Abstract
Background and Objectives
Genitourinary syndrome of menopause (GSM) is a common condition affecting of most postmenopausal women, which greatly impacks the quality of life,and need to treat. This prospective multicenter cohort study aimed to compare the efficacy and safety of the fractional carbon dioxide (CO2) laser with that of topical estrogen for vaginal treatment and relieving symptoms of genitourinary syndrome of menopause (GSM).
Study Design/Materials and Methods
This study included 162 postmenopausal patients who received vaginal laser or topical Estriol cream therapy between January 2017 and May 2019 at eight study centers in China. The degree of GSM‐related symptoms (vaginal burning, dryness, and dyspareunia) was evaluated using the Vaginal Health Index score (VHIS) and Visual Analog Scale (VAS) at baseline, 1, 3, 6, and 12 months posttreatment. The primary endpoint was the improvement in vaginal burning, dryness, and dyspareunia at 6 months after treatment. Multivariate logistic regression was used to compare the rate of improvement in the two groups.
Results
At baseline, the laser and control groups showed no significant difference in the mean age, time after menopause, and the VHIS (all P > 0.05). In the laser group, compared with baseline, significant differences were seen in the VHIS after the first or second treatment session and at 1, 3, 6, and 12 months posttreatment (P < 0.01). In the control group, compared with baseline, the VHIS showed significant differences after 1, 3, and 6 months of treatment (P < 0.05). However, there was no significant difference after 3 and 6 months of follow‐up between the two groups (P > 0.05). The VHIS scores were significantly higher after 1 month (16.63 ± 2.79 vs. 15.57 ± 2.43) and 12 months (15.72 ± 2.59 vs. 12.12 ± 4.08) of treatment in both the groups (P < 0.05). At 6 months after treatment, both groups showed improvement in vaginal burning, vaginal dryness, and dyspareunia (P > 0.05). The VAS findings at 6 months posttreatment were significantly different when compared with the pretreatment findings (P < 0.001). There were no significant adverse effects in the two groups.
Conclusions
Fractional CO2 laser vaginal treatment could be a safe and effective option for treating symptoms of GSM, including vaginal burning, dryness, and dyspareunia. The improvement in symptoms was comparable with that seen with topical estrogen therapy and lasted for at least 6–12 months posttreatment. Lasers Surg. Med. © 2020 Wiley Periodicals LLC
Keywords: genitourinary syndrome of menopause, vulvovaginal atrophy, vaginal burning, fractional CO2 laser, vaginal laser treatment
INTRODUCTION
Genitourinary syndrome of menopause (GSM) is a series of symptoms and clinical signs associated with the female genital tract caused by estrogen deficiency. It is characterized by atrophy of the mucous membranes and skin of the vulva, vagina, urethra, and bladder [1]. It is also known as vulvovaginal atrophy (VVA) and atrophic vaginitis. However, since VVA does not include the effects of estrogen deficiency on the urinary system, this condition has currently been named GSM [2]. With the increasing life expectancy in China, postmenopausal women are likely to live in an estrogen‐deficient state for more than one‐third of their lives. GSM is a common condition affecting 50%–80% of postmenopausal women. It greatly impacts the quality of life, sexual function, and causes significant emotional distress [3, 4]. The incidence of GSM increases with living longer after menopause, and the symptoms tend to worsen over time. Although the symptoms are unlikely to resolve without treatment, only around 20% of patients seek medical attention [5]. However, the number of postmenopausal women seeking treatment for GSM is rising with increasing requirements for a better quality of life.
With the decline in estrogen levels, the tissue of the vagina undergoes various changes, including thinning of the vaginal epithelium, narrowing of the vaginal canal, and loss of elasticity and rugations. Meanwhile, the vulvar area, particularly the mucous membrane of the vestibular, becomes atrophic and more vulnerable to bleeding and tearing in response to minimal trauma. The common manifestations include itching, burning, dryness, irritation, dysuria, and dyspareunia associated with the reduction of lubrication and elasticity of the vagina [1, 2]. At present, there are several treatment options for alleviating GSM symptoms. Symptomatic treatment is the mainstay therapy and includes vaginal hormonal medications, nonhormonal products, and behavioral modifications. Topical hormonal treatment is the first‐line therapy for improving vaginal symptoms and restoring epithelial integrity and vaginal flora [6]. However, hormonal therapy may also have side effects, such as the increased risk of venous thromboembolism and estrogen‐dependent gynecological malignancies, especially with oral hormones. Therefore, there is an increasing need for other treatments for GSM.
