Abstract
Pharmacokinetics, pharmacodynamics, and safety/tolerability of iberdomide (CC‐220), a highly potent oral cereblon E3 ligase modulator (CELMoD), were evaluated in escalating single‐dose (0.03, 0.1, 0.3, 1, 2, 4, 6 mg) and multiple‐dose (0.3 mg once daily for 14 days, 1 mg once daily for 28 days, 0.3 mg once daily for 28 days, or 1 mg once daily for 7 days with a 7‐day washout, then once daily for 7 more days) studies in healthy subjects (n = 99). Iberdomide exposure increased in a dose‐proportional manner. Terminal half‐life was 9‐13 hours after a single dose. Iberdomide decreased peripheral CD19+ B lymphocytes (Emax, 92.4%; EC50, 0.718 ng/mL), with modest reductions in CD3+ T lymphocytes (Emax, 34.8%; EC50, 0.932 ng/mL). Lipopolysaccharide‐stimulated proinflammatory cytokines (IL‐1α, IL‐1β) were reduced, but anti‐CD3‐stimulated IL‐2 and interferon‐γ were increased. Iberdomide 1 mg once daily partially decreased T‐cell‐independent antibody responses to PPV23 but did not change tetanus toxoid recall response. Pharmacodynamic data suggest dose‐dependent, differential immunomodulatory effects on B and T lymphocytes. Iberdomide was tolerated up to 6 mg as a single dose and at 0.3 mg once daily for 4 weeks. Grade 3 asymptomatic neutropenia was observed following 1 mg once daily for 21 days; a 7‐day drug holiday alleviated neutropenia. Further investigation of iberdomide in autoimmune and hematological diseases is warranted.
Keywords: autoimmune diseases, cereblon, hematological malignancies, iberdomide, immunomodulation
Iberdomide (CC‐220) is a novel, orally available, highly potent cereblon modulator. 1 , 2 Iberdomide co‐opts cereblon, a component of the Cul4A/DDB1/Roc1 ubiquitin E3 ligase complex involved in protein degradation, and induces ubiquitination and degradation of Ikaros and Aiolos by proteasomes. 1 , 3 Ikaros and Aiolos are zinc‐finger transcription factors that critically regulate immune cell development and homeostasis. 4 , 5
In vitro, iberdomide, at concentrations as low as 1 nM, reduced Ikaros and Aiolos protein levels in B lymphocytes, T lymphocytes, and monocytes in assays using peripheral blood mononuclear cells (PBMCs) or whole blood from healthy volunteers and patients with systemic lupus erythematosus (SLE). 1 , 6 Iberdomide inhibited the differentiation of B lymphocytes into antibody‐secreting plasmablasts and plasma cells, reducing production of immunoglobulin G (IgG), immunoglobulin M (IgM), and anti‐dsDNA and antiphospholipid antibodies. In addition, iberdomide demonstrated potent antiproliferative effects on B‐lymphocyte‐derived tumor cell lines in vitro, 7 as well as antitumor activity in multiple myeloma (MM) models (data on file, Bristol‐Myers Squibb).
Currently, iberdomide is in development for the treatment of hematological malignancies and autoimmune diseases. Degradation of Ikaros and Aiolos disrupts the c‐Myc/IRF4 axis, which is implicated in the pathogenesis of MM. 7 , 8 In addition, polymorphisms in genes encoding Ikaros and Aiolos have been associated with the risk of developing SLE. 6
Here, we report the results of the first‐in‐human, single‐ascending‐dose (SAD) and multiple‐ascending‐dose (MAD) studies conducted in healthy subjects to characterize the pharmacokinetics (PK), pharmacodynamics (PD), safety, and tolerability of iberdomide.
Methods
The protocols and informed consent documentation were reviewed and approved by the Schulman Associates institutional review board (Sunrise, Florida). Written informed consent was obtained from each individual participating in the studies. The studies were performed at Covance Clinical Research Unit (Madison, Wisconsin) according to good clinical practice and in accordance with the principles of the Declaration of Helsinki. The ClinicalTrials.gov registration numbers are NCT01733875 for the SAD study (CC‐220‐CP‐001) and NCT02034773 for the MAD study (CC‐220‐CP‐002).
