Summary
Background
Certolizumab pegol (CZP) is an Fc‐free, PEGylated anti‐tumour necrosis factor biologic.
Objectives
To report 3‐year safety data from three phase III trials of CZP in adults with plaque psoriasis.
Methods
Data were pooled from CIMPASI‐1 (NCT02326298), CIMPASI‐2 (NCT02326272) and CIMPACT (NCT02346240). Included patients had moderate‐to‐severe plaque psoriasis of ≥ 6 months’ duration; had been randomized to CZP 200 mg every 2 weeks (Q2W) (400 mg at weeks 0, 2 and 4) or CZP 400 mg Q2W; and had received at least one dose of CZP with up to 144 weeks of exposure. Treatment‐emergent adverse events (TEAEs) were classified using MedDRA v18·1. Reported incidence rates (IRs) are incidence of new cases per 100 patient‐years (PY).
Results
Over 144 weeks, 995 patients received at least one dose of CZP (exposure: 2231·3 PY); 731 and 728 received at least one dose of CZP 200 mg Q2W (1211·4 PY) and/or 400 mg Q2W (1019·9 PY), respectively. The IR [95% confidence interval (CI)] of TEAEs was 144·9 (135·3–155·0) for all patients, 134·1 (123·2–145·7) for CZP 200 mg Q2W and 158·3 (145·5–171·9) for CZP 400 mg Q2W. The IR (95% CI) of serious TEAEs for all patients was 7·5 (6·4–8·8); the IRs were 6·7 (5·2–8·3) and 8·7 (6·9–10·8) for CZP 200 mg and 400 mg Q2W, respectively. Overall, 3·2% of patients reported serious infections (2·2% within each of the CZP 200 and 400 mg Q2W groups). Overall, there was one case of active tuberculosis, 16 malignancies in 14 patients and seven deaths (two considered treatment‐related). The cumulative IR of TEAEs did not increase over time.
Conclusions
No new safety signals were identified compared with previously reported data. Risk did not increase with longer or higher CZP exposure.
Short abstract
What is already known about this topic?
Certolizumab pegol is an Fc‐free, PEGylated, anti‐tumour necrosis factor biologic approved for adults with moderate‐to‐severe plaque psoriasis.
Safety data from phase III trials in plaque psoriasis have found the incidence of adverse events to be generally similar over 16 weeks of treatment between the evaluated certolizumab pegol doses 200 mg and 400 mg every 2 weeks and placebo.
Additionally, the safety profile was in line with the class over 48 weeks.
What does this study add?
Plaque psoriasis is a chronic disease for which patients require lifetime management; long‐term safety data are important to understand the benefits and risks of prolonged treatment.
Here, 3‐year data from a pooled analysis of three phase III trials of certolizumab pegol in plaque psoriasis are presented, representing 2231·3 patient‐years of exposure.
No new safety signals were identified and the risk of treatment‐emergent adverse events did not increase with longer or higher certolizumab pegol exposure.
Plaque psoriasis is an immune‐mediated inflammatory disease affecting approximately 2–6% of adults in Western countries. 1 , 2 , 3 Anti‐tumour necrosis factor (TNF) agents are among the most widely used biologic therapies for plaque psoriasis. 4 Although their benefit–risk profile is well established across a range of immune‐mediated inflammatory diseases, 4 , 5 , 6 , 7 prolonged treatment raises concerns regarding potential risk of serious infections [including tuberculosis (TB)], malignancies, lupus‐like syndromes, paradoxical inflammation and demyelinating disorders. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Long‐term data from clinical trials provide a valuable means to further understand the benefits and risks of prolonged treatment.
Certolizumab pegol (CZP) is an Fc‐free, PEGylated, anti‐TNF biologic currently approved for the treatment of adults with moderate‐to‐severe plaque psoriasis, rheumatoid arthritis, psoriatic arthritis, Crohn disease and axial spondyloarthritis, which comprises ankylosing spondylitis and nonradiographic axial spondyloarthritis. 16 , 17 Because CZP lacks the IgG Fc domain, unlike other anti‐TNF biologics it does not bind the neonatal Fc receptor. 18 Two prospective studies on pregnant and postpartum women have shown no to minimal placental transfer of CZP from mothers to infants, 19 and minimal transfer into breast milk. 20
Short‐term safety data have been reported for CIMPASI‐1 (NCT02326298), CIMPASI‐2 (NCT02326272) and CIMPACT (NCT02346240), which are phase III, randomized, multicentre trials of CZP in plaque psoriasis conducted in North America and Europe. These analyses found a similar safety profile between CZP and placebo after 16 weeks of treatment. 21 Additionally, no new safety signals associated with CZP dosed at 200 mg or 400 mg every two weeks (Q2W) over 48 weeks were identified compared with other anti‐TNF biologics and CZP in other indications. 22 , 23 More recently, the long‐term safety of CZP was examined for 11 317 patients pooled across clinical trials in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, plaque psoriasis and Crohn disease. 24 This analysis, which included psoriasis data up to August 2017, did not identify any new safety signals and suggested that the varying incidence of certain adverse events (AEs) between indications may be attributable to underlying inflammatory disease and other patient‐related factors. 24
Here we present an in‐depth analysis of the long‐term safety of CZP in moderate‐to‐severe plaque psoriasis using final data from the CIMPASI‐1, CIMPASI‐2 and CIMPACT trials, representing approximately 3 years of treatment. The data from these trials are available on request (see Appendix 2).
Patients and methods
Data sources and patient population
Data were pooled from the CIMPASI‐1 and CIMPASI‐2 double‐blinded and placebo‐controlled studies, 22 and the CIMPACT double‐blinded, placebo‐controlled and single‐blinded active‐controlled study. 23 Safety data were assessed for all patients receiving at least one dose of CZP with up to 144 weeks of exposure prior to study completion (CIMPASI‐1: 24 October 2018; CIMPASI‐2: 12 September 2018; CIMPACT: 17 December 2018). Each trial enrolled adults (≥ 18 years of age) with moderate‐to‐severe plaque psoriasis of ≥ 6 months’ duration with Psoriasis Area and Severity Index (PASI) ≥ 12, ≥ 10% body surface area affected, and Physician’s Global Assessment ≥ 3 on a five‐point scale. All participants were candidates for systemic psoriasis therapy, phototherapy and/or photochemotherapy. The full exclusion criteria have been published previously. 22 , 23
Study designs
All three trials included a 48‐week double‐blinded, placebo‐controlled phase, followed by an open‐label phase to week 144. 22 , 23 Patients were initially randomized to CZP 200 mg Q2W (CZP 400‐mg loading dose at weeks 0, 2 and 4), CZP 400 mg Q2W or placebo Q2W. In CIMPACT only, patients were also randomized to an etanercept treatment arm. After an initial 16 weeks of treatment, patients in all three studies received double‐blinded treatment with either CZP or placebo to week 48. At week 48, all patients who entered the open‐label phase and had achieved ≥ 50% improvement in PASI (PASI 50) received CZP dosed at 200 mg Q2W; dosing adjustment was permitted through to week 144 depending on PASI response and/or investigator discretion. The full study designs are provided in Figure S1 (see Supporting Information).
The placebo and etanercept treatment arms were not included in this analysis; across all three studies, placebo treatment was continued by 11 patients after week 16. The week 16 placebo and etanercept safety data have been described elsewhere. 22 , 23
Tuberculosis screening
All patients were screened for TB through an interferon‐γ release assay test (QuantiFERON‐TB Gold; Cellestis, Chadstone, Australia), a chest X‐ray, physical examination and a questionnaire to assess TB signs, symptoms and possible exposure. Patients with latent TB at screening received a full course of latent TB treatment initiated at least 4 weeks prior to the first dose of study treatment. Patients unable to complete a minimum of 4 weeks of TB treatment during the screening period were permitted one rescreening. Throughout the studies, patients were assessed at least annually for TB. Study treatment was stopped immediately for any patient with suspected new latent or active TB infection.
Safety evaluations
An assessment of treatment‐emergent AEs (TEAEs) is presented, defined as AEs occurring while treatment was ongoing or ≤ 70 days after the last CZP dose, regardless of the dose received. TEAEs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) version 18·1.