In recent years, fractional laser technology has been introduced and rapidly applied in various fields of medicine. It has become the most popular cosmetic (skin) and female reproductive function recovery technology. Laser technology has also been applied for the treatment of VVA. Previous studies have shown that fractional carbon dioxide (CO2) laser is effective and safe for improving vaginal symptoms associated with GSM [7, 8, 9, 10, 11]. A small, controlled study has shown that Er:YAG laser treatment successfully relieved symptoms of GSM, and the results were more pronounced and longer‐lasting compared with a topical Estriol treatment [12]. However, the previous study had some limitations, including small sample size, short follow‐up time, lack of large‐sample multicenter research, and the complications following vaginal laser treatment. We, therefore, conducted this multicenter prospective cohort study to compare the efficacy and safety of fractional CO2 laser treatment with topical estrogen therapy for vaginal symptoms and treatment of GSM.
MATERIALS AND METHODS
Study Design and Subjects
This prospective multicenter cohort study recruited 162 postmenopausal women who received vaginal fractional CO2 laser (laser group) or topical Estriol cream (control group) therapy between January 2017 and May 2019 at eight study centers (Peking University People's Hospital, Peking University Shenzhen Hospital, Changsha Hospital for Maternity and Child Healthcare, Sun Yat‐sen Memorial Hospital of Sun Yat‐sen University, West China Second University Hospital, the Third Affiliated Hospital of Zhengzhou University, Puyang Oilfield General Hospital, Liuzhou Maternity, and Child Healthcare) in China. The study protocol was approved by the Ethics Committee of the Peking University People's Hospital (2017PHB004‐01), and informed consent was obtained from each participant.
Inclusion and Exclusion Criteria
Patients who (i) were postmenopausal (ii) had GSM‐related symptoms, including vaginal dryness, vaginal burning, and dyspareunia with moderate or severe intensity, (iii) were not satisfied with previous local estrogen treatment, or (iv) had breast cancer treatment accompanied by GSM were included in the study. Patients who (i) received hormonal replacement therapy within 6 months, (ii) received vaginal medication within 1 month, (iii) had acute or recurrent genital tract or urinary tract infection, (iv) had chronic diseases affecting study compliance, and (v) suffered from mental disorders affecting the assessment were excluded from the study. Beyond these, the two groups also had individual exclusion criteria. Patients with pelvic organ prolapse greater than stage II or previous vaginal mesh implantation for pelvic floor reconstruction were excluded from the laser group. Patients with contraindications for the use of estrogen were excluded from the control group.
Pretreatment Examination
The patients underwent a gynecological examination, discharging test, and thin‐prep cytologic test for genital tract inflammation, herpes and vegetation, human papillomavirus, herpes simplex virus, cancer, and precancerous lesions before the treatment. In the laser group, a HiScan V2LR laser (10,600 nm wavelength) scanning system with a 360° probe (Monalisa Touch; DEKA Laser, Florence, Italy) was used for the vaginal wall treatment. One course of laser treatment included two or three laser sessions at an interval of approximately 4 ± 1 weeks. The treatment parameters were: power of 35–40 W, dwell time of 800–1,000 seconds, dot spacing of 800–1,000 µm, and single or double stack. The treatment was performed in the outpatient clinic without analgesia and anesthesia. Sexual intercourse was not permitted for 7 days after the laser treatment. In the control group, 0.5 g of vaginal, topical Estriol cream (15 g:15 ml; Organon (Dublin, Ireland) Ltd.) was administered once a day for the first week, then twice a week. The course of treatment was for 3 months.