Subjects
For both studies, healthy male subjects and female subjects of nonchildbearing potential (aged 18‐55 years) with body mass index (BMI) of 18‐33 kg/m2 were eligible for enrollment. Health was determined by clinical laboratory tests, physical examination, and electrocardiography. Exclusion criteria included a history of alcohol and drug abuse, smoking >10 cigarettes per day, use of prescription drugs or any investigational therapy within 30 days or 5 half‐lives (whichever is longer) of dosing, and use of nonprescription drugs within 14 days, with the exception of acetaminophen. In the CC‐220‐CP‐002 study, subjects enrolled in the iberdomide 1‐mg cohort were required to have a baseline tetanus titer of ≥0.1 IU/mL to ensure prior immunity to tetanus toxoid.
Design
Single‐Ascending‐Dose Study (CC‐220‐CP‐001)
CC‐220‐CP‐001 was a phase 1 single‐center, randomized, double‐blind, placebo‐controlled SAD study enrolling 65 healthy subjects. Dose escalation was conducted in sequential cohorts; 6 subjects received iberdomide, and 2 subjects received placebo in each cohort. The doses were 0.03, 0.1, 0.3, 1, 2, 4, and 6 mg. The starting iberdomide dose was based on data from animal toxicity studies; 0.03 mg of iberdomide was selected, with a resulting safety margin of approximately 26‐fold to the human equivalent dose of the monkey no‐observed‐adverse‐effect level. Iberdomide was administered 1 dose level at a time, and subsequent dose levels were administered only after the safety and tolerability of the preceding dose were deemed acceptable based on the Toxicity Grading Scale for Healthy Volunteers described in the U.S. Food and Drug Administration guidance. Escalation to the next dose would not continue if any of the following treatment‐related findings were observed in the previous cohort: serious adverse events (AEs) in ≥1 subject; grade ≥3 AEs in ≥2 subjects in the same system organ class; grade ≥2 AEs in ≥3 subjects in the same system organ class; ≥2 subjects with ophthalmological‐related events; ≥2 subjects with neurologically related events of moderate to severe intensity; ≥2 subjects with absolute neutrophil count (ANC) <1 × 109/L, absolute lymphocyte count <0.5 × 109/L, platelet (PLT) count <50 × 109/L, or hemoglobin (Hgb) <8 g/dL; or ≥3 subjects with ANC <1.5 × 109/L, absolute lymphocyte count <0.8 × 109/L, PLT <75.5 × 109/L, or Hgb <10 g/dL.
The CC‐220‐CP‐001 study also included an evaluation of food effects, which was an open‐label, randomized, 2‐period, 2‐way crossover study exploring the effect of a standard high‐fat breakfast on the bioavailability of iberdomide. The standard high‐fat breakfast was equal or equivalent to 2 eggs fried in butter, 2 strips of bacon, 2 slices of buttered toast or bread, 4 ounces of hash brown potatoes, and 8 ounces of whole milk (800‐1000 total calories, of which 500‐600 were provided by fat). Subjects (n = 9) received a single oral dose of iberdomide (1 mg) in each of 2 study periods, once without food (ie, fasted) and once with food (ie, fed), in random treatment sequence. The fasted and fed study periods were separated by a washout of 11‐14 days.
Multiple‐Ascending‐Dose Study (CC‐220‐CP‐002)
CC‐220‐CP‐002 was a phase 1 randomized, double‐blind, placebo‐controlled MAD study enrolling 34 healthy subjects. Subjects were enrolled into 4 cohorts: (1) iberdomide 0.3 mg once daily for 14 days (n = 6) or placebo (n = 3); (2) iberdomide 1 mg once daily for 28 days (n = 6) or placebo (n = 3); the next cohort was initially planned to be dosed at 2 mg once daily for 14 days, but because of dose‐limiting toxicity, it was amended and was replaced by cohorts; (3) iberdomide 0.3 mg once daily for 28 days (n = 6) or placebo (n = 2); and (4) iberdomide 1 mg (n = 6) or placebo (n = 2) once daily for 7 days with a 7‐day washout, then once daily for 7 additional days.
