Abstract
Background
Dermal fillers have become an integral part of any aesthetic physician's intervention.
Aims
To assess, by means of ultrasounds, the tissue integration of the hyaluronic acid (HA) dermal filler VYC‐25L in chin and jaw.
Methods
Prospective, noncomparative, open‐label, and multicenter study conducted on healthy subjects, with age comprised between 30 and 60 years old, who attended to the clinic to perform a facial rejuvenation treatment of the lower third of the face. VYC‐25L was injected using a 27G needle (supraperiosteal bolus, from 0.2 to 0.3 mL per bolus) in the chin and with canula (retrograde threads, from 0.4 to 0.6 mL) in the jaw. Ultrasound examinations (UE) were performed at each study center by the same experienced observer at baseline, immediately after injection, 48 hours, and 30 days after treatment.
Results
Thirty patients (10 per center) were included in the study. At baseline, UE found a characteristic heterogeneous pattern of subcutaneous cellular tissue, with alternation of soft anechoic and hyperechoic images. The UE, performed immediately after treatment, showed a poorly defined globular ultrasound pattern, with anechoic images indicative of liquid content. Forty‐eight hours after treatment, UE are still showing a globular pattern, with well‐defined anechoic areas. Thirty days after treatment, a thickening of the subcutaneous cellular tissue was observed in all the evaluated zones, with a total integration of the HA into the tissue.
Conclusion
VYC‐25L might represent a significant advance in volumization/restoration of the lower face. Its biointegration was total at day 30 and practically complete at 48 hours of treatment.
Keywords: biointegration, hyaluronic acid, lowerface, minimally invasive aesthetic procedures, ultrasound
1. INTRODUCTION
Dermal fillers have become an integral part of any aesthetic physician's intervention. 1 , 2 In fact, minimally invasive aesthetic procedures, such as fillers, increased by 10.4%, significantly more than aesthetic operations, which showed a slight decrease of 0.6%. 1 Over the past several years, hyaluronic acid (HA) injectable dermal fillers have become the most popular agents for soft tissue contouring and volumizing. 1 , 2 , 3
Due to its physicochemical properties, HA is one of the most hygroscopic molecules in nature and hydrated HA can contain up to 1000‐fold more water than its own weight. 4
Different manufacturing related factors, including HA concentration; polymer chain length; crosslinking degree; or crosslinking technology are going to influence significantly on different filler properties, such as requisite needle size; particle size; duration; extrusion force; and elastic Modulus (G'), which will critically influence product selection and indication. 5 , 6
Among the different aforementioned factors, crosslinking is essential to slow down the enzymatic degradation rate of the HA by endogenous hyaluronidase and therefore to prolong the product's half‐life. 6 , 7 However, the degree of crosslinking appeared to potentially affect filler biocompatibility, which might have clinical implications. 3 The extent and amount of crosslinking critically impact, not only on the biophysical, but also on the biological properties of a particular filler, including tissue integration, water uptake (swelling), and resistance to degradation. 6 , 7 Additionally, the type and density of HA crosslinkage, as well as the manufacturing technology, may influence not only the in vivo persistence but also the safety profile of dermal fillers. 6 , 7
When HA filer is injected, the effect of soft tissue volume and shape enhancement is in theory limited to 6‐18 months depending on the type of filler used, the anatomical site, and the individual patient's genetics. 3 Nevertheless, there is evidence suggesting that the effects of VYC‐20L (Juvéderm Voluma®; Allergan plc, Dublin, Ireland) may last for a longer time. 8
One of the latest generation of fillers was created with a patented Vycross® technology (Allergan, Inc, Irvine, CA, USA), which utilizes a proprietary mix of low and high molecular weight HA. 9 Due to its unique composition and crosslinking process, Vycross® produces less hygroscopic fillers than those made with former technologies, which entails to absorb less water, minimal gel swelling, and a longer duration. 10 Additionally, this technology has allowed the creation of diverse fillers, with different G' and cohesivity that were especially designed for treating every need and area of the face. 11
The latest innovation of the Vycross® range was VYC‐25L (Juvéderm Volux®; Allergan plc, Dublin, Ireland), with 25 mg/mL of HA. VYC‐25L combines a high G’ (resistance to deformation) and high cohesivity, 12 which makes it an ideal HA filler for creating and restoring facial volume.
VYC‐25L has been successfully used for aesthetic management of the facial lower third. 13 , 14 Ogilvie et al 13 in a prospective, single‐blind, randomized, and controlled study evaluated the efficacy and safety of VYC‐25L for restoring and creating facial volume in the chin and jaw area of subjects with chin retrusion. The results of this study suggested a significant improvement in a Global Aesthetic Improvement Scale in more than 90% of patients at month 3. This improvement was reported by both patients and investigators. 13
Moreover, the physical improvements in the chin and jaw area lasted beyond 18 months and were similar between initial and repeat VYC‐25L treatments. 14 Ogilvie et al also observed that the VYC‐25L treatment injected 18 months after initial treatment required less injection volume to achieve similar aesthetic results. 14
However, these studies did not provide any information about the tissue integration of the product. Since tissue integration may significantly influence the clinical outcomes, it would be highly desirable to know, in a clinical setting, whether the new fillers show a good tissue integration profile.