Evaluation of Indicators and Follow‐Up
The primary endpoints of the study was improvement in vaginal burning, dryness, and dyspareunia at 6 months after treatment, and evaluate the vaginal health index score (VHIS) [6]. A Visual Analog Scale (VAS) (0–100 points) was used to evaluate the intensity of each GSM symptom before and after treatment. The VAS was also used to assess the degree of pain during the laser probe insertion and treatment. The symptoms and severity of GSM were evaluated by the doctor at each multicenter hospital, and complete regression was defined as a VAS of 0 points. The improvement in symptoms was defined as VAS < 10 points after treatment.
Patients were followed up at 1, 3, 6, and 12 months after the last treatment. Data on general information and medical history, including age, marital status, residence, education, sexual life, and the onset of menopausal, were collected. The VHIS and VAS scores for different postmenopausal symptoms, as well as adverse events before and after treatment, were recorded and evaluated for both the groups.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (x ± s) or median (interquartile range), and the count data were expressed using counts and percentages. The χ 2 test was used to compare the rate of symptom relief between the two groups, and multivariate logistic regression was used to compare the rate of relief from burning pain at 6 months after treatment between the two groups. t Test or Mann–Whitney U test and multiple linear regression were used to compare the VHIS scores at 6 months after treatment between the two groups. P < 0.05 was considered statistically significant.
RESULTS
Patient Characteristics
A total of 185 patients with GSM symptoms were eligible for inclusion in this study. Of these, while 13 patients received only one session of laser treatment, four and six patients in the laser and control groups, respectively, had no follow‐up. Finally, 162 patients (laser treatment: 108 and cream treatment: 54) were included in the analysis. The mean age and time after menopause were 56.56 ± 7.59 (38–80) and 8.16 ± 7.08 (1–33) years, respectively. The patient characteristics at baseline showed no significant difference between the two groups (P > 0.05) (Table 1).
Table 1.
Patient Characteristics
| Laser group | Control group | t Value | P value | |
|---|---|---|---|---|
| Age (year ± SD) | 56.07 ± 7.41 | 57.54 ± 7.91 | −1.158 | 0.249 |
| Time after menopause (year ± SD) | 7.68 ± 7.13 | 9.18 ± 6.94 | −1.238 | 0.218 |
| Education | ||||
| High school and below | 71/108 (65.7%) | 36/54 (66.7%) | 0.014 | 0.526 |
| University and above | 37/108 (34.3%) | 18/54 (33.3%) | ||
| Residence address | ||||
| City | 86/94 (91.5%) | 53/54 (98.1%) | 2.663 | 0.097 |
| Rural | 8/94 (8.5%) | 1/54 (1.9%) | ||
| Sexually active | ||||
| Yes | 66/94 (70.2%) | 36/52 (69.2%) | 0.015 | 0.523 |
| No | 28/94 (29.8%) | 16/52 (30.8%) |
SD, standard deviation.
In the laser group, 12 patients had urinary incontinence (UI), 12 had hypertension or coronary heart disease, 7 were postoperative cancer patients (3, 3, and 1 case of cervical, breast, and thyroid cancers, respectively), and 7 patients had recurrent GSM. In the control group, 4 patients had UI, 12 had hypertension or coronary heart disease, 1 had thyroid cancer, and 5 patients had recurrent GSM. In the laser group, 98 and 10 patients received 3 and 2 treatment sessions, respectively. A total of 99 (91.7%), 93 (86.2%), and 64 (59.3%) patients were followed up for 3, 6, and 12 months after laser treatment, respectively, while 35 patients (64.8%) were followed up for 12 months in the control group.
Efficacy of Fractional CO2 Laser Treatment
Improvement in VAS at 6 months posttreatment
The improvement rate for vaginal burning 6 months after treatment was 76.93% (60/78) in the laser group, while it was 77.78% (14/18) in the control group. No significant differences were seen between the two groups posttreatment (P > 0.05). The logistic regression analysis model showed that these rates were significantly associated with the pretreatment VAS for vaginal burning (P < 0.05), after adjusting for the time after menopause and pretreatment VHIS (P > 0.05) (Table 2).
Table 2.