Serious TEAEs (SAEs) included all medical occurrences that were life‐threatening or led to death, hospitalization, congenital anomalies or birth defects, or resulted in persistent or significant disability. 25 Events deemed medically significant by the clinical investigator could also be recorded as SAEs, regardless of severity. Infections requiring treatment with intravenous antibiotics were classified as serious.
TEAEs of interest in this analysis included serious infectious events (SIEs) including opportunistic infections, malignancies, major adverse cardiovascular events (MACEs; inclusive of fatal and nonfatal myocardial infarction, serious cerebrovascular events and congestive heart failure, regardless of seriousness), demyelinating‐like disorders, new or worsening psoriasis, haematopoietic cytopenia, lupus and lupus‐like illness, serious skin reactions, hypersensitivity or anaphylactic reactions, serious bleeding events and hepatic events.
Pregnancy outcomes were summarized for pregnancies with known maternal CZP exposure, prospectively reported up to 22 September 2019.
Statistical analysis
Safety data were analysed for the combined CZP‐treated group (all CZP) and separately for each CZP dose. For patients who were exposed to both CZP doses during the course of the studies, TEAEs were assigned to the dose the patient was receiving at the time of event onset. The duration of exposure to each CZP dose is summarized under the respective treatment group, but each patient is included only once in the overall population count for the all CZP group. Exposure‐adjusted incidence rates (IRs) with 95% confidence intervals (CIs) were calculated as the number of first occurrences of a particular TEAE per 100 patient‐years (PY). Statistical analyses were specified within an integrated statistical analysis plan and were performed in SAS version 9·4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient population
Across all three studies, a total of 995 patients received at least one dose of CZP (total exposure 2231·3 PY), including 731 patients treated with CZP 200 mg Q2W (1211·4 PY) and 728 patients treated with CZP 400 mg Q2W (1019·9 PY) (Table 1). The mean CZP exposure is shown in Table 2 and the total exposure in Table 3. Baseline patient characteristics were comparable across CZP dose groups (Table 1).
Table 1.
Pooled demographics and baseline characteristics of patients treated with certolizumab pegol (CZP)
| All CZP (N = 995) | CZP 200 mg Q2W (N = 731) | CZP 400 mg Q2W (N = 728) | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ± SD | 45·6 ± 13·2 | 45·3 ± 13·1 | 45·7 ± 13·1 |
| Male, n (%) | 652 (65·5) | 491 (67·2) | 472 (64·8) |
| White, n (%) | 939 (94·4) | 694 (94·9) | 683 (93·8) |
| BMI (kg m−2), mean ± SD | 30·4 ± 7·0 | 30·2 ± 6·7 | 30·6 ± 7·1 |
| Weight (kg), mean ± SD | 90·7 ± 22·7 | 90·2 ± 21·4 | 91·4 ± 23·2 |
| Geographical region, n (%) | |||
| Central/Eastern Europe | 454 (45·6) | 336 (46·0) | 323 (44·4) |
| Western Europe | 181 (18·2) | 140 (19·2) | 151 (20·7) |
| North America | 360 (36·2) | 255 (34·9) | 254 (34·9) |
| Baseline disease characteristics | |||
| Psoriasis disease duration (years), mean ± SD | 18·2 ± 12·5 | 18·4 ± 12·6 | 18·2 ± 12·4 |
| Concomitant PsA, n (%) | 174 (17·5) | 127 (17·4) | 125 (17·2) |
| PASI, mean ± SD | 20·2 ± 7·8 | 20·1 ± 7·8 | 20·2 ± 7·7 |
| BSA (%), mean ± SD | 25·7 ± 15·3 | 25·5 ± 15·2 | 25·7 ± 15·0 |
| PGA score, n (%) | |||
| 3: moderate | 692 (69·5) | 509 (69·6) | 503 (69·1) |
| 4: severe | 303 (30·5) | 222 (30·4) | 225 (30·9) |
| Prior treatments, n (%) | |||
| Number of prior biologic therapies | |||
| 0 | 696 (69·9) | 510 (69·8) | 508 (69·8) |
| 1 | 228 (22·9) | 171 (23·4) | 166 (22·8) |
| 2 | 70 (7·0) | 50 (6·8) | 53 (7·3) |
| ≥ 3 | 1 (0·1) | 0 | 1 (0·1) |
| Anti‐TNF | 123 (12·4) | 94 (12·9) | 88 (12·1) |
| Anti‐IL‐17 | 149 (15·0) | 109 (14·9) | 106 (14·6) |
| Anti‐IL‐12/IL‐23 | 49 (4·9) | 30 (4·1) | 43 (5·9) |
| Prior chemotherapy or phototherapy | 475 (47·7) | 352 (48·2) | 360 (49·5) |
| Any prior systemic therapy for psoriasis | 714 (71·8) | 529 (72·4) | 532 (73·1) |
Patients who received both CZP 200 mg every 2 weeks (Q2W) and CZP 400 mg Q2W are included once in the population count for the ‘all CZP’ group. BMI, body mass index; BSA, body surface area; IL, interleukin; PASI, Psoriasis Area and Severity Index; PGA, Physician’s Global Assessment; PsA, psoriatic arthritis; SD, standard deviation; TNF, tumour necrosis factor.
Table 2.
Overall summary of treatment‐emergent adverse events (TEAEs) in patients treated with certolizumab pegol (CZP)
| All CZP (N = 995) | CZP 200 mg Q2W (N = 731) | CZP 400 mg Q2W (N = 728) | |
|---|---|---|---|
| Total exposure, PY | 2231·3 | 1211·4 | 1019·9 |
| Exposure (days) | |||
| Mean ± SD | 768·1 ± 314·0 | 569·7 ± 328·6 | 477·7 ± 306·3 |
| Median (range) | 897·0 (14·0–1035·0) | 671·0 (12·0–1022·0) | 343·0 (12·0–1014·0) |
| n (%)a | IR/100 PY (95% CI) | n (%)a | IR/100 PY (95% CI) | n (%)a | IR/100 PY (95% CI) | |
|---|---|---|---|---|---|---|
| All TEAEs | 847 (85·1) | 144·9 (135·3–155·0) | 557 (76·2) | 134·1 (123·2–145·7) | 563 (77·3) | 158·3 (145·5–171·9) |
| Mild | 700 (70·4) | 81·7 (75·8–88·0) | 436 (59·6) | 76·7 (69·7–84·3) | 446 (61·3) | 88·8 (80·8–97·5) |
| Moderate | 627 (63·0) | 52·7 (48·6–57·0) | 395 (54·0) | 53·6 (48·4–59·1) | 373 (51·2) | 59·9 (54·0–66·3) |
| Severe | 132 (13·3) | 6·3 (5·3–7·5) | 66 (9·0) | 5·7 (4·4–7·2) | 70 (9·6) | 7·2 (5·6–9·1) |
| TEAEs leading to death | 7 (0·7) | 0·3 (0·1–0·7) | 4 (0·5) | 0·3 (0·1–0·9) | 3 (0·4) | 0·3 (0·1–0·9) |
| SAEs | 154 (15·5) | 7·5 (6·4–8·8) | 76 (10·4) | 6·7 (5·2–8·3) | 82 (11·3) | 8·7 (6·9–10·8) |
| TEAEs leading to discontinuation | 88 (8·8) | 4·0 (3·2–4·9) | 41 (5·6) | 3·4 (2·5–4·6) | 48 (6·6) | 4·7 (3·5–6·3) |
| Drug‐related TEAEs | 264 (26·5) | 14·6 (12·9–16·5) | 151 (20·7) | 14·6 (12·4–17·2) | 148 (20·3) | 16·9 (14·3–19·9) |
Patients who received both CZP 200 mg every 2 weeks (Q2W) and CZP 400 mg Q2W are included once in the population count for the ‘all CZP’ group. CI, confidence interval; IR, incidence rate; PY, patient‐years; SD, standard deviation; SAE, serious TEAE. aThe number of patients who reported at least one TEAE in the category.
Table 3.
Total exposure to study medication
| Placebo | CZP 200 mg Q2W | CZP 400 mg Q2W | |
|---|---|---|---|
| Week 16 | 46·9 PY | 106·5 PY | 104·5 PY |
| Week 48 | – | 310·8 PY | 418·0 PY |
| Week 96 | – | 771·7 PY | 699·6 PY |
| Week 144 | – | 1211·4 PY | 1019·9 PY |
CZP, certolizumab pegol; Q2W, every 2 weeks; PY, patient‐years.