The main purpose of this real‐life study was to assess, by means of ultrasounds, the tissue integration of the HA dermal filler VYC‐25L in chin and jaw. Additionally, this study also evaluated, as secondary outcomes, the treatment efficacy and safety profile, as well as, treatment patient satisfaction.
2. METHODS
2.1. Design
Prospective, noncomparative, open‐label, and multicenter study conducted at 3 Aesthetic Spanish Centers located in 3 different cities (Málaga, Castellón, and Bilbao) from September to December of 2019.
The study protocol was approved by an independent ethics committee. This study was performed according to the Helsinki Declaration and Good Clinical Practice guidelines. Written informed consent was obtained before enrolment.
2.2. Patients
Study sample included healthy subjects (either men or women) with age comprised between 30 and 60 years old who attended to the clinic to perform a facial rejuvenation treatment of the lower third of the face.
Patients with systemic lupus erythematosus, bleeding disorders, uncontrolled hypertension, allergies to any of its component's, and/or pregnant or nursery women were excluded of the study. Patients underwent anticoagulant treatment like aspirin, clopidogrel, or warfarin were no excluded, but treatment must be discontinued for, at least, 7 days before the procedure. 15
2.3. Technique
2.3.1. HA filler
VYC‐25L (Juvéderm Volux®; Allergan plc, Dublin, Ireland) is a highly cohesive HA dermal filler (25 mg/mL of HA) with lidocaine 0.3%. In the chin, 0.2 to 0.4 ml of VYC‐25L were injected superficial to the depressor anguli oris, at the deep subcutaneous level, into the C6 point of the de Maio MD Codes® 16 by means a 27G needle or a blunt microcannula, as needed, with a fanning technique (Figure 1). Regarding the administration into the jaw (jw), 0.3 to 0.6 ml of VYC‐25L were administered, at the deep subcutaneous level, with a blunt microcannula and retrograde threads, into the jw3 and jw4 points of the de Maio MD Codes® 16 (Figure 1).
FIGURE 1.
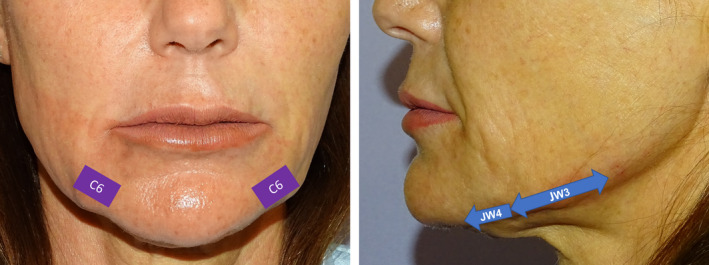
Different treatment points. Adapted from de Maio et al 16 Purple rectangles: The lateral point of the chin (C6). Blue arrows: Points for treat the jawline Jw3 (mandible body) and Jw4 (lower prejowl)
2.3.2. Ultrasonography
Ultrasound examinations (UE) were performed at each study center by the same experienced observer (FU, JB, and SC, respectively). The equipments used during the study were the ECO 6 with a 10 MHz linear array transducer (CHISON Medical Technologies Co., LTD. Seattle, WA, USA) in Málaga; The Z6 with a 12 MHz linear array transducer (MINDRAY Medical International Co., Ltd. Shenzhen 518057, P. R. China) in Castellón de la Plana; and the DP 20 with a 7.5 MHz linear array transducer (MINDRAY Medical International Co., Ltd. Shenzhen 518 057, P. R. China) in Bilbao. The transducer was applied to the Jw3, Jw4, and Chin (C)6 zones 16 using a coupling gel; taking care to avoid any pressure on the study zone.
UE were performed (in the right site) at the Jw3, Jw4, and C6 zones 16 at baseline (before VYC‐25L was injected), immediately after injection, 48 hours after injections, and 30 days after treatment.
2.4. Patient satisfaction
A five‐point Likert scale (Very Dissatisfied; Dissatisfied; Neither satisfied nor dissatisfied; Satisfied; and Very satisfied) was used to assess the degree of patient satisfaction with the treatment. The scale included the following question: Are you satisfied with the treatment results?
2.5. Outcomes
Primary study variable was the ultrasound pattern observed at the different study zones.
Secondary end‐points included the treatment efficacy (assessed by means photographs), safety profile, and patients’ satisfaction.
2.6. Statistical analysis
A standard statistical analysis was performed using MedCalc Statistical software version 19.2 (MedCalc software bv, Ostend, Belgium; https://www.medcalc.org; 2019).
Data are expressed as median [95% confidence interval (95% CI)], mean (range), or percentages as appropriate.
3. RESULTS
Thirty patients (10 at each study center) were included in the study. All patients concluded the study. Mean (95% confidence interval) age was 47.3 (44.1‐50.6), years and 26 (86.7%) were woman.
At baseline, UE found a characteristic heterogeneous pattern of subcutaneous cellular tissue, with alternation of soft anechoic and hyperechoic images. It was possible to differentiate the different echogenic tissue densities according to its composition (fat, amorphous fundamental substance, connective tissue, and liquids) (Figure 2).
FIGURE 2.