Analysis of Factors That Influence VAS at 6 Months Posttreatment
| Exp (B) | 95% CI | ||||||
|---|---|---|---|---|---|---|---|
| B | SE | Wals | Sig. | Exp (B) | Upper | Lower | |
| Groups | −1.369 | 1.019 | 1.804 | 0.179 | 0.254 | 0.034 | 1.876 |
| Time after menopause | −0.019 | 0.155 | 0.015 | 0.903 | 0.981 | 0.724 | 1.329 |
| Pretreatment VHIS | −0.313 | 0.225 | 1.939 | 0.164 | 0.731 | 0.471 | 1.136 |
| VAS for vaginal burning pretreatment | 0.056 | 0.028 | 3.970 | 0.046 | 1.057 | 1.001 | 1.117 |
| Constant value | 2.027 | 2.997 | 0.457 | 0.499 | 7.590 |
CI, confidence interval; VAS, Visual Analog Scale; VHIS, Vaginal Health Index score.
VHIS before and after treatment
The VHIS after one, two, and three sessions and 3, 6, and 12 months posttreatment were significantly different compared with the pretreatment scores in both groups (P < 0.01). In the laser group, no significant difference was seen between 3 and 6 months posttreatment, and between 6 and 12 months posttreatment (P > 0.05). In the control group, the VHIS at 1, 3, and 6 months posttreatment was significantly increased compared with the pretreatment scores (P < 0.05). Additionally, the VHIS at 12 months posttreatment showed a significant decline compared with the scores at 3 and 6 months posttreatment (P < 0.05). In the laser group, the VHIS increased after the first session, further improving significantly at 3 months posttreatment, and lasting up to 6–12 months posttreatment. However, the VHIS at 12 months posttreatment had declined compared with the score at 3 months posttreatment (P < 0.05). Both approaches resulted in a significant improvement in GSM‐related symptoms, as shown in Table 3.
Table 3.
Comparison of the VHIS Before and After Treatment (χ ± s)
| VHIS | ||||||
|---|---|---|---|---|---|---|
| Group | Pretreatment | 1 month after the first session | 1 month after the second session | 1‐month posttreatment | 3 months posttreatment | 6 months posttreatment |
| Laser group | 11.19 ± 2.84 | 13.42 ± 2.94 | 15.35 ± 3.17 | 16.63 ± 2.79 | 17.01 ± 3.02 | 16.79 ± 3.55 |
| Control group | 11.24 ± 2.49 | 13.64 ± 1.94 | 14.95 ± 2.07 | 15.57 ± 2.43 | 16.23 ± 2.88 | 17.07 ± 2.97 |
| t Value | −0.122 | −0.443 | 0.721 | 2.140 | 1.334 | −0.28 |
| P value | 0.903 | 0.695 | 0.472 | 0.034 | 0.184 | 0.780 |
In the laser group, the VHIS was compared between (i) posttreatment and pretreatment as well as 1 month after the first session, P < 0.01, and (ii) 3 and 12 months posttreatment, P < 0.05.
In the control group, the VHIS was compared between (i) posttreatment and pretreatment, P < 0.05, (ii) 3 and 12 months posttreatment, P < 0.05, and (iii) 6 and 12 months posttreatment, P < 0.05.
VHIS, Vaginal Health Index score.
VHIS in the two groups
While a significant difference was seen in the VHIS between the two groups 1 month after treatment (P < 0.05), no significant difference was seen at 3 and 6 months posttreatment (P > 0.05). The VHIS at 12 months posttreatment was better in the laser group (15.72 ± 2.59) compared with the control group (12.12 ± 4.08) (P < 0.05). Linear regression analysis showed that the VHIS at 6 months posttreatment had a significant association with the scores before treatment (P < 0.05), after adjusting for age, time after menopause, vaginal burning, vaginal dryness, and dyspareunia (P > 0.05) (Table 4).
Table 4.
