Abstract
The necessary reduction of greenhouse gas (GHG) emissions may lead in the future to an increase in solar irradiance (solar brightening). Anthropogenic aerosols (and their precursors) that cause solar dimming are in fact often co‐emitted with GHGs. While the reduction of GHG emissions is expected to slow down the ongoing increase in the greenhouse effect, an increased surface irradiance due to reduced atmospheric aerosol load might occur in the most populated areas of the earth. Increased irradiance may lead to air warming, favour the occurrence of heatwaves and increase the evaporative demand of the atmosphere. This is why effective and sustainable solar radiation management strategies to reflect more light back to space should be designed, tested and implemented together with GHG emission mitigation. Here we propose that new plants (crops, orchards and forests) with low‐chlorophyll (Chl) content may provide a realistic, sustainable and relatively simple solution to increase surface reflectance of large geographical areas via changes in surface albedo. This may finally offset all or part of the expected local solar brightening. While high‐Chl content provides substantial competitive advantages to plants growing in their natural environment, new plants with low‐Chl content may be successfully used in agriculture and silviculture and be as productive as the green wildtypes (or even more). The most appropriate strategies to obtain highly productive and highly reflective plants are discussed in this paper and their mitigation potential is examined together with the challenges associated with their introduction in agriculture.
Keywords: climate mitigation, land surface albedo, low‐Chl plants, solar brightening, solar radiation management
Climate change mitigation policies aimed at the reduction of greenhouse gas (GHG) emissions might cause an increase in solar irradiance in the most populated areas of the planet (solar brightening). Anthropogenic aerosols causing solar dimming are co‐emitted with GHGs. New plants with low‐chlorophyll (Chl) content may offset such brightening. Their introduction increases surface reflectance (albedo) of large geographical areas, thus reducing the shortwave radiative forcing (RFSW). Additional benefits such as reducing transpiration and/or mitigating local effects of extreme temperatures may be obtained. Genome editing aims to create those new plants targeting both appropriate optical properties and enhanced productivity.
1. INTRODUCTION: CLEAN THE AIR, HEAT THE PLANET
Any change in the atmospheric transmission of solar irradiance is defined as solar brightening or dimming depending on whether more or less light reaches the surface due to light scattering and absorption. Those atmospheric properties depend on the burden of different species of aerosols. Large changes in atmospheric transmittance have been documented over the past decades. Substantial dimming was recorded from the 1950s in many locations, whereas brightening has occurred since the 1980s and is still ongoing. In Italy, the annual mean irradiance increased by 6.4 ± 0.7 W m−2 per decade for the northern part of the country over the period of more rapid brightening (1981–2013), but such an increase was much larger during summer months (9.3 ± 1.1 W m−2 per decade; Manara et al., 2016). It is now well understood that at least half of those changes in surface irradiance was caused by changes in atmospheric shortwave absorption (Philipona et al., 2009; Schwarz et al., 2020). The recent lockdown due to the SARS‐CoV‐2 pandemic has led to a significant reduction of industrial activity and transport in the most densely populated regions of the world: anthropogenic emissions of GHG decreased (Le Quéré et al., 2020) together with co‐emitted species such as aerosols and their precursors (Bao & Zhang, 2020; Broomandi et al., 2020; Ranjan et al., 2020; Tobías et al., 2020; Venter et al., 2020). A detailed study based on ground and satellite observations, and on a radiative transfer model experiment, estimated that in some Western European countries affected by the pandemic (United Kingdom, Belgium, The Netherlands and Germany), the lockdown‐driven increase in irradiance in spring 2020 (March–May) was on average 2.3 W m−2, corresponding to a 1.3% increase compared to the same period in 2010–2019 (van Heerwaarden et al., 2020). The Global Carbon Project (Le Quéré et al., 2020) estimated that during the same period the CO2 emission of those countries was reduced by 68.1 Mt CO2. By means of those two numbers, a brightening rate of 0.033 W m−2 MtCO2 −1 can be roughly estimated. Such a calculation is rather simplistic as it does not consider, among other things, circulation‐driven aerosol advection in the troposphere. On the other hand, Turnock et al. (2016) estimated that near‐surface temperature in central Europe increases up to 0.2°C per W m−2 of additional irradiance.
Significant brightening during the recent lockdown is confirmed in this paper for another region: Friuli‐Venezia‐Giulia in the north‐east of Italy. The relationship between atmospheric moisture content and solar irradiance in clear‐sky days during April 2020 was different from that observed in the same period of the previous 20 years (Figure 1). Solar brightening increased by 1% during the lockdown.
FIGURE 1.
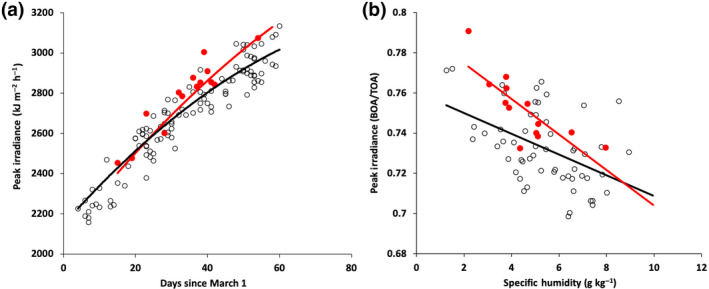
(a) Peak irradiance (kJ m−2) at Brugnera meteorological station (45.9189°, 12.5458°) in clear‐sky days for 2 months after 1st March 2020 (red dots, red line) and for the time series 2002–2019 (empty dots, black line). The data are fitted by cubic splines; (b) peak irradiance ratio (peak at the surface BOA/peak at the top of atmosphere TOA) at Brugnera station vs. specific humidity (g kg−1) for the same period of time. The data are fitted by linear regression (see Data S1 for methods)
Brightening associated with the pandemic is obviously an extreme case, but it anticipates what might happen in the future in some areas of the planet in response to policies aimed at a rapid and effective reduction of GHG emissions. This reduction will also unavoidably reduce the atmospheric burden of co‐emitted pollutants, including light absorbing and light scattering aerosols and their balance (Arneth et al., 2009). Brightening will translate into extra energy that will be partly absorbed by the surface with consequences on local warming, evaporative demand and atmospheric circulation. Climate change mitigation policies must urgently consider risks associated with brightening and investigate, develop and test the most appropriate solutions to avoid potential associated dangerous effects.