Overall safety
Over 144 weeks, TEAEs were experienced by 847 (85·1%) CZP‐treated patients; most events were mild or moderate in intensity (Table 2). The overall IR of SAEs was 7·5 per 100 PY for all CZP‐treated patients, 6·7 per 100 PY for the CZP 200 mg Q2W group and 8·7 per 100 PY for the CZP 400 mg Q2W group (Table 2). In total, 324 (32·6%) CZP‐treated patients discontinued treatment, 88 of whom (8·8% overall) discontinued treatment due to TEAEs (Table 2). Other reasons for discontinuation included withdrawn consent (7·6%), mandatory withdrawal due to lack of PASI 50 response (6·4%), lack of efficacy (1·5%), loss to follow‐up (4·8%) and protocol violation (0·3%). The IRs of TEAEs, drug‐related TEAEs and TEAEs leading to study discontinuation were similar between the two CZP dose groups (Table 2).
The cumulative IRs of all TEAEs and SAEs were higher during the first 16 weeks of CZP exposure, decreasing by 48 weeks and remaining stable through 144 weeks of cumulative exposure (Figures 1 and 2). The most commonly reported TEAEs by MedDRA system organ class were infections and infestations, musculoskeletal and connective tissue disorders, and skin and subcutaneous tissue disorders. No single system organ class showed a difference > 5% in the incidence of TEAEs between the two CZP dose groups (Table 4).
Figure 1.
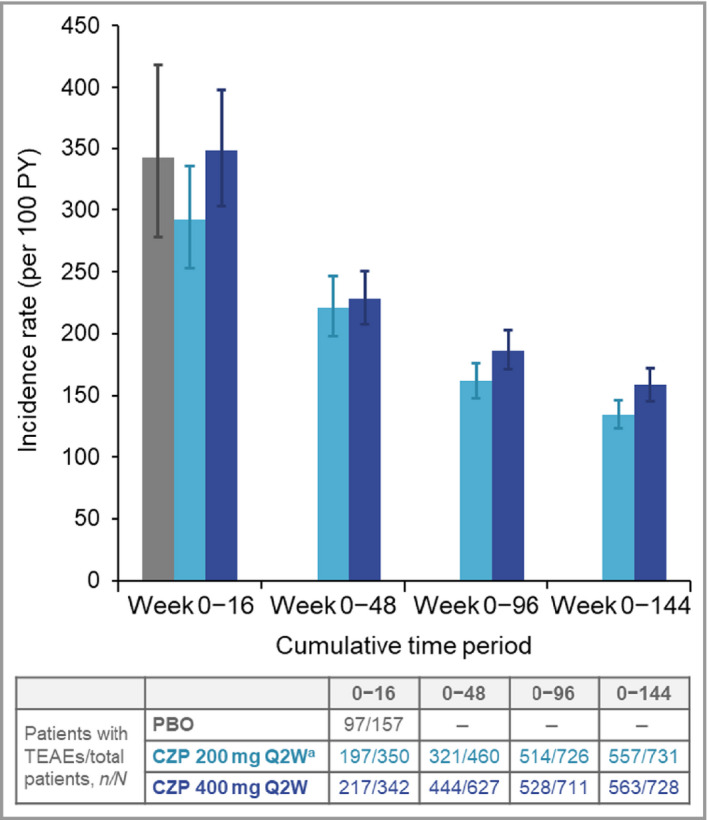
Cumulative incidence rates of all treatment‐emergent adverse events (TEAEs) to week 144. CZP, certolizumab pegol; PBO, placebo; PY, patient‐years; Q2W, every 2 weeks. aPatients on CZP 200 mg Q2W received a loading dose of CZP 400 mg at weeks 0, 2 and 4. Error bars correspond to 95% confidence intervals.
Figure 2.
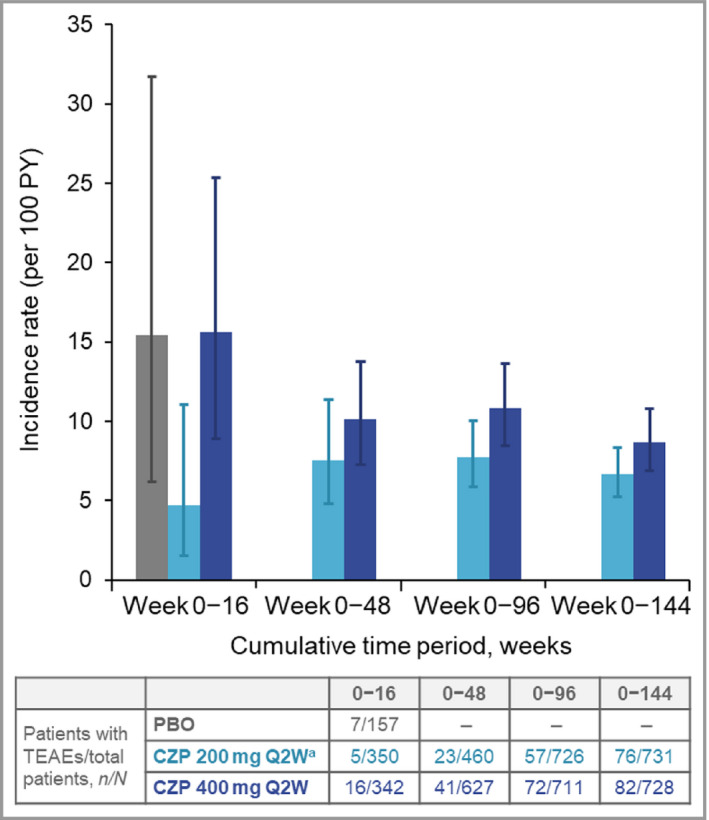
Cumulative incidence rates of serious treatment‐emergent adverse events (SAEs) to week 144. CZP, certolizumab pegol; PBO, placebo; PY, patient‐years; Q2W, every 2 weeks. aPatients on CZP 200 mg Q2W received a loading dose of CZP 400 mg at weeks 0, 2 and 4. Error bars correspond to 95% confidence intervals.
Table 4.