Analysis of Factors That Influence VHIS at 6 Months Posttreatment
| Nonstandardized coefficient | Standard coefficient | ||||
|---|---|---|---|---|---|
| B | Standard error | Trial version | t | Sig. | |
| Constant value | 5.440 | 4.978 | 1.093 | 0.294 | |
| Groups | −0.595 | 1.347 | −0.086 | −0.442 | 0.666 |
| Time after menopause | −0.151 | 0.254 | −0.137 | −0.594 | 0.563 |
| Pretreatment VHIS for vaginal burning | −0.059 | 0.048 | −0.322 | −1.229 | 0.241 |
| Pretreatment VHIS for vaginal dryness | 0.040 | 0.049 | 0.195 | 0.805 | 0.435 |
| Pretreatment VHIS for dyspareunia | 0.054 | 0.062 | 0.285 | 0.879 | 0.395 |
| Pretreatment VHIS | 1.050 | 0.274 | 0.804 | 3.838 | 0.002 |
Trial version: it shows in results of statistical software SPSS.
VHIS, Vaginal Health Index score.
Regression in GSM‐related symptoms after treatment
Regression in GSM‐related symptoms showed no significant differences at 3 and 6 months posttreatment (P > 0.05) in the laser group when compared with the control group (Fig. 1). At 12 months postlaser treatment, vaginal burning, dryness, and dyspareunia were seen in 66.0% (31/47), 56.3% (26/47), and 57.9% (22/38) patients, respectively.
Figure 1.
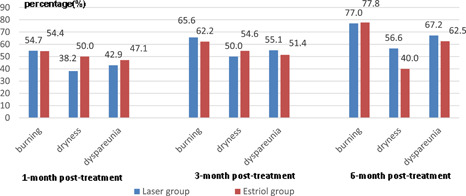
Comparison of improvement in symptoms of genitourinary syndrome of menopause 1, 3, and 6 months posttreatment in the two groups. Foundation item: Chinese Association of Plastics and Aesthetics (FRPR201601‐01).
Comparison of Side Effects Between the Two Groups
In the laser group, the mean VAS score for pain associated with the probe entering introitus, moving in the vagina, and during treatment was 38.93 ± 21.19, 28.06 ± 19.61, and 24.21 ± 15.96, respectively. At 6 months posttreatment, 85.3% (81/95) of the patients were satisfied with the treatment in the laser group. There were no significant adverse effects in the two groups. The laser treatment was well‐tolerated, with no serious adverse events, such as vaginal infection, adhesion, stenosis, and ulcer during and after the treatment.
DISCUSSION
Since the theory of fractional laser treatment was put forward by laser medical experts at Harvard University [7, 8], it has been rapidly applied in most fields of medicine. Because of its safety and effectiveness for tissue remodeling, fractional laser therapy has been a mainstay of aesthetic medicine in recent years, especially facial skin and vulvovaginal mucosa [13, 14]. Based on the selectivity theory, different laser wavelengths, pulse durations, and energy parameters are selected for tissues with different biological characteristics to decrease the side effects.
Laser technology takes advantage of the thermal effects of the laser. Laser treatment of the vaginal mucosa epithelium is a well‐defined process characterized by inflammation. The laser's thermal effect stimulates the heat shock response, leading to a rapid alteration in the cellular metabolism, release of heat shock protein 70 and transforming growth factor, inflammation and hyperplasia of fibroblasts, collagen, and extracellular matrix. Therefore, CO2 laser treatment increases the thickness of the vaginal epithelium and the fibrillary component of the extracellular matrix, thereby promoting tissue restoration and remodeling [15, 16], which becomes apparent 1–2 months after treatment and positive outcomes that persist over time [7, 10, 17, 18].
With the advances in laser instruments, damage to the surrounding normal tissue is minimized [19]. A computer graphics generator in fractional technical innovation was used to form the matrix arranged with fine laser dots. The retained unacted area, superficial tissue exfoliation, and lamellar tiny column damage with proper depth led to rapid tissue regeneration and recovery, all of which greatly reduced the occurrence of adverse reactions [7, 8, 9, 10, 11, 12, 19]. The fractional technology used in our study has unique advantages. In the design of laser emission mode, the difference in water retention between the atrophic mucous membrane and skin tissue makes this technology more suitable for mucosal therapy.
