Summary
Background
Reflectance confocal microscopy (RCM) is a noninvasive method for skin assessment, allowing entire lesion evaluation up to the papillary dermis. RCM is a potentially attractive alternative to punch biopsy (PB) in basal cell carcinoma (BCC).
Objectives
To determine the diagnostic accuracy of RCM vs. PB in diagnosing and subtyping BCC, and to study patient satisfaction and preferences.
Methods
Patients with a clinically suspected primary BCC were randomized between RCM and biopsy. Conventional surgical excision or follow‐up were used as reference. Sensitivity and specificity for BCC diagnosis and subtyping were calculated for both methods. BCC subtype was stratified based on clinical relevance: aggressive (infiltrative/micronodular) vs. nonaggressive (superficial/nodular) histopathological subtype and superficial vs. nonsuperficial BCC. Data on patient satisfaction and preferences were collected using a questionnaire and a contingent valuation method.
Results
Sensitivity for BCC diagnosis was high and similar for both methods (RCM 99·0% vs. biopsy 99·0%; P = 1·0). Specificity for BCC diagnosis was lower for RCM (59·1% vs. 100·0%; P < 0·001). Sensitivity for aggressive BCC subtypes was lower for RCM (33·3% vs. 77·3%; P = 0·003). Sensitivity for nonsuperficial BCC was not significantly different (RCM 88·9% vs. biopsy 91·0%; P = 0·724). Patient satisfaction and preferences were good and highly comparable for both methods.
Conclusions
Biopsy outperforms RCM in diagnosing and subtyping clinically suspected primary BCC. This outcome does not support routine clinical implementation of RCM, as a replacement for PBs in this patient group.
Short abstract
What is already known about this topic?
Expert groups have demonstrated the potency of in vivo diagnosing and subtyping of basal cell carcinoma (BCC) using confocal imaging. However, the diagnostic accuracy and financial consequences remain unclear, especially regarding correct subtyping.
What does this study add?
Confocal imaging was tested on performance in a real‐world clinical setting, as an alternative to diagnostic punch biopsies (PBs). In this setting, we concluded that for clinically suspicious primary BCC in daily practice, a PB remains preferred above confocal imaging, as it provides a superior accuracy for diagnosing and subtyping.
Linked Comment: Patalay. Br J Dermatol 2021; 184:590.
Since the 1990s, reflectance confocal microscopy (RCM) – also called confocal imaging – became known for its noninvasive skin imaging potential. Preliminary studies of RCM used for basal cell carcinoma (BCC) focused on the correlation of histopathological and RCM features. 1 , 2 , 3 The high and increasing incidence of BCC, 4 , 5 , 6 accompanied by a persistent increasing pressure on our already heavily overloaded healthcare systems, justify the interest in this field. Ultimately, RCM could be a quick, patient‐friendly and economically interesting alternative to the current standard punch biopsy (PB) diagnosis. A PB is an invasive procedure, with risks of pain, scar formation and sampling error. 7 , 8 , 9 , 10 , 11 The latter might lead to undertreatment followed by a recurrent tumour requiring additional (costly) therapy. Furthermore, PB does not give an immediate result owing to the time needed for tissue processing and assessment.
In contrast, RCM provides a noninvasive cellular‐level view and facilitates direct diagnosing with the possibility of complete lesion assessment, minimizing the risk of sampling error. Therefore, replacing PB with RCM would potentially save time, patient discomfort and money. Also, it might facilitate diagnosis and treatment in one visit (‘one‐stop shop’). 12
As recently concluded, clinical evidence on the implementation of RCM for regular BCC diagnostics is currently too premature. Furthermore, most work was performed by experts, 13 and cost‐effectiveness has yet to be evaluated. Ideally, RCM should at least have similar diagnostic accuracy in diagnosing BCC as the currently used biopsies. Furthermore, the BCC subtype should be identified correctly, to select the most appropriate treatment. Also, costs of RCM preferably should not exceed costs of PB, and implementation should be feasible. Furthermore, the patient’s experience should, ideally, be superior with RCM or at least similar to PB. All of these outcomes were assessed in this randomized controlled trial (RCT).
The primary objective was to investigate whether a correct diagnosis and subtype could be determined with RCM in patients with a clinically suspected primary BCC, in a real‐world setting. The secondary objectives were to study patient satisfaction and patient preferences.
Patients and methods
Study design
In this multicentre RCT, patients with a clinically suspected primary BCC were randomized between two index tests: RCM and diagnostic PB. If RCM was negative for BCC, a PB was taken to confirm this diagnosis. Also, in cases of inconclusive RCM imaging the patient was offered a PB. PBs taken from patients randomized to RCM were not included in the calculation of the diagnostic accuracy of PB.