2. SOLAR RADIATION MANAGEMENT STRATEGIES
The Radiative Forcing (RF) concept is commonly used by the Intergovernmental Panel on Climate Change (IPCC) to ‘evaluate and compare the strength of the various mechanisms affecting the Earth's radiation balance and thus causing climate change’ (Myhre et al., 2013). RF is the net change in the energy balance of the earth surface expressed in W m−2 which is caused by any perturbation of incoming and/or outgoing shortwave (SW) and longwave (LW) radiation. Measures aimed at reducing the Shortwave Radiative Forcing (RFSW) fall within the broad category of Solar Radiation Management (SRM) strategies. The majority of SRMs aims to reduce the amount of incoming solar radiation reaching the surface: Stratospheric Aerosol Injection, Marine Cloud Brightening or Cirrus Cloud Thinning are among those strategies and some are already at the concept proof stage of their development (Tollefson, 2018). Recent studies highlighted the risks and the potential climatic feedback of some of those SRMs, with emphasis on major changes in storm track and storm intensity (Gertler et al., 2020), as well as on their social implications (Reynolds, 2019). Seneviratne et al. (2018) while reviewing the main concerns raised by SRMs, concluded that climate engineering may indeed have a global impact but could lead to strong regional disparities and affect rainfall patterns. This is why the concept of regional Land Radiative Management (LRMreg) was proposed as the ‘intentional modification of radiative properties of the land surface’ at the regional scale (Seneviratne at al., 2018). LRMreg implies the modification of the optical properties of urban, agricultural and forest land uses. When surface reflectance is intentionally increased, an energy imbalance is created as less energy is absorbed by the surface; this is the negative RFSW which can be subtracted from the positive RFLW caused by the increasing GHG concentrations in the atmosphere.
Sustainable LRMreg strategies, their implementation and the potential effects on RFSW have been recently reviewed in a number of publications (Bright et al., 2017; Hirsch et al., 2017, 2018; Lugato et al., 2020; Seneviratne et al., 2018). LRMreg options can be briefly summarized in three categories of albedo modification, (i) with artificial solutions, (ii) with land/crop management approaches and (iii) with bio‐based strategies:
A large number of studies considered the effect of albedo enhancement in cities and industrial areas using the so‐called ‘white roofs’ concept to mitigate urban heat‐island effects. Local effects of changes in roof and pavements reflectivity were examined using models (Akbari et al., 2009; Hamwey, 2007; Jacobson & Ten Hoeve, 2012) to conclude that this solution basically leads to a reduction of urban heat islands and of interior temperatures in summer (Seneviratne et al., 2018). More recently, the effect of this type of albedo changes was confirmed for a number of metropolitan areas in the USA (Sacramento, Houston and Chicago) where models predicted that urban albedo enhancement from 0.2 to 0.65 could lead to a mean decrease in air temperature of 1.52 ± 0.87°C with substantial differences between urban and suburban areas (Jandaghian & Akbari, 2018). When the same approach was used for the metropolitan area of Montreal in Canada, the predicted decrease in air temperature was lower (0.5°C; Jandaghian & Akbari, 2020).
Changes in land use and agronomic practices can modify surface albedo on a much larger scale. Soil mulching with crop residues and no‐tillage management can reflect more incoming light and mitigate the impact of heatwaves (Davin et al., 2014). Other practices aimed at keeping the soil surface covered by vegetation in all instances, such as double cropping or cover‐crops, are considered to have a beneficial impact on both the local and global climate, via changes in surface albedo and increases in carbon sequestration (Hirsch et al., 2018; Lugato et al., 2020). In spite of its high complexity, afforestation undoubtedly plays a role in climate mitigation. It is generally agreed that tree plantations or re‐naturalization can reduce land surface albedo, especially in the case of coniferous trees (Luyssaert et al., 2018). Such critical biophysical influence is also due to the modification that forests exert on snow cover. Dense forests cause a decrease of the contribution of snow to the overall land surface reflectance, especially at high latitudes. This is why Lutz and Howarth (2014) proposed a decrease in the rotation period of high‐latitude forests (i.e. the time between two consecutive harvests in a managed forest), showing that this can increase the fraction of shortwave radiation reflected by the surface via the enhancement of the relative contribution of snow cover to the overall surface reflectance.
Bio‐based strategies to enhance surface albedo stem from the fact that the reflectance properties of leaves show a high variability among plants, with traits which are often under genetic control. For instance, some mutants of common crop species bear leaves which are much lighter than the more widespread cultivars (Figure 2). Hirsch et al. (2018) used a state‐of‐the‐art climate model (Hurrel et al., 2013) to explore how changes in albedo of croplands can affect weather extremes which were indicated as the most relevant Climate Change Detection indices (Zhang et al., 2011). They also examined changes in the mean monthly day‐time maximum and night‐time minimum temperatures to evaluate changes in temperature distribution. They assumed that albedo can be intentionally varied (increased) in croplands by selecting existing cultivars differing in leaf pubescence (density, distribution and structure of leaf trichomes) and glaucousness (characteristics of leaf waxes; Doughty et al., 2011). A meaningful range of variability in albedo was derived from Breuer et al. (2003) for important crop species such as wheat, barley, corn, oats, rye, soybean and sunflower. The study concluded that plausible crop albedo could vary from 0.02 to 0.1. Model results based on such a variability did not differ from earlier studies (Ridgwell et al., 2009; Singarayer et al., 2009; Wilhelm et al., 2015) leading to the conclusion that the increase in crop albedo can have substantial effects on the global climate, when scaled to the entire planetary cropland area. These studies, however, neither considered the possibility of increasing the reflectance of leaves by genetically manipulating their pigment content, nor examined changes in yield that could be associated with the albedo modification.
FIGURE 2.

Minngold (right) and Eiko (left) soybean varieties cultivated in Italy in 2016
3. LOW‐CHLOROPHYLL PLANTS AND RADIATIVE FORCING
The idea of enhancing the reflectance of leaves in the visible and near‐infrared range of the solar spectrum by reducing the concentration of light‐absorbing pigments in leaves is attracting increasing attention. The photosynthetic rates of plant leaves have been considered to depend directly on their Chl content (Croft et al., 2017; Gitelson et al., 2014). A few recent studies have shown that mutants with reduced leaf Chl content exhibit similar or higher gross Carbon uptake rates and biomass accumulation (Gu et al., 2017; Paixão et al., 2019; Sakowska et al., 2018; Slattery et al., 2017), even at the canopy scale (Kirst et al., 2017). This is definitely not a universal response as in some cases, plants with low‐Chl content also have lower leaf‐scale photosynthetic rates (Zhang et al., 2019) and reduced yields (Genesio et al., 2020).