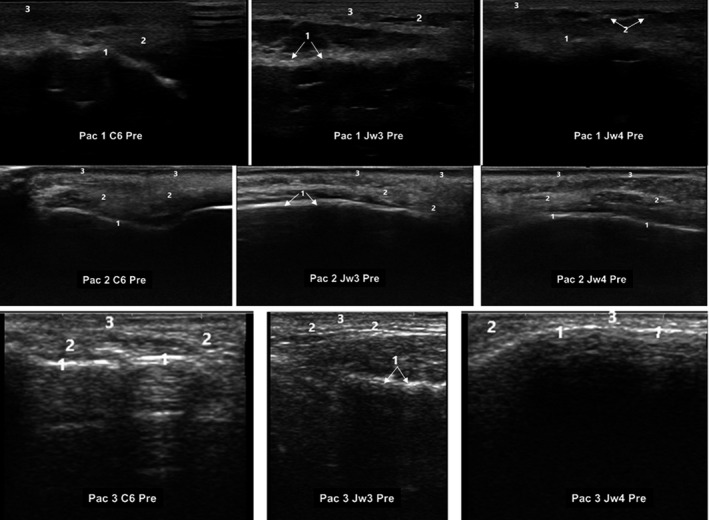
Ultrasound images of three different patients at baseline. Ultrasound images corresponded to different MD Codes® (de Maio et al 16 ). C6; Jw3, and Jw4. C: Chin; Jw: Jaw. C6. 1: Supraperiostic; 2: Subcutaneous cellular tissue; 3: Epidermis/Dermis. Jw3.1: Masseter muscle; 2: Subcutaneous cellular tissue; 3: Epidermis/Dermis. Jw4. 1: Supraperiostic; 2: Subcutaneous cellular tissue; 3: Epidermis/Dermis
The different ultrasound patterns of the VYC‐25L and its degree of biointegration throughout the study follow‐up are shown in Table 1.
TABLE 1.
Overview of the different ultrasound patterns of the VYC‐25L® filler and its degree of integration throughout the study follow‐up
| Time | Ultrasound findings |
|---|---|
| Before treatment | Characteristic heterogeneous pattern of a normal (healthy) subcutaneous tissue, with alternating soft anechoic and hyperechoic images. It was possible to differentiate the different echogenic densities depending on the tissue |
| Immediately after treatment | Poorly defined globular ultrasound pattern, with anechoic images indicative of liquid content. It was possible to see the presence of an edematous subcutaneous cellular tissue, with a clear subsequent echogenic reinforcement in all the study areas, indicating the existence of a liquid content |
| 48 hours after treatment | The presence of the globular pattern remained, with well‐defined anechoic areas, which indicate an increase in volume in all the treated areas |
| 30 days after treatment | The ultrasound images showed a complete typical heterogeneous pattern, without residual anechoic areas, which was indicative of a total integration of VYC‐25L® into the tissue. There was a thickening of the subcutaneous cellular tissue in all the evaluated areas, with a total integration of the hyaluronic acid filler into the tissue |
The UE performed immediately after treatment showed a globular and poorly defined ultrasound pattern, with anechoic images indicative of liquid content. Additionally, there was a subcutaneous cellular tissue edema, with clear posterior echogenic reinforcement in all the study areas, which was indicative of the existence of a liquid content (sonic waves experienced an accelerate passage, which were slowed‐down when they found normal tissue) (Figure 3).
FIGURE 3.
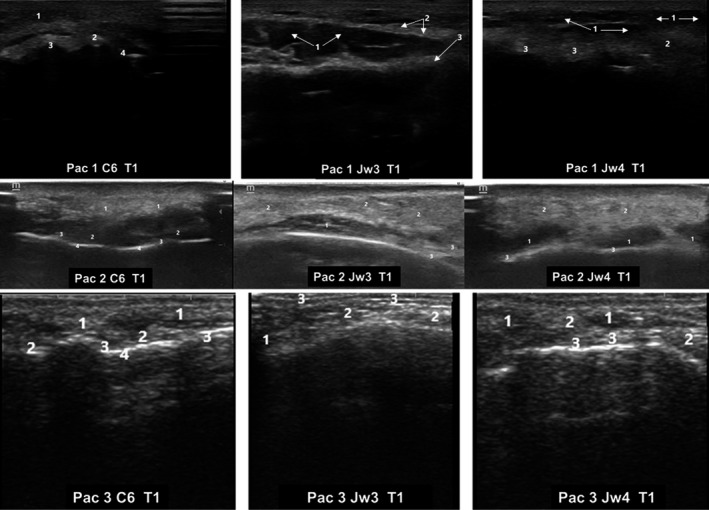
Ultrasound images of three different patients immediately after hyaluronic acid filler injection (T1). Ultrasound images corresponded to different MD Codes® (de Maio et al 16 ). C6; Jw3, and Jw4. C: Chin; Jw: Jaw. C6. 1: Subcutaneous tissue edema; 2: Increased echogenicity of subcutaneous cell tissue adjacent to the periosteum; 3: Supraperiostic; 4: Periosteum with increased echogenic density. Jw3.1: Masseter muscle; 2: Subcutaneous cellular tissue edema; 3: Increased tissue echogenicity at the supraperiosteal level. Jw4. 1: Anechoic supraperiosteal images and increased echogenicity of subcutaneous cellular tissue; 2: Subcutaneous cellular tissue edema; 3: Echogenic reinforcement
Forty‐eight hours after treatment, ultrasound images are still showing a globular pattern, with well‐defined anechoic areas, which indicate an increase in volume in all the MD Codes® evaluated. The presence of small and painless nodules, of semi‐soft consistency in the treated areas, was perceived, by palpation, in many patients. Additionally, the nodules were visible view at different study locations, which might indicate a lack of full integration of the HA filler into the tissue 48 hours after its injection (Figure 4).