As the reference standards, only conventional surgical excision or follow‐up (when the PB did not show BCC) were accepted. Figure 1 illustrates the study design.
Figure 1.
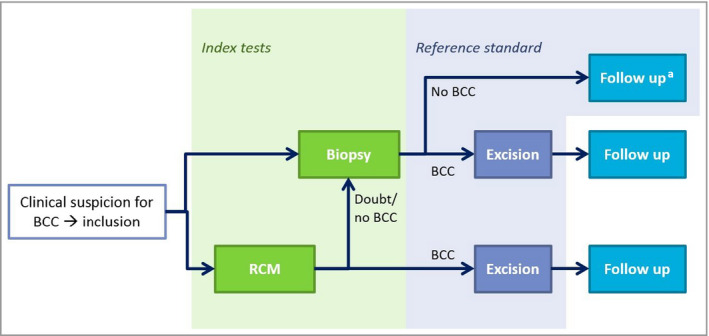
Study design of the randomized controlled trial investigating in vivo reflectance confocal microscopy (RCM) vs. a diagnostic punch biopsy in patients with a clinically suspected primary basal cell carcinoma (BCC). aIn cases where biopsy did not show BCC, but another diagnosis could be made, patients were treated accordingly when indicated and follow‐up was performed.
This study was approved by the regional medical ethical committee (CMO Regio Arnhem‐Nijmegen, Dossier number 2015‐1963, NL number 5449·091·15) and conducted according to the principles of the Declaration of Helsinki. The protocol was registered at ClinicalTrials.gov (NCT02623101), and has also been published previously. 14
Patients
Patients from four hospitals in the Netherlands were included: Radboud University Medical Centre (coordinating academic hospital, Nijmegen); Netherlands Cancer Institute (tertiary oncological reference centre, Amsterdam); Canisius Wilhelmina Hospital (general hospital, Nijmegen); Rijnstate Hospital (general hospital, Velp). Patients with a clinically suspected primary BCC were eligible for inclusion and referred by their treating clinician. Patients had to be ≥ 18 years old, able and willing to give written informed consent, and to adhere to the study requirements. Lesions were only included if conventional surgery was the preferred treatment. In cases where Mohs surgery, radiotherapy or topical treatment was considered, patients were excluded. All anatomical sites were included; however, lesions had to be accessible to either of the RCM devices. Patients participating in another trial – currently or in the previous 28 days – were excluded. Patients from the Netherlands Cancer Institute with BCC in the head‐and‐neck area were not included as they had already participated in another study.
The participating hospitals defined the four strata used for the variable computer‐generated block randomization, with block sizes of four, six or eight. Patients were randomized between RCM and PB by one of the researchers, using an automated assignment system (Castor Electronic Data Capture; Castor, Hoboken, NJ, USA), with a randomization weight of 1 for both groups. The researcher entered the patient’s details into the system and diagnostic allocation was returned. Afterwards, the same researcher performed RCM assessment or PB. The allocation sequence was concealed from the researcher. Excision and follow‐up were performed in regular daily clinical practice, by physicians not involved in patient inclusion, randomization or diagnostic trial intervention. Owing to the nature of the intervention the participating patients, researchers and treating physicians could not be blinded to the diagnostic intervention performed (e.g. visible PB scar). The pathologists examining the tissue after PB and/or excision were blinded to the study group.
Sample size
Based on an extensive local database of previously collected cases (n >200), we assumed a percentage of correctly identified BCC subtypes of 71% using PB and 85% using RCM, when compared with conventional surgical excision as the gold standard in this study. Consequently, 148 patients were needed per group to obtain a power of 80% (Fisher’s exact, two‐sided, α = 0·05). In addition, it was expected that 10% of patients in whom a physician had a clinical suspicion of BCC would not have a histopathologically confirmed BCC. Therefore, the initial goal was to include 329 patients with a clinical suspicion of primary BCC. More details of the initial sample size calculation can be found in the previously published study protocol. 14
Owing to the limited progress of the study, mainly caused by technical problems with the RCM after 288 patients, the continuation of the study would only be possible with major changes in the staffing of the study team. Before taking such a major decision it was decided to analyse whether the 41 remaining patients to be included would have an impact on the primary outcome. For specificity, a clear statistically significant difference had already been found (P < 0·001), while the high levels of sensitivity (99% for both methods) precluded the possibility of finding any difference. This premature end of inclusion was authorized by the medical ethical committee that approved the study (CMO Regio Arnhem–Nijmegen)
Diagnostic methods
Index test: reflectance confocal microscopy
For imaging we used the commercially available VivaScope 1500® and 3000® [Caliber ID (Henrietta, NY, USA); Mavig GmbH (München, Germany)] devices. The VivaScope 3000 is a handheld RCM device that allows imaging of lesions that were inaccessible by the VivaScope 1500.