Most frequent treatment‐emergent adverse events (TEAEs) [reported in > 5% of all patients treated with certolizumab pegol (CZP)] by MedDRA system organ class and preferred term
| All CZP (N = 995) | CZP 200 mg Q2W (N = 731) | CZP 400 mg Q2W (N = 728) | |
|---|---|---|---|
| Total exposure, PY | 2231·3 | 1211·4 | 1019·9 |
|
System organ classa Preferred terma |
n (%)c | IR/100 PY (95% CI) | n (%)c | IR/100 PY (95% CI) | n (%)c | IR/100 PY (95% CI) |
|---|---|---|---|---|---|---|
| Infections and infestations | 646 (64·9) | 59·1 (54·6–63·8) | 406 (55·5) | 60·8 (55·0–67·0) | 385 (52·9) | 63·3 (57·1–69·9) |
| Bronchitis | 57 (5·7) | 2·7 (2·0–3·4) | 29 (4·0) | 2·5 (1·7–3·5) | 30 (4·1) | 3·0 (2·0–4·3) |
| Nasopharyngitis | 251 (25·2) | 14·2 (12·5–16·0) | 144 (19·7) | 14·2 (12·0–16·7) | 152 (20·9) | 17·7 (15·0–20·8) |
| Upper respiratory tract infection | 156 (15·7) | 7·9 (6·7–9·3) | 79 (10·8) | 7·2 (5·7–9·0) | 89 (12·2) | 9·7 (7·8–11·9) |
| Urinary tract infection | 50 (5·0) | 2·3 (1·7–3·0) | 25 (3·4) | 2·1 (1·4–3·1) | 28 (3·8) | 2·8 (1·9–4·1) |
| Musculoskeletal and connective tissue disorders | 237 (23·8) | 12·8 (11·2–14·6) | 136 (18·6) | 13·0 (10·9–15·3) | 124 (17·0) | 14·1 (11·7–16·8) |
| Arthralgia | 67 (6·7) | 3·1 (2·4–4·0) | 37 (5·1) | 3·2 (2·2–4·4) | 30 (4·1) | 3·0 (2·1–4·3) |
| Back pain | 55 (5·5) | 2·6 (1·9–3·3) | 32 (4·4) | 2·7 (1·9–3·9) | 25 (3·4) | 2·5 (1·6–3·7) |
| Skin and subcutaneous tissue disorders | 224 (22·5) | 11·7 (10·2–13·4) | 117 (16·0) | 10·7 (8·8–12·8) | 127 (17·4) | 14·0 (11·7–16·7) |
| Psoriasisb | 61 (6·1) | 2·8 (2·1–3·6) | 35 (4·8) | 2·9 (2·0–4·1) | 35 (4·8) | 3·5 (2·4–4·9) |
| Gastrointestinal disorders | 211 (21·2) | 11·1 (9·7–12·7) | 109 (14·9) | 10·2 (8·3–12·2) | 115 (15·8) | 12·8 (10·6–15·4) |
| Injury, poisoning and procedural complications | 194 (19·5) | 10·0 (8·7–11·6) | 107 (14·6) | 9·9 (8·1–11·9) | 99 (13·6) | 10·8 (8·8–13·1) |
| Investigations | 178 (17·9) | 9·1 (7·8–10·5) | 84 (11·5) | 7·5 (6·0–9·3) | 109 (15·0) | 12·0 (9·8–14·5) |
| Nervous system disorders | 158 (15·9) | 8·1 (6·9–9·4) | 71 (9·7) | 6·4 (5·0–8·0) | 92 (12·6) | 10·0 (8·1–12·3) |
| Headache | 64 (6·4) | 3·0 (2·3–3·9) | 32 (4·4) | 2·7 (1·9–3·9) | 36 (4·9) | 3·7 (2·6–5·1) |
| General disorders and administration site conditions | 136 (13·7) | 6·8 (5·7–8·0) | 55 (7·5) | 4·8 (3·6–6·3) | 84 (11·5) | 9·1 (7·3–11·3) |
| Respiratory, thoracic and mediastinal disorders | 125 (12·6) | 6·2 (5·1–7·3) | 59 (8·1) | 5·2 (3·9–6·7) | 76 (10·4) | 8·1 (6·3–10·1) |
| Metabolism and nutrition disorders | 105 (10·6) | 5·1 (4·1–6·1) | 58 (7·9) | 5·1 (3·9–6·6) | 54 (7·4) | 5·6 (4·2–7·3) |
| Vascular disorders | 99 (9·9) | 4·8 (3·9–5·8) | 47 (6·4) | 4·1 (3·0–5·4) | 56 (7·7) | 5·9 (4·4–7·6) |
| Hypertension | 70 (7·0) | 3·3 (2·6–4·2) | 33 (4·5) | 2·9 (2·0–4·0) | 38 (5·2) | 3·9 (2·8–5·4) |
| Renal and urinary disorders | 64 (6·4) | 3·0 (2·3–3·8) | 34 (4·7) | 2·9 (2·0–4·0) | 36 (4·9) | 3·6 (2·5–5·0) |
| Eye disorders | 55 (5·5) | 2·6 (1·9–3·3) | 23 (3·1) | 1·9 (1·2–1·9) | 35 (4·8) | 3·5 (2·5–4·9) |
| Neoplasms benign, malignant and unspecified | 52 (5·2) | 2·4 (1·8–3·2) | 29 (4·0) | 2·5 (1·6–3·5) | 27 (3·7) | 2·7 (1·8–4·0) |
| Psychiatric disorders | 52 (5·2) | 2·4 (1·8–3·2) | 25 (3·4) | 2·1 (1·4–3·1) | 28 (3·8) | 2·8 (1·9–4·1) |
| Reproductive system and breast disorders | 50 (5·0) | 2·3 (1·7–3·1) | 30 (4·1) | 2·5 (1·7–3·6) | 23 (3·2) | 2·3 (1·5–3·5) |
Patients who received both CZP 200 mg every 2 weeks (Q2W) and CZP 400 mg Q2W are included once in the population count for the ‘all CZP’ group. CI, confidence interval; IR, incidence rate of new cases; PY, patient‐years. aMedical Dictionary for Regulatory Activities (MedDRA) version 18·1. bNew or worsening psoriasis. cThe number of patients who reported at least one TEAE in the category.
Deaths
Seven deaths were reported (Table 2), two of which were considered related to study treatment by the investigator. One patient had acute myocardial infarction; they were obese (body mass index ≥ 30 kg m−2) at the time of the event, had pre‐existing hypertension and atherosclerosis, and had ongoing tobacco use. The second patient had a cardiac arrest due to liver failure and vasodilatory shock in association with haemorrhagic pancreatic necrosis (the patient was obese and had pre‐existing hepatic cirrhosis and a history of gastritis). The other five deaths were considered unrelated to study treatment by the investigators, reported under the following MedDRA preferred terms (each reported by one patient): (i) pneumonia Legionella in a patient > 65 years of age with multiple comorbidities (asthma, previous congestive heart failure, coronary artery disease on dual antiplatelet therapy, hypertension, hyperlipidaemia and history of pneumonia; this event was also considered an SIE); (ii) cirrhosis alcoholic; (iii) chronic obstructive pulmonary disease; (iv) craniocerebral injury (due to motor vehicle accident); and (v) multiple injuries (due to motor vehicle accident).
Serious infectious events and opportunistic infections
There were 41 SIEs reported in 32 patients receiving CZP; the IRs of SIEs were similar between the groups receiving CZP 200 mg Q2W and CZP 400 mg Q2W (Table 5). The cumulative IR of SIEs remained stable over time, regardless of dose (Figure 3). The most commonly reported SIE was pneumonia, reported in four patients (two each within the CZP 200 mg Q2W and CZP 400 mg Q2W dose groups). The other most common SIEs were bronchitis, cellulitis, endophthalmitis, erysipelas and infected bite, each reported in two patients.
Table 5.
