Abstract
Background
Prosthetic joint infection (PJI) is a potentially limb-threatening complication of total knee arthroplasty. Phage therapy is a promising strategy to manage such infections including those involving antibiotic-resistant microbes, and to target microbial biofilms. Experience with phage therapy for infections associated with retained hardware is limited. A 62-year-old diabetic man with a history of right total knee arthroplasty 11 years prior who had suffered multiple episodes of prosthetic knee infection despite numerous surgeries and prolonged courses of antibiotics, with progressive clinical worsening and development of severe allergies to antibiotics, had been offered limb amputation for persistent right prosthetic knee infection due to Klebsiella pneumoniae complex. Intravenous phage therapy was initiated as a limb-salvaging intervention.
Methods
The patient received 40 intravenous doses of a single phage (KpJH46Φ2) targeting his bacterial isolate, alongside continued minocycline (which he had been receiving when he developed increasing pain, swelling, and erythema prior to initiation of phage therapy). Serial cytokine and biomarker measurements were performed before, during, and after treatment. The in vitro anti-biofilm activity of KpJH46Φ2, minocycline and the combination thereof was evaluated against a preformed biofilm of the patient’s isolate and determined by safranin staining.
Results
Phage therapy resulted in resolution of local symptoms and signs of infection and recovery of function. The patient did not experience treatment-related adverse effects and remained asymptomatic 34 weeks after completing treatment while still receiving minocycline. A trend in biofilm biomass reduction was noted 22 hours after exposure to KpJH46Φ2 (P = .063). The addition of phage was associated with a satisfactory outcome in this case of intractable biofilm-associated prosthetic knee infection. Pending further studies to assess its efficacy and safety, phage therapy holds promise for treatment of device-associated infections.
Keywords: phage therapy, bacteriophage therapy, arthroplasty, prosthetic joint infection, biofilm
Treatment with intravenous phage therapy targeting Klebsiella in an end-stage prosthetic knee joint infection resulted in sustained clinical improvement and decreased key inflammatory cytokines. Analysis of in vitro anti-biofilm activity of KpJH46Φ2 showed a trend toward reduction in biofilm biomass.
Total knee arthroplasty (TKA) is a life-enhancing option for degenerative joint disease, with 700 000 primary TKAs performed in 2014 in the United States alone [1]. Prosthetic joint infection (PJI) is a complication of TKA associated with substantial morbidity and healthcare expense [2]; the estimated incidence ranges from 2.05 to 2.18% per procedure. The cost to treat PJI in the United States was estimated to be $1.6 billion/year in 2017 [3] and is likely to be higher today. Taking into consideration current surgical site infection rates and the aging population, surgical site infections related to total joint arthroplasty are projected to increase [4].
Biofilms are surface-associated, structured communities of bacteria encased in extracellular polymeric matrix that confers distinct survival benefits to bacteria against host defenses and antimicrobial agents [5]. Bone and joint infections, particularly those that involve prosthetic materials, represent prototypic biofilm-associated infections that pose treatment challenges with conventional antibiotics [6].
Phage therapy has reemerged as a potential alternative to antibiotics for the treatment of complex infections, including bone and joint infections, in the Western world [7–9]. Phages demonstrate in vitro activity against clinically-relevant bacteria, including antibiotic-resistant strains; their clinical utility may be especially impactful in settings where antibiotics fail [10].
Promising results of phage activity have been reported against bacterial biofilms including those formed by Pseudomonas aeruginosa [11], Enterococcus faecalis [12], and Staphylococcus aureus in animal models [13]. In this regard, phage therapy may be useful in the setting of biofilm-associated infections in humans. There are a limited number of reported human cases describing phage therapy for bone and joint [14] and device-associated [15, 16] infections; results published to date, however, have been promising. Few significant adverse events have been reported to be associated with phage therapy to date [17]. A recent case report describes transient phage-associated transaminitis [18]. Here we present the successful treatment of a chronic PJI using intravenous phage therapy in a man who had failed multiple surgical interventions and prolonged therapy with conventional antibiotics. We describe the clinical and therapeutic features of the case and highlight challenges and opportunities of phage therapy for PJI.