With the VivaScope 1500, VivaBlocks of 4 × 4 mm were made at the level of the stratum corneum, stratum spinosum, dermoepidermal junction and dermis in order to find RCM features for BCC and its subtype.
The VivaScope 3000 was used when a lesion was not accessible by the VivaScope 1500. With either the VivaScope 1500 or VivaScope 3000, VivaStacks were made in the areas of interest and movies were recorded to document vascularization. The number of imaging sites was determined during the clinical situation. When BCC characteristics were captured in the first series of images no additional images were needed.
Lesion assessment was performed unblinded to clinical information, using previously published RCM features as a guideline. 1 , 2 , 3 , 15 , 16 , 17 However, as there is currently no consensus on specific subtype criteria, inter‐reader variability was expected.
Reflectance confocal microscopy readers
To mimic realistic clinical use, RCM images were assessed real time by on‐demand available readers dedicated to working with RCM. This enabled further diagnostics (e.g. PB in case of inconclusive RCM) or treatment without any delay. Most lesions were included, randomized and assessed by experienced readers (M.P./Y.S.E.). Experienced readers met all of the following criteria: (i) trained at the University of Modena (organized by the VivaScope distributor Mavig GmbH); (ii) having 2–4 years of RCM experience before initiation of the study; and (iii) having assessed at least 200 BCC before study initiation. In‐house trained readers with less RCM experience (W.WvdW./K.N.; < 6 months for BCC) assessed a minority (< 10%) of patients included in the diagnostic analysis. Diagnostic accuracy of RCM was calculated for all readers. In addition, a separate analysis was performed for the most experienced readers only.
Index test: punch biopsy
Patients allocated to the PB group received standard diagnostic care: a 3‐mm PB taken from the clinically most suspected area after local anaesthesia with 1% Xylocaine/adrenaline. PBs were paraffin embedded after at least 4 h of formaldehyde fixation. Paraffin sections (6 μm) were processed and stained with haematoxylin and eosin by a standard protocol. Histopathological classification of BCC was performed by experienced pathologists according to Dutch guidelines. 18
Reference test: excision
Conventional surgical excision was performed according to the current Dutch recommendations, 18 with a 3–5‐mm margin for low‐ and high‐risk BCC, respectively. Excision specimens were processed using the standard protocol: tumours were inspected and measured by a technician by the naked eye and excision margins were inked. The specimen was then bread‐loaf cut every 3 mm, followed by paraffin embedding, processing of paraffin sections (6 μm) and haematoxylin and eosin staining. Histopathological classification was performed as for biopsies.
Reference test: follow‐up
For patients not diagnosed with a BCC, regular clinical follow‐up was used as reference test, in line with the recent Cochrane review (Figure 1). 13 The diagnosis of ‘no BCC’ was classified as correct in cases where no reported signs for BCC in the treated area developed during follow‐up. In cases where an alternative diagnosis was made by PB (e.g. squamous cell carcinoma), the patient was treated when indicated and followed up.
Statistical analysis
For each group baseline characteristics were summarized for descriptive statistics.
For all sensitivity and specificity estimates, cross‐tables were used and 95% confidence intervals (CIs) were calculated using the exact binomial calculation. Comparison between diagnostic groups was compared using two‐tailed unconditional exact tests (α = 0·05). Imputation of missing observations was not performed. All analyses were performed on a complete case basis, with completeness being restricted to the variables needed for any specific analysis. Patients without a reference test (e.g. did not undergo a conventional surgical excision after BCC diagnosis) were excluded from the diagnostic accuracy analysis. Data preparation was performed in SPSS (version 25; IBM, Armonk, NY, USA), tests and calculations of CIs were performed using the Exact library (version 2·0) in R (version 3·6·1).
Primary outcome: basal cell carcinoma diagnosis
Sensitivity and specificity for BCC diagnosis (‘BCC’ or ‘no BCC’) was calculated by comparing the outcome of the index test (PB or RCM) with the reference test (conventional surgical excision or follow‐up).
Primary outcome: basal cell carcinoma subtyping
According to the Dutch guidelines, 18 four BCC subtypes were used for initial classification by the pathologist: superficial (sBCC), nodular (nBCC), infiltrative (iBCC) and micronodular (mnBCC), as well as mixed‐type BCC. Classifying index test subtypes as correct or incorrect was further guided by clinical relevance. This led to assessment of aggressiveness and superficial growth pattern, resulting in two methods for subtype stratification: (i) based on aggressiveness – as aggressiveness determines treatment choices, lesions were divided between ‘low risk’ (only sBCC and/or nBCC) and ‘aggressive’ (iBCC or mnBCC, and mixed‐type lesions including these subtypes); (ii) based on superficial growth pattern – as noninvasive treatments are available for sBCC (e.g. imiquimod cream), superficial growth pattern was assessed separately. BCCs with only a superficial growth pattern were classified as ‘only superficial’. All others were classified as ‘(partially) nonsuperficial’.
