 (1.5) | 1.0 (0.3) | 3.2 (0.5) |
| Some of the time | 1351 | 26.3 (1.3) | 7.9 (1.2) | 4.0 (0.4) | 1.0 (0.5) | 2.8 (0.6) |
| A little of the time | 707 | 13.6 (0.8) | 5.9 (1.3) | 3.6 (1.2) | 0.3 (0.2) | 2.0 (0.7) |
| None of the time | 710 | 12.8 (2.1) | 5.3 (0.7) | 3.0 (0.9) | 0.1 (0.1) | 2.3 (0.5) |
| Modified Rao–Scott (R–S) χ2 test or Fisher‐Exact (F‐E) test | R–S χ 2(4) = 13.38, p = .010* | R–S χ 2(4) = 10.86, p = .028* | F‐E p = .072 | F‐E p = .363 | ||
| Changes at work | ||||||
| Changed to specific COVID‐19‐related work location | 1679 | 31.9 (2.2) | 10.7 (1.1) | 6.6 (0.7) | 1.5 (0.2) | 2.7 (0.4) |
| Changed of team or assigned functionsb | 1316 | 25.5 (1.0) | 8.5 (1.2) | 4.9 (1.0) | 0.7 (0.2) | 2.9 (0.4) |
| No changes in assigned functions, team, or work location | 2169 | 42.6 (1.6) | 6.5 (0.6) | 3.6 (0.4) | 0.4 (0.2) | 2.5 (0.4) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(2) = 19.86, p < .001* | R–S χ 2(2) = 11.82, p = .003* | F‐E p < .001* | R–S χ 2(2) = 0.74, p = .692 | ||
| Frequency of working at home | ||||||
| Always | 195 | 2.5 (0.8) | 3.3 (1.0) | 1.9 (0.8) | 0.0 (0.0) | 1.5 (0.8) |
| Almost always | 158 | 2.3 (0.6) | 5.3 (2.1) | 3.8 (1.8) | 1.1 (1.2) | 0.5 (0.5) |
| Often | 266 | 4.6 (0.6) | 2.4 (1.1) | 2.0 (1.0) | 0.1 (0.1) | 0.2 (0.2) |
| Sometimes | 452 | 8.1 (1.1) | 7.0 (1.3) | 2.6 (0.7) | 0.9 (0.5) | 3.5 (0.9) |
| Seldom | 471 | 8.3 (0.7) | 7.4 (1.7) | 3.5 (0.6) | 1.0 (0.5) | 2.9 (1.3) |
| Never | 3622 | 74.2 (2.9) | 9.2 (1.0) | 5.6 (0.6) | 0.8 (0.2) | 2.8 (0.4) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(5) = 20.13, p = .001*, a | R–S χ 2(5) = 28.61, p < .001*, a | R–S χ 2(4) = 1.31, p = .859a | R–S χ 2(5) = 9.36, p = .095a | ||
| COVID‐19 infection history | ||||||
| Having been hospitalized for COVID‐19 | 55 | 1.3 (0.2) | 12.6 (4.8) | 4.5 (2.4) | 0.0 (0.0) | 8.1 (4.6) |
| Positive COVID‐19 test or medical COVID‐19 diagnosisc | 845 | 16.6 (2.0) | 10.0 (2.6) | 5.9 (1.8) | 1.0 (0.5) | 3.2 (0.6) |
| None of the above | 4264 | 82.0 (2.2) | 7.9 (0.6) | 4.7 (0.4) | 0.8 (0.2) | 2.5 (0.3) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | F‐E p = .058 | F‐E p = .556 | F‐E p = .789 | F‐E p = .004* | ||
| Isolation or quarantine because of COVID‐19 | ||||||
| Having been isolated or quarantined | 1258 | 23.3 (1.3) | 8.7 (1.7) | 4.6 (1.0) | 1.1 (0.4) | 2.9 (0.5) |
| Not having been isolated or quarantined | 3906 | 76.7 (1.3) | 8.3 (0.6) | 5.0 (0.5) | 0.7 (0.1) | 2.6 (0.2) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(1) = 0.08, p = .782 | R–S χ 2(1) = 0.13, p = .722 | F‐E p = .856 | R–S χ 2(1) = 0.44, p = .509 | ||
| Close ones infected with COVID‐19 | ||||||
| Partner, children, or parents | 730 | 14.4 (2.0) | 9.5 (1.9) | 5.5 (1.5) | 0.5 (0.2) | 3.5 (0.7) |
| Other family, friends, or othersd | 3175 | 60.3 (1.3) | 8.5 (0.7) | 4.8 (0.5) | 0.9 (0.2) | 2.8 (0.4) |
| None of the above | 1259 | 25.3 (1.9) | 7.3 (0.7) | 4.6 (0.5) | 0.8 (0.4) | 1.9 (0.3) |
| Modified Rao–Scott (R–S) χ 2 test or Fisher‐Exact (F‐E) test | R–S χ 2(2) = 2.59, p = .273 | R–S χ 2(2) = 0.53, p = .766 | F‐E p = .273 | R–S χ 2(2) = 3.91, p = .141 |
Abbreviation: COVID‐19, coronavirus disease 2019.
Statistically significant (α = .05).
a Fisher‐Exact test could not be estimated due to computational limitations; the modified Rao–Scott χ 2 test is shown.
The category “changed of team or assigned functions” excludes those that changed to a specific COVID‐19‐related work location.
The category “positive COVID‐19 test or medical COVID‐19 diagnosis” excludes those having been hospitalized for COVID‐19.
The category “other family, friends, or others” excludes having a partner, children, or parents infected with COVID‐19.
In multivariable analysis of individual characteristics (Table 2), STB remained strongly associated with pre‐existing mood and anxiety disorders (any STB OR range 2.12–3.22; plan or attempt OR range 2.62–4.64). Detailed analysis of age showed that odds for STB increased slightly between age 30 and 49, and declined for those aged 50 or more. Those with female gender, married or having children in care all had lower odds for any STB (OR range 0.62–0.80). Female gender and being married was also protective for plan or attempt (OR range = 0.40–0.68). No clear associations were found between STB and professional status. The frequency of direct exposure to COVID‐19 patients was positively associated with any STB (OR = 1.11), and passive suicidal ideation only (OR = 1.17). Those working more frequently at home had generally lower odds for STB (OR range 0.78–0.82). No significant associations were found with COVID‐19 infection history and with having been isolated or quarantined.
Table 2.