FIGURE 4.
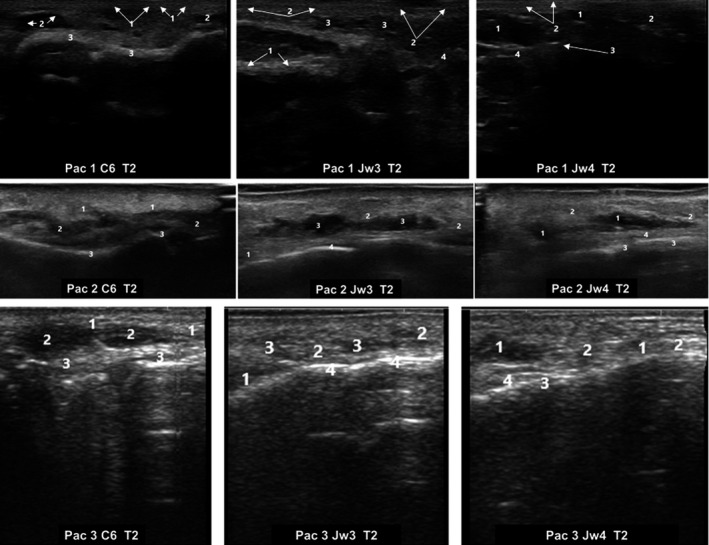
Ultrasound images of three different patients 48‐hours after hyaluronic acid filler injection (T2). Ultrasound images corresponded to different MD Codes® (de Maio et al 16 ). C6; Jw3, and Jw4. C: Chin; Jw: Jaw. C6. 1: Edema and thickening of subcutaneous cell tissue; 2: Typical anechoic images of globular pattern; 3: Supraperiosteum and posterior echogenic reinforcement. Jw3.1: Masseter muscle; 2: Thickening of subcutaneous cell tissue; 3: Anechoic images with globular pattern; 4: Supraperiosteum. Jw4. 1: Typical anechoic images of globular pattern; 2: Thickening of subcutaneous cell tissue; 3. Supraperiosteum; 4: Posterior echogenic reinforcement
At day 30 after treatment, a thickening of the subcutaneous cellular tissue was observed at all the evaluated zones, with a total integration of the HA into the tissue (the palpable nodules observed at 48 hours after treatment had disappeared). Ultrasound images presented a typical complete heterogeneous pattern, without residual anechoic areas, which was indicative of a total integration of the VYC‐25L into the tissue (Figure 5).
FIGURE 5.
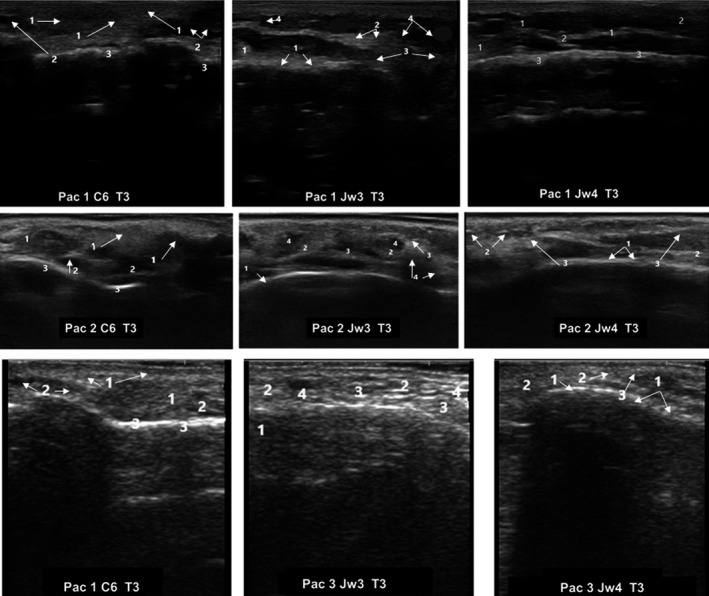
Ultrasound images of three different patients 30 days after hyaluronic acid filler injection (T3). Ultrasound images corresponded to different MD Codes® (de Maio et al 16 ). C6; Jw3, and Jw4. C: Chin; Jw: Jaw. C6. 1: Heterogeneous pattern; 2: Small anechoic areas; 3: Supraperiosteum and posterior echogenic reinforcement. Jw3.1: Masseter muscle; 2: Thickening and increased density of subcutaneous cell tissue; 3: Heterogeneous pattern of tissue integration. 4: Small anechoic images. Jw4. 1: Heterogeneous pattern; 2: Thickening and increased density of subcutaneous cell tissue; 3. Supraperiosteum
Figure 6 shows the evolution of ultrasound patterns in a patient throughout the study.
FIGURE 6.