Sensitivity of RCM and PB for aggressiveness and for (partially) nonsuperficial BCC were calculated by comparing index test results with the reference test (conventional surgical excision). Cases with PB‐confirmed BCC without residual BCC in the excisional specimen were excluded from this analysis.
Subset analysis
Diagnostic accuracy by experienced readers and VivaScope 1500 only
Diagnostic accuracy for BCC diagnosis (sensitivity and specificity) and subtyping (sensitivity) were also calculated in the patient subset imaged with RCM by the most experienced readers (M.P./Y.S.E.). As most previous studies used the non‐handheld VivaScope 1500 device, additional analyses for the patient subset imaged with this device only were also performed.
Secondary outcomes: patient satisfaction and patient preferences
Patient satisfaction was measured using a 7‐point Likert scale just after the index test (T1) and during follow‐up (T2). In patients who had experience of both PB and RCM (gained before or during this study), patient preferences were measured using a multiple‐choice questionnaire and a contingent valuation method (CVM). More details are provided in the Supporting Information.
Results
Participants and histopathology
Details on patient inclusion, allocation and study finalization are shown in Figure 2. In summary, between 24 February 2016 and 1 February 2019, a total of 288 patients were included (RCM, n = 145; PB, n = 143). Of the included patients, 119 of 145 (82·1%) in the RCM group and 123 of 143 (86·0%) in the PB group received both the index and reference test and were included in the diagnostic accuracy calculations. Patients in whom the PB did not show BCC and who underwent nonsurgical treatment and/or clinical follow‐up because an alternative diagnosis was made (n = 41) had a median follow‐up time of 30 months (range 3–47) without any change in initial diagnoses during follow‐up. Baseline characteristics per test group are comparable and given in Table 1.
Figure 2.
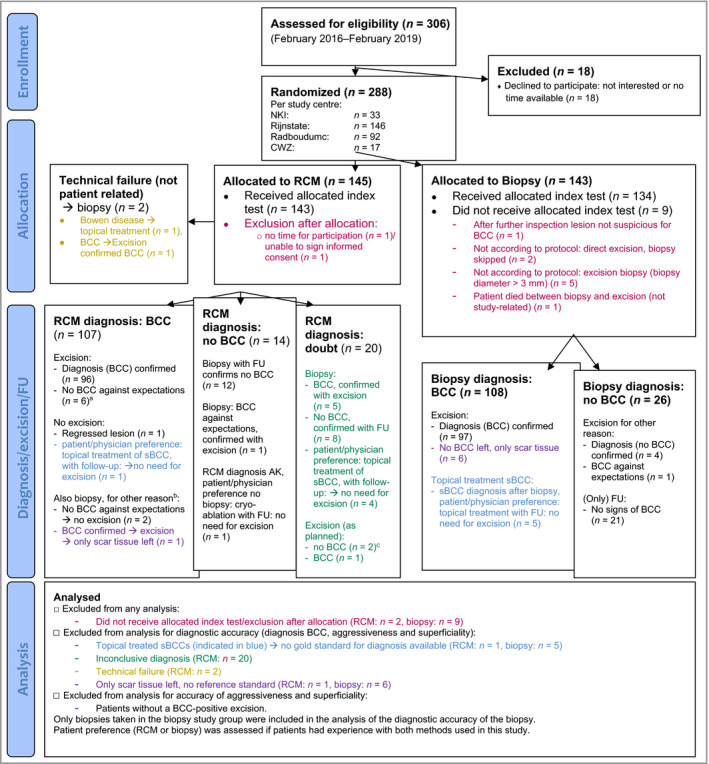
Overview of study participant inclusion, allocation and study finalization. NKI, Nederlands Kanker Instituut (Netherlands Cancer Institute); CWZ, Canisius Wilhelmina Ziekenhuis (Canisius Wilhelmina Hospital); RCM, reflectance confocal microscopy; FU, follow‐up; sBCC, superficial BCC. aLichenoid keratosis (n = 1), actinic keratosis (n = 2), seborrhoeic keratosis (n = 1), squamous cell carcinoma (n = 1), Merkel cell carcinoma (n = 1). bBecause of Mohs indication/doubt about subtype based on RCM. cActinic keratosis (n = 1), dermal melanocytic naevus (n = 1).
Table 1.