Multivariable associations between 30‐day suicidal thoughts and behaviors (STB) and individual characteristics (n = 5164)
| % (SE) or Med (SE) (IQR) | Any STB (n = 395) | Passive ideation only (n = 243) | Active ideation without plan or attempt (n = 41) | Active ideation with plan or attempt (n = 111) | ||
|---|---|---|---|---|---|---|
| n | OR (95% CI)a | OR (95% CI)a | OR (95% CI)a, b | OR (95% CI)a | ||
| Age | ||||||
| Spline 18–29 years | 0.92 (0.83–1.02) | 0.94 (0.84–1.05) | 0.84 (0.74–0.95)* | 0.95 (0.82–1.09) | ||
| Spline 30–49 years | 1.03 (1.00–1.06)* | 1.03 (0.99–1.07) | 1.02 (0.96–1.08) | 1.05 (1.01–1.10)* | ||
| Spline 50+ years | 0.93 (0.88–0.97)* | 0.94 (0.88–1.01) | 0.77 (0.62–0.95)* | 0.91 (0.85–0.97)* | ||
| Gender—female (vs. male) | 4167 | 77.3 (1.3) | 0.80 (0.67–0.97)* | 1.46 (1.05–2.04)* | 0.42 (0.23–0.78)* | 0.40 (0.28–0.58)* |
| Marital status—married (vs. single, divorced, or legally separated, or widowed) | 2411 | 47.9 (2.0) | 0.75 (0.59–0.95)* | 0.82 (0.54–1.24) | 0.69 (0.31–1.58) | 0.68 (0.46–0.99)* |
| Children in care (vs. no children in care) | 1974 | 38.7 (0.8) | 0.62 (0.43–0.89)* | 0.62 (0.42–0.91)* | 0.46 (0.20–1.06) | 0.63 (0.37–1.10) |
| Lifetime mood disorder before onset COVID‐19 outbreak | 568 | 12.1 (0.7) | 3.22 (2.67–3.89)* | 2.34 (1.65–3.30)* | 5.03 (2.69–9.38)* | 4.64 (3.00–7.18)* |
| Lifetime anxiety disorder before onset COVID‐19 outbreak | 1893 | 37.9 (1.1) | 2.12 (1.59–2.83)* | 1.82 (1.41–2.36)* | 2.64 (1.41–4.93)* | 2.62 (1.49–4.62)* |
| Lifetime substance use disorder before onset COVID‐19 outbreak | 67 | 1.4 (0.2) | 0.62 (0.30–1.30) | 0.21 (0.04–1.26) | 0.24 (0.02–2.97) | 1.34 (0.68–2.63) |
| Other lifetime mental disorder before onset COVID‐19 outbreak | 135 | 2.4 (0.3) | 1.63 (0.96–2.77) | 1.03 (0.38–2.80) | 0.56 (0.09–3.44) | 3.71 (1.90–7.23)* |
| Profession | ||||||
| Medical doctor | 1372 | 20.8 (0.4) | 0.78 (0.48–1.27) | 0.64 (0.37–1.13) | 2.60 (0.64–10.58) | 1.00 (0.43–2.31) |
| Nurse | 1635 | 30.6 (0.6) | 0.66 (0.43–1.00) | 0.54 (0.34–0.87)* | 1.94 (0.46–8.27) | 0.86 (0.45–1.65) |
| Auxiliary nurse | 681 | 18.8 (1.6) | 0.80 (0.56–1.14) | 0.59 (0.38–0.91)* | 3.78 (0.89–16.04) | 1.23 (0.62–2.46) |
| Other professions involved in patient care | 568 | 12.8 (0.7) | 0.67 (0.49–0.93)* | 0.54 (0.28–1.05) | 1.05 (0.22–5.10) | 1.01 (0.52–1.96) |
| Other profession not involved in patient care | 908 | 17.0 (1.6) | (ref) | (ref) | (ref) | (ref) |
| Frequency of direct exposure to COVID‐19 patients (scaled 0–4) | 1.9 (0.1) (0.9‐3.1) | 1.11 (1.01–1.23)* | 1.17 (1.04–1.31)* | 0.98 (0.73–1.30) | 1.04 (0.76–1.44) | |
| Changes at work | ||||||
| Changed to specific COVID‐19‐related work location | 1679 | 31.9 (2.2) | 1.27 (0.88–1.85) | 1.40 (0.92–2.12) | 1.68 (0.76–3.73) | 0.91 (0.48–1.75) |
| Changed of team or assigned functionsc | 1316 | 25.5 (1.0) | 1.17 (0.93–1.48) | 1.22 (0.85–1.75) | 1.24 (0.54–2.81) | 1.09 (0.67–1.76) |
| No changes in assigned functions, team or work location | 2169 | 42.6 (1.6) | (ref) | (ref) | (ref) | (ref) |
| Frequency of working at home (scaled 0–5) | 0.0 (0.2) (0.0–0.1) | 0.82 (0.75–0.90)* | 0.82 (0.70–0.96)* | 1.07 (0.80–1.44) | 0.78 (0.64–0.94)* | |
| COVID‐19 infection history | ||||||
| Having been hospitalized for COVID‐19 | 55 | 1.3 (0.2) | 1.43 (0.46–4.44) | 1.05 (0.33–3.38) | 1.37 (0.10–18.32) | 2.30 (0.71–7.42) |
| Positive COVID‐19 test or medical COVID‐19 diagnosisd | 845 | 16.6 (2.0) | 1.32 (0.84–2.07) | 1.44 (0.78–2.63) | 0.94 (0.40–2.20) | 1.24 (0.75–2.05) |
| None of the above | 4264 | 82.0 (2.2) | (ref) | (ref) | (ref) | (ref) |
| Having been isolated or quarantined because of COVID‐19 | 1258 | 23.3 (1.3) | 0.80 (0.63–1.01) | 0.71 (0.49‐1.04) | 1.30 (0.63–2.68) | 0.78 (0.50–1.23) |
| Close ones infected with COVID‐19 | ||||||
| Partner, children, or parents | 730 | 14.4 (2.0) | 1.32 (0.80–2.18) | 1.28 (0.65–2.51) | 0.68 (0.24–1.94) | 1.72 (0.80–3.68) |
| Other family, friends or otherse | 3175 | 60.3 (1.3) | 1.26 (0.94–1.69) | 1.15 (0.84–1.58) | 1.02 (0.51–2.01) | 1.60 (0.81–3.17) |
| None of the above | 1259 | 25.3 (1.9) | (ref) | (ref) | (ref) | (ref) |
Abbreviations: COVID‐19, coronavirus disease 2019; IQR, interquartile range.
Statistically significant (α = .05).
All analyses adjust for time of survey (weeks), hospital membership, and all predictors shown in the rows.
Due to data sparseness, the model was estimated using Penalized Maximum Likelihood Estimation (Firth‐type Estimation).
The category “changed of team or assigned functions” excludes those that changed to a specific COVID‐19‐related work location.