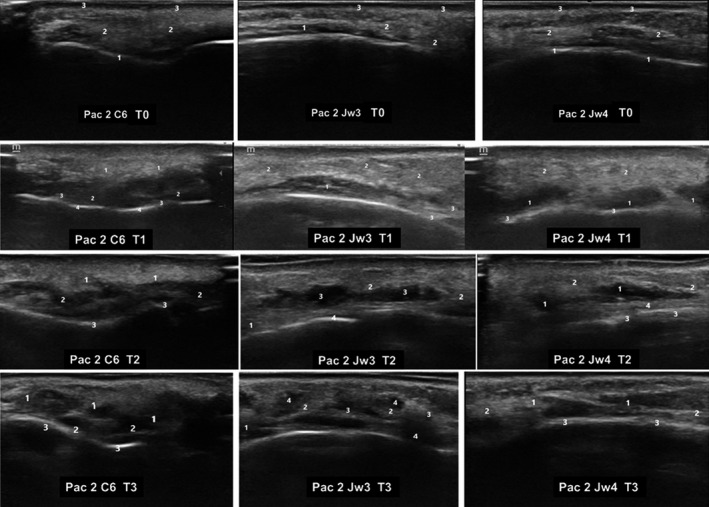
Ultrasound images of a patient treated with VYC‐25L over the course of the study. Ultrasound images corresponded to different MD Codes® (de Maio et al 16 ). T0. Before the study. C6. 1: Supraperiostic; 2: Subcutaneous cellular tissue; 3: Epidermis/Dermis. Jw3.1: Masseter muscle; 2: Subcutaneous cellular tissue; 3: Epidermis/Dermis. Jw4. 1: Supraperiostic; 2: Subcutaneous cellular tissue; 3: Epidermis/Dermis. T1. Immediately after treatment. C6. 1: Subcutaneous tissue edema; 2: Increased echogenicity of subcutaneous cell tissue adjacent to the periosteum; 3: Supraperiostic; 4: Periosteum with increased echogenic density. Jw3.1: Masseter muscle; 2: Subcutaneous cellular tissue edema; 3: Increased tissue echogenicity at the supraperiosteal level. Jw4. 1: Anechoic supraperiosteal images and increased echogenicity of subcutaneous cellular tissue; 2: Subcutaneous cellular tissue edema; 3: Echogenic reinforcement. T2. Forty‐eight hours after treatment. C6. 1: Edema and thickening of subcutaneous cell tissue; 2: Typical anechoic images of globular pattern; 3: Supraperiosteum and posterior echogenic reinforcement. Jw3.1: Masseter muscle; 2: Thickening of subcutaneous cell tissue; 3: Anechoic images with globular pattern; 4: Supraperiosteum. Jw4. 1: Typical anechoic images of globular pattern; 2: Thickening of subcutaneous cell tissue; 3: Supraperiosteum; 4: Posterior echogenic reinforcement. T3. Thirty days after treatment. C6. 1: Heterogeneous pattern; 2: Small anechoic areas; 3: Supraperiosteum and posterior echogenic reinforcement. Jw3.1: Masseter muscle; 2: Thickening and increased density of subcutaneous cell tissue; 3: Heterogeneous pattern of tissue integration. 4: Small anechoic images. Jw4. 1: Heterogeneous pattern; 2: Thickening and increased density of subcutaneous cell tissue; 3: Supraperiosteum. C: Chin; Jw: Jaw
Clinical results, assessed by means photographs, have shown a significant aesthetic improvement (Figures 7, 8, 9).
FIGURE 7.
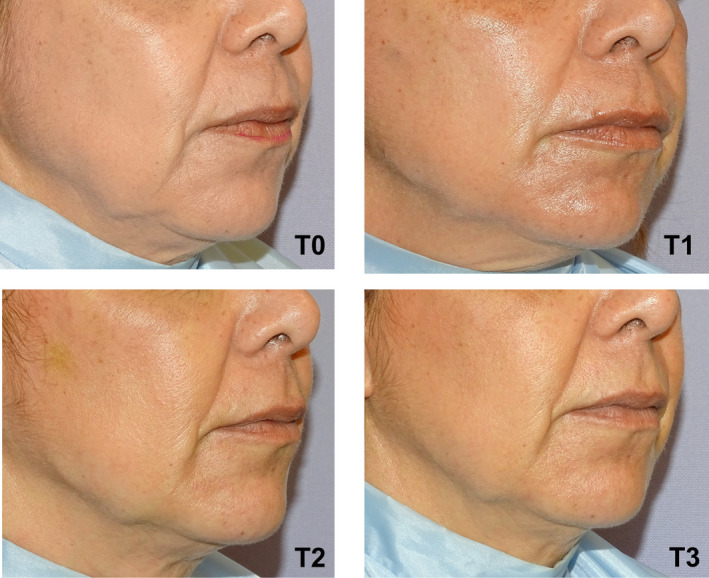
Right oblique projection of a patient face before and after being treated with VYC‐25L®. T0: Before treatment. T1: Immediately after treatment. T2: At 48 hours after treatment administration. T3: 30 days after treatment administration
FIGURE 8.