Pharmacokinetic Assessments
Approximately 3 mL of human whole blood at specified times (see Supplementary Appendix) was collected and centrifuged to obtain plasma. The harvested plasma was transferred to precharged citric acid tubes to obtain acidified plasma (40 mM) for measurement of iberdomide (a single stereoisomer with the S‐configuration) and its R‐enantiomer (CC‐17195). Concentrations in plasma were measured using a validated human plasma liquid chromatography‐tandem mass spectrometry assay (LC‐MS/MS). Iberdomide and its R‐enantiomer along with their stable labeled internal standards (iberdomide‐d8 and its R‐enantiomer‐d8) were extracted from acidified human plasma by solid‐phase extraction (SPE) with Waters Oasis Elution HLB cartridges. The SPE eluent with 0.1% formic acid in 30:70 acetonitrile/water was directly injected onto an LC‐MS/MS system (SCIEX API 4000 mass spectrometer; Framingham, Massachusetts). Mobile phase A consisted of 5 mM ammonium acetate in water, and mobile phase B consisted of acetonitrile. The analytical column used was a Chiral Tech, Chiral‐CBH column (100 × 3 mm, 5 µm). A gradient elution was used at a flow rate of 1.2 mL/min with run time of 5.2 minutes. The retention times were approximately 2.5 minutes for both iberdomide and its internal standard, and approximately 1.5 minutes for both CC‐17195 and its internal standard. The multiple‐reaction monitoring transition in the electrospray ionization positive ionization mode was as follows: m/z 450.1 → m/z 104.0 for iberdomide and its R‐enantiomer; m/z 458.1 → m/z 104.0 for the internal standards. The calibration curve for both iberdomide and its R‐enantiomer ranged from 0.1 to 100 ng/mL in acidified human plasma, with the lower limit of quantification of 0.1 ng/mL. In both method validation and study sample analysis, the precision and accuracy data for standard/quality control samples were well within acceptance criteria for both iberdomide and its R‐enantiomer.
Pharmacokinetic parameters were derived from plasma concentration‐time profiles by noncompartmental analysis with Phoenix WinNonlin version 6.4 (Pharsight Corp., Mountain View, California) and included area under the concentration‐versus‐time curve from time zero to the last detectable concentration (AUCt), calculated using the linear trapezoidal method; AUC from time zero extrapolated to infinity (AUC∞); AUC from time zero to 24 hours postdose (AUC24); maximum plasma concentration (Cmax); time to maximum plasma concentration (Tmax); terminal elimination half‐life (t1/2, z); and apparent total plasma clearance (CL/F). In the MAD study, the accumulation ratio was calculated for Cmax and AUCt.
Pharmacodynamic Assessments
Effects of iberdomide on CD19+ B‐ and CD3+ T‐lymphocyte counts were evaluated using flow cytometry. Whole blood (4 mL per time) was collected from subjects in the CC‐220‐CP‐002 study at specified times (see Supplemental Table 1). The assay was conducted at LabCorp Clinical Trials (Cranford, New Jersey). Briefly, 100 µL of well‐mixed sodium heparin anticoagulated whole‐blood specimen was pipetted into each of the tubes. All red blood cells were lysed with ammonium chloride lysing reagent for 5 minutes in the dark at room temperature. The tubes were then centrifuged, decanted, and gently rack‐raked to disperse the cell pellets. The cells were washed twice with phosphate‐buffered saline (PBS)‐1% bovine serum albumin (BSA). The appropriate volume of antibody cocktail, including CD19 antibody (biotinylated, BD Pharmingen catalog number 555411; BD Pharmingen, San Jose, California) and CD3 antibody (AF700; Analytical Systems), were added to the labeled tubes. The tubes were vortexed and incubated for 30 minutes in the dark at room temperature. After incubation, all cells were washed with PBS‐1% BSA. The appropriate volume of Qdot Streptavidin 605 Conjugate (Thermo Fisher Scientific, Waltham, Massachusetts) was added to all tubes. The tubes were vortexed and incubated for 20 minutes in the dark at room temperature. After incubation, the cells were washed with PBS‐1% BSA and finally fixed with 1% paraformaldehyde solution. The samples were acquired on the FACSCanto II flow cytometer (BD Biosciences), collecting 250 000 events with PerCP‐Cy5.5 V450 compensation settings applied. Data were reported as absolute values (cells/µL), and absolute values were used to calculate percentage of baseline at each time. To determine maximum effect (Emax) and half maximal effective concentration (ie, the concentration that reduces the cell count to 50% within the range between 100% and [100 – Emax]; EC50) of iberdomide with regard to counts of CD19+ B lymphocytes and CD3+ T lymphocytes in peripheral blood, a sigmoid effect model was selected using PD modeling in Phoenix WinNonlin. The equation used was: E = E0 − (Emax × Cγ)/(Cγ+ EC50 γ). The concentration is 0 at E0, and the maximum treatment effect was measured as E0 ‒ Emax.