The category “positive COVID‐19 test or medical COVID‐19 diagnosis” excludes those having been hospitalized for COVID‐19.
e The category “other family, friends or others” excludes having a partner, children, or parents infected with COVID‐19.
Next, we analyzed each of the potentially modifiable contextual factors separately, each time adjusting for individual characteristics (Table 3). The perceived lack of coordination, communication, personnel, or supervision at work as well as the degree of financial stress were consistently associated with all 30‐day STB outcomes (OR range 1.18–1.87). Perceived frequency of lack of protective material (OR = 1.18), and significant loss in personal or familial income (OR = 1.35) were also associated with any STB, but only with passive ideation in the detailed analyses. Having to make decisions regarding prioritizing care among COVID‐19 patients was only associated with active suicidal ideation with plan or attempt (OR = 1.57).
Table 3.
Multivariable associations between 30‐day suicidal thoughts and behaviors (STB) and potentially modifiable contextual factors (n = 5164)
| Any STB (n = 395) | Passive ideation only (n = 243) | Active ideation without plan or attempt (n = 41) | Active ideation with plan or attempt (n = 111) | ||||
|---|---|---|---|---|---|---|---|
| n | % (SE) or Med (SE) (IQR) | OR (95% CI)a | OR (95% CI)a | OR (95% CI)a, b | OR (95% CI)a | ||
| Work‐related factors | Average weekly hours worked | ||||||
| 51 h or more | 775 | 13.4 (1.1) | 0.78 (0.49–1.25) | 0.80 (0.42–1.55) | 1.12 (0.50–2.50) | 0.61 (0.32–1.17) | |
| 41–50 h | 1354 | 25.6 (2.2) | 0.78 (0.71–0.84)* | 0.84 (0.72–0.99)* | 0.75 (0.38–1.45) | 0.61 (0.43–0.87)* | |
| 40 h or less | 3035 | 61.0 (3.0) | (ref) | (ref) | (ref) | (ref) | |
| Perceived lack of communication, coordination, personnel, or supervision (scaled 0.0–4.0)c | 1.9 (0.1) (1.0–2.5) | 1.48 (1.33–1.64)* | 1.36 (1.20–1.53)* | 1.87 (1.37–2.56)* | 1.67 (1.44–1.94)* | ||
| Perceived lack of coordination (scaled 0–4) | 1.6 (0.1) (0.8–2.4) | 1.36 (1.18–1.57)* | 1.27 (1.08–1.49)* | 1.53 (1.16–2.03)* | 1.54 (1.27–1.86)* | ||
| Perceived lack of communication (scaled 0–4) | 1.5 (0.1) (0.7–2.3) | 1.37 (1.22–1.53)* | 1.22 (1.07–1.40)* | 1.76 (1.35–2.31)* | 1.59 (1.30–1.94)* | ||
| Perceived lack of personnel (scaled 0–4) | 1.5 (0.2) (0.3–2.6) | 1.22 (1.11–1.33)* | 1.18 (1.10–1.27)* | 1.49 (1.16–1.91)* | 1.24 (1.02–1.50)* | ||
| Perceived lack of supervision (scaled 0–4) | 1.1 (0.1) (0.0–2.1) | 1.28 (1.22–1.34)* | 1.24 (1.13–1.36)* | 1.39 (1.11–1.75)* | 1.34 (1.21–1.49)* | ||
| Perceived frequency of lack of protective equipment (scaled 0–4) | 1.8 (0.1) (1.1–2.6) | 1.18 (1.05–1.34)* | 1.21 (1.07–1.36)* | 1.23 (0.92–1.64) | 1.08 (0.90–1.30) | ||
| Perceived inefficiency of protective equipment (scaled 0–3) | 0.3 (0.0) (0.0–1.1) | 1.06 (0.85–1.33) | 1.04 (0.79–1.38) | 1.05 (0.79–1.39) | 1.05 (0.80–1.37) | ||
| Having to make decisions regarding prioritizing care among COVID‐19 patients | 858 | 14.9 (1.3) | 1.18 (0.79–1.75) | 1.07 (0.67–1.70) | 0.96 (0.43–2.16) | 1.57 (1.07–2.32)* | |
| Having patient(s) in care that died from COVID‐19 | 1993 | 37.8 (2.7) | 1.07 (0.85–1.36) | 1.46 (0.79–2.71) | 0.99 (0.50–1.99) | 0.64 (0.40–1.02) | |
| Financial factors | Significant loss in personal or familial income | 1058 | 20.4 (0.9) | 1.32 (1.03–1.69)* | 1.43 (1.05–1.95)* | 0.97 (0.51–1.84) | 1.13 (0.65–1.95) |
| Financial stress (scaled 0.0–4.0)d,e | 0.7 (0.0) (0.0–1.7) | 1.39 (1.31–1.48)* | 1.32 (1.16–1.51)* | 1.14 (0.90–1.46) | 1.60 (1.28–2.00)* | ||
| Stress related to job or income loss due to COVID‐19 (scaled 0–4)e | 0.2 (0.0) (0.0–1.4) | 1.27 (1.18–1.37)* | 1.21 (1.04–1.42)* | 1.01 (0.81–1.25) | 1.44 (1.16–1.79)* | ||
| Stress related to financial situation (scaled 0–4)e | 0.6 (0.0) (0.0–1.6) | 1.38 (1.31–1.45)* | 1.32 (1.21–1.45)* | 1.27 (1.01–1.61)* | 1.52 (1.30–1.78)* |
Abbreviations: COVID‐19, coronavirus disease 2019; IQR, interquartile range.
Statistically significant (α = .05).
Each cell represents a separate regression model, each time adjusting for time of survey (weeks), hospital membership, and all individual characteristics (i.e., age, gender, marital status, having children in care, lifetime mental disorders before onset COVID‐19 outbreak, profession, frequency of direct exposure to COVID‐19 patients, changes in assigned functions, team or work location, frequency of working at home, COVID‐19 infection history, having been isolated or quarantined related to COVID‐19, and COVID‐19 infection of close ones).
Due to data sparseness, the model was estimated using Penalized Maximum Likelihood Estimation (Firth‐type Estimation).
The Perceived lack of communication, coordination, personnel, or supervision scale is created using the four 5‐level Likert‐type items shown in the rows below (see Section 2.2).
The Financial stress scale is created using the two 5‐level Likert‐type items shown in the rows below (see Section 2.2).
Likelihood ratio tests for linearity of effect were significant, and models including polynomials suggest a better model fit when including a quadratic term. For ease of interpretability, we present here the models including the linear term only; models including the quadratic term are presented in Table S5.