The upper layers of green plants absorb more light than they can efficiently use. Pale green‐genotypes ensure instead a better distribution of light in the depth of the canopy space due to higher transmittance (Long et al., 2006). The consequence is that more leaves absorb light at a range of intensities that they can use more efficiently, and the overall light absorption of a pale‐green canopy might not be necessarily lower than that of a fully green canopy. Nevertheless, reduced light absorption and increased reflectance have been documented for a spontaneous pale‐green soybean mutant under field conditions (Sakowska et al., 2020), thus supporting the view that a better distribution of light in the canopy space may limit, but not prevent, an overall increase in surface albedo. This last experiment also showed that under steady‐state conditions, the Gross Primary Productivity (GPP) of the pale‐green and the ‘green’ canopies were similar in spite of a detectable difference in the fraction of absorbed photosynthetically active radiation (fAPAR).
Such a counter‐intuitive relationship between canopy Chl content, light absorbance and photosynthesis in higher plants raises two basic questions on the green nature of leaves: why do plants have a dominant green colour? And why is the intensity of this colour so high in nature? Answers involve the evolutionary trajectory of plants. Higher plants derive from green algae which have evolved by competing with prokaryotic purple and cyanobacteria. The light absorption of those autotrophic microorganisms peaks in the green region of the solar spectrum (Figure 3). Their outer antenna structure, called phycobilisome, reaches the highest efficiency in transferring excitonic energy to (bacterio)‐Chl pigments whose photosynthetic reaction centres catalyse primary charge separation and electrogenic reactions. As a consequence, eukaryotic green algae competed for light by evolving antenna systems based on Chl and xanthophyll‐proteins whose pigments were tuned for absorption of wavelengths not absorbed by prokaryotic antenna systems (Morosinotto et al., 2003; Wientjies et al., 2012). This was achieved by developing a multigene superfamily of light harvesting complexes (LHC; Engelken et al., 2010). Competition in the oligotrophic conditions of shallow marine environments played a major role in the evolution of such maximization of photon capture.
FIGURE 3.
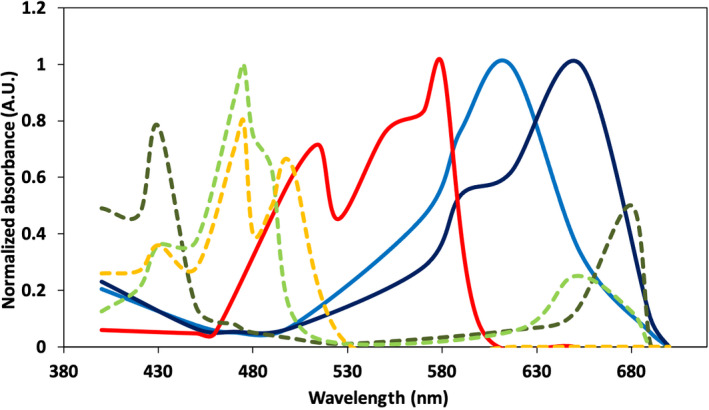
Normalized absorption spectra (in Arbitrary Units [A.U.]) of eukaryotic pigments (dashed lines): Chl a (dark green), Chl b (pale green), carotenoids (orange) and prokaryotic pigments (solid lines): Phycoerythrin (red), Phycocyanin (light blue), Allophycocyanin (dark blue)
The reason why plants contain such a large amount of Chl in their leaves remains more elusive. As cell volume increases, internal optical path‐length also increases, thus limiting the absorptive efficiency of Chl‐a molecules through spectral modifications due to self‐shading. As a result, larger cells tend to have relatively lower intracellular concentrations of Chl‐a compared to smaller cells, to limit shading effects due to the packaging of pigments (Finkel et al., 2004). This translates into a size dependence of Chl‐a content which was documented in the laboratory (Key et al., 2010) and in field studies (Maraóón et al., 2007). On short time scales, sunflecks are produced by leaf/branch movements, thus increasing the heterogeneity of photon fluence experienced by individual chloroplasts and cells. This makes the photosynthetic light harvesting efficiency of Chls highly dependent on their localization within leaves and canopies. Consistently, within natural canopies (Bauerle et al., 2020) and crops (Bonelli & Andrade, 2020), deep leaf layers lie below the light compensation point and limit biomass production (Dall’Osto et al., 2005; Pérez‐Bueno et al., 2008). Such light gradient‐dependent limitation of photosynthetic productivity is a problem for agriculture and algal culture in photobioreactors (Dall’Osto et al., 2019). As already pointed out above, the assumption that photosynthesis can be estimated from Chl content is too simplistic (Li, He, et al., 2018). Chl is an expensive chemical to be synthesized, chiefly for its high N content (Nunes‐Nesi et al., 2010), making it problematic to understand why plants accumulated high levels of Chl beyond those required for maximal growth rate. Yet, the sessile nature of plants makes the competition for light and nutrients a major factor for reproductive success. Differences in light‐intercepting ability conferred a disproportionate competitive advantage, thereby confirming that under productive conditions competition between species for this resource is size asymmetric (Vojtech et al., 2007). Extensive analysis of Chl content of leaves in natural environment has shown that it may vary over 20 folds depending on species, life form and geographical distribution (Li, Liu, et al., 2018). A large part of the variability is associated with the coexistence of different plant species (Freschet et al., 2011). Genetic factors and interaction with competing species are determinants in defining pigment content and optical properties of the vegetation.
On the other hand, the genetic basis for pigment content opens the possibility of engineering canopy structure and reflectivity of domesticated species through genetic manipulation. There are pale‐green varieties of a large number of trees, herbs, grasses, crops and ornamental species. An extensive study using 67 soybean mutants with lower‐than‐wild‐type Chl content showed that a reduction in Chl content reduces photon absorption and translates into reduced C‐assimilation at the leaf scale (Walker et al., 2018). The averaged biomass accumulation was however not reduced proportionally, and the reduction in biomass in the Y11y11 genotype, which showed a large reduction in Chl (>50%), was only modest (Slattery et al., 2017). Compensatory effects were attributed to a better distribution of light within the canopy space but also to increased photosynthetic efficiency due to a favourable change in the vertical distribution of nitrogen and its partitioning from excess Chl into more ‘beneficial investments’ such as carboxylation (V cmax) and substrate regeneration (J max) efficiencies (Walker et al., 2018). The observed modest decrease in biomass accumulation and yield was mainly explained by pleiotropic effects associated with the nature of the genetic lesions induced by the mutations, in addition to the decrease in pigment content. This is consistent with reports of enhanced productivity of pale‐green mutants in different plant species (Gu et al., 2017; Kirst et al., 2017) and algae (Cazzaniga et al., 2014; Dall’Osto et al., 2019), together with the enhanced light sensitivity of Ch1 mutants of Arabidopsis (Havaux et al., 2004). These observations suggest that a careful choice of genetic loci to be targeted is required for selection of genetic strains with high photoprotection capacity (Dall’Osto et al., 2019). A large variety of pale‐green mutants have been associated with changes in the composition of the light harvesting systems whose biosynthesis requires the coordinated action of at least two pathways for chromophore biosynthesis, namely for Chl and carotenoids and the involvement of both nuclear and plastid genomes (Dall’Osto et al., 2015). Major classes of pale‐green mutants include Chl a, Chl b and xanthophyll biosynthetic enzymes, the genes encoding members of the LHC family binding these chromophores, the genes encoding the import of nuclear precursors into the chloroplast and the assembly of the plastid‐encoded and nuclear‐encoded subunits of photosystems. As an example, we focus on chromophore biosynthesis for LHC antenna proteins, which bind most of the chlorophyll on Earth: a pale‐green phenotype can be observed upon mutation of either Chl a, Chl b or xanthophyll biosynthetic enzymes and yet the phenotypes strongly depend on the precise locus targeted by the mutation. Two mutations on the same enzymes, the Mg‐Chelatase, yielded a high‐light‐resistant phenotype when subunit H was targeted (Chekounova et al., 2001) while mutation on the gun4 regulative subunit yielded a photosensitive phenotype (Formighieri et al., 2012). Also, the Ch1 mutant of Arabidopsis thaliana, targeting Chl b synthase, yielded a highly photosensitive phenotype despite causing a strong reduction of the LHC functional antenna size and of the complement of LHC proteins, due to the promiscuous binding of Chl a on Chl b binding sites which affects efficiency of energy transfer reactions (Croce et al., 2002).