Summary of selected treatment‐emergent adverse events (TEAEs) of interest
| Total exposure, PY | All CZP (N = 995) | CZP 200 mg Q2W (N = 731) | CZP 400 mg Q2W (N = 728) |
|---|---|---|---|
| 2231·3 | 1211·4 | 1019·9 |
| n (%) | IR (95% CI) | n (%) | IR (95% CI) | n (%) | IR (95% CI) | |
|---|---|---|---|---|---|---|
| Serious infectious events | 32 (3·2) | 1·5 (1·0–2·1) | 16 (2·2) | 1·3 (0·8–2·2) | 16 (2·2) | 1·6 (0·9–2·6) |
| Opportunistic infections | 4 (0·4) | 0·2 (0·1–0·5) | 1 (0·1) | 0·1 (0·0–0·5) | 3 (0·4) | 0·3 (0·1–0·9) |
| Active tuberculosis | 1 (0·1) | 0·0 (0·0–0·3) | 0 | 0 | 1 (0·1) | 0·1 (0·0–0·6) |
| Fungal oesophagitis | 1 (0·1) | 0·0 (0·0–0·3) | 1 (0·1) | 0·1 (0·0–0·5) | 0 | 0·0 |
| Pneumonia Legionella | 1 (0·1) | 0·0 (0·0–0·3) | 0 | 0·0 | 1 (0·1) | 0·1 (0·0–0·6) |
| Bacteraemia | 1 (0·1) | 0·0 (0·0–0·3) | 0 | 0·0 | 1 (0·1) | 0·1 (0·0–0·6) |
| All malignancies | 14 (1·4) | 0·6 (0·3–1·1) | 8 (1·1) | 0·7 (0·3–1·3) | 8 (1·1) | 0·8 (0·3–1·6) |
| Malignancies excluding NMSC | 10 (1·0) | 0·5 (0·2–0·8) | 7 (1·0)a | 0·6 (0·2–1·2) | 4 (0·5)b | 0·4 (0·1–1·0) |
| NMSC | 5 (0·5) | 0·2 (0·1–0·5) | 1 (0·1)c | 0·1 (0·0–0·5) | 4 (0·5)d | 0·4 (0·1–1·0) |
| MACEs | 9 (0·9) | 0·4 (0·2–0·8) | 5 (0·7)e | 0·4 (0·1–1·0) | 4 (0·5)f | 0·4 (0·1–1·0) |
| Congestive heart failure | 1 (0·1) | 0·0 (0·0–0·3) | 0 | 0 | 1 (0·1) | 0·1 (0·0–0·6) |
| Demyelinating‐like disorders | 2 (0·2) | 0·1 (0·0–0·3) | 1 (0·1) | 0·1 (0·0–0·5) | 1 (0·1) | 0·1 (0·0–0·6) |
| Serious psoriatic conditionsg | 6 (0·6) | 0·3 (0·1–0·6) | 3 (0·4) | 0·3 (0·1–0·7) | 3 (0·4) | 0·3 (0·1–0·9) |
| Psoriasis | 3 (0·3) | 0·1 (0·0–0·4) | 2 (0·3) | 0·2 (0·0–0·6) | 1 (0·1) | 0·1 (0·0–0·6) |
| Erythrodermic psoriasis | 1 (0·1) | 0·0 (0·0–0·3) | 0 | 0 | 1 (0·1) | 0·1 (0·0–0·6) |
| Guttate psoriasis | 1 (0·1) | 0·0 (0·0–0·3) | 0 | 0 | 1 (0·1) | 0·1 (0·0–0·6) |
| Pustular psoriasis | 1 (0·1) | 0·0 (0·0–0·3) | 1 (0·1) | 0·1 (0·0–0·5) | 0 | 0 |
| Serious haematopoietic cytopenia | 1 (0·1) | 0·0 (0·0–0·3) | 1 (0·1)h | 0·1 (0·0–0·5) | 0 | 0 |
| Serious bleeding events | 11 (1·1) | 0·5 (0·3–0·9) | 7 (1·0)i | 0·6 (0·2–1·2) | 4 (0·5)j | 0·4 (0·1–1·0) |
Patients who received both certolizumab pegol (CZP) 200 mg every 2 weeks (Q2W) and CZP 400 mg Q2W are included once in the population count for the ‘all CZP’ group; n (%) refers to the number of patients who reported at least one TEAE in the category. CI, confidence interval; IR, incidence rate of new cases per 100 patient years; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer. aIncludes one each of breast cancer, glioblastoma, Hodgkin disease, laryngeal cancer, non‐small cell lung cancer, oropharyngeal squamous cell carcinoma and prostate cancer. bIncludes one each of adenocarcinoma of colon, anaplastic oligodendroglioma, prostate cancer and clear cell renal cell carcinoma. cOne basal cell carcinoma. dIncludes three basal cell carcinomas and one keratoacanthoma. eIncludes one each of acute myocardial infarction, angina pectoris and cerebrovascular accident, and two transient ischaemic attack. fIncludes one each of heart failure, congestive heart failure, acute coronary syndrome and extradural haematoma. gNew or worsening psoriasis events classified as serious. hOne abnormal blood count. iOne each of disseminated intravascular coagulation, splenic haematoma, haemorrhagic necrotic pancreatitis, rectal haemorrhage, urinary bladder haemorrhage, menorrhagia, genital haemorrhage, epistaxis and haemothorax (can be more than one per patient). jOne each of haemorrhoidal haemorrhage, upper gastrointestinal haemorrhage, haematoma infection, extradural haematoma, contusion and purpura (can be more than one per patient).
Figure 3.
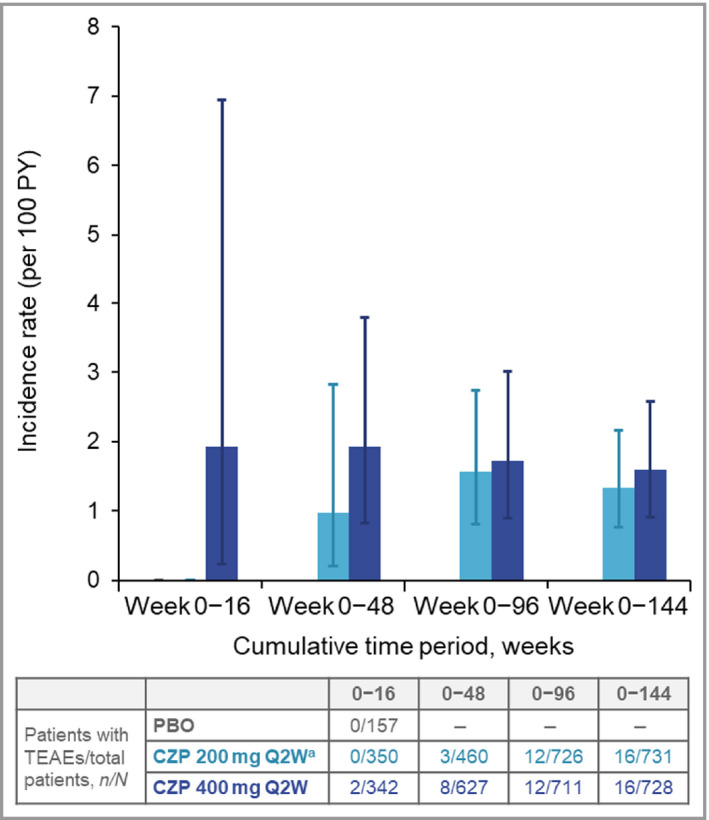
Cumulative incidence rates of serious infections to week 144. CZP, certolizumab pegol; PBO, placebo; PY, patient‐years; Q2W, every 2 weeks; TEAE, treatment‐emergent adverse event. aPatients on CZP 200 mg Q2W received a loading dose of CZP 400 mg at weeks 0, 2 and 4. Error bars correspond to 95% confidence intervals.
The IR of opportunistic infections was 0·2 per 100 PY, with a total of four events reported (Table 5). These were the fatal case of pneumonia Legionella described above, one case of severe bacteraemia secondary to Eggerthella lenta assessed as not related to study treatment, one case of fungal oesophagitis and one case of active TB. This case of TB was reported in a patient enrolled in CIMPACT who received etanercept during the initial 16‐week period before escaping to CZP 400 mg Q2W. TB was diagnosed 172 days after etanercept initiation and 60 days after CZP initiation, and the patient was discontinued from the study. Before study entry the QuantiFERON‐TB Gold test was negative and chest X‐ray was normal; the patient lived in a country with a high prevalence of TB.
Malignancies
There were 16 malignancies in 14 CZP‐treated patients (IR 0·6 per 100 PY). Excluding nonmelanoma skin cancer (NMSC), 11 malignancies were reported in 10 patients (IR 0·5 per 100 PY), including one case of Hodgkin lymphoma (in a patient receiving CZP 200 mg Q2W) (Table 5). The incidences of malignancies excluding NMSC were comparable between the two CZP doses (Table 5) and across cumulative exposure periods (Figure 4). No cases of melanoma were reported. The five cases of NMSC (IR 0·2 per 100 PY) comprised four basal cell carcinomas and one keratoacanthoma (Table 5).
Figure 4.
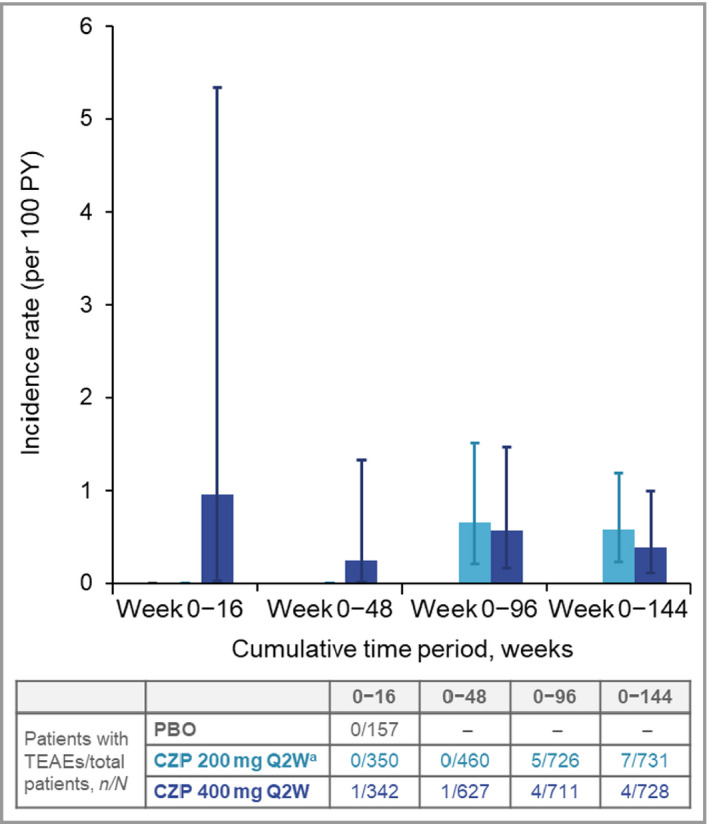
Cumulative incidence rates of malignancies (excluding nonmelanoma skin cancer) to week 144. CZP, certolizumab pegol; PBO, placebo; PY, patient‐years; Q2W, every 2 weeks; TEAE, treatment‐emergent adverse event. aPatients on CZP 200 mg Q2W received a loading dose of CZP 400 mg at weeks 0, 2 and 4. Error bars correspond to 95% confidence intervals.