In multivariable models including the variables selected after lasso shrinkage (Table 4), 30‐day STB outcomes remained significantly associated with pre‐existing mood and anxiety disorders (OR range 1.63–5.05), the perceived lack of coordination, communication, personnel, or supervision at work (OR range 1.35–1.85), and the degree of financial stress (OR range 1.28–1.50), with OR generally being higher for active ideation, plan, or attempt.
Table 4.
Final multivariable models predicting 30‐day suicidal thoughts and behaviors (STB; n = 5164)
| % (SE) or Med (SE) (IQR) | Any STB (n = 395) | Passive ideation only (n = 243) | Active ideation without plan or attempt (n = 41) | Active ideation with plan or attempt (n = 111) | |||
|---|---|---|---|---|---|---|---|
| n | OR (95% CI)a | OR (95% CI)a | OR (95% CI)a, b | OR (95% CI)a | |||
| Individual characteristics | Age | ||||||
| Spline 18‐29 years | 0.91 (0.82–1.01) | 0.94 (0.84–1.04) | 0.81 (0.71–0.92)* | 0.94 (0.82–1.07) | |||
| Spline 30‐49 years | 1.04 (1.01–1.07)* | 1.03 (0.99–1.08) | 1.02 (0.96–1.08) | 1.06 (1.03–1.10)* | |||
| Spline 50+ years | 0.93 (0.89–0.98)* | 0.95 (0.88–1.03) | 0.76 (0.61–0.96)* | 0.92 (0.86–0.98)* | |||
| Gender—female (vs. male) | 4167 | 77.3 (1.3) | 0.82 (0.69–0.98)* | 1.52 (1.04–2.22)* | 0.45 (0.24–0.83)* | 0.38 (0.29–0.51)* | |
| Marital status—married (vs. single, divorced, or legally separated, or widowed) | 2411 | 47.9 (2.0) | 0.73 (0.55–0.96)* | 0.78 (0.48–1.25) | 0.71 (0.29–1.74) | 0.65 (0.44–0.96)* | |
| Children in care (vs. no children in care) | 1974 | 38.7 (0.8) | 0.61 (0.42–0.89)* | 0.60 (0.41–0.87)* | 0.56 (0.23–1.38) | 0.61 (0.35–1.06) | |
| Lifetime mood disorder before onset COVID‐19 outbreak | 568 | 12.1 (0.7) | 2.92 (2.40–3.54)* | 2.09 (1.46–2.99)* | 5.05 (2.68–9.52)* | 4.21 (2.70–6.58)* | |
| Lifetime anxiety disorder before onset COVID‐19 outbreak | 1893 | 37.9 (1.1) | 1.90 (1.40–2.58)* | 1.63 (1.24–2.14)* | 2.24 (1.17–4.29)* | 2.39 (1.30–4.40)* | |
| Frequency of direct exposure to COVID‐19 patients (scaled 0–4) | 1.9 (0.1) (0.9–3.1) | 1.05 (0.95–1.16) | 1.10 (1.02–1.19)* | 1.07 (0.82–1.39) | 0.95 (0.71–1.25) | ||
| Frequency of working at home (scaled 0–5) | 0.0 (0.2) (0.0–0.1) | 0.84 (0.73–0.96)* | 0.84 (0.75–0.95)* | 0.94 (0.69–1.26) | 0.78 (0.60–1.00)* | ||
| Potentially modifiable contextual factors | Perceived lack of communication, coordination, personnel, or supervision (scaled 0.0–4.0) | 1.9 (0.1) (1.0–2.5) | 1.44 (1.27–1.63)* | 1.35 (1.13–1.61)* | 1.85 (1.34–2.56)* | 1.59 (1.34–1.90)* | |
| Perceived inefficiency of protective equipment (scaled 0–3) | 0.3 (0.0) (0.0–1.1) | 0.89 (0.73–1.08) | 0.90 (0.71–1.15) | 0.86 (0.64–1.15) | 0.83 (0.62–1.11) | ||
| Financial stress (scaled 0.0–4.0)c | 0.7 (0.0) (0.0–1.7) | 1.34 (1.26–1.43)* | 1.28 (1.14–1.44)* | 1.10 (0.86–1.41) | 1.50 (1.24–1.83)* |
Note: Individual characteristics and potentially modifiable contextual factors included in the final multivariable models are selected using the lasso shrinkage method, optimizing the Bayesian Information Criterion.
Abbreviations: COVID‐19, coronavirus disease 2019; IQR, interquartile range.
Statistically significant (α = .05).
All analyses adjust time of survey (weeks), hospital membership, and all predictors shown in the rows.
Due to data sparseness, the model was estimated using Penalized Maximum Likelihood Estimation (Firth‐type Estimation).
Likelihood ratio tests for linearity of effect were significant, and models including polynomials suggest a better model fit when including a quadratic term. For ease of interpretability, we present here the models including the linear term only; models including the quadratic term are presented in Table S6.
Approximately 50.5% of 30‐day STB was potentially attributable to a perceived lack of coordination, communication, personnel, or supervision at work (Figure 1), and approximately 44.1% of STB was potentially attributable to financial stress. Interventions that completely eliminate both these risk factors could potentially eliminate 73.3% of any 30‐day STB (61.5%–61.6% of 30‐day passive or active ideation only, and 81.3% of active ideation with plan or attempt). PARP for pre‐existing mental disorders were 35.8% for any 30‐day STB, 24.8% for passive ideation only, and 47.7%–49.5% for active ideation, plan, or attempt. Additionally eliminating pre‐existing mental disorders could increase reductions in 30‐day STB to 83.5% (71.2% for passive ideation only, and 80.1%–90.4% for active ideation, plan, or attempt).
Figure 1.
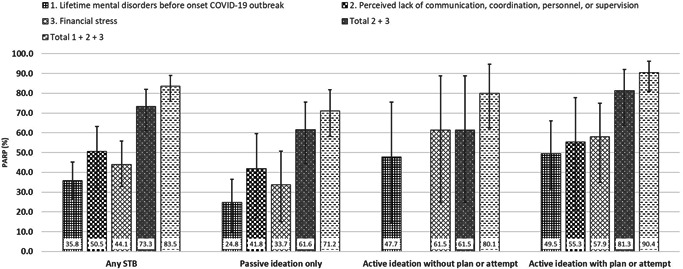
Multivariable PARPs (All analyses adjust for time of survey [weeks], hospital membership, age, gender, marital status, having children in care, frequency of direct exposure to COVID‐19 patients, frequency of working at home, perceived inefficiency of protective equipment, and the predictors shown in the figure.) for 30‐day suicidal thoughts and behaviors (STB; n = 5164). Individual characteristics and potentially modifiable contextual factors included in the final multivariable models are selected using the lasso shrinkage method, optimizing the Bayesian Information Criterion. Variables included for PARP analysis are potentially modifiable contextual factors, and lifetime mental disorders before the onset of the COVID‐19 outbreak; only PARPs that are statistically different from zero are shown. See Tables S7 and S8 for more details on PARP analysis. PARP of individual risk factors do not sum to 100% because of the multifactorial etiology of STB, that is, one risk factor can be part of multiple risk factor constellations leading to STB. COVID‐19, coronavirus disease 2019; PARP, population‐attributable risk proportion; STB, suicidal thoughts and behavior
4. DISCUSSION
Approximately 8.4% of hospital workers had 30‐day STB during the first wave of the Spain COVID‐19 pandemic. Of those, about 4 out of 10 had active suicidal ideation (3.5% in total), including 6 hospital workers with a 30‐day suicide attempt. Substantial proportions of 30‐day STB were associated with perceived lack in communication, coordination, personnel, or supervision (50.5%) and with financial stress (44.1%).