The effects of iberdomide on T‐lymphocyte, B‐lymphocyte, and myeloid cell production of cytokines (including interleukin [IL]‐1α, IL‐1β, IL‐2, and interferon [IFN]‐γ) were evaluated using ex vivo assays. Cytokine production was assessed following anti‐CD3 or lipopolysaccharide (LPS) stimulation of whole blood ex vivo. For each subject, blood samples were collected at each nominal time in a TruCulture null tube (without stimulus; Myriad Rules‐Based Medicine [RBM] catalog number 782–001086; Myriad RBM, Austin, Texas), a TruCulture anti‐CD3 stimulation tube (Myriad RBM catalog number 782–001202), and a TruCulture LPS‐stimulation tube (Myriad RBM catalog number 782–001087) predose on days 1, 2, 5, 8, 11, 14, 18, and 21 and at follow‐up for the iberdomide 0.3‐mg once‐daily × 14‐day cohort of the MAD study, and predose on days 1, 2, 5, 8, 11, 14, 21, 28, 32, and 35 and at follow‐up for the iberdomide 1‐mg once‐daily × 28‐day cohort of the MAD study. Briefly, following blood collection, the TruCulture tube was gently mixed by inverting and placed with cap pointing up in a preheated block thermostat at 37°C. LPS‐stimulation tubes and null tubes were incubated for 18 ± 4 hours. For the CD3 antibody stimulation, tubes were incubated for 42 ± 4 hours. The screw cap was removed from each tube, and the Seraplas valve slowly inserted until it was approximately 5 mm (1/4 inch) above the cell sediment level. The stick from the valve was disconnected and removed with a counterclockwise turn. The supernatant was gently decanted into the 3‐mL labeled cryovial without disturbing the valve. The 3‐mL cryovial was immediately frozen in a −70°C freezer until shipment to Myriad RBM and analyzed using the TruCulture Multi‐Analyte Profile version 1.0 (Myriad RBM), measuring cytokines and chemokines. Reported values were used to calculate background‐corrected values, using the following equations, in which NULL indicates the background (ie, no stimulus): (1) LPS background‐corrected value = LPS value as measured – NULL at nominal time and (2) CD3 background‐corrected value = CD3 value as measured – NULL at nominal time. These background‐corrected values were then used to calculate the percentage of baseline at each time.
In the MAD study, the effect of iberdomide on antibody responses to vaccines was assessed in subjects receiving iberdomide 1 mg once daily or placebo for 28 days. Subjects in this cohort received a tetanus toxoid adsorbed vaccine (Aventis Pasteur, Swiftwater, Pennsylvania) and 23‐valent pneumococcal polysaccharide vaccine (PPV23; Pneumovax 23, Merck and Co., Inc., Whitehouse Station, New Jersey), administered according to their respective package inserts on day 14 approximately 4 hours following administration of iberdomide. Both tetanus toxoid adsorbed vaccine (0.5 mL) and PPV23 (0.5 mL) were injected intramuscularly on different sides of limbs 10 minutes apart. IgG against tetanus toxoid and IgG against pneumococcal serotypes were assessed at screening, at baseline, and on days 7, 14, 21, and 28 of the treatment phase, as well as on day 38/follow‐up visit. The antibody