Since fractional CO2 laser treatment was used for the management of vaginal atrophy in combination with platelet‐rich plasma for the first time in 2011, by Gaspar et al. [19] several clinical studies have shown that CO2 and Er:YAG laser result in rapid and obvious effects, as well as rapid recovery with fewer side effects [20, 21, 22, 23, 24, 25, 26]. In a study on 50 postmenopausal women with moderate to severe VVA, fractional CO2 laser therapy was shown to increase the VHIS during a 12‐week follow‐up [7]. It has also been shown to significantly improve the quality of life, sexual function, and psychological score with no serious adverse events [9]. In 2015, the fractional CO2 laser was used to treat GSM at the Peking University People's Hospital and significant benefits were reported [27].
This is the first prospective, multicenter, cohort study of fractional CO2 laser for the treatment of GSM in China. Our findings confirm the safety and effectiveness of fractional CO2 laser therapy for GSM symptoms. In 2018, a randomized controlled study showed that laser treatment alone and in combination with estrogen significantly improved dyspareunia, vaginal burning, and dryness in the 20th week after treatment, while estrogen treatment alone only improved vaginal dryness (P < 0.001) [11], highlighting the long‐lasting advantages of laser treatment. We found a significant difference in the VHIS at 1 and 12 months after treatment, while no significant difference was seen at 3 and 6 months posttreatment between the two groups, suggesting that laser treatment might have longer‐lasting benefits compared with topical estrogen therapy. These results will add to the growing body of evidence supporting the efficacy of vaginal lasers as an alternative treatment for GSM [4]. The improvement in GSM symptoms following laser treatment is related to changes in the histology and ultrastructure of the vaginal mucosa, including a thicker vaginal mucosal layer, higher glucose load of the vagina, changes in the extracellular matrix fiber components, fibroblast activity, angiogenesis, and restructuring of the lamina propria [15, 16]. This process results in the emergence of new, healthy, and more youthful mucosa. In particular, the heavier atrophy related to burning, dyspareunia on vaginal opening, and lower one‐third of the vagina could be markedly improved [14]. So, based on these changes, the urinary symptoms and sexual function and vaginal laxity were significantly effect in the intravaginal fractional CO2 laser groups [28, 29], which laser group showed a statistically significant reduction in nocturia compared with that of the promestriene group [28].
The number of sessions was mainly based on the response of the vaginal tissue to the laser treatment. The satisfaction with the three‐session laser procedure was 84%–91.7% in most previous studies [7, 8, 27]. We also found significant improvement in vaginal burning and dyspareunia at 6 months posttreatment with two or three sessions of laser treatment. However, since our study included only 10 cases with two sessions, our findings should be confirmed using a larger sample size. Previous studies have shown that the effects of laser treatment may have a laser dose‐response manner [30]. Four weeks interval was selected because mainly the second session could be given to vaginal tissues, when it had recovered after laser treatment, to increase the stimulation of laser on collagen in the atrophic tissue, and minimizing the side effects.
A pilot comparative cohort study in which GSM patients received three sessions of Er:YAG laser therapy after a 2‐week vaginal Estriol treatment showed alleviation of GSM symptoms up to 18 months posttreatment. Furthermore, a significant improvement in the maturation value and a decrease in pH was detected in the laser group up to 12 months after treatment [12]. Laser treatment can have different effects in different patient groups. These results suggest that the long‐term effects of laser therapy might be better than those of estrogen therapy. In the present study, only 59.3% of the patients were followed up to 12 months postlaser treatment. Long‐term effects, therefore, need to be confirmed in longer follow‐up studies.
In this study, waveforms for the vaginal mucosa tissue were selected. Multiple small laser dots were evenly distributed, and the unacted tissue was preserved around it, allowing the epithelium to regenerate rapidly with a minor injury. In addition, the CO2 laser wavelength due to its affinity for water (Er:YAG, 2,940 nm, CO2, 10,600 nm) penetrates deeper, and the resultant micro‐ablation is better for the vaginal mucosa tissue. So, it has no adverse events or is minimal during and after treatment in this and previous studies [7, 8, 9, 10, 11, 30]. Most patients generally experience mild discomfort during laser probe insertion, movement, and laser treatment, most of which disappears after a few minutes of treatment. Local mild and temporary discomfort, slight congestion, or swelling recovers naturally within 24–48 hours. However, the probe must be taken carefully into the vagina, especially in patients with a narrow, atrophied vaginal opening [13]. Although complications are rare, it is still important to optimize the wavelengths and energy, depending on the tissue type, to prevent injuries. The indications and contraindications should be carefully monitored before making a choice. Physicians should be cautious to potential complications [31].