Major adverse cardiovascular events
The incidence of MACEs over 144 weeks of CZP exposure was similar between the two dose groups, with nine cases reported overall (IR 0·4 per 100 PY). This included one case of congestive heart failure in the CZP 400 mg Q2W group (Table 5).
Demyelinating disorders
There were two demyelinating events: one case of multiple sclerosis reported in the CZP 200 mg Q2W group and one case of primary progressive multiple sclerosis in the CZP 400 mg Q2W group (the patient’s medical history indicated that the symptoms pre‐dated study entry) (Table 5).
New or worsening psoriasis
Overall, there were 95 cases of new or worsening psoriasis reported in 73 patients (IR 3·4 per 100 PY, 95% CI 2·6–4·2), with a similar incidence between CZP 200 mg Q2W (IR 3·4 per 100 PY, 95% CI 2·5–4·6) and CZP 400 mg Q2W (IR 4·2 per 100 PY, 95% CI 3·0–5·7). Six of these events were classified as serious (Table 5).
There were two cases of dermatitis psoriasiform, one in each CZP dose group. Eczema was reported in 12 patients (IR 0·5 per 100 PY, 95% CI 0·3–1·0), with a similar incidence between CZP dose groups: 0·6 per 100 PY (95% CI 0·2–1·2) for CZP 200 mg Q2W and 0·6 per 100 PY (95% CI 0·2–1·3) for CZP 400 mg Q2W. All cases of dermatitis psoriasiform and eczema were mild to moderate in intensity and none was classified as serious.
Other treatment‐emergent adverse events of interest
Overall, there was one serious event of haematopoietic cytopenia (blood count abnormal in the CZP 200 mg Q2W group), no lupus or lupus‐like events, and no serious skin reactions. One case of anaphylactoid reaction was reported in the CZP 400 mg Q2W group (considered related to study drug). There were no cases of hypersensitivity reaction. The overall incidence of serious bleeding events was low (IR 0·5 per 100 PY), with a total of 15 events reported in 11 patients (IRs were similar between the two dose groups) (Table 5).
In total, 101 CZP‐treated patients reported 212 hepatic events (IR 4·8 per 100 PY, 95% CI 3·9–5·9). Except for one patient in the CZP 200 mg Q2W group who had an SAE of drug‐induced liver injury (associated with TEAEs of chronic pancreatitis and cholecystitis), most hepatic events were nonserious, mild or moderate in intensity, considered not related to the study drug by the investigator, and did not result in study discontinuation. Thirty‐five patients met liver function test criteria of elevated bilirubin [≥ 1·5 × upper limit of normal (ULN)] and 65 patients had either elevated alanine transaminase (ALT) or elevated aspartate transaminase (AST) (≥ 3 × ULN). None met Hy’s Law criteria (bilirubin elevation ≥ 2 × ULN and ALT or AST elevation ≥ 3 × ULN at the same visit), except for the patient described above who died from cardiac arrest in association with hepatic failure.
Six pregnancies with known maternal CZP exposure were reported across the three studies. The pregnancy outcomes included four live births: three without malformation (two with maternal CZP exposure in the first trimester only and one in the first and second trimesters) and one with ankyloglossia congenital (a familial/genetic disorder; maternal CZP exposure during the first trimester). The other two pregnancies resulted in one elective termination and one spontaneous abortion (both with maternal CZP exposure in the first and second trimesters).
Discussion
Control of psoriasis requires long‐term treatment. The safety data presented here, comprising 2231·3 PY of exposure, represent the longest report to date of CZP safety in patients with moderate‐to‐severe plaque psoriasis. No new safety signals were identified compared with the safety profile of CZP in other approved indications, or other anti‐TNF medications approved for the treatment of moderate‐to‐severe plaque psoriasis. 26 , 27 , 28
At week 144, IRs of TEAEs and SAEs were lower than the cumulative rates at weeks 16 and 48, suggesting that the risk of AEs does not increase with longer CZP exposure. Furthermore, IRs of TEAEs and SAEs were comparable between CZP 200 mg Q2W and CZP 400 mg Q2W; no individual system organ class showed a difference > 5% in incidence between dose groups. These data indicate that there is no increased risk associated with exposure to CZP 400 mg Q2W compared with CZP 200 mg Q2W.
Infections were the most common TEAE, consistent with other biologics in psoriasis. 4 , 29 , 30 , 31 , 32 , 33 , 34 The overall IR of SIEs reported here (1·5 per 100 PY) was within the range of serious infection rates reported for biologics in real‐world psoriasis registries (< 0·6 to 2·0 per 100 PY). 35 , 36 Treatment with certain biologics has been specifically linked to greater risk of opportunistic infection, 37 , 38 and current psoriasis treatment guidelines recommend ongoing assessment of patients treated with biologics for risk factors, signs and symptoms of infection, particularly TB. 39 , 40 Here, four cases of opportunistic infection were reported overall, including one case of active TB in a patient from a country with a high prevalence of TB (a known risk factor), and a fatal case of pneumonia Legionella in a patient with multiple risk factors for serious infection (including older age, chronic asthma and prior pneumonia).
The risk of malignancy is particularly relevant for patients with psoriasis, in whom higher incidences of malignancies such as lymphoma, NMSC and cancers of the oropharynx, larynx, liver and bladder have been reported compared with the general population. 41 , 42 , 43 , 44 , 45 The IR of NMSC reported here (0·2 per 100 PY) was not increased compared with the general population of people with psoriasis (0·35–1·29 per 100 PY), 46 , 47 and was comparable with that reported for other biologics. 30 , 48 The IRs of malignancies excluding NMSC (0·6 per 100 PY and 0·4 per 100 PY for the CZP 200 mg and 400 mg Q2W groups, respectively) remained stable over time and were comparable with the estimated IR of first malignancy among patients with psoriasis in the Psoriasis Longitudinal Assessment and Registry (0·55 per 100 PY). 49 This result was also consistent with the previous safety analysis of CZP across indications, which reported IRs of 0·41–0·77 per 100 PY. 24 Compared with the general population, patients with psoriasis tend to have a higher exposure to ultraviolet radiation through receipt of phototherapy, which may increase the risk of melanoma. Although 48% of CZP‐treated patients in this analysis reported prior phototherapy or chemophototherapy at baseline, no cases of melanoma were reported.
Seven deaths were reported in this analysis (IR 0·3 per 100 PY), including two cases due to cardiac causes in patients with pre‐existing cardiovascular risk factors. This is in line with the estimated standardized mortality rate for an age‐ and sex‐matched population (IR 0·35 per 100 PY), 24 and similar findings have been reported for adalimumab. 50 The IRs of other TEAEs of interest, including MACEs, were consistent with those previously reported for psoriasis in the CZP cross‐indication study. 24
Although previous week 16 analyses of these three CZP trials in plaque psoriasis have shown no differences between placebo and CZP in the number and type of AEs, 21 due to the study design, placebo data were not available after week 16. As with all clinical trials, interpretation of the data presented here is further limited by the strict inclusion and exclusion criteria whereby this patient population might be expected to have fewer comorbidities than would be seen in the real world. 51 , 52 The safety of CZP in psoriasis should be continually assessed in clinical practice.