CASE PRESENTATION
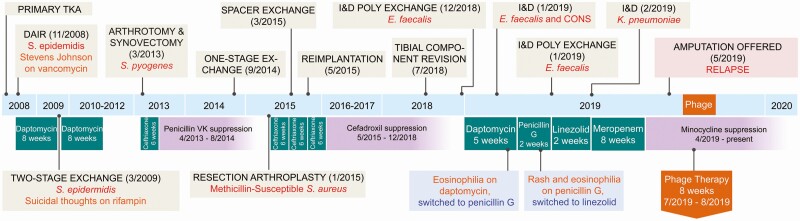
A 62 year-old male with a history of obesity, diabetes mellitus, and bilateral knee osteoarthritis underwent primary TKA in 2008, complicated shortly after by right TKA PJI due to Staphylococcus epidermidis. He failed an attempt at debridement, irrigation, antibiotics, and implant retention (DAIR) and required a 2-stage exchange in 2009. This course was complicated by Stevens-Johnson syndrome with vancomycin and suicidal thoughts with rifampin. In 2013, he suffered from a Streptococcus pyogenes right TKA PJI, initially treated with arthrotomy and synovectomy, followed by a 1-stage exchange in 2014. This was followed by a right methicillin-susceptible Staphylococcus aureus (MSSA) TKA PJI for which he underwent 2-stage exchange in 2015 after which he was placed on oral cefadroxil. He then developed progressive pain, swelling, and loosening of the tibial component and underwent a revision in July 2018. Intraoperative cultures were negative.
In November 2018, he developed a large fluid collection and dehiscence at the surgical site and underwent incision and drainage (I&D) and polyethylene liner exchange on 20 December 2018 with intraoperative cultures positive for Enterococcus faecalis (susceptible to penicillin, vancomycin, minocycline, and linezolid). He was started on oral penicillin VK. However, because of continued drainage, he had another debridement on 10 January 2019; intraoperative cultures were again positive for E. faecalis. He had persistent serosanguinous drainage and underwent repeat I&D on 28 January 2019 and replacement of the distal femoral modular component. Intraoperative cultures were again positive for E. faecalis and also in 1 specimen yielded Staphylococcus devriesei/haemolyticus (susceptible to vancomycin, doxycycline, and minocycline, and resistant to oxacillin); doxycycline was added to penicillin VK.
On 24 February 2019, he developed pruritis and eosinophilia attributed to penicillin and was started on daptomycin but developed eosinophilia and was then transitioned to oral linezolid; doxycycline was discontinued. He was admitted on 25 February 2019 for continued drainage and underwent I&D. Intraoperative cultures grew Klebsiella pneumoniae complex (susceptible to ampicillin-sulbactam, ceftriaxone, ciprofloxacin, meropenem, trimethoprim-sulfamethoxazole, and minocycline.) There was no growth of E. faecalis or S. devriesei/haemolyticus, and linezolid was discontinued. He was discharged on meropenem (due to allergies) for coverage of K. pneumoniae complex. After 8 weeks of meropenem, he was transitioned to oral minocycline for life-long suppression. The rationale for the minocycline was that he had not had a history of allergy to tetracyclines. An additional benefit was to provide coverage for all three of the recently cultured bacteria.
Unfortunately, 4 weeks after starting oral minocycline, he developed increasing pain, swelling, and erythema. Because he was not a candidate for further revision surgery, amputation was recommended. As a result of the size of his prosthesis, the level of the amputation was projected to be high, with hip disarticulation a possibility (Figure 1).
Figure 1.
Summary of the patient’s clinical course, timeline of surgical interventions, intraoperative cultures, antibiotics administered, and adverse events.
At this time, the decision was made to attempt phage therapy to salvage his leg by targeting the most recent organism cultured from his knee, K. pneumoniae complex. Neither E. faecalis nor S. devriesei/haemolyticus had grown from these intraoperative cultures. Although K. pneumoniae complex was presumed to be the major driver of the recurrence, this could not be confirmed because of patient refusal of arthrocentesis due to a history of multiple painful and unsuccessful attempts. Because residual E. faecalis and S. devriesei/haemolyticus was not proven eradicated, chronic suppressive minocycline was continued in the hope thatthe patient’s infection could be controlled with combination therapy and amputation avoided. Another reason to continue minocycline was that we did not feel comfortable discontinuing standard of care, especially with the patient tolerating it, in the setting of an experimental therapy.