A major strength of this study is the large probability sample of hospital workers, opposed to the high amount of small samples used in mental health research among healthcare professionals during virus outbreaks (median n = 333 [IQR 131–769]; Kisely et al., 2020). This enabled us to provide detailed analyses of all presumed precursor states in the suicidal spectrum (Ribeiro et al., 2016). Despite the large sample size, few cases of 30‐day suicide attempt were included, precluding detailed analysis of this outcome. An important limitation of our study is the low survey response rate (11.8%), in line with the low and declining survey response rates among healthcare professionals worldwide (Cho et al., 2013). While nonresponse bias may affect prevalence estimates in either direction, the expected bias in associations is substantially lower (Amaya & Presser, 2016). We adjusted all estimates for potential nonresponse bias using poststratification weights. Differences in poststratifying variables between our sample and the census population were small, and COVID‐19 infection rates in our sample were very similar to official prevalence estimates among healthcare professionals. Two other limitations of this study are worth mentioning: (1) only those hospital workers with institutional email addresses were eligible, which excludes hospital workers with jobs that are potentially subcontracted to external services (e.g., food and cleaning services), and those who lost their job by time of survey invitation; and (2) although we adjusted all analyses for time of survey, we did not investigate how potentially modifiable contextual factors (e.g., hospital preparedness) changed over time. To optimize intervention planning efforts, future research should provide more qualitative insight into these contextual factors (e.g., operationalizing hospital preparedness with clear definitions), and investigate changes over time in these factors in response to viral outbreaks.
To our knowledge, we present the first study that estimated STB among hospital workers during a virus outbreak. Over the past century, Spain consistently had relatively low rates of suicide (range 3.5–9.0/100,000 for the years 1906–2016; Alfonso‐Sánchez et al., 2020), with 12‐month suicidal ideation and plans among Spanish adults also being consistently low, that is, 0.7%–0.9%, and 0.2%–0.4%, respectively (Bernal et al., 2007; Kovess‐Masfety et al., 2011; Miret et al., 2014). Against this, we found a 30‐day prevalence of 3.5% for active ideation, plans, or attempts, suggesting that STB among hospital workers during the COVID‐19 outbreak is at least three times higher—but likely much higher—than in the general Spanish population before the COVID‐19 outbreak. One previous study among 375 neurosurgeons recruited through snowball sampling found a prevalence of 5.1% of active suicidal ideation during the COVID‐19 pandemic (Sharif et al., 2020), somewhat higher than our estimate (3.5%). No previous studies report on STB prevalence among Spanish hospital or healthcare workers under normal working conditions. Few previous studies outside of Spain report on suicidal ideation among physicians under normal working conditions, and found 12‐month estimates in the range 2.6%–14.3% (Fridner et al., 2009; Hem et al., 2000), and 30‐day estimates of 4.3% (Brooks et al., 2018; Loas et al., 2018). However, these studies were restricted to female physicians or report data from countries with higher suicide rates (Sweden, Norway, Belgium) than Spain.
To the extent that STB among hospital workers during the COVID‐19 outbreak was effectively increased, and given the associations between STB and future suicide (Ribeiro et al., 2016), our study may warn for an expected increase in suicides among Spanish hospital workers. We identified three important STB correlates that provide empirical evidence for suicide intervention preventions.
First, a large proportion of 30‐day STB (50.5%) was associated with a perceived inadequate response to the COVID‐19 outbreak in terms of coordination, supervision, communication, and available healthcare personnel. To the extent that this perception reflects the objective hospitals' response, this finding underscores the need of increasing hospital preparedness for virus outbreaks (European Centre for Disease Prevention and Control, 2020). But this finding also highlights the importance of societal efforts to reduce the spread of the virus to avoid healthcare systems being overwhelmed, and to avoid adverse mental health among hospital workers facing a sudden lack of hospital preparedness. Second, considerable proportions of 30‐day STB (44.1%) were associated with financial stress, including stress related to potential income or job loss due to the COVID‐19 virus pandemic. Spain was among those countries hit hard by the 2007–2008 economic crisis, with subsequent cuts in healthcare expenditure (Thomson et al., 2013). This may exacerbate fears among Spanish hospital workers related to the future economic impact of the COVID‐19 pandemic, and points to the need of increasing financial security for hospital workers active during the COVID‐19 outbreak. Third, our results suggest that those hospital workers with a history of mental disorder are at increased risk for STB during high‐stress periods such as a virus outbreak, in line with a stress‐diathesis etiological model of suicide (van Heeringen & Mann, 2014). Importantly, a recent meta‐analysis documented an absolute lack of research on effective interventions for mental disorders and STB among healthcare personnel, both on the individual‐ and on the organizational level (Petrie et al., 2019). Our study points to the need for adequate mental health support for hospital workers active during virus outbreaks (Vieta et al., 2020). When suicidal ideation is present, suicide prevention guidelines recommend additional support for healthcare workers such as crisis helplines or online interventions (Gunnell et al., 2020; Moutier, 2020; Wasserman et al., 2020), which should take into account specific experiences such as moral injury and COVID‐19‐related traumatic experiences.
5. CONCLUSION
This study provides the first empirical evidence for a potential increase of STB among hospital workers during virus outbreaks, and suggests important associations with a lack in hospital preparedness, financial stress, and pre‐existing adverse mental health. Future research should confirm our findings by including a non‐pandemic control condition, and by using a prospective study design including objective markers of hospital functioning and hospital workers' financial situation. The COVID‐19 pandemic has revealed how dependent we are as a society on a well‐functioning healthcare system. Improving future mental health and promoting fair financial and working conditions among hospital workers should therefore be an absolute priority.