In synthesis, engineering Chl content has implications for environment, biomass production and crop yield. Yet, application to crop engineering requires a profound analysis of phenotypes to identify the genetic targets for the construction of high‐yield phenotypes with strong capacity for photoprotection against high irradiance.
The advent of such a new generation of low‐Chl plants is promising. But an effective RFSW not only depends on albedo but it is also site‐ and time‐dependent. It is a combination of surface optical properties, solar zenith angle (Sieber et al., 2019) and irradiance. In a mitigation perspective, equivalent anthropogenic carbon emissions can be converted to RFLW by knowing the radiative efficiency or the absolute global warming potential of CO2‐equivalents in the atmosphere (Myhre et al., 2013). Similarly, changes in surface albedo of a given area can be converted into CO2‐equivalents when surface irradiance and its seasonal/diurnal course are also known or can be predicted with sufficient accuracy. Such conversion gained much attention in the debate about the net mitigation potentials of afforestation; planting trees has been considered an effective way to sequester atmospheric carbon in the biomass and in the soil while the decrease in albedo, which is often associated with afforestation of grasslands and croplands, may cause substantial positive RFSW (Luyssaert et al., 2018; Sieber et al., 2019). The Time‐Dependent Emission Equivalent (TDEE) is a metric proposed to estimate emission equivalence of albedo changes. It makes use of (i) the warming potentials of CO2‐equivalents, (ii) the duration of the change in albedo (permanency), (iii) the absolute mass of CO2‐equivalents in the atmosphere and (iv) the RFSW magnitude (Bright et al., 2016). A simplified version of this metric was applied to evaluate the effect of the substitution of a conventional ‘green’ soybean cultivar with a low‐Chl mutant (Genesio et al., 2020). This showed that a permanent change of surface albedo for this crop would have differential impacts in different areas of the world, being dependent on the fraction of productive soybean area, on crop duration and the local/seasonal irradiance. The minimum global annual mean RFSW was estimated as −1.69 W m−2 in the regions where soybean is the dominant crop and weather conditions are the most favourable in terms of irradiance. The global substitution of this crop finally led to a negligible global estimated RFSW of −0.003 W m−2 which is equivalent to 4.4Gt CO2‐eq., globally.
Increased crop reflectance translates into reduced light absorbance and, hence, into an overall reduction of net radiation. If less energy is absorbed by the canopy, less energy is available for photochemistry, but this may be compensated by the increased efficiency in carbon fixation (Sakowska et al., 2018). On the other hand, less energy is dissipated as latent and/or sensible heat with obvious consequences on crop water use (transpiration) and/or on the local climate (cooling). Transpiration rates of a pale green soybean mutant measured in the field by Sakowska et al. (2018) using heat‐balance sap flow gauges were reduced by approximately 25%. In a subsequent experiment, a significant decrease in air temperature was observed at 2 m above the pale‐green canopy during daytime hours (Genesio et al., 2020) in response to a decrease in the sensible heat flux (Figure 4). These ‘local’ effects of the introduction of low‐Chl crops are of importance and may certainly provide positive side effects to climate change mitigation policies.
FIGURE 4.
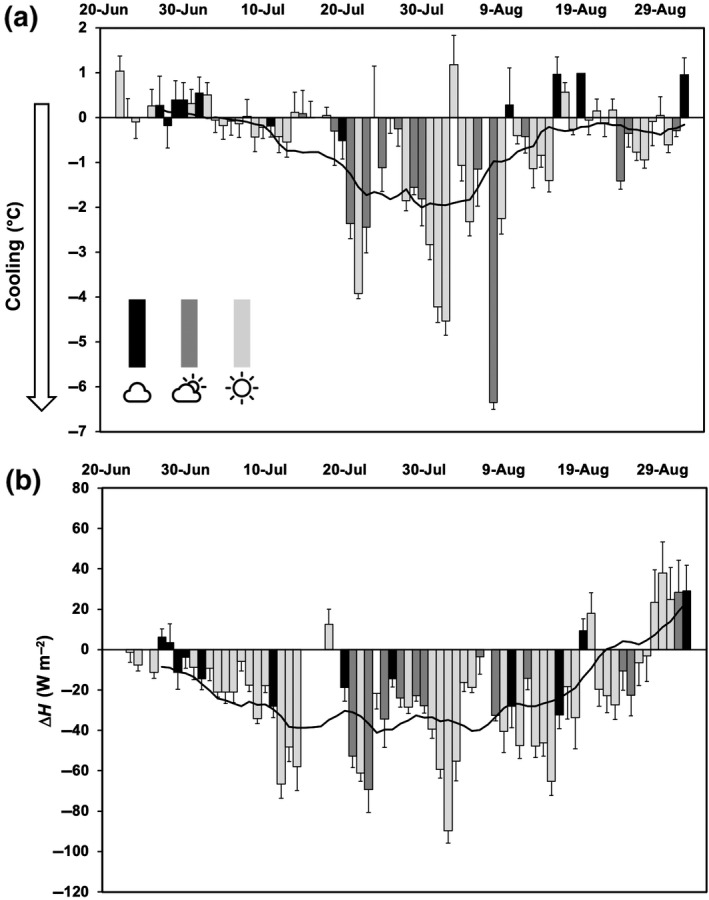
(a) Difference in the mean daily daytime air temperature in °C (10:00–16:00 h) measured at 2 m height above the soil between plots cultivated with the soybean mutant MinnGold and the commercial variety DekaBig in Ariis (Udine, Italy) in 2017. (b) Difference in the mean daily daytime sensible heat flux (H) measured for the same plots by means of eddy covariance in W m−2. The bars are coloured to outline weather conditions (legend in the figure). The 15‐day running means of air temperature and sensible heat flux differences are shown as solid lines
4. MITIGATION OF SOLAR BRIGHTENING
Our opinion is that low‐Chl crops can contribute to the mitigation of the expected solar brightening while having additional benefits. Brightening is already ongoing in most industrialized areas and is expected to be further increased in the future as a result of measures aiming to reduce GHG emissions. Such an effect is rarely considered in the climate change debate. It is worth reiterating here that brightening will inevitably be associated with the decarbonization of our society. The warming effect of increased shortwave irradiance (positive RFSW) will then offset, at the local scale, a fraction of the negative RFLW due to reduced GHG emissions. Field experiments made with a pale‐green soybean mutant in northern Italy showed that RFSW was reduced locally by 4.1 ± 0.6 W m−2 during the crop cycle (May–September) and by 1 ± 0.1 W m−2 on a full year scale (Genesio et al., 2020). We believe therefore that the introduction of novel low‐Chl content crop species will have a critical importance in future climate change mitigation (Arneth et al., 2009; Seneviratne et al., 2018).