Expanded access was granted by the Food and Drug Administration (FDA) (eIND number 19122) and Mayo Clinic Institutional Review Board (IRB 19-003978). Consent for Emergency Use Authorization was obtained and filed, and the treatment protocol reviewed by IRB. Upon review, the full IRB committee affirmed that the institutional procedure for emergency use reporting was followed and deemed the emergency use acceptable in accordance with 21CFR56.102(d) and 21CFR56.104(c).
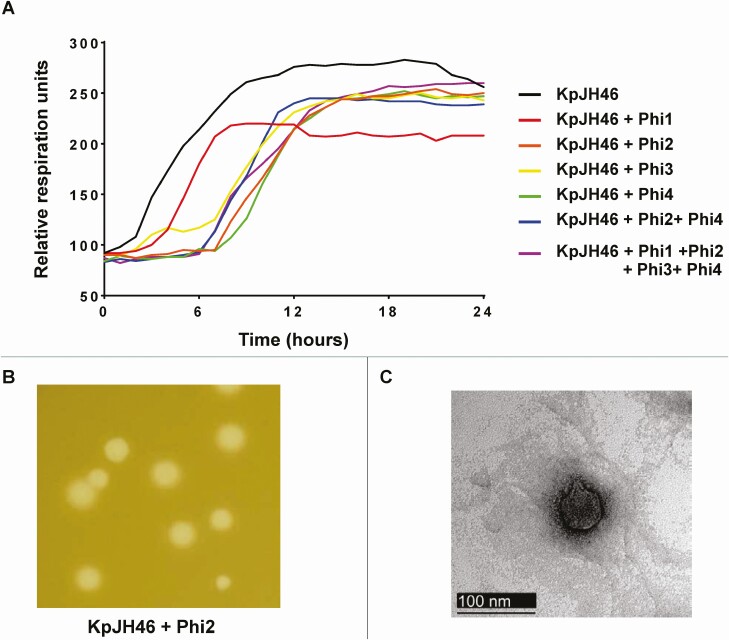
Diverse and well-characterized phages found in the PhageBankTM collection were screened against the patient’s K. pneumoniae complex (KpJH46), as described in the Supplementary Material. None of the PhageBankTM phages tested lysed KpJH46 for extended periods of time; therefore, we proceeded to perform de novo isolation of phages specific to KpJH46. Four environmentally isolated phages were able to repetitively hold growth of the bacteria as measured by the Host Range Quick Test (HRQT) assay (Figure 2A), but only 2, phages Φ2 and Φ4, produced clear plaques of good size (Figure 2B). No synergism was observed by combining phages; thus amplification and production of Φ2 (KpJH46Φ2) was pursued for treatment.
Figure 2.
Characterization of de novo isolated bacteriophages against KpJH46. A, 24-hour growth analysis of Klebsiella pneumoniae complex (4 × 105 bacterial cells, strain KpJH4) in the presence of several phages, Phi1 to Phi4, at an MOI of 1 using the OmniLog system. B, Plaque of phage 2 against KpJH4 using the double agar layer technique. C, Electron microscopy of phage KpJH46Φ2. Abbreviation: MOI, multiplicity of infection.
Purified and GMP-grade preparation of KpJH46Φ2 was obtained from Adaptive Phage Therapeutics (APT) in Gaithersburg, MD. The endotoxin content of this preparation was 22 EU/mL, which met the endotoxin requirements set by the FDA (≤5 EU/mL/kg). For additional information on phage isolation, production and purification, please see the Supplementary Material.
The patient received daily infusions of 6.3 × 1010 phages in 50 mL of normal saline intravenously in the Mayo Clinic Clinical Research and Trials Unit (CRTU) each weekday for a total of 40 doses. Infusions were given over 30 minutes with peripheral intravenous access established daily. Weekly complete blood count (CBC) with differential count, complete metabolic panel (CMP), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were obtained over time. Additionally, serum cytokines were measured weekly before, during, and at the end of phage therapy, as described in the Supplementary Material.