The MINDCOVID Working Group is formed by
Itxaso Alayo, Jordi Alonso, Manuel Alonso, Mar Álvarez‐Villalba, Benedikt Amann, Franco F. Amigo, Gerard Anmella, Andrés Aragón, Núria Aragonès, Enric Aragonès, Ana Isabel Arizón, Angel Asunsolo, Alfons Ayora, Laura Ballester, Puri Barbas, Josep Basora, Elena Bereciartua, Inés Bravo, Ignasi Bolíbar, Xavier Bonfill, Ronny Bruffaerts, Alberto Cotillas‐Rodero, Paula Cristóbal‐Narváez, Andrés Cuartero, Concha de Paz, Isabel del Cura‐González, Maria Jesús del Yerro, Joke De Vocht, Domingo Díaz, Joan Domènech‐Abella, José Luís Domingo, José I. Emparanza, Mireia Espallargues, Meritxell Espuga, Patricia Estevan‐Burdeus, Mireia Félez‐Nobrega, M. Isabel Fernández, Tania Fernández, Montse Ferrer, Yolanda Ferreres, Giovanna Fico, María João Forjaz, Rosa García‐Barranco, Carles García‐Ribera, J. Manuel García‐Torrecillas, Araceli Garrido‐Barral, Elisa Gil, María Giola‐Insigna, Marta Gómez, Javier Gómez, Ana González‐Pinto, Josep Maria Haro, Margarita Hernando, Milagros Iriberri, Leontien Jansen, Núria Jiménez, Xavi Jiménez, Ronald C. Kessler, Amparo Larrauri, Fernando León‐Vázquez, Mayte López‐Atanes, Nieves López‐Fresneña, Carmen López‐Rodríguez, Juan A. López‐Rodríguez, Germán López‐Cortacans, Alba Marcos, Jesús Martín, Vicente Martín, Mercedes Martínez‐Cortés, Raquel Martínez‐Martínez, Alma D. Martínez de Salázar, Isabel Martínez, Marco Marzola, Nelva Mata, Josep María Molina, Juan D. Molina, Emilia Molinero, Philippe Mortier, Carmen Muñoz‐Ruipérez, Andrea Murru, Lydia Navarro, Beatriz Olaya, Jorge Olmedo‐Galindo, Rafael M. Ortí‐Lucas, Rafael Padrós, Meritxell Pallejà, Raúl Parra, Julio Pascual, José María Pelayo‐Terán, Rosa Pla, Nieves Plana, Coro Pérez‐Aznar, Beatriz Pérez‐Gómez, Víctor Pérez‐Solà, Aurora Pérez‐Zapata, José Ignacio Pijoan, Elena Polentinos‐Castro, Beatriz Puértolas, María Teresa Puig, Álex Quílez, María Jesús Quintana, Antonio Quiroga, David Rentero, Cristina Rey, Cristina Rius, Carmen Rodríguez‐Blázquez, M. José Rojas‐Giraldo, Yamina Romero‐Barzola, Gabriel Rubio, Pedro Ruiz, Mercedes Rumayor, Margarita Sáenz, Jesús Sánchez, Ignacio Sánchez‐Arcilla, Ferran Sanz, Consol Serra, Victòria Serra‐Sutton, Manuela Serrano, Sílvia Solà, Sara Solera, Miguel Soto, Alejandra Tarragó, Natividad Tolosa, Mireia Vázquez, Margarita Viciola, Eduard Vieta, Gemma Vilagut, Wouter Voorspoels, Sara Yago‐González, Jesús Yáñez‐Sánchez, Yolanda Zapico, Luís María Zorita, Iñaki Zorrilla, and Saioa L. Zurbano.
CONFLICT OF INTEREST STATEMENT
Eduard Vieta reports personal fees from Abbott, personal fees from Allergan, personal fees from Angelini, grants from Novartis, grants from Ferrer, grants and personal fees from Janssen, personal fees from Lundbeck, personal fees from Sage, personal fees from Sanofi, outside the submitted work. Juan D. Molina reports personal fees from Janssen, personal fees and nonfinancial support from Otsuka, personal fees and nonfinancial support from Lundbeck, personal fees from Angelini, personal fees and nonfinancial support from Accord, outside the submitted work. In the past 3 years, Ronald C. Kessler was a consultant for Datastat, Inc., Sage Pharmaceuticals, and Takeda. All other authors reported no conflict of interest.
AUTHOR CONTRIBUTIONS
Philippe Mortier, Gemma Vilagut, and Jordi Alonso reviewed the literature. Philippe Mortier, Gemma Vilagut, Montse Ferrer, Jordi Alonso, Enric Aragonès, Víctor Pérez‐Solà, Josep M. Haro, Ronald C. Kessler, and Ronny Bruffaerts conceived and designed the study. Consol Serra, Juan D. Molina, Nieves López‐Fresneña, Teresa Puig, José M. Pelayo‐Terán, José I. Pijoan, José I. Emparanza, Meritxell Espuga, Nieves Plana, Ana González‐Pinto, Consol Serra, Enric Aragonès, Isabel del Cura‐González, Andrés Aragón‐Peña, Mireia Campos, Aurora Pérez‐Zapata, Eduard Vieta, and Víctor Pérez‐Solà acquired the data. Philippe Mortier and Gemma Vilagut cleaned and analyzed the data. Philippe Mortier, Gemma Vilagut, and Jordi Alonso drafted the initial version of the manuscript. All authors reviewed the initial draft and made critical contributions to the interpretation of the data and approved the manuscript. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors would like to sincerely thank Puri Barbas and Franco Amigo for the management of the project as well as all hospital workers that participated in the study. This study was supported by Fondo de Investigación Sanitaria, Instituto de Salud Carlos III (Ministerio de Ciencia e Innovación)/FEDER (COV20/00711); ISCIII (Sara Borrell, CD18/00049) (PM); FPU (FPU15/05728) (LB); ISCIII (PFIS, FI18/00012) (BP); Generalitat de Catalunya (2017SGR452).
Mortier P, Vilagut G, Ferrer M, et al. Thirty‐day suicidal thoughts and behaviors among hospital workers during the first wave of the Spain COVID‐19 outbreak. Depression Anxiety. 2020;38:528–544. 10.1002/da.23129
Contributor Information
Philippe Mortier, Email: pmortier@imim.es.
Jordi Alonso, Email: jalonso@imim.es.
DATA AVAILABILITY STATEMENT
The de‐identified participant data as well as the study protocol and statistical analysis plan used for this study are available upon reasonable request from the corresponding authors (Philippe Mortier and Jordi Alonso) as long as the main objective of the data sharing request is replicating the analysis and findings as reported in this paper.