5. CONCLUSIONS
The recent SARS‐CoV‐2 pandemic indirectly provided a clear example of the local environmental implications of a rapid decarbonization of our society. The slowdown of industrial activity and transport during the generalized lockdown led to reduced GHG emissions and increased RFSW. This was a preview of what is expected to happen in response to the implementation of the mitigation policies central to the Paris Agreement. A rapid reduction of GHG emissions is likely to be associated with solar brightening in the most densely populated areas of the world.
This message should be conveyed to policymakers together with the idea that new low‐Chl highly reflective plants may be used in agriculture and forestry to mitigate local impacts while not endangering crop and fibre yields. Genetic targets for the creation of those new plants are partly identified, but more research and extensive testing are still required to address numerous challenges. Full evidence should be provided that (i) genetically modified low‐Chl plants can sustain or even increase mean crop yields and ensure effective reduction in RFSW under different climates; (ii) local atmospheric feedback, that is, the impact of low‐Chl plants on the local climate, does not impair growth and the susceptibility of crops to biotic and abiotic stress; and (iii) current farming practices are adequate to support the expected changes in surface reflectance and to ensure yields and food quality of the new crops.
A new generation of highly reflective plants and their introduction in agriculture and forestry need to be examined together with their acceptability to farmers and society. Farmers may be concerned especially if benefits are not directly tied to productivity while changes in the colour of traditional landscapes may conflict with priorities of landscape attractiveness. However, it is worth noting here that profound past changes in agriculture and silviculture have already modified farmers’ perspectives and landscapes, without noticeable impact.
We finally recommend that new interdisciplinary research is designed, involving molecular biologists to foster the creation of new plants, climate modellers to carefully assess their potential impact on radiative forcing from local‐to‐regional scale, and agronomists and eco‐physiologists to assess their performance in different environments.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This paper originates from discussions made on the occasion of the International Congress on ‘Biophysics of Photosynthesis: from molecules to the field’ 2–4 October 2019, Accademia dei Lincei, Rome. Giorgio Alberti, Gemini delle Vedove and Alessandro Peressotti (University of Udine) are acknowledged for critical support in the experimental activities and data management. We also thank the De Eccher Agricultural Farm in Ariis, Udine, Italy. R.B. is the recipient of the RIBA 2017 grant from University of Verona dedicated to the construction of pale green plants.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Akbari, H. , Menon, S. , & Rosenfeld, A. (2009). Global cooling: Increasing world‐wide urban albedos to offset CO2 . Climatic Change, 94(3–4), 275–286. 10.1007/s10584-008-9515-9 [DOI] [Google Scholar]
- Arneth, A. , Unger, N. , Kulmala, M. , & Andreae, M. O. (2009). Clean the air, heat the planet? Science, 326(5953), 672–673. [DOI] [PubMed] [Google Scholar]
- Bao, R. , & Zhang, A. (2020). Does lockdown reduce air pollution? Evidence from 44 cities in northern China. Science of the Total Environment, 731, 139052. 10.1016/j.scitotenv.2020.139052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle, W. L. , McCullough, C. , Iversen, M. , & Hazlett, M. (2020). Leaf age and position effects on quantum yield and photosynthetic capacity in hemp crowns. Plants, 9(2), 271. 10.3390/plants9020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli, L. E. , & Andrade, F. H. (2020). Maize radiation use‐efficiency response to optimally distributed foliar‐nitrogen‐content depends on canopy leaf‐area index. Field Crops Research, 247, 107557. 10.1016/j.fcr.2019.107557 [DOI] [Google Scholar]
- Breuer, L. , Eckhardt, K. , & Frede, H. G. (2003). Plant parameter values for models in temperate climates. Ecological Modelling, 169(2–3), 237–293. 10.1016/S0304-3800(03)00274-6 [DOI] [Google Scholar]
- Bright, R. M. , Bogren, W. , Bernier, P. , & Astrup, R. (2016). Carbon‐equivalent metrics for albedo changes in land management contexts: Relevance of the time dimension. Ecological Applications, 26(6), 1868–1880. 10.1890/15-1597.1 [DOI] [PubMed] [Google Scholar]
- Bright, R. M. , Davin, E. , O’Halloran, T. , Pongratz, J. , Zhao, K. , & Cescatti, A. (2017). Local temperature response to land cover and management change driven by non‐radiative processes. Nature Climate Change, 7(4), 296–302. [Google Scholar]
- Broomandi, P. , Karaca, F. , Nikfal, A. , Jahanbakhshi, A. , Tamjidi, M. , & Kim, J. R. (2020). Impact of COVID‐19 event on the air quality in Iran. Aerosol and Air Quality Research, 20(8), 1793–1804. 10.4209/aaqr.2020.05.0205 [DOI] [Google Scholar]
- Cazzaniga, S. , Dall’Osto, L. , Scibilia, L. , Szaub, J. , Ballottari, M. , Purton, S. , & Bassi, R. (2014). Domestication of the green alga Chlorella sorokiniana: Reduction of antenna size improves light‐use efficiency in a photobioreactor. Biotechnology for Biofuels, 7(1), 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekounova, E. , Voronetskaya, V. , Papenbrock, J. , Grimm, B. , Beck, C. (2001). Characterization of Chlamydomonas mutants defective in the H subunit of Mg‐chelatase. Molecular Genetics and Genomics, 266(3), 363–373. 10.1007/s004380100574 [DOI] [PubMed] [Google Scholar]