The patient was maintained on 100 mg oral minocycline twice daily over the course of phage therapy. The patient reported minor and intermittent pruritus of the right lower extremity about 2 weeks into the course of therapy but experienced no apparent adverse events from the infusions.
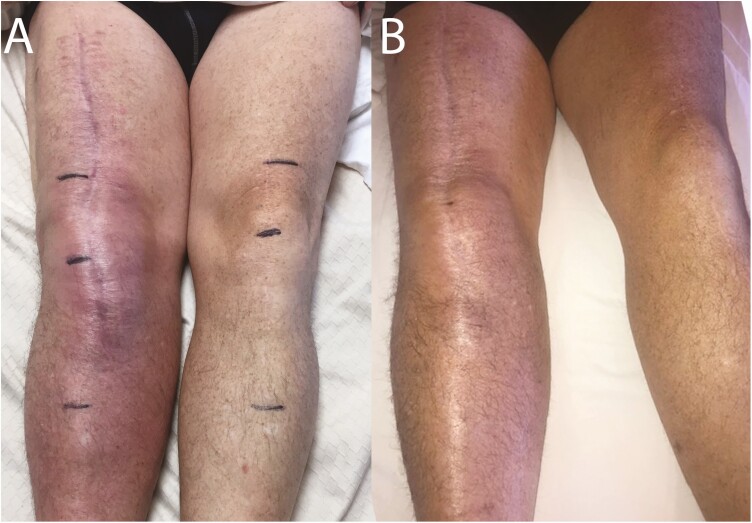
Over the course of phage therapy, the patient experienced improvement in erythema, swelling, pain, range of motion, and function of the right lower extremity (Figure 3). Erythema improved rapidly after 2 doses. Pain levels were 8–9 on a scale of 1–10 (1 = minimal, 10 = severe) prior to phage therapy, decreasing over time; he reported no pain at the end of therapy. After undergoing phage therapy, he described being able to drive his tractor, sit comfortably in a restaurant, and perform his daily routine to an extent that had not been possible prior to phage therapy. He was followed for 34 weeks after completion of therapy without incident and with apparent resolution of symptoms.
Figure 3.
Phage therapy resulted in reduced erythema and swelling. Images are shown of the patient’s lower extremities (A) before and (B) after completion of phage therapy.
In conjunction with the healthcare team, the patient and his family decided to continue suppressive minocycline because of the success that had been realized with the combination of therapies and the unknown risk that might jeopardize the patient’s hard-won return to function and resolution of pain.
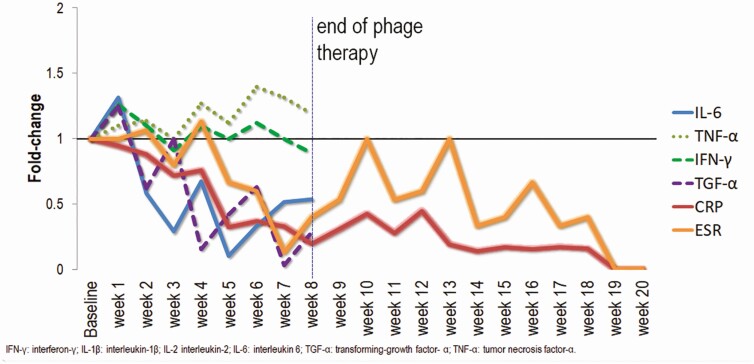
Inflammatory markers (ESR and CRP) and proinflammatory cytokines, including interleukin 6 (IL-6), interferon-gamma (IFN-γ), and transforming growth factor-alpha (TGF-α) decreased over the treatment course while TNF-α levels rose slightly (Figure 4). Additional cytokines were measured over time, the results of which are shown in the Supplementary Material. Anti-phage antibodies were measured, as described in the Supplementary Material, with no appreciable change over time, suggesting that immunity to the phages was not mounted over the course of phage therapy.
Figure 4.
Fold-change in inflammatory cytokines and biomarkers over the course of and 20 weeks beyond completion of phage therapy. Weekly serum samples collected prior to phage administration (baseline) and during phage therapy were assessed for serum inflammatory markers and cytokines. Additional cytokine data are shown in Figure S1. Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IFN, interferon; IL, interleukin; TGF, transforming growth factor; TNF, tumor necrosis factor.