REFERENCES
- Alfonso‐Sánchez, J. L. , Martin‐Moreno, J. M. , Martinez, I. M. , & Martinez, A. A. (2020). Epidemiological study and cost analysis of suicide in Spain: Over 100 years of evolution. Archives of Suicide Research, 24(suppl 2), S356–S369. 10.1080/13811118.2019.1612802 [DOI] [PubMed] [Google Scholar]
- Amaya, A. , & Presser, S. (2016). Nonresponse bias for univariate and multivariate estimates of social activities and roles. Public Opinion Quarterly, 81(1), nfw037. 10.1093/poq/nfw037 [DOI] [Google Scholar]
- Arango, C. (2020). Lessons learned from the coronavirus health crisis in Madrid, Spain: How COVID‐19 has changed our lives in the last 2 weeks. Biological Psychiatry, 88(7), e33–e34. 10.1016/j.biopsych.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal, M. , Haro, J. M. , Bernert, S. , Brugha, T. , de Graaf, R. , Bruffaerts, R. , Lépine, J. P. , de Girolamo, G. , Vilagut, G. , Gasquet, I. , Torres, J. V. , Kovess, V. , Heider, D. , Neeleman, J. , Kessler, R. , & Alonso, J. (2007). Risk factors for suicidality in Europe: Results from the ESEMED study. Journal of Affective Disorders, 101(1–3), 27–34. 10.1016/j.jad.2006.09.018 [DOI] [PubMed] [Google Scholar]
- Brooks, E. , Gendel, M. H. , Early, S. R. , & Gundersen, D. C. (2018). When doctors struggle: Current stressors and evaluation recommendations for physicians contemplating suicide. Archives of Suicide Research, 22(4), 519–528. 10.1080/13811118.2017.1372827 [DOI] [PubMed] [Google Scholar]
- Cho, Y. I. , Johnson, T. P. , & VanGeest, J. B. (2013). Enhancing surveys of health care professionals. Evaluation & the Health Professions, 36(3), 382–407. 10.1177/0163278713496425 [DOI] [PubMed] [Google Scholar]
- Dohrenwend, B. S. , Krasnoff, L. , Askenasy, A. R. , & Dohrenwend, B. P. (1978). Exemplification of a method for scaling life events: the Peri Life Events Scale. Journal of Health and Social Behavior, 19(2), 205–229. 10.2307/2136536 [DOI] [PubMed] [Google Scholar]
- Dutheil, F. , Aubert, C. , Pereira, B. , Dambrun, M. , Moustafa, F. , Mermillod, M. , Baker, J. S. , Trousselard, M. , Lesage, F. X. , & Navel, V. (2019). Suicide among physicians and health‐care workers: A systematic review and meta‐analysis. PLOS One, 14(12), e0226361. 10.1371/journal.pone.0226361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control . (2020). Preparedness for COVID‐19. Retrieved from https://www.ecdc.europa.eu/en/covid-19/preparedness-and-response
- Eysenbach, G. (2004). Improving the quality of web surveys: The Checklist for Reporting Results of Internet E‐Surveys (CHERRIES). Journal of Medical Internet Research, 6(3), e34. 10.2196/jmir.6.3.e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, J. C. , Ribeiro, J. D. , Fox, K. R. , Bentley, K. H. , Kleiman, E. M. , Huang, X. , Musacchio, K. M. , Jaroszewski, A. C. , Chang, B. P. , & Nock, M. K. (2017). Risk factors for suicidal thoughts and behaviors: A meta‐analysis of 50 years of research. Psychological Bulletin, 143(2), 187–232. 10.1037/bul0000084 [DOI] [PubMed] [Google Scholar]
- Fridner, A. , Belkic, K. , Marini, M. , Minucci, D. , Pavan, L. , & Schenck‐Gustafsson, K. (2009). Survey on recent suicidal ideation among female university hospital physicians in Sweden and Italy (the HOUPE study): Cross‐sectional associations with work stressors. Gender Medicine, 6(1), 314–328. 10.1016/j.genm.2009.04.006 [DOI] [PubMed] [Google Scholar]
- Gunnell, D. , Appleby, L. , Arensman, E. , Hawton, K. , John, A. , Kapur, N. , … Yip, P. S. (2020). Suicide risk and prevention during the COVID‐19 pandemic. The Lancet Psychiatry, 7(6), 468–471. 10.1016/S2215-0366(20)30171-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie, T. , Tibshirani, R. , & Friedman, J. (2009). Springer series in statistics. The elements of statistical learning—Data mining, inference, and prediction. Springer. 10.1007/b94608 [DOI] [Google Scholar]
- Hawton, K. , Agerbo, E. , Simkin, S. , Platt, B. , & Mellanby, R. J. (2011). Risk of suicide in medical and related occupational groups: A national study based on Danish case population‐based registers. Journal of Affective Disorders, 134(1–3), 320–326. 10.1016/j.jad.2011.05.044 [DOI] [PubMed] [Google Scholar]
- Hem, E. , Grønvold, N. , Aasland, O. , & Ekeberg, Ø. (2000). The prevalence of suicidal ideation and suicidal attempts among Norwegian physicians. Results from a cross‐sectional survey of a nationwide sample. European Psychiatry, 15(3), 183–189. 10.1016/S0924-9338(00)00227-3 [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , & Üstün, T. B. (2004). The World Mental Health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). International Journal of Methods in Psychiatric Research, 13(2), 93–121. 10.1002/mpr.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisely, S. , Warren, N. , McMahon, L. , Dalais, C. , Henry, I. , & Siskind, D. (2020). Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta‐analysis. BMJ, 369, m1642. 10.1136/bmj.m1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovess‐Masfety, V. , Boyd, A. , Haro, J. M. , Bruffaerts, R. , Villagut, G. , Lépine, J. P. , Gasquet, I. , & Alonso, J. (2011). High and low suicidality in Europe: A fine‐grained comparison of France and Spain within the ESEMeD surveys. Journal of Affective Disorders, 133(1–2), 247–256. 10.1016/j.jad.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysinska, K. , & Martin, G. (2009). The struggle to prevent and evaluate: Application of population attributable risk and preventive fraction to suicide prevention research. Suicide and Life‐Threatening Behavior, 39(5), 548–557. 10.1521/suli.2009.39.5.548 [DOI] [PubMed] [Google Scholar]
- Litz, B. T. , Stein, N. , Delaney, E. , Lebowitz, L. , Nash, W. P. , Silva, C. , & Maguen, S. (2009). Moral injury and moral repair in war veterans: A preliminary model and intervention strategy. Clinical Psychology Review, 29(8), 695–706. 10.1016/j.cpr.2009.07.003 [DOI] [PubMed] [Google Scholar]
- Loas, G. , Lefebvre, G. , Rotsaert, M. , & Englert, Y. (2018). Relationships between anhedonia, suicidal ideation and suicide attempts in a large sample of physicians. PLOS One, 13(3), 1–23. 10.1371/journal.pone.0193619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIND/COVID‐19: Mental health Impact and NeeDs associated with COVID‐19: A comprehensive national evaluation in Spain (COV20/00711) . (2020). Retrieved from http://eu-isciii.es/covidfundinspain/mind-covid-19-mental-health-impact-and-needs-associated-with-covid-19-a-comprehensive-national-evaluation-in-spain/
- Miret, M. , Caballero, F. F. , Huerta‐Ramírez, R. , Moneta, M. V. , Olaya, B. , Chatterji, S. , Haro, J. M. , & Ayuso‐Mateos, J. L. (2014). Factors associated with suicidal ideation and attempts in Spain for different age groups. Prevalence before and after the onset of the economic crisis. Journal of Affective Disorders, 163, 1–9. 10.1016/j.jad.2014.03.045 [DOI] [PubMed] [Google Scholar]
- Moutier Christine (2020). Suicide Prevention in the COVID‐19 Era. JAMA Psychiatry. 10.1001/jamapsychiatry.2020.3746 [DOI] [PubMed] [Google Scholar]
- Muller, A. E. , Hafstad, E. V. , Himmels, J. P. W. , Smedslund, G. , Flottorp, S. , Stensland, S. Ø. , Stroobants, S. , Van de Velde, S. , & Vist, G. E. (2020). The mental health impact of the covid‐19 pandemic on healthcare workers, and interventions to help them: A rapid systematic review. Psychiatry Research, 293(August), 113441. 10.1016/j.psychres.2020.113441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock, M. K. , Borges, G. , & Ono, Y. (Eds.). (2012). Suicide: Global perspectives from the WHO World Mental Health Surveys. Cambridge University Press.