Baseline characteristicsa
| RCM group (n = 145) | Biopsy group (n = 143) | |
|---|---|---|
| Patient characteristics | ||
| Median (range) age (years) | 70 (21–90) | 70 (33–87) |
| Sex | ||
| Male | 78 (53·8) | 89 (62·2) |
| Female | 67 (46·2) | 54 (37·8) |
| History of cutaneous (pre)malignancyb | 111 (76·6) | 96 (67·1) |
| History of BCC | 94 (64·8) | 79 (55·2) |
| Lesion characteristics | ||
| Location | ||
| Head/neck area (H‐zone) | 3 (2·1) | 5 (3·5) |
| Head/neck area (no H‐zone) | 37 (25·5) | 32 (22·4) |
| Trunk (frontal side) | 47 (32·4) | 40 (28·0) |
| Trunk (backside) | 35 (24·1) | 42 (29·4) |
| Upper limb | 12 (8·3) | 15 (10·5) |
| Lower limb | 11 (7·6) | 9 (6·3) |
Data are n (%) unless otherwise indicated. Data may not add up owing to rounding. No missing variables. RCM, reflectance confocal microscopy. aOf patient allocated in this randomized controlled trial; ball types of cutaneous (pre)malignancies, including basal cell carcinoma (BCC).
Primary outcome: basal cell carcinoma diagnosis
Results are shown in Tables 2 and 3.
Table 2.
Cross‐tables of index [reflectance confocal microscopy (RCM) or biopsy] and reference test [conventional excision/follow‐up (FU)]a
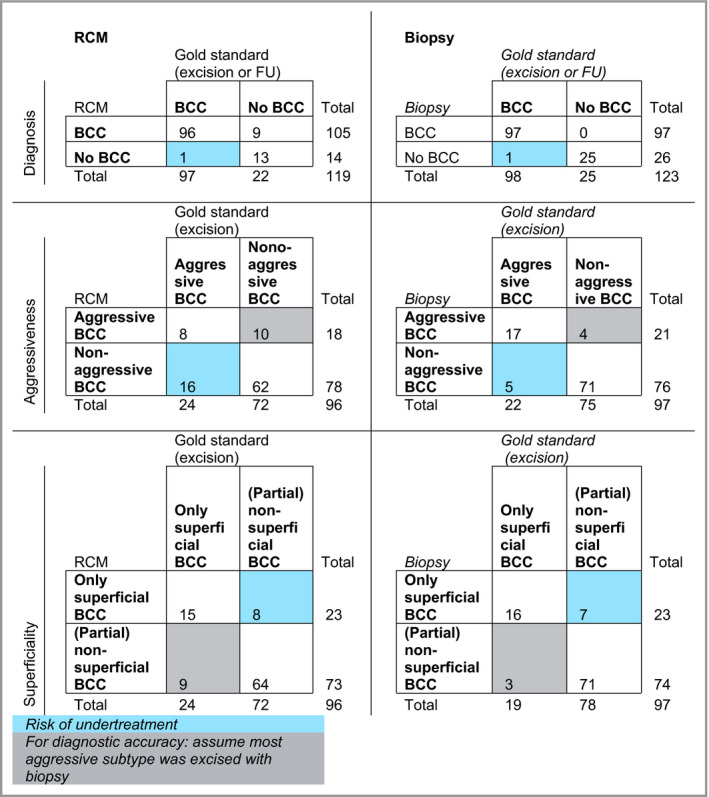
BCC, basal cell carcinoma. aOnly patients who received both the index test (RCM or biopsy) and reference test (conventional surgical excision or FU) were included in this analysis (n = 242).
Table 3.
Measures of diagnostic accuracy for diagnosing and subtyping basal cell carcinoma (BCC)a
| RCM | Biopsy | P‐value difference | 95% CI for difference | |
|---|---|---|---|---|
| Sensitivity diagnosis BCC (95% CI) | 99·0% (94·4–100) | 99·0% (94·4–100) | 1·00 | –4·9 to 4·9 |
| Specificity diagnosis BCC (95% CI) | 59·1% (36·4–79·3) | 100% (86·3–100) | < 0·001a | –61·8 to –22·4 |
| Sensitivity aggressive BCC (95% CI) | 33·3% (15·6–55·3) | 77·3% (54·6–92·2) | 0·003a | –67·1 to –14·6 |
| Sensitivity nonsuperficial BCC (95%CI) | 88·9% (79·3–95·1) | 91·0% (82·3–96·3) | 0·724 | –13·0 to 8·3 |
CI, confidence interval; RCM, reflectance confocal microscopy. aOnly patients who received both the index test (RCM or biopsy) and reference test (conventional surgical excision or follow‐up) were included in this analysis (n = 242). Comparison of RCM and biopsy was performed using two‐tailed unconditional exact tests (α = 0·05).