- Croce, R. , Canino, G. , Ros, F. , & Bassi, R. (2002). Chromophores organization of the higher plants photosynthetic antenna complex CP26. Biochemistry, 41, 7334–7343. [DOI] [PubMed] [Google Scholar]
- Croft, H. , Chen, J. M. , Luo, X. , Bartlett, P. , Chen, B. , & Staebler, R. M. (2017). Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Global Change Biology, 23(9), 3513–3524. 10.1111/gcb.13599 [DOI] [PubMed] [Google Scholar]
- Dall’Osto, L. , Cazzaniga, S. , Guardini, Z. , Barera, S. , Benedetti, M. , Mannino, G. , Maffei, M. E. , & Bassi, R. (2019). Combined resistance to oxidative stress and reduced antenna size enhances light‐to‐biomass conversion efficiency in Chlorella vulgaris cultures. Biotechnology for Biofuels, 16(12), 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Osto, L. , Bressan, M. , & Bassi, R. (2015). Biogenesis of light harvesting proteins. Biochimica et Biophysica Acta (BBA)‐Bioenergetics, 1847(9), 861–871. 10.1016/j.bbabio.2015.02.009 [DOI] [PubMed] [Google Scholar]
- Dall'Osto, L. , Caffarri, S. , & Bassi, R. (2005). A mechanism of non‐photochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. The Plant Cell, 17(4), 1217–1232. 10.1105/tpc.104.030601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davin, E. L. , Seneviratne, S. I. , Ciais, P. , Olioso, A. , & Wang, T. (2014). Preferential cooling of hot extremes from cropland albedo management. Proceedings of the National Academy of Sciences of the United States of America, 111(27), 9757–9761. 10.1073/pnas.1317323111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty, C. E. , Field, C. B. , & McMillan, A. M. (2011). Can crop albedo be increased through the modification of leaf trichomes, and could this cool regional climate? Climatic Change, 104(2), 379–387. 10.1007/s10584-010-9936-0 [DOI] [Google Scholar]
- Engelken, J. , Brinkmann, H. , & Adamska, I. (2010). Taxonomic distribution and origins of the extended LHC (light‐harvesting complex) antenna protein superfamily. BMC Evolutionary Biology, 10(1), 233. 10.1186/1471-2148-10-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel, Z. V. , Irwin, A. J. , & Schofield, O. (2004). Resource limitation alters the 3/4 size scaling of metabolic rates in phytoplankton. Marine Ecology Progress Series, 273, 269–279. 10.3354/meps273269 [DOI] [Google Scholar]
- Formighieri, C. , Ceol, M. , Bonente, G. , Rochaix, J. D. , & Bassi, R. (2012). Retrograde signaling and photoprotection in a gun4 mutant of Chlamydomonas reinhardtii . Molecular Plant, 5(6), 1242–1262. 10.1093/mp/sss051 [DOI] [PubMed] [Google Scholar]
- Freschet, G. T. , Dias, A. T. C. , Ackerly, D. D. , Aerts, R. , van Bodegom, P. M. , Cornwell, W. K. , Dong, M. , Kurokawa, H. , Liu, G. , Onipchenko, V. G. , Ordoñez, J. C. , Peltzer, D. A. , Richardson, S. J. , Shidakov, I. I. , Soudzilovskaia, N. A. , Tao, J. , & Cornelissen, J. H. C. (2011). Global to community scale differences in the prevalence of convergent over divergent leaf trait distributions in plant assemblages. Global Ecology and Biogeography, 20(5), 755–765. 10.1111/j.1466-8238.2011.00651.x [DOI] [Google Scholar]
- Genesio, L. , Bright, R. M. , Alberti, G. , Peressotti, A. , Delle Vedove, G. , Incerti, G. , Toscano, P. , Rinaldi, M. , Muller, O. , & Miglietta, F. (2020). A chlorophyll‐deficient, highly reflective soybean mutant: Radiative forcing and yield gaps. Environmental Research Letters, 15(7), 074014. 10.1088/1748-9326/ab865e [DOI] [Google Scholar]
- Gertler, C. G. , O'Gorman, P. A. , Kravitz, B. , Moore, J. C. , Phipps, S. J. , & Watanabe, S. (2020). Weakening of the extratropical storm tracks in solar geoengineering scenarios. Geophysical Research Letters, 47(11). 10.1029/2020GL087348 [DOI] [Google Scholar]
- Gitelson, A. A. , Peng, Y. , Arkebauer, T. J. , & Schepers, J. (2014). Relationships between gross primary production, green LAI, and canopy chlorophyll content in maize: Implications for remote sensing of primary production. Remote Sensing of Environment, 144, 65–72. 10.1016/j.rse.2014.01.004 [DOI] [Google Scholar]
- Gu, J. , Zhou, Z. , Li, Z. , Chen, Y. , Wang, Z. , & Zhang, H. (2017). Rice (Oryza sativa L.) with reduced chlorophyll content exhibit higher photosynthetic rate and efficiency, improved canopy light distribution, and greater yields than normally pigmented plants. Field Crops Research, 200, 58–70. 10.1016/j.fcr.2016.10.008 [DOI] [Google Scholar]
- Hamwey, R. M. (2007). Active amplification of the terrestrial albedo to mitigate climate change: An exploratory study. Mitigation and Adaptation Strategies for Global Change, 12(4), 419–439. 10.1007/s11027-005-9024-3 [DOI] [Google Scholar]
- Havaux, M. , Dall'Osto, L. , Cuiné, S. , Giuliano, G. , & Bassi, R. (2004). The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana . Journal of Biological Chemistry, 279(14), 13878–13888. [DOI] [PubMed] [Google Scholar]
- Hirsch, A. L. , Prestele, R. , Davin, E. L. , Seneviratne, S. I. , Thiery, W. , & Verburg, P. H. (2018). Modelled biophysical impacts of conservation agriculture on local climates. Global Change Biology, 24(10), 4758–4774. 10.1111/gcb.14362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, A. L. , Wilhelm, M. , Davin, E. L. , Thiery, W. , & Seneviratne, S. I. (2017). Can climate‐effective land management reduce regional warming? Journal of Geophysical Research: Atmospheres, 122(4), 2269–2288. 10.1002/2016JD026125 [DOI] [Google Scholar]
- Hurrell, J. W. , Holland, M. M. , & Gent, P. R. (2013). The community earth system model: A framework for collaborative research. Bulletin of the American Meteorological Society, 94(9), 1339–1360. 10.1175/BAMS-D-12-00121.1 [DOI] [Google Scholar]
- Jacobson, M. Z. , & Ten Hoeve, J. E. (2012). Effects of urban surfaces and white roofs on global and regional climate. Journal of Climate, 25(3), 1028–1044. [Google Scholar]