DETERMINATION OF IN VITRO ANTI-BIOFILM ACTIVITY
The individual and combined activity of the phage and minocycline was determined against in vitro KpJH biofilms. The patient’s isolate was passaged twice from MicrobankTM vials (Pro-Lab Diagnostics, Round Rock, Texas, USA) stored at −80°C onto BBLTM TrypticaseTM Soy Agar with 5% Sheep Blood (TSA II) (Becton, Dickinson) and incubated at 37°C in room air overnight. A mid-log phase culture was grown from 3–5 colonies suspended in tryptic soy broth at 37°C room air with shaking (120 rpm) and diluted in cation-adjusted Mueller-Hinton broth (CAMHB) to a concentration of 1.5 × 104 CFU/mL. One hundred fifty microliters of bacterial suspension were added to wells of a Falcon® 96-well, flat-bottom, nontissue culture treated plate (Corning Life Sciences) and incubated for 24 hours at 37°C room air with shaking (120 rpm). Wells were evacuated and rinsed once with sterile 1X phosphate-buffered saline (PBS). Six wells each were treated with 200 μL of phage KpJH46Φ2 at a concentration of 1.26 × 109 PFU/mL, minocycline (10 μg/mL), phage plus minocycline (per the above specified concentrations), or CAMHB (controls) for 22 hours at 37°C room air with shaking (80 rpm). Wells were again evacuated, rinsed once with PBS, dried overnight at room temperature, stained with 0.1% safranin, evacuated, rinsed, dried, and resuspended in 30% glacial acetic acid, at which point the optical density (OD492) was determined [19]. OD492 values of the treatment groups were compared using Kruskall-Wallis test. Analysis was conducted using R software (R Core Team, 2014), and figures were produced using the package ggplot2 (Wickham, 2009) and Microsoft Excel (2010).
RESULTS
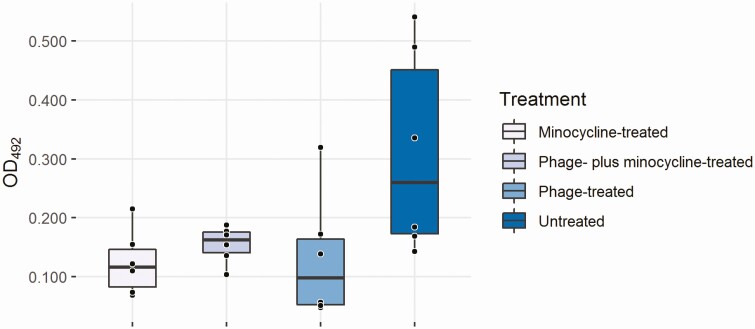
Median OD492 values for the untreated, phage-only, minocycline-only and combination-treated wells were 0.260 (95% CI .143–.540), 0.098 (95% CI .048–.319), 0.116 (95% CI .069–.215), and 0.163 (95% CI .104–.188), respectively (P = .063) (Figure 5) indicating trend in biofilm biomass reduction. There was a trend toward lower median OD492 values in treated versus untreated wells; however, this difference was not statistically significant.
Figure 5.
In vitro biofilm biomass (determined by safranin staining) after treatment with phage, minocycline, or the 2 combined.
DISCUSSION
Chronic PJI is among the most difficult infections to clear with available antibiotics. Although there are growing numbers of case reports describing phage therapy in the setting of soft tissue infections, reports of phage application to musculoskeletal infections with retained medical devices are limited. Here we describe a single patient treated with monophage therapy for hardware-associated K. pneumoniae complex infection with a retained TKA, with resolution of local signs and symptoms of infection, reduction of pain and inflammation, increased range of motion, and recovery of function despite retention of hardware. He remained asymptomatic 34 weeks after completing treatment and experienced no adverse effects. Our experience affirms 3 reports of phage therapy in treatment of PJI with DAIR. Causative agents in these cases were S. aureus [20] and multidrug-resistant P. aeruginosa [16]. In those studies, phages were administered with surgical intervention along with intravenous antibiotics. Together, these data suggest that phage therapy may hold promise for treatment of device-associated infections.