Sensitivity for BCC diagnosis was high and comparable in both groups [99·0% vs. 99·0%; 95% CI –4·9 to 4·9 (P = 1·0)]. The specificity for diagnosing BCC was significantly lower for RCM than for PB [59·1% vs. 100%; 95% CI –61·8 to –22·4% (P < 0·001)].
Primary outcome: basal cell carcinoma subtyping
BCC subtyping results are also shown in Tables 2 and 3.
Aggressiveness
The sensitivity for aggressiveness was significantly lower for RCM than for PB [33·3% vs. 77·3%; 95% CI –67·1 to –14·6 (P = 0·003)].
Superficial growth pattern
The sensitivity for diagnosing a nonsuperficial subtype was similar for RCM and PB [88·9% vs. 91·0%; 95% CI –13·0 to 8·3 (P = 0·724)].
Additional analyses
Experienced users
The primary outcomes did not change when only including data from experienced readers (Table S1 and S2; see Supporting Information).
Non‐handheld VivaScope 1500
The primary outcomes did not change when only data obtained from this device were included (Tables S3 and S4; see Supporting Information).
Secondary outcome: patient satisfaction and patient preferences
Patient satisfaction was good and highly comparable between groups over the different timepoints. Most patients who had experience with both methods said they preferred RCM over PB, under the assumption of comparable diagnostic accuracy [n = 50/58 (86%)]. The CVM showed no significant difference in the price these patients were willing to pay for their preferred diagnostic method. Detailed results are included in the Supporting Information (Tables S5 and S6).
Discussion
In this RCT comparing BCC diagnosis and subtyping by RCM and PB, we studied RCM in a real‐world setting that mirrored possible future implementation in primary BCC‐suspected lesions. Our dedicated RCM readers were available on demand in daily clinical practice and performed on‐the‐spot RCM evaluation and/or PB. In this setting, sensitivity remained high, as was expected from previous studies. 15 , 19 , 20 However, specificity (59·1%, 95% CI 36·4–79·3) was insufficient compared with PB and relatively low compared with other studies. 19 Further investigation of this difference revealed that the specificity for RCM in general hospitals (50·0%, 95% CI 18·7–81·3) seemed to be lower than in tertiary centres (66·7%, 95% CI 34·9–90·1; P = 0·42). This might be owing to the higher chance of diagnosing a BCC in general hospitals because of different patient populations (65·9% and 78·6% of the included patients had a pathologically confirmed BCC in tertiary centres and general hospitals, respectively).
Regarding BCC subtyping, our data showed that PB is more accurate in BCC subtyping than RCM, especially in identifying an aggressive growth pattern, which is essential for proper risk stratification and treatment selection. Therefore, a PB is preferred over RCM in routine daily practice in BCC‐suspected lesions.
Despite inferior diagnostic accuracy, RCM could still be relevant for specific patients. For example, previous data have shown that handheld RCM could play a role in eyelid margin tumours, 21 as well as large lesion mapping. 22
Although a real‐world clinical setting was needed to obtain realistic data, this approach comes with its disadvantages. It resulted in multiple deviations from the study protocol (Figure 2). Also, in these busy, real‐world clinical settings, physicians sometimes forgot to refer eligible patients for inclusion or chose the quickest option (clinical routine without study inclusion). On the one hand this hampered the progress but on the other hand this would probably also happen after further implementation and points out a potentially important threshold. Furthermore, as conventional excision was used as the gold standard in this study, which is the most commonly used treatment option in BCC, BCC treated with other treatment modalities were not included. This might have led to a selection bias and might have led to an underestimation of the diagnostic accuracy potential of RCM in these subgroups and the accompanying financial effects. For example, RCM diagnosis in superficial BCC treated with a nonsurgical modality could be beneficial over PB because of the potential prevention of nonresponse due to a partial nonsuperficial part of the BCC in combination with PB sampling error.
Aiming for a financially attractive implementation, we chose to study replacement of regular diagnostic PB. Another strategy could be to use RCM additionally, before deciding on a PB or excision. This is the way dermoscopy is currently being used. 23 , 24 , 25 , 26 In a population with different skin tumours, Yelamos et al. 27 showed that RCM improves diagnostic confidence and accuracy in equivocal tumours.
This strategy of use only for equivocal tumours is most attractive for high‐volume clinics, or if RCM and knowledge about assessment are already available (e.g. because of other use, such as imaging of suspicious melanocytic lesions).
Besides this, other developments (e.g. combining in vivo RCM with optical coherence tomography) 28 might increase the diagnostic accuracy and clinical applicability of the technique.