- Nock, M. K. , Stein, M. B. , Heeringa, S. G. , Ursano, R. J. , Colpe, L. J. , Fullerton, C. S. , Hwang, I. , Naifeh, J. A. , Sampson, N. A. , Schoenbaum, M. , Zaslavsky, A. M. , & Kessler, R. C. (2014). Prevalence and correlates of suicidal behavior among soldiers. JAMA Psychiatry, 71(5), 514. 10.1001/jamapsychiatry.2014.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa, S. , Ntella, V. , Giannakas, T. , Giannakoulis, V. G. , Papoutsi, E. , & Katsaounou, P. (2020). Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID‐19 pandemic: A systematic review and meta‐analysis. Brain, Behavior, and Immunity, 88(January), 901–907. 10.1016/j.bbi.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie, K. , Crawford, J. , Baker, S. T. E. , Dean, K. , Robinson, J. , Veness, B. G. , Randall, J. , McGorry, P. , Christensen, H. , & Harvey, S. B. (2019). Interventions to reduce symptoms of common mental disorders and suicidal ideation in physicians: A systematic review and meta‐analysis. The Lancet Psychiatry, 6(3), 225–234. 10.1016/S2215-0366(18)30509-1 [DOI] [PubMed] [Google Scholar]
- Posner, K. , Brown, G. K. , Stanley, B. , Brent, D. A. , Yershova, K. V. , Oquendo, M. A. , Currier, G. W. , Melvin, G. A. , Greenhill, L. , Shen, S. , & Mann, J. J. (2011). The Columbia—Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. American Journal of Psychiatry, 168(12), 1266–1277. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red Nacional de Vigilancia Epidemiólogica (RENAVE). Ministerio de Sanidad, España . (2020). Retrieved from https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/home.htm
- Ribeiro, J. D. , Franklin, J. C. , Fox, K. R. , Bentley, K. H. , Kleiman, E. M. , Chang, B. P. , & Nock, M. K. (2016). Self‐injurious thoughts and behaviors as risk factors for future suicide ideation, attempts, and death: A meta‐analysis of longitudinal studies. Psychological Medicine, 46(2), 225–236. 10.1017/S0033291715001804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roser, M. , Ritchie, H. , Ortiz‐Ospina, E. , & Hasell, J. (2020). Coronavirus Pandemic (COVID‐19). Retrieved from https://ourworldindata.org/coronavirus
- Salazar de Pablo, G. , Vaquerizo‐Serrano, J. , Catalan, A. , Arango, C. , Moreno, C. , Ferre, F. , Shin, J. I. , Sullivan, S. , Brondino, N. , Solmi, M. , & Fusar‐Poli, P. (2020). Impact of coronavirus syndromes on physical and mental health of health care workers: Systematic review and meta‐analysis. Journal of Affective Disorders, 275, 48–57. 10.1016/j.jad.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif, S. , Amin, F. , Hafiz, M. , Benzel, E. , Peev, N. , & Dahlan, R. H. , … World Spinal Column Society Executive Board . (2020). COVID 19—Depression and Neurosurgeons. World Neurosurgery, 21(1), 1–9. 10.1016/j.wneu.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson, S. , Figueras, J. , Evetovits, T. , Jowett, M. , Mladovsky, P. , Maresso, A. , … Kluge, H. (2013). Economic Crisis, Health Systems and Health in Europe Impact and implications for policy. European Observatory on Health Systems and Policies Series IV–16. [PubMed] [Google Scholar]
- Tyssen, R. , Vaglum, P. , Grønvold, N. T. , & Ekeberg, Ø. (2001). Suicidal ideation among medical students and young physicians: a nationwide and prospective study of prevalence and predictors. Journal of Affective Disorders, 64(1), 69–79. 10.1016/S0165-0327(00)00205-6 [DOI] [PubMed] [Google Scholar]
- van Buuren, S. (2012). Flexible imputation of missing data. Chapman & Hall. 10.1201/b11826 [DOI] [Google Scholar]
- van Heeringen, K. , & Mann, J. J. (2014). The neurobiology of suicide. The Lancet Psychiatry, 1(1), 63–72. 10.1016/S2215-0366(14)70220-2 [DOI] [PubMed] [Google Scholar]
- Vieta, E. , Pérez, V. , & Arango, C. (2020). Psychiatry in the aftermath of COVID‐19. Revista de Psiquiatría y Salud Mental, 13(2), 105–110. 10.1016/j.rpsm.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindegaard, N. , & Benros, M. E. (2020). COVID‐19 pandemic and mental health consequences: Systematic review of the current evidence. Brain, Behavior, and Immunity, 89, 531–542. 10.1016/j.bbi.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman, D. , Iosue, M. , Wuestefeld, A. , & Carli, V. (2020). Adaptation of evidence‐based suicide prevention strategies during and after the COVID‐19 pandemic. World Psychiatry, 19(3), 294–306. 10.1002/wps.20801 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The de‐identified participant data as well as the study protocol and statistical analysis plan used for this study are available upon reasonable request from the corresponding authors (Philippe Mortier and Jordi Alonso) as long as the main objective of the data sharing request is replicating the analysis and findings as reported in this paper.