In this pooling of phase III trial data to assess the long‐term safety of CZP in plaque psoriasis over 3 years, no new safety signals were identified compared with previously reported data for CZP in other indications, and with other anti‐TNF medications approved for psoriasis. Risk of TEAEs did not increase with longer CZP exposure, and the safety profiles of the two dose groups were similar. While anti‐TNF biologics remain a mainstay of treatment for moderate‐to‐severe plaque psoriasis due to their well‐established safety and efficacy profiles, further comparative studies are needed to investigate fully the risk and benefit of all biologics, which could be utilized to support the development of treatment guidelines by professional organizations.
Supporting information
Figure S1 Study design of the CIMPASI‐1, CIMPASI‐2 and CIMPACT phase III trials.
Powerpoint S1 Journal Club Slide Set.
Acknowledgments
The authors thank the patients, and the investigators and their teams who took part in this study. The authors also acknowledge Cécile Ecoffet, PharmD, of UCB Pharma, Brussels, Belgium, for critical review during the development of this manuscript; Sarah Kavanagh, MPH, for statistical analyses; Amelia Frizell‐Armitage, PhD, and Ricardo Milho, PhD, from Costello Medical, Cambridge, UK, for medical writing and editorial assistance in preparing this manuscript for publication, based on the authors’ input and direction; and Susanne Wiegratz, Dipl‐Biol, MSc, of UCB Pharma, Monheim, Germany, for publication coordination. Richard Warren is supported by the Manchester NIHR Biomedical Research Centre.
Cumulative incidence rates of malignanciesAppendix
Conflicts of interest. A.B. has served as a scientific adviser and/or clinical study investigator for AbbVie, Aclaris, Allergan, Almirall, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, Eli Lilly and Company, FLX Bio, Forte, Galderma, Janssen, LEO, Novartis, Ortho, Pfizer, Regeneron, Sandoz, Sanofi Genzyme, Sun Pharma and UCB Pharma; and as a paid speaker for AbbVie. C.P. has served as a scientific adviser–consultant and/or clinical study investigator for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dermira, Eli Lilly and Company, GlaxoSmithKline, Janssen, LEO, Merck Sharp & Dohme, Novartis, Pierre Fabre, Pfizer, Regeneron, Sandoz, Sanofi Genzyme and UCB Pharma. P.v.d.K. has received fees for consultancy services or lectureships from Celgene, Almirall, AbbVie, Amgen, Eli Lilly, Novartis, Janssen, LEO Pharma, Bristol Myers Squibb and Dermavant. R.B.W. has acted as a consultant and/or speaker for AbbVie, Almirall, Amgen, Avillion, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly and Company, GlaxoSmithKline, Janssen, LEO Pharma, Novartis, Sanofi Genzyme and UCB Pharma. A.B.G. has current consulting or advisory board agreements with Janssen, Celgene Corporation, Beiersdorf, Bristol Myers Squibb Co., AbbVie, UCB Pharma, Novartis, Incyte, Lilly, Reddy Labs, Valeant, Dermira Inc., Allergan, Sun Pharmaceutical Industries, XBiotech (no personal compensation), LEO, Avotres Therapeutics and Boehringer Ingelheim; and research and educational grants from Janssen, Incyte, Novartis, XBiotech, UCB Pharma and Boehringer Ingelheim. R.G.L. has served as a principal investigator, on scientific advisory boards and/or as a speaker for AbbVie, Amgen, Centocor, Pfizer, Janssen, LEO Pharma, Boehringer Ingelheim International GmbH, Eli Lilly and Company, UCB Pharma, Novartis, Celgene and Valeant Pharmaceuticals. F.B., C.A. and M.B. are employees of UCB Pharma. M.L. has received honoraria from Allergan, Aqua, LEO Pharma and Promius; and is an employee of Mount Sinai, which receives research funds from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen/Johnson & Johnson, Kadmon, MedImmune/AstraZeneca, Novartis, Pfizer, Valeant and Vidac. K.R. has received honorarium from AbbVie, Affibody, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Covagen, Eli Lilly, Forward Pharma, GSK, Janssen‐Cilag, Kyowa Kirin, LEO Pharma, Medac, Merck Sharp & Dohme Corp., Novartis, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Takeda, UCB Pharma, Valeant and Xenoport.
Appendix 2.
Data sharing statement. The underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient‐level data and redacted trial documents, which may include analysis‐ready datasets, study protocols, annotated case report forms, statistical analysis plans, dataset specifications and clinical study reports. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password‐protected portal.
Funding sources
This article was based on the original studies CIMPASI‐1 (NCT02326298) and CIMPASI‐2 (NCT02326272) sponsored by Dermira, Inc. and UCB Pharma. UCB is the regulatory sponsor of certolizumab pegol in plaque psoriasis. Support for third‐party writing assistance for this article, provided by Amelia Frizell‐Armitage, PhD, and Ricardo Milho, PhD, of Costello Medical, Cambridge, UK, was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Conflicts of interest statements can be found in Appendix 1.
References
- 1. Danielsen K, Olsen AO, Wilsgaard T et al. Is the prevalence of psoriasis increasing? A 30‐year follow‐up of a population‐based cohort. Br J Dermatol 2013; 168:1303–10. [DOI] [PubMed] [Google Scholar]
- 2. Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol 2009; 60:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 2014; 70:512–16. [DOI] [PubMed] [Google Scholar]
- 4. Sbidian E, Chaimani A, Garcia‐Doval I et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta‐analysis. Cochrane Database Syst Rev 2017; 12:CD011535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ash Z, Gaujoux‐Viala C, Gossec L et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta‐analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012; 71:319–26. [DOI] [PubMed] [Google Scholar]
- 6. Miligkos M, Papamichael K, Vande Casteele N et al. Efficacy and safety profile of anti‐tumor necrosis factor‐α versus anti‐integrin agents for the treatment of Crohn’s disease: a network meta‐analysis of indirect comparisons. Clin Ther 2016; 38:1342–58. [DOI] [PubMed] [Google Scholar]
- 7. Singh JA, Hossain A, Tanjong Ghogomu E et al. Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease‐modifying anti‐rheumatic drugs: a systematic review and network meta‐analysis. Cochrane Database Syst Rev 2016; 2016:CD012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bongartz T, Sutton AJ, Sweeting MJ et al. Anti‐TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta‐analysis of rare harmful effects in randomized controlled trials. JAMA 2006; 295:2275–85. [DOI] [PubMed] [Google Scholar]
- 9. Burmester GR, Mease P, Dijkmans BA et al. Adalimumab safety and mortality rates from global clinical trials of six immune‐mediated inflammatory diseases. Ann Rheum Dis 2009; 68:1863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cleynen I, Vermeire S. Paradoxical inflammation induced by anti‐TNF agents in patients with IBD. Nat Rev Gastroenterol Hepatol 2012; 9:496. [DOI] [PubMed] [Google Scholar]
- 11. Girolomoni G, Altomare G, Ayala F et al. Safety of anti‐TNFα agents in the treatment of psoriasis and psoriatic arthritis. Immunopharmacol Immunotoxicol 2012; 34:548–60. [DOI] [PubMed] [Google Scholar]
- 12. Guinard E, Bulai Livideanu C, Barthélémy H et al. Active tuberculosis in psoriasis patients treated with TNF antagonists: a French nationwide retrospective study. J Eur Acad Dermatol Venereol 2016; 30:1336–41. [DOI] [PubMed] [Google Scholar]
- 13. Kamata M, Tada Y. Safety of biologics in psoriasis. J Dermatol 2018; 45:279–86. [DOI] [PubMed] [Google Scholar]
- 14. Kemanetzoglou E, Andreadou E. CNS demyelination with TNF‐α blockers. Curr Neurol Neurosci Rep 2017; 17:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winthrop KL. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat Rev Rheumatol 2006; 2:602. [DOI] [PubMed] [Google Scholar]
- 16. Electronic Medicines Compendium . Cimzia 200 mg solution for injection in pre‐filled pen. Available at: http://www.medicines.org.uk/emc/medicine/32367 (last accessed 25 June 2020).