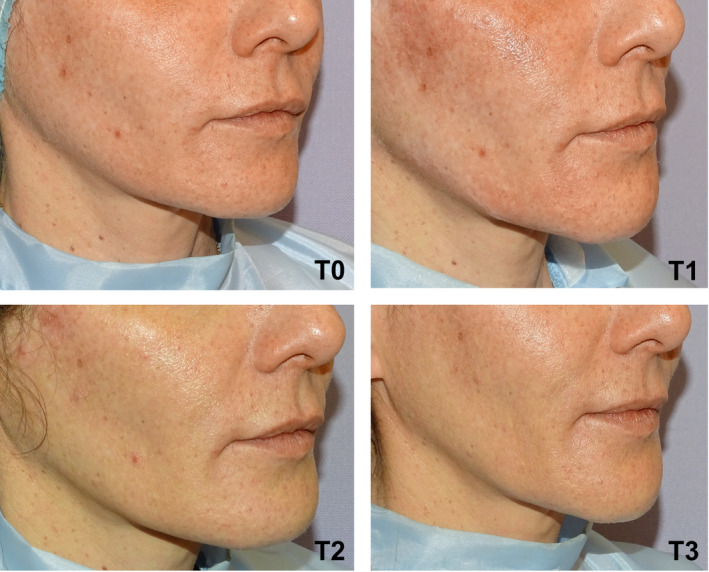
Right oblique projection of a patient face before and after being treated with VYC‐25L®. T0: Before treatment. T1: Immediately after treatment. T2: At 48 hours after treatment administration. T3: 30 days after treatment administration
FIGURE 9.
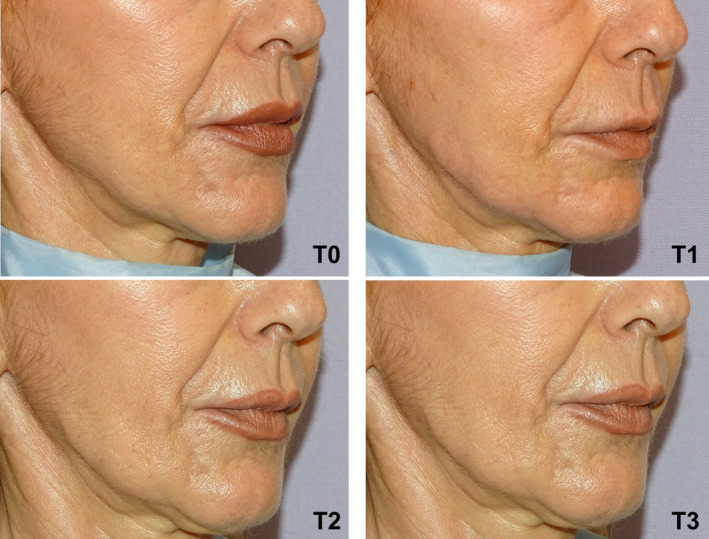
Right oblique projection of a patient face before and after being treated with VYC‐25L®. T0: Before treatment. T1: Immediately after treatment. T2: At 48 hours after treatment administration. T3: 30 days after treatment administration
Patient satisfaction survey showed very good results, with 27 (90%) patients reporting to be “Very satisfied” or “Satisfied,” with only 3 patients “dissatisfied” with the treatment results (they waited better results).
Regarding safety, there were no serious reported adverse events. Four patients had bruising in C6 at 48 hours, maybe due to the use of a needle in that area. All the reported adverse events were mild in severity and all were fully recovered without treatment.
4. DISCUSSION
This study has sequentially illustrated the VYC‐25L integration process. The results of the current real‐life study found a partial biointegration 48 hours after treatment administration and a total integration of the HA into the tissue 30 days after injection. Ultrasound images showed a complete heterogeneous pattern, without residual anechoic/hypoechoic areas, which was indicative of a total integration of the VYC‐25L into the tissue.
Additionally, the clinical outcomes obtained in a clinical setting, suggested the good efficacy and safety profile of VYC‐25L.
Over the past several years, injectable HA dermal fillers have become one of the most frequently performed aesthetic minimally invasive procedures worldwide. 1 , 2 Due to the high demand for soft tissue augmentation procedures, the number of HA fillers currently available in the market has continuously increased. Thus, it is extremely important to know, not only their efficacy/safety profile, but also their bio‐integration after injection into the skin/subcutaneous cellular tissue. 6
Various chemical reactions have been used to modify HA hydrogel to control their mechanical properties, including their elasticity and degradation resistance. 17 HA can be chemically modified by two main different ways: crosslinking or conjugation. 17 In addition, there are different types of crosslinking procedures. 17 , 18 Crosslinking is attempted to improve biomechanical properties while maintaining biocompatibility and biological activity. 17 , 18 The most common crosslinker used in dermal fillers manufacturing process is 1,4 butanediol diglycidyl ether (BDDE), producing intermolecular bonds for enhanced stability of the HA matrix. 17 , 18
HA dermal fillers are classified into two types, monophasic and biphasic. 18 The monophasic fillers are prepared by mixing the high‐molecular‐weight HA and the low‐molecular‐weight HA. 18 Monophasic and biphasic fillers have different behaviors after their injection. 19 , 20 Monophasic fillers have exhibited a better tissue integration profile and, as a consequence, they provide a more natural aesthetic appearance. 19 , 20
VYC‐25L is a monophasic HA filler, performed with the Vycross® technology, that combines a high amount of HA (25 mg/mL) with the higher elastic modulus (G') and higher cohesivity currently available in the market (based on preclinical comparative research with currently available fillers). 12 Additionally, including short‐chain HA allows more crosslinkers to attach to HA chains at both ends, thereby resulting in a longer product duration than fillers with only long‐chain HA (11,21). Vycross® technology, unlike sizing technology used in other fillers, does not break up the crosslinked HA by passing the product through sizing screens via sieves, but instead produces monophasic gels. 5 , 6 , 21
As far as we know, this is the first study evaluating the bio‐integration of VYC‐25L. Micheels et al 22 evaluated the integration properties of a Vycross® technology HA filler with ultrasounds. At day 0, they reported the presence of a mixture of iso‐ and hypo‐echogenic pools, with areas where the product distribution was homogeneous, while in other ones it was heterogeneous distributed, as compared with the nontreated surrounding areas. 22 In our study, ultrasound examinations performed immediately after treatment showed a globular, poorly defined ultrasound pattern, with anechoic images indicative of liquid content. Similar to Micheels et al, 22 our study found a posterior echogenic reinforcement in all the study areas, which was indicative of the existence of a liquid content.