Our case is notable for several reasons. First, phage therapy was administered intravenously and not intraoperatively, as was the case in the prior DAIR cases. This suggests a potential avenue to treat infected hardware with phage therapy that circumvents surgery, implantation of a delivery device, and hardware removal. Another advantage of intravenous therapy is that the patient can receive infusions at an outpatient infusion center (or conceivably at home).
Second, in our study, as in a recent publication (PMID 32397354), a single phage was used in conjunction with an oral antibiotic. Our result suggests that it may be possible to treat hardware infections with conventional antibiotics plus a single phage. Although it remains to be defined whether a phage cocktail or monophage therapy is more efficacious, a monophage approach is attractive given the labor-intensive nature of identifying, developing and purifying even a single phage species for personalized therapy. A streamlined approach like that taken here may make the availability of phage therapy more feasible than using cocktails.
The ideal duration of intravenous phage therapy for PJI remains unclear. We observed that CRP normalized on the last day of therapy, suggesting long courses to be potentially necessary. However, a case series was recently published describing 4 cases of chronic osteomyelitis successfully treated with 7–10 days of locally applied phage cocktails [9]. Although the management differed from that of our patient in that these patients underwent hardware removal, it is possible that a shorter duration of therapy would have been sufficient for our patient. Clinical trials will be essential to determine the optimal duration of phage therapy for particular clinical indications.
This case is also notable as we monitored the patient’s inflammatory response before, during, and at the end of phage therapy noticing normalization of ESR and CRP at the end of therapy and decreasing values of other inflammatory cytokines over the treatment course. In particular, we observed a decrease in IL-6, IFN-γ, and TGF-α, markers often used in concert to evaluate PJI in clinical settings [21, 22]. TNF-α was slightly increased after phage therapy, although the clinical relevance of this observation is unclear. A total of 76 cytokines and chemokines were measured (Figure S1); most are as yet understudied and/or poorly understood due to the complex nature of interactions within the immune system. More studies prospectively examining these cytokines during treatment of bone and joint infections with or without phage therapy might be helpful to determine the clinical relevance of such markers.
Interestingly, there appeared to be continued reduction in ESR and CRP through the last measurement, suggesting a benefit even 57 days (40 doses) after the start of therapy. After the end of therapy, there was a continued downward trend of CRP and stability of ESR. Twenty weeks after completion of phage therapy, ESR (6 mm/1 h; normal ≤ 22 mm/1 h) and CRP (5.7 mg/L; normal ≤ 8.0 mg/L) remained normal (Figure 4).
We observed phage-specific antibodies in our patient’s serum prior to initiation of phage therapy, and these remained stable over time. This echoes observations made by investigators in Poland [23] where phage-specific antibodies were found in healthy volunteers not treated with phage. The presence of phage-specific antibodies has been largely hypothesized to result from exposure to environmental or normal microbiome-associated phages. The same investigators also showed that the induction of phage-specific antibodies was highly variable in their cohort [24] and that the presence of antibodies did not preclude clinical success [25]. Several studies, including those performed by the group in Poland, use the K rate of phage inactivation in their methods [26] to predict phage neutralization by different concentrations of patient sera containing phage-specific antibody. This functional assay was not performed in our case, and the degree of phage inactivation is unknown at the titers observed of phage-specific antibodies in this patient’s serum. The role of measuring phage-specific antibodies in clinical cases remains to be determined.
It is notable that phage therapy succeeded in this instance despite the likely presence of hardware-associated K. pneumoniae biofilm [27]. A trend toward reduced biomass was noted in all treatment groups in the in vitro studies. More experiments are needed to determine the antibiofilm activity of phage. However, our successful single outcome in conjunction with emerging clinical data suggest that phage therapy may be a promising emerging alternative or adjunct to conventional antibiotics in biofilm-associated infections [28].