In our experience, a fast and easy‐to‐use device (limiting the human resources required and increasing user acceptance), and balancing frequency of use (influencing price per patient) and device costs are crucial for successful implementation and a positive cost–benefit ratio. In the hypothetical case of similar diagnostic accuracy, RCM would be borderline competitive with a PB regarding cost in the Netherlands with the assessment of 1000 patients annually per device and only using the device for BCC diagnostics (Table S6; see Supporting Information). Diagnostic costs are highly dependent on the volume of patients imaged with the RCM owing to the relatively large amount of capital cost vs. PB (Table S6; see Supporting Information). Furthermore, although in this study the experienced user subset demonstrated a similar accuracy to the complete dataset, it needs to be mentioned that an important number of RCM assessments were inconclusive and additional PB was performed in these cases according to the study protocol (n = 20/143; 14·0%), which was more often the case in less experienced users. These inconclusive patients were not included in the accuracy calculations but would probably increase diagnostic costs and hamper clinical productivity in real‐world clinical practice. Also, this emphasizes the importance and potential impact of user experience in relation to the cost–benefit effects of daily practice implementation.
PB outperforms RCM in diagnosing and subtyping clinically suspected primary BCC. This outcome does not support routine clinical implementation of RCM as a replacement for PBs in this patient group.
Supporting information
Table S1 Results of only experience reflectance confocal microscopy readers.
Table S2 Diagnostic accuracy of experienced reflectance confocal microscopy readers compared with biopsy.
Table S3 Reflectance confocal microscopy cross‐tables including only the VivaScope 1500.
Table S4 Diagnostic accuracy of the VivaScope 1500 compared with biopsy.
Table S5 Patient satisfaction.
Table S6 Diagnostic costs of biopsy and reflectance confocal microscopy diagnostics.
Acknowledgments
This study could not have been conducted without the contribution of the involved patients and we are grateful to them. We also thank T.K.P. Nguyen (K.N.) for her contribution to patient inclusion. We highly appreciate the advice of pathologist W.A.M. Blokx and statistician J.C.M. Hendriks regarding the project and study design.
W.W–vdW and M.P. contributed equally.
Plain language summary available online
References
- 1. Gonzalez S, Tannous Z. Real‐time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol 2002; 47:869–74. [DOI] [PubMed] [Google Scholar]
- 2. Peppelman M, Wolberink EA, Blokx WA et al. In vivo diagnosis of basal cell carcinoma subtype by reflectance confocal microscopy. Dermatology 2013; 227:255–62. [DOI] [PubMed] [Google Scholar]
- 3. Longo C, Lallas A, Kyrgidis A et al. Classifying distinct basal cell carcinoma subtype by means of dermatoscopy and reflectance confocal microscopy. J Am Acad Dermatol 2014; 71:716–24. [DOI] [PubMed] [Google Scholar]
- 4. Peggy A, Wu M. Epidemiology, pathogenesis, and clinical features of basal cell carcinoma. Available at: https://www.uptodate.com/contents/epidemiology‐pathogenesis‐and‐clinical‐features‐of‐basal‐cell‐carcinoma (last accessed 22 May 2019).
- 5. Flohil SC, de Vries E, Neumann HA et al. Incidence, prevalence and future trends of primary basal cell carcinoma in the Netherlands. Acta Derm Venereol 2011; 91:24–30. [DOI] [PubMed] [Google Scholar]
- 6. Flohil SC, Seubring I, van Rossum MM et al. Trends in basal cell carcinoma incidence rates: a 37‐year Dutch observational study. J Invest Dermatol 2013; 133:913–18. [DOI] [PubMed] [Google Scholar]
- 7. Cameron MC, Lee E, Hibler BP et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol 2019; 80:321–39. [DOI] [PubMed] [Google Scholar]
- 8. Wolberink EA, Pasch MC, Zeiler M et al. High discordance between punch biopsy and excision in establishing basal cell carcinoma subtype: analysis of 500 cases. J Eur Acad Dermatol Venereol 2013; 27:985–9. [DOI] [PubMed] [Google Scholar]
- 9. van Delft LCJ, Nelemans PJ, Jansen MHE et al. Histologic subtype of treatment failures after noninvasive therapy for superficial basal cell carcinoma: an observational study. J Am Acad Dermatol 2019; 80:1022–8. [DOI] [PubMed] [Google Scholar]