Our study has some limitations. First, there was no strict randomized control. Second, the follow‐up time and study objects representativeness at 12 months posttreatment were not enough. Therefore, a long‐term, multicenter trial with larger sample size is needed to further evaluate the long‐term efficacy and safety of CO2 laser therapy for GSM.
In conclusion, fractional CO2 laser therapy is an effective, improved, and alternative method to alleviate vaginal burning, vaginal dryness, and dyspareunia associated with menopause, especially in patients who are survivors of an estrogen‐dependent malignancy [32]. At 1 month posttreatment, it was more effective than a topical Estriol treatment, and the effects lasted for 6–12 months. We have established the indications, evaluation criteria, and steps to avoid complications associated with this treatment method. The operation is relatively simple and can be performed without anesthesia at an outpatient clinic.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Contract grant sponsor: Chinese Association of Plastics and Aesthetics; Contract grant number: FRPR201601‐01.
REFERENCES
- 1. Santos I, Clissold S. Urogenital disorders associated with oestrogen deficiency: The role of promestriene as topical oestrogen therapy. Gynecol Endocrinol 2010;26(9):644–651. [DOI] [PubMed] [Google Scholar]
- 2. Portman DJ, Gass ML, Vulvovaginal Atrophy Terminology Consensus Conference Panel . Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and The North American Menopause Society. Menopause 2014;21(10):1063–1068. [DOI] [PubMed] [Google Scholar]
- 3. Cagnacci A, Carbone MM, Palma F. Prevalence and association between objective signs and subjective symptoms of vaginal atrophy: The AGATA study. Menopause 2016;23(10):1139–1145. [DOI] [PubMed] [Google Scholar]
- 4. Flint R, Cardozo L, Grigoriadis T, Rantell A, Pitsouni E, Athanasiou S. Rationale and design for fractional microablative CO2 laser versus photothermal non‐ablative erbium:YAG laser for the management of genitourinary syndrome of menopause: A non‐inferiority, single‐blind randomized controlled trial. Climacteric 2019;22(3):307–311. [DOI] [PubMed] [Google Scholar]
- 5. Szymański J, Zaręba K, Jakiel G, Słabuszewska‐Jóźwiak A. Genitourinary syndrome of menopause—Is the problem solved? State of the art 2018. Prz Menopauzalny 2018;17(4):168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: An overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol 2016;215(6):704–711. [DOI] [PubMed] [Google Scholar]
- 7. Salvatore S, Nappi RE, Zerbinati N, et al. A 12‐week treatment with fractional CO2 laser for vulvovaginal atrophy: A pilot study. Climacteric 2014;17(4):363–369. [DOI] [PubMed] [Google Scholar]
- 8. Perino A, Calligaro A, Forlani F, et al. Vulvo‐vaginal atrophy: A new treatment modality using thermo‐ablative fractional CO2 laser. Maturitas 2015;80(3):296–301. [DOI] [PubMed] [Google Scholar]
- 9. Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric 2015;18(2):219–225. [DOI] [PubMed] [Google Scholar]
- 10. Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause 2016;23(10):1102–1107. [DOI] [PubMed] [Google Scholar]
- 11. Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double‐blind, placebo‐controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical Estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018;25(1):21–28. [DOI] [PubMed] [Google Scholar]
- 12. Gaspar A, Brandi H, Gomez V, Luque D. Efficacy of Erbium:YAG laser treatment compared to topical Estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med 2017;49(2):160–168. 10.1002/lsm.22569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Streicher LF. Vulvar and vaginal fractional CO2 laser treatments for genitourinary syndrome of menopause. Menopause 2018;25(5):571–573. [DOI] [PubMed] [Google Scholar]