However, the findings of the subsequent examination of the current study significantly differ from those reported by Micheels et al 22 At day 15 and Day 90, Micheels et al reported that there were no variations as compared to day 0. 22 Nevertheless, our study found significant differences in ultrasound appearance between day 0 and day 30 (we did not perform an examination either day 15 or day 90). Moreover, at day 30, ultrasound images findings suggested a total integration of the VYC‐25L into the tissue.
Due to the differences in study protocol and product characteristics (they evaluated a 15 mg/mL HA filler), it is not easy to compare our results with those of Micheels et al 22
VYC‐25L has been successfully and safely use for volumizing and contouring the chin and jaw area. 13 , 14 About this aspect, our results are in line with those reported by Ogilvie et al 13 , 14 From a clinical perspective, VYC‐25L achieved good aesthetic results, with a satisfactory safety profile. Additionally, patient satisfaction was really very high, with the 90% of patients being “Very satisfied” or “Satisfied” with the treatment results.
Finally, although the incidence of hyaluronic acid dermal filler complications observed in the current study was low and all the adverse events were mild, early and late complications have been described in the literature. 23 , 24 Several complications have been associated with the use of dermal fillers, including injection site reactions (erythema, edema, pain/tenderness, etc); infection (erythema, edema, nodule/access, etc); hypersensitivity (erythema, edema, etc); technical and placement errors (asymmetries, contour irregularities, dysesthesias, etc); skin discoloration (redness, whiteness, and/or hyperpigmentation); and vascular compromise (blurred vision, loss of vision, etc). 23 , 24
This study has some limitations that should be taken into consideration. The first one is its open‐label design. A double‐blind and comparative design would have been desirable. Nevertheless, the purpose of our study was not to compare different HA fillers, but rather to provide a first view of the bio‐integration a new HA filler with a high amount of HA (25 mg/mL) and high G' and cohesivity. The second limitation is the length of follow‐up. Although 30 days after treatment the HA filler was totally integrated into the tissue, a longer follow‐up time would have provided information about the product behavior 12‐18 months after its administration. Other limitation might be the fact that the current study did not perform "objective" measurements, such as dermis thickness, which might have provided valuable information about the product. Additionally, the current study did not measure the volume change after VYC‐25L administration, but only measured the amount of product injected in the different locations. Nevertheless, due to the small amount of VYC‐25L injected it is impossible to establish correlations between the ultrasonography findings and the amount of filler. An additional limitation of this study is the fact that three different ultrasound devices have been used (one in each study center). Nevertheless, it should be highlighted that throughout the study the patients were examined always with the same device and by the same observer, which obviously reduced the variability. Moreover, ultrasound images were used for qualitative assessment rather than for quantitative measurements.
Despite these limitations, based on the results of this study, VYC‐25L might represent a significant advance in Volumization/Restoration of the lower face. Its bio‐integration was total at 30 days of treatment, this integration being practically complete from 48 hours post‐treatment. Additionally, the study findings may help to explain the patients the behavior of the hyaluronic acid filler having been administered.
Although it is not too much appropriate to speak about predictability when speaking about medical treatments, according to the ultrasound images and clinical results, VYC‐25L may appear as a predictable and reproducible treatment option for managing lowerface aesthetic procedures.
Further studies are needed to elucidate the long‐term behavior of VYC‐25L.
CONFLICT OF INTEREST
Dr Urdiales‐Gálvez has received a Grant from Allergan SA for covering the medical writing services and the publication fees.
All the coauthors declare that they have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Fernando Urdiales‐Gálvez involved in conception and design of the study, acquisition of data, interpretation of data, and revision of the work. Jordan Barres‐Caballer: involved in conception and design of the study, acquisition data, and drafting the manuscript. Sara Carrasco‐Sánchez: involved in conception and design of the study, acquisition data, and interpretation of data.
ACKNOWLEDGMENTS
Medical writing and Editorial assistant services have been provided by Ciencia y Deporte SL Support for this assistance was funded by Allergan SA at the request of the investigator.
Urdiales‐Gálvez F, Barres‐Caballer J, Carrasco‐Sánchez S. Ultrasound assessment of tissue integration of the crosslinked hyaluronic acid filler VYC‐25L in facial lower‐third aesthetic treatment: A prospective multicenter study. J Cosmet Dermatol. 2021;20:1439–1449. 10.1111/jocd.13632
Funding information
Medical writing services have been provided by Allergan. Allergan did not participate in either data collection, analysis, or redaction of the manuscript. Neither honoraria nor payments were made for authorship.