- 10. Kadouch DJ, van Haersma de With A, Limpens J et al. Is a punch biopsy reliable in subtyping basal cell carcinoma? A systematic review. Br J Dermatol 2016; 175:401–3. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen KP, Knuiman GJ, van Erp PE et al. Standard step sectioning of skin biopsy specimens diagnosed as superficial basal cell carcinoma frequently yields deeper and more aggressive subtypes. J Am Acad Dermatol 2017; 76:351–53. [DOI] [PubMed] [Google Scholar]
- 12. Kadouch DJ, Elshot YS, Zupan‐Kajcovski B et al. One‐stop‐shop with confocal microscopy imaging vs. standard care for surgical treatment of basal cell carcinoma: an open‐label, noninferiority, randomized controlled multicentre trial. Br J Dermatol 2017; 177:735–41. [DOI] [PubMed] [Google Scholar]
- 13. Dinnes J, Deeks JJ, Chuchu N et al. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev 2018; 12:CD013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peppelman M, Nguyen KP, Alkemade HA et al. Diagnosis of basal cell carcinoma by reflectance confocal microscopy: study design and protocol of a randomized controlled multicenter trial. JMIR Res Protoc 2016; 5:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nori S, Rius‐Diaz F, Cuevas J et al. Sensitivity and specificity of reflectance‐mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol 2004; 51:923–30. [DOI] [PubMed] [Google Scholar]
- 16. Ulrich M, Roewert‐Huber J, Gonzalez S et al. Peritumoral clefting in basal cell carcinoma: correlation of in vivo reflectance confocal microscopy and routine histology. J Cutan Pathol 2011; 38:190–5. [DOI] [PubMed] [Google Scholar]
- 17. Longo C, Farnetani F, Ciardo S et al. Is confocal microscopy a valuable tool in diagnosing nodular lesions? A study of 140 cases. Br J Dermatol 2013; 169:58–67. [DOI] [PubMed] [Google Scholar]
- 18. Kelleners‐Smeets NWJea. Dutch Evidence‐based Guidelines Basal Cell Carcinoma. Utrecht: Nederlandse Vereniging voor Dermatologie en Venerologie, 2015. [Google Scholar]
- 19. Kadouch DJ, Leeflang MM, Elshot YS et al. Diagnostic accuracy of confocal microscopy imaging vs. punch biopsy for diagnosing and subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol 2017; 31:1641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kadouch DJ, Schram ME, Leeflang MM et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol 2015; 29:1890–7. [DOI] [PubMed] [Google Scholar]
- 21. Cinotti E, Perrot JL, Campolmi N et al. The role of in vivo confocal microscopy in the diagnosis of eyelid margin tumors: 47 cases. J Am Acad Dermatol 2014; 71:912–18. [DOI] [PubMed] [Google Scholar]
- 22. Peppelman M, Wolberink EA, Koopman RJ et al. In vivo reflectance confocal microscopy: a useful tool to select the location of a punch biopsy in a large, clinically indistinctive lesion. Case Rep Dermatol 2013; 5:129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Menzies SW, Westerhoff K, Rabinovitz H et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol 2000; 136:1012–16. [DOI] [PubMed] [Google Scholar]
- 24. Pan Y, Chamberlain AJ, Bailey M et al. Dermatoscopy aids in the diagnosis of the solitary red scaly patch or plaque‐features distinguishing superficial basal cell carcinoma, intraepidermal carcinoma, and psoriasis. J Am Acad Dermatol 2008; 59:268–74. [DOI] [PubMed] [Google Scholar]
- 25. Witkowski AM, Ludzik J, DeCarvalho N et al. Non‐invasive diagnosis of pink basal cell carcinoma: how much can we rely on dermoscopy and reflectance confocal microscopy? Skin Res Technol 2016; 22:230–7. [DOI] [PubMed] [Google Scholar]
- 26. Borsari S, Pampena R, Lallas A et al. Clinical Indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol 2016; 152:1093–8. [DOI] [PubMed] [Google Scholar]
- 27. Yelamos O, Manubens E, Jain M et al. Improvement of diagnostic confidence and management of equivocal skin lesions by integration of reflectance confocal microscopy in daily practice: prospective study in two referral skin cancer centers. J Am Acad Dermatol 2019; S0190–9622:30969–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahu A, Yelamos O, Iftimia N et al. Evaluation of a combined reflectance confocal microscopy‐optical coherence tomography device for detection and depth assessment of basal cell carcinoma. JAMA Dermatol 2018; 154:1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Results of only experience reflectance confocal microscopy readers.
Table S2 Diagnostic accuracy of experienced reflectance confocal microscopy readers compared with biopsy.
Table S3 Reflectance confocal microscopy cross‐tables including only the VivaScope 1500.
Table S4 Diagnostic accuracy of the VivaScope 1500 compared with biopsy.
Table S5 Patient satisfaction.
Table S6 Diagnostic costs of biopsy and reflectance confocal microscopy diagnostics.