- Jandaghian, Z. , & Akbari, H. (2018). The effect of increasing surface albedo on urban climate and air quality: A detailed study for Sacramento, Houston, and Chicago. Climate, 6(2), 19. [Google Scholar]
- Jandaghian, Z. , & Akbari, H. (2020). Effects of increasing surface reflectivity on aerosol, radiation, and cloud interactions in the urban atmosphere. Theoretical and Applied Climatology, 139(3–4), 873–892. [Google Scholar]
- Key, T. , McCarthy, A. , Campbell, D. A. , Six, C. , Roy, S. , & Finkel, Z. V. (2010). Cell size trade‐offs govern light exploitation strategies in marine phytoplankton. Environmental Microbiology, 12(1), 95–104. [DOI] [PubMed] [Google Scholar]
- Kirst, H. , Gabilly, S. T. , Niyogi, K. K. , Lemaux, P. G. , & Melis, A. (2017). Photosynthetic antenna engineering to improve crop yields. Planta, 245, 1009–1020. [DOI] [PubMed] [Google Scholar]
- Le Quéré, C. , Jackson, R. B. , Jones, M. W. , Smith, A. J. , Abernethy, S. , Andrew, R. M. , & Friedlingstein, P. (2020). Temporary reduction in daily global CO2 emissions during the COVID‐19 forced confinement. Nature Climate Change, 1–7. [Google Scholar]
- Li, Y. , He, N. , Hou, J. , Xu, L. , Liu, C. , Zhang, J. , & Wu, X. (2018). Factors influencing leaf chlorophyll content in natural forests at the biome scale. Frontiers in Ecology and Evolution, 6, 64. [Google Scholar]
- Li, Y. , Liu, C. , Zhang, J. , Yang, H. , Xu, L. , Wang, Q. , Sack, L. , Wu, X. , Jihua, H. , & He, N. (2018). Variation in leaf chlorophyll concentration from tropical to cold‐temperate forests: Association with gross primary productivity. Ecological Indicators, 85, 383–389. [Google Scholar]
- Long, S. P. , Zhu, X.‐G. , Naidu, S. L. , & Ort, D. R. (2006). Can improvement in photosynthesis increase crop yields? Plant, Cell and Environment, 29, 315–330. [DOI] [PubMed] [Google Scholar]
- Lugato, E. , Cescatti, A. , Jones, A. , Ceccherini, G. , & Duveiller, G. (2020). Maximising climate mitigation potential by carbon and radiative agricultural land management with cover crops. Environmental Research Letters. 10.1088/1748-9326/aba137 [DOI] [Google Scholar]
- Lutz, D. A. , & Howarth, R. B. (2014). Valuing albedo as an ecosystem service: Implications for forest management. Climatic Change, 124(1–2), 53–63. 10.1007/s10584-014-1109-0 [DOI] [Google Scholar]
- Luyssaert, S. , Marie, G. , Valade, A. , Chen, Y. Y. , Djomo, S. N. , Ryder, J. , Otto, J. , Naudts, K. , Lansø, A. S. , Ghattas, J. , & McGrath, M. J. (2018). Trade‐offs in using European forests to meet climate objectives. Nature, 562(7726), 259–262. 10.1038/s41586-018-0577-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manara, V. , Brunetti, M. , Celozzi, A. , Maugeri, M. , Sanchez‐Lorenzo, A. , & Wild, M. (2016). Detection of dimming/brightening in Italy from homogenized all‐sky and clear‐sky surface solar radiation records and underlying causes (1959–2013). Atmospheric Chemistry and Physics, 16(17), 11145–11161. [Google Scholar]
- Maraóón, E. , Cermeóo, P. , Rodríguez, J. , Zubkov, M. V. , & Harris, R. P. (2007). Scaling of phytoplankton photosynthesis and cell size in the ocean. Limnology and Oceanography, 52(5), 2190–2198. 10.4319/lo.2007.52.5.2190 [DOI] [Google Scholar]
- Morosinotto, T. , Breton, J. , Bassi, R. , & Croce, R. (2003). The nature of a chlorophyll ligand in Lhca proteins determines the far red fluorescence emission typical of photosystem I. Journal of Biological Chemistry, 278(49), 49223–49229. 10.1074/jbc.M309203200 [DOI] [PubMed] [Google Scholar]
- Myhre, G. , Shindell, D. , Bréon, F. M. , Collins, W. , Fuglestvedt, J. , Huang, J. , Koch, D. , Lamarque, J. F. , Lee, D. , Mendoza, B. , Nakajima, T. , Robock, A. , Stephens, G. , Takemura, T. , & Zhang, H. (2013). Anthropogenic and Natural Radiative Forcing. In Stocker T. F., Qin D., Plattner G.‐K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., & Midgley P. M. (Eds.), Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (pp. 659–740). Cambridge University Press. Retrieved from https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_Chapter08_FINAL.pdf [Google Scholar]
- Nunes‐Nesi, A. , Fernie, A. R. , & Stitt, M. (2010). Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Molecular Plant, 3(6), 973–996. 10.1093/mp/ssq049 [DOI] [PubMed] [Google Scholar]
- Paixão, J. S. , Da Silva, J. R. , Ruas, K. F. , Rodrigues, W. P. , Filho, J. A. M. , Bernado, W. D. P. , Abreu, D. P. , Ferreira, L. S. , Gonzalez, J. C. , Griffin, K. L. , Ramalho, J. C. , & Campostrini, E. (2019). Photosynthetic capacity, leaf respiration and growth in two papaya (Carica papaya) genotypes with different leaf chlorophyll concentrations. Aob Plants, 11(2), plz013. 10.1093/aobpla/plz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Bueno, M. L. , Johnson, M. P. , Zia, A. , Ruban, A. V. , & Horton, P. (2008). The Lhcb protein and xanthophyll composition of the light harvesting antenna controls the ΔpH‐dependency of non‐photochemical quenching in Arabidopsis thaliana . FEBS Letters, 582(10), 1477–1482. [DOI] [PubMed] [Google Scholar]
- Philipona, R. , Behrens, K. , & Ruckstuhl, C. (2009). How declining aerosols and rising greenhouse gases forced rapid warming in Europe since the 1980s. Geophysical Research Letters, 36(2). 10.1029/2008GL036350 [DOI] [Google Scholar]
- Ranjan, A. K. , Patra, A. K. , & Gorai, A. K. (2020). Effect of lockdown due to SARS COVID‐19 on aerosol optical depth (AOD) over urban and mining regions in India. Science of the Total Environment, 745, 141024. 10.1016/j.scitotenv.2020.141024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, J. L. (2019). Solar geoengineering to reduce climate change: A review of governance proposals. Proceedings of the Royal Society A, 475(2229), 20190255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgwell, A. , Singarayer, J. S. , Hetherington, A. M. , & Valdes, P. J. (2009). Tackling regional climate change by leaf albedo bio‐geoengineering. Current Biology, 19(2), 146–150. 10.1016/j.cub.2008.12.025 [DOI] [PubMed] [Google Scholar]