Our case also outlines some of the challenges of phage therapy, including the as yet unestablished optimal routes of administration, dosing, and duration of therapy. Also unclear is the role of antibiotics and whether phage therapy may be an alternative to antibiotics or simply an adjunct. Finally, we did not feel it was ethical to withdraw the chronic suppressive minocycline because of the possibility that this was contributing to the patient’s success and have thus not demonstrated eradication of infection. Nonetheless, our case demonstrates a clear clinical turnaround brought about by the addition of phage therapy, resulting in a tangible and life-altering benefit for our patient.
CONCLUSIONS
This case illustrates how novel antibiofilm therapies might potentially reshape the way we treat infections in the era of increasing medical device use. Although more studies are needed and there are reasons for vigilance, reports such as this one suggest that phage therapy may be safe, effective, and well tolerated. More research on the subject will enhance our understanding of the value of phage therapy in clinical practice.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. From Mayo Clinic the authors thank Drs Andrew Badley, Keith Stewart, Elie Berbari, Douglas Osmon, Aaron Tande, and Talha Riaz, for their critical advice. A special thanks to Jennifer Weis and Melinda Shea, RN, and the nursing and pharmacy staff of the NCATS supported Clinical Research and Trials Unit (CRTU), and Kerryl E. Greenwood-Quaintance for her technical expertise. From APT lab, the authors thank Jarrar Haider, Brittany Sisson, Martin Lee, Joseph Tewell, Viet Dang, Gustavo Aguilar, and Anna Kawa.
Financial support. This work was supported and funded in part by the Congressionally Directed Medical Research Program (work unit number A1427), Naval Medical Research Center. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the US Government. This publication was also made possible by the Mayo Clinic CTSA through grant number UL1TR002377 from the National Center for Advancing Translational Sciences (NCATS), and T32 funding, grant number AR56950 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, both components of the National Institutes of Health (NIH).
Potential conflicts of interest. B. B. is an employee of the US Government and holds patent 10357522. This work was prepared as part of their official duties. Title 17 U.S.C. § 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. § 101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties. M. H. and F. M. are, respectively, Geneva Foundation and Leidos contractor employees working for the US Government. M. B. reports equity in Adaptive Phage Therapeutics. D. L. reports personal fees from Zimmer Biomet, Mako/Stryker; research support from Corin USA; stock/stock options from Acuitive Technologies and Ketai Medical Devices; and board/committee membership from American Joint Replacement Registry and Orthopedic Research and Education Foundation, outside the submitted work. M. H. holds patent 10357522. R. P. reports grants from CD Diagnostics, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, ContraFect, TenNor Therapeutics Limited and Shionogi. R. P. is a consultant to Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST and Qvella; monies are paid to Mayo Clinic. In addition, R. P. has a patent on Bordetella pertussis/parapertussis polymerase chain reaction issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued. R. P. receives travel reimbursement from ASM and IDSA, an editor’s stipend from IDSA, and honoraria from the NBME, Up-to-Date, and the Infectious Diseases Board Review Course. G. S. and R. P. report an equity and royalty-bearing know-how agreement with Adaptive Phage Therapeutics (APT). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the US, 2014 to 2030. J Bone Joint Surg Am 2018; 100:1455–60. [DOI] [PubMed] [Google Scholar]
- 2. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014; 27:302–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beringer DC. CORR Insights®: what is the long-term economic societal effect of periprosthetic infections after THA? A Markov analysis. Clin Orthop Relat Res 2017; 475:1901–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolford HM, Hatfield KM, Paul P, Yi SH, Slayton RB. The projected burden of complex surgical site infections following hip and knee arthroplasties in adults in the United States, 2020 through 2030. Infect Control Hosp Epidemiol 2018; 39:1189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther 2007; 82:204–9. [DOI] [PubMed] [Google Scholar]
- 6. Tzeng A, Tzeng TH, Vasdev S, et al. Treating periprosthetic joint infections as biofilms: key diagnosis and management strategies. Diagn Microbiol Infect Dis 2015; 81:192–200. [DOI] [PubMed] [Google Scholar]