- 14. Preti M, Vieira‐Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: An ICS/ISSVD best practice consensus document. J Low Genit Tract Dis 2019;23(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginalmucosa after fractional carbon dioxide laser treatment. Lasers Med Sci 2015;30(1):429–436. [DOI] [PubMed] [Google Scholar]
- 16. Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: An ex vivo study. Menopause 2015;22(8):845–849. [DOI] [PubMed] [Google Scholar]
- 17. Sipos AG, Kozma B, Poka R, Larson K, Takacs P. The effect of fractional CO2 laser treatment on the symptoms of pelvic floor dysfunctions: Pelvic floor distress inventory‐20 questionnaire. Lasers Surg Med 2019;51(10):882–886. [DOI] [PubMed] [Google Scholar]
- 18. Tovar‐Huamani J, Mercado‐Olivares F, Grandez‐Urbina JA, Pichardo‐Rodriguez R, Tovar‐Huamani M, García‐Perdomo H. Efficacy of fractional CO2 laser in the treatment of genitourinary syndrome of menopause in Latin‐American population: First Peruvian experience. Lasers Surg Med 2019;51(6):509–515. [DOI] [PubMed] [Google Scholar]
- 19. Gaspar A, Addamo G, Brandi H. Vaginal fractional CO2 laser: A minimally invasive option for vaginal rejuvenation. Am J Cosmet Surg 2011;28(3):156–162. [Google Scholar]
- 20. Lang P, Dell JR, Rosen L, Weiss P, Karram M. Fractional CO2 laser of the vagina for genitourinary syndrome of menopause: Is the out‐of‐pocket cost worth the outcome of treatment? Lasers Surg Med 2017;49(10):882–885. [DOI] [PubMed] [Google Scholar]
- 21. Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: The second‐generation thermotherapy for the genitourinary syndrome of menopause. Climacteric 2015;18(5):757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gambacciani M, Torelli MG, Martella L, et al. Rationale and design for the Vaginal Erbium Laser Academy Study (VELAS): An international multicenter observational study on genitourinary syndrome of menopause and stress urinary incontinence. Climacteric 2015;18(Suppl 1):43–48. [DOI] [PubMed] [Google Scholar]
- 23. Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1‐year outcomes. Menopause 2017;24(7):810–814. [DOI] [PubMed] [Google Scholar]
- 24. Lee MS. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90° and 360° scanning scopes: A pilot study & short‐term results. Laser Ther 2014;23(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogrinc UB, Senčar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med 2015;47(9):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salvatore S, Pitsouni E, Del Deo F, Parma M, Athanasiou S, Candiani M. Sexual function in women suffering from genitourinary syndrome of menopause treated with fractionated CO2 laser. Sex Med Rev 2017;5(4):486–494. [DOI] [PubMed] [Google Scholar]
- 27. Miao Y, Li J, Wang J. The curative effect and feasibility analysis of fractional CO2 laser in the treatment of vulvovaginal atrophy in postmenopausal women. Chin J Clin Obstet Gynecol 2016;17(4):294–297. [Google Scholar]
- 28. Aguiar LB, Politano CA, Costa‐Paiva L, Juliato CRT. Efficacy of fractional CO2 laser, promestriene, and vaginal lubricant in the treatment of urinary symptoms in postmenopausal women: A randomized clinical trial. Lasers Surg Med 2020;52(8):713–720. [DOI] [PubMed] [Google Scholar]
- 29. Lauterbach R, Dabaja H, Matanes E, Gruenwald I, Lowenstein L. The efficacy and safety of CO<sub>2</sub> laser treatment for sexual function and vaginal laxity improvement in pre‐menopausal women [published online ahead of print May 26, 2020]. Lasers Surg Med. 10.1002/lsm.23263 [DOI] [PubMed] [Google Scholar]
- 30. Athanasiou S, Pitsouni E, Falagas ME, Salvatore S, Grigoriadis T. CO2‐laser for the genitourinary syndrome of menopause. How many laser sessions? Maturitas 2017;104:24–28. [DOI] [PubMed] [Google Scholar]
- 31. Al‐Badr A, Alkhamis WH. Laser vaginal tightening complications: Report of three cases. Lasers Surg Med 2019;51(9):757–759. 10.1002/lsm.23110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments: A retrospective study. Menopause 2018;25(6):657–662. [DOI] [PubMed] [Google Scholar]