- Sakowska, K. , Alberti, G. , Genesio, L. , Peressotti, A. , Delle Vedove, G. , Gianelle, D. , Colombo, R. , Rodeghiero, M. , Panigada, C. , Juszczak, R. , Celesti, M. , Rossini, M. , Haworth, M. , Campbell, B. W. , Mevy, J. P. , Vescovo, L. , Cendrero‐Mateo, M. P. , Rascher, U. , & Miglietta, F. (2018). Leaf and canopy photosynthesis of a chlorophyll deficient soybean mutant. Plant, Cell & Environment, 41(6), 1427–1437. 10.1111/pce.13180 [DOI] [PubMed] [Google Scholar]
- Sakowska, K. , Cendrero‐Mateo, M. P. , van der Tol, C. , Celesti, M. , Alberti, G. , Juszczak, R. , Miglietta, F. , & Rascher, U. ; and the other members of the SOYFLEX campaign team . (2020). Exploring the scattering and reabsorption of chlorophyll fluorescence: Implications for remote sensing of photosynthesis. EGU General Assembly 2020, Online, 4–8 May 2020, EGU2020‐18194. 10.5194/egusphere-egu2020-18194 [DOI]
- Schwarz, M. , Folini, D. , Yang, S. , Allan, R. P. , & Wild, M. (2020). Changes in atmospheric shortwave absorption as important driver of dimming and brightening. Nature Geoscience, 13(2), 110–115. 10.1038/s41561-019-0528-y [DOI] [Google Scholar]
- Seneviratne, S. I. , Phipps, S. J. , Pitman, A. J. , Hirsch, A. L. , Davin, E. L. , Donat, M. G. , Hirschi, M. , Lenton, A. , Wilhelm, M. , & Kravitz, B. (2018). Land radiative management as contributor to regional‐scale climate adaptation and mitigation. Nature Geoscience, 11(2), 88–96. 10.1038/s41561-017-0057-5 [DOI] [Google Scholar]
- Sieber, P. , Ericsson, N. , & Hansson, P. A. (2019). Climate impact of surface albedo change in Life Cycle Assessment: Implications of site and time dependence. Environmental Impact Assessment Review, 77, 191–200. 10.1016/j.eiar.2019.04.003 [DOI] [Google Scholar]
- Singarayer, J. S. , Ridgwell, A. , & Irvine, P. (2009). Assessing the benefits of crop albedo bio‐geoengineering. Environmental Research Letters, 4(4), 045110. 10.1088/1748-9326/4/4/045110 [DOI] [Google Scholar]
- Slattery, R. A. , VanLoocke, A. , Bernacchi, C. J. , Zhu, X. G. , & Ort, D. R. (2017). Photosynthesis, light use efficiency, and yield of reduced‐chlorophyll soybean mutants in field conditions. Frontiers in Plant Science, 8, 549. 10.3389/fpls.2017.00549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobías, A. , Carnerero, C. , Reche, C. , Massagué, J. , Via, M. , Minguillón, M. C. , Alastuey, A. , & Querol, X. (2020). Changes in air quality during the lockdown in Barcelona (Spain) one month into the SARS‐CoV‐2 epidemic. Science of the Total Environment, 726, 138540. 10.1016/j.scitotenv.2020.138540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson, J. (2018). The sun dimmers. Nature, 563, 613–615. [DOI] [PubMed] [Google Scholar]
- Turnock, S. T. , Butt, E. W. , Richardson, T. B. , Mann, G. W. , Reddington, C. L. , Forster, P. M. , Haywood, J. , Crippa, M. , Janssens‐Maenhout, G. , Johnson, C. E. , Bellouin, N. , Carslaw, K. S. , & Spracklen, D. V. (2016). The impact of European legislative and technology measures to reduce air pollutants on air quality, human health and climate. Environmental Research Letters, 11(2), 024010. 10.1088/1748-9326/11/2/024010 [DOI] [Google Scholar]
- van Heerwaarden, C. C. , Mol, W. B. , Veerman, M. A. , Benedict, I. B. , Heusinkveld, B. G. , Knap, W. H. , Kazadzis, S. , Kouremeti, N. , & Fiedler, S. (2020). COVID‐19 lockdown contribution to spring surface solar irradiance record in Western Europe. arXiv preprint arXiv:2008.13497.
- Venter, Z. S. , Aunan, K. , Chowdhury, S. , & Lelieveld, J. (2020). COVID‐19 lockdowns cause global air pollution declines. Proceedings of the National Academy of Sciences of the United States of America, 117(32), 18984–18990. 10.1073/pnas.2006853117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtech, E. , Turnbull, L. A. , & Hector, A. (2007). Differences in light interception in grass monocultures predict short‐term competitive outcomes under productive conditions. PLoS One, 2, e499. 10.1371/journal.pone [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, B. J. , Drewry, D. T. , Slattery, R. A. , VanLoocke, A. , Cho, Y. B. , & Ort, D. R. (2018). Chlorophyll can be reduced in crop canopies with little penalty to photosynthesis. Plant Physiology, 176(2), 1215–1232. 10.1104/pp.17.01401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wientjes, E. , Roest, G. , & Croce, R. (2012). From red to blue to far‐red in Lhca4: How does the protein modulate the spectral properties of the pigments? Biochimica et Biophysica Acta (BBA)‐Bioenergetics, 1817(5), 711–717. 10.1016/j.bbabio.2012.02.030 [DOI] [PubMed] [Google Scholar]
- Wilhelm, M. , Davin, E. L. , & Seneviratne, S. I. (2015). Climate engineering of vegetated land for hot extremes mitigation: An Earth system model sensitivity study. Journal of Geophysical Research: Atmospheres, 120(7), 2612–2623. [Google Scholar]
- Zhang, S. , Wu, X. , Cui, J. , Zhang, F. , Wan, X. , Liu, Q. , Zhong, Y. U. , & Lin, T. (2019). Physiological and transcriptomic analysis of yellow leaf coloration in Populus deltoides Marsh. PLoS One, 14(5), e0216879. 10.1371/journal.pone.0216879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Alexander, L. , Hegerl, G. C. , Jones, P. , Tank, A. K. , Peterson, T. C. , Trewin, B. , & Zwiers, F. W. (2011). Indices for monitoring changes in extremes based on daily temperature and precipitation data. Wiley Interdisciplinary Reviews‐Climate Change, 2, 851–870. 10.1002/wcc.147 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