REFERENCES
- 1.The international study on aesthetic/cosmetic procedures performed in 2018. https://www.isaps.org/wp‐content/uploads/2019/12/ISAPS‐Global‐Survey‐2018‐Press‐Release‐English.pdf. Accessed April 22, 2020.
- 2. Devgan L, Singh P, Durairaj K. Minimally invasive facial cosmetic procedures. Otolaryngol Clin North Am. 2019;52:443‐459. [DOI] [PubMed] [Google Scholar]
- 3. Fallacara A, Manfredini S, Durini E, Vertuani S. Hyaluronic acid fillers in soft tissue regeneration. Facial Plast Surg. 2017;33:87–96. Erratum. In: Facial Plast Surg. 2017;33:244. [DOI] [PubMed] [Google Scholar]
- 4. Laurent TC, Fraser JRE. Hyaluronan. FASEB J. 1992;6:2397‐2404. [PubMed] [Google Scholar]
- 5. Mansouri Y, Goldenberg G. Update on hyaluronic acid fillers for facial rejuvenation. Cutis. 2015;96:85‐88. [PubMed] [Google Scholar]
- 6. Micheels P, Sarazin D, Tran C, Salomon D. Effect of different crosslinking technologies on hyaluronic acid behavior: a visual and microscopic study of seven hyaluronic acid gels. J Drugs Dermatol. 2016;15:600‐606. [PubMed] [Google Scholar]
- 7. Guarise C, Barbera C, Pavan M, Panfilo S, Beninatto R, Galesso D. HA‐based dermal filler: downstream process comparison, impurity quantitation by validated HPLC‐MS analysis, and in vivo residence time study. J Appl Biomater Funct Mater. 2019;17:2280800019867075. [DOI] [PubMed] [Google Scholar]
- 8. Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;20:81‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Philipp‐Dormston WG, Hilton S, Nathan M. A prospective, open‐label, multicenter, observational, postmarket study of the use of a 15 mg/mL hyaluronic acid dermal filler in the lips. J Cosmet Dermatol. 2014;13:125‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Few J, Cox SE, Paradkar‐Mitragotri D, Murphy DK. A multicenter, single‐blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient‐reported outcomes at 2 years. Aesthet Surg J. 2015;35:589‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(5 Suppl):139S‐148S. [DOI] [PubMed] [Google Scholar]
- 12. Pierre S, Liew S, Bernardin A. Basics of dermal filler rheology. Dermatol Surg. 2015;41(Suppl 1):S120‐S126. [DOI] [PubMed] [Google Scholar]
- 13. Ogilvie P, Sattler G, Gaymans F, et al. Safe, effective Chin and Jaw restoration with VYC‐25L hyaluronic acid injectable gel. Dermatol Surg. 2019;45:1294‐1303. [DOI] [PubMed] [Google Scholar]
- 14. Ogilvie P, Benouaiche L, Philipp‐Dormston WG, et al. VYC‐25L hyaluronic acid injectable gel is safe and effective for long‐term restoration and creation of volume of the lower face. Aesthet Surg J. 2020:sjaa013. 10.1093/asj/sjaa013. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Urdiales‐Gálvez F, Delgado NE, Figueiredo V, et al. Preventing the complications associated with the use of dermal fillers in facial aesthetic procedures: an expert group consensus report. Aesthetic Plast Surg. 2017;41:667‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Maio M. MD Codes™: a methodological approach to facial aesthetic treatment with injectable hyaluronic acid fillers. Aesthetic Plast Surg. 2020. 10.1007/s00266-020-01762-7. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schante CE, Zuber G, Herlin C, Vandamme TF. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr Polym. 2011;85:469‐489. [Google Scholar]
- 18. Park KY, Kim HK, Kim BJ. Comparative study of hyaluronic acid fillers by in vitro and in vivo testing. J Eur Acad Dermatol Venereol. 2014;28:565‐568. [DOI] [PubMed] [Google Scholar]
- 19. Flynn TC, Sarazin D, Bezzola A, Terrani C, Micheels P. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637‐643. [DOI] [PubMed] [Google Scholar]
- 20. Hee CK, Shumate GT, Narurkar V, Bernardin A, Messina DJ. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(Suppl 1):S373‐S381. [DOI] [PubMed] [Google Scholar]
- 21. Micheels P, Besse S, Sarrazin D. Visual, ultrasonographic, and microscopic study on hyaluronic acid‐based gel. J Drugs Dermatol. 2016;15:1092‐1098. [PubMed] [Google Scholar]
- 22. Micheels P, Besse S, Sarazin D. Two crosslinking technologies for superficial reticular dermis injection: a comparative ultrasound and histologic study. J Clin Aesthet Dermatol. 2017;10:29‐36. [PMC free article] [PubMed] [Google Scholar]
- 23. Park TH, Seo SW, Kim JK, Chang CH. Clinical experience with hyaluronicacid‐filler complications. J Plast Reconstr Aesthet Surg. 2011;64:892‐896. [DOI] [PubMed] [Google Scholar]
- 24. Urdiales‐Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42:498‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]