- 7. Kortright KE, Chan BK, Koff JL, Turner PE. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 2019; 25:219–32. [DOI] [PubMed] [Google Scholar]
- 8. Furfaro LL, Payne MS, Chang BJ. Bacteriophage therapy: clinical trials and regulatory hurdles. Front Cell Infect Microbiol 2018; 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Onsea J, Soentjens P, Djebara S, et al. Bacteriophage application for difficult-to-treat musculoskeletal infections: development of a standardized multidisciplinary treatment protocol. Viruses 2019; 11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gordillo Altamirano FL, Barr JJ. Phage therapy in the postantibiotic era. Clin Microbiol Rev 2019; 32:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alves DR, Perez-Esteban P, Kot W, et al. A novel bacteriophage cocktail reduces and disperses Pseudomonas aeruginosa biofilms under static and flow conditions. Microb Biotechnol 2016; 9:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khalifa L, Brosh Y, Gelman D, et al. Targeting Enterococcus faecalis biofilms with phage therapy. Appl Environ Microbiol 2015; 81:2696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morris J, Kelly N, Elliott L, et al. Evaluation of bacteriophage anti-biofilm activity for potential control of orthopedic implant-related infections caused by Staphylococcus aureus. Surg Infect (Larchmt) 2019; 20:16–24. [DOI] [PubMed] [Google Scholar]
- 14. Patey O, McCallin S, Mazure H, Liddle M, Smithyman A, Dublanchet A. Clinical indications and compassionate use of phage therapy: personal experience and literature review with a focus on osteoarticular infections. Viruses 2018; 11:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan BK, Turner PE, Kim S, Mojibian HR, Elefteriades JA, Narayan D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health 2018; 2018:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tkhilaishvili T, Winkler T, Müller M, Perka C, Trampuz A. Bacteriophages as adjuvant to antibiotics for the treatment of periprosthetic joint infection caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 2019; 64:ee00924–19. doi: 10.1093/cid/ciy947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Haddad L, Harb CP, Gebara MA, Stibich MA, Chemaly RF. A systematic and critical review of bacteriophage therapy against multidrug-resistant ESKAPE organisms in humans. Clin Infect Dis 2019; 69:167–78. [DOI] [PubMed] [Google Scholar]
- 18. Doub JB, Ng VY, Johnson AJ, et al. Salvage bacteriophage therapy for a chronic MRSA prosthetic joint infection. Antibiotics (Basel) 2020; 9:241–7. PMID:32397354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Toole GA. Microtiter dish biofilm formation assay. J Vis Exp 2011; 30:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferry T, Leboucher G, Fevre C, et al. Salvage debridement, antibiotics and implant retention (“DAIR”) with local injection of a selected cocktail of bacteriophages: is it an option for an elderly patient with relapsing Staphylococcus prosthetic-joint infection? Open Forum Infect Dis 2018; 5:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saleh A, George J, Faour M, Klika AK, Higuera CA. Serum biomarkers in periprosthetic joint infections. Bone Joint Res 2018; 7:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoon JR, Yang SH, Shin YS. Diagnostic accuracy of interleukin-6 and procalcitonin in patients with periprosthetic joint infection: a systematic review and meta-analysis. Int Orthop 2018; 42:1213–26. [DOI] [PubMed] [Google Scholar]
- 23. Łusiak-Szelachowska M, Zaczek M, Weber-Dąbrowska B, et al. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol 2014; 27:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Żaczek M, Łusiak-Szelachowska M, Jończyk-Matysiak E, et al. Antibody production in response to staphylococcal MS-1 phage cocktail in patients undergoing phage therapy. Front Microbiol 2016; 7:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Łusiak-Szelachowska M, Żaczek M, Weber-Dąbrowska B, et al. Antiphage activity of sera during phage therapy in relation to its outcome. Future Microbiol 2017; 12:109–17. [DOI] [PubMed] [Google Scholar]
- 26. Adams MH. Bacteriophages. New York: Interscience Publishers, 1959. [Google Scholar]
- 27. Seifi K, Kazemian H, Heidari H, et al. Evaluation of biofilm formation among Klebsiella pneumoniae isolates and molecular characterization by ERIC-PCR. Jundishapur J Microbiol 2016; 9:e30682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burrowes B, Harper DR, Anderson J, McConville M, Enright MC. Bacteriophage therapy: potential uses in the control of antibiotic-resistant pathogens. Expert Rev Anti Infect Ther 2011; 9:775–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.