- 17. UCB. CIMZIA . Highlights of prescribing information. Available at: https://www.cimzia.com/themes/custom/cimzia/docs/CIMZIA_full_prescribing_information.pdf (last accessed 24 June 2020).
- 18. Baker T, Kevorkian L, Nesbitt A. Investigation into the binding affinity of certolizumab pegol to FcRn and functional consequences for FcRn‐mediated transcytosis: comparison to infliximab, adalimumab and etanercept. Ann Rheum Dis 2013; 72:A426. [Google Scholar]
- 19. Mariette X, Forger F, Abraham B et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis 2018; 77:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clowse ME, Forger F, Hwang C et al. Minimal to no transfer of certolizumab pegol into breast milk: results from CRADLE, a prospective, postmarketing, multicentre, pharmacokinetic study. Ann Rheum Dis 2017; 76:1890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blauvelt A, Reich K, Lebwohl M et al. Certolizumab pegol for the treatment of patients with moderate‐to‐severe chronic plaque psoriasis: pooled analysis of week 16 data from three randomized controlled trials. J Eur Acad Dermatol Venereol 2019; 33:546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gottlieb AB, Blauvelt A, Thaçi D et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double‐blinded, placebo‐controlled studies (CIMPASI‐1 and CIMPASI‐2). J Am Acad Dermatol 2018; 79:302–14. [DOI] [PubMed] [Google Scholar]
- 23. Lebwohl M, Blauvelt A, Paul C et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks of a phase 3, multicenter, randomized, double‐blind, etanercept‐and placebo‐controlled study (CIMPACT). J Am Acad Dermatol 2018; 79:266–76. [DOI] [PubMed] [Google Scholar]
- 24. Curtis JR, Mariette X, Gaujoux‐Viala C et al. Long‐term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn’s disease: a pooled analysis of 11 317 patients across clinical trials. RMD Open 2019; 5:e000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. US Food and Drug Administration . Reporting serious problems to the FDA: what is a serious adverse event? 2012. Available at: https://www.fda.gov/safety/medwatch/howtoreport/ucm053087.htm (last accessed 24 June 2020).
- 26. Gordon K, Papp K, Poulin Y et al. Long‐term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open‐label extension study for patients from REVEAL. J Am Acad Dermatol 2012; 66:241–51. [DOI] [PubMed] [Google Scholar]
- 27. Reich K, Nestle FO, Papp K et al. Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet 2005; 366:1367–74. [DOI] [PubMed] [Google Scholar]
- 28. Tyring S, Gordon KB, Poulin Y et al. Long‐term safety and efficacy of 50mg of etanercept twice weekly in patients with psoriasis. JAMA Dermatol 2007; 143:719–26. [DOI] [PubMed] [Google Scholar]
- 29. Reich K, Warren RB, Iversen L et al. Long‐term efficacy and safety of tildrakizumab for moderate‐to‐severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br J Dermatol 2020; 182:605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leonardi C, Papp K, Strober B et al. Comprehensive long‐term safety of adalimumab from 18 clinical trials in adult patients with moderate‐to‐severe plaque psoriasis. Br J Dermatol 2019; 180:76–85. [DOI] [PubMed] [Google Scholar]
- 31. Deodhar A, Mease PJ, McInnes IB et al. Long‐term safety of secukinumab in patients with moderate‐to‐severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post‐marketing surveillance data. Arthritis Res Ther 2019; 21:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puig L, Lebwohl M, Bachelez H et al. Long‐term efficacy and safety of brodalumab in the treatment of psoriasis: 120‐week results from the randomized, double‐blind, placebo‐ and active comparator‐controlled phase 3 AMAGINE‐2 trial. J Am Acad Dermatol 2020; 82:352–9. [DOI] [PubMed] [Google Scholar]
- 33. Langley RG, Kimball AB, Nak H et al. Long‐term safety profile of ixekizumab in patients with moderate‐to‐severe plaque psoriasis: an integrated analysis from 11 clinical trials. J Eur Acad Dermatol Venereol 2019; 33:333–9. [DOI] [PubMed] [Google Scholar]
- 34. Reich K, Papp KA, Armstrong AW et al. Safety of guselkumab in patients with moderate‐to‐severe psoriasis treated through 100 weeks: a pooled analysis from the randomized VOYAGE 1 and VOYAGE 2 studies. Br J Dermatol 2019; 180:1039–49. [DOI] [PubMed] [Google Scholar]
- 35. Kalb RE, Fiorentino DF, Lebwohl MG et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol 2015; 151:961–9. [DOI] [PubMed] [Google Scholar]
- 36. Strober B, Crowley J, Langley RG et al. Systematic review of the real‐world evidence of adalimumab safety in psoriasis registries. J Eur Acad Dermatol Venereol 2018; 32:2126–33. [DOI] [PubMed] [Google Scholar]
- 37. Singh JA, Wells GA, Christensen R et al. Adverse effects of biologics: a network meta‐analysis and Cochrane overview. Cochrane Database Syst Rev 2011; 2011:CD008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Winthrop KL, Novosad SA, Baddley JW et al. Opportunistic infections and biologic therapies in immune‐mediated inflammatory diseases: consensus recommendations for infection reporting during clinical trials and postmarketing surveillance. Ann Rheum Dis 2015; 74:2107–16. [DOI] [PubMed] [Google Scholar]
- 39. Menter A, Strober BE, Kaplan DH et al. Joint AAD‐NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol 2019; 80:1029–72. [DOI] [PubMed] [Google Scholar]
- 40. Smith CH, Jabbar‐Lopez ZK, Yiu ZZ et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol 2017; 177:628–36. [DOI] [PubMed] [Google Scholar]
- 41. Boffetta P, Gridley G, Lindelof B. Cancer risk in a population‐based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol 2001; 117:1531–7. [DOI] [PubMed] [Google Scholar]
- 42. Chen YJ, Wu CY, Chen TJ et al. The risk of cancer in patients with psoriasis: a population‐based cohort study in Taiwan. J Am Acad Dermatol 2011; 65:84–91. [DOI] [PubMed] [Google Scholar]
- 43. Frentz G, Olsen JH. Malignant tumours and psoriasis: a follow‐up study. Br J Dermatol 1999; 140:237–42. [DOI] [PubMed] [Google Scholar]
- 44. Gelfand JM, Shin DB, Neimann AL et al. The risk of lymphoma in patients with psoriasis. J Invest Dermatol 2006; 126:2194–201. [DOI] [PubMed] [Google Scholar]
- 45. Olsen JH, Møller H, Frentz G. Malignant tumors in patients with psoriasis. J Am Acad Dermatol 1992; 27:716–22. [DOI] [PubMed] [Google Scholar]
- 46. Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A et al. The risk of cancer in patients with psoriasis: a population‐based cohort study in the Health Improvement Network. JAMA Dermatol 2016; 152:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kimball AB, Schenfeld J, Accortt NA et al. Incidence rates of malignancies and hospitalized infectious events in patients with psoriasis with or without treatment and a general population in the U.S.A.: 2005–09. Br J Dermatol 2014; 170:366–73. [DOI] [PubMed] [Google Scholar]
- 48. Reich K, Mrowietz U, Radtke MA et al. Drug safety of systemic treatments for psoriasis: results from The German Psoriasis Registry PsoBest. Arch Dermatol Res 2015; 307:875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fiorentino D, Ho V, Lebwohl MG et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol 2017; 77:845–54. [DOI] [PubMed] [Google Scholar]
- 50. Burmester GR, Panaccione R, Gordon KB et al. Adalimumab: long‐term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis 2013; 72:517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kirsten N, Bulai Livideanu C, Richard MA et al. Inclusion and exclusion criteria in phase III trials with systemic agents in psoriasis: the external validity of drug development. Br J Dermatol 2016; 175:636–8. [DOI] [PubMed] [Google Scholar]
- 52. Masson Regnault M, Castañeda‐Sanabria J, Diep Tran MHT et al. Users of biologics in clinical practice: would they be eligible for phase III clinical studies? Cohort study in the French psoriasis registry PSOBIOTEQ. J Eur Acad Dermatol Venereol 2020; 34:293–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Study design of the CIMPASI‐1, CIMPASI‐2 and CIMPACT phase III trials.
Powerpoint S1 Journal Club Slide Set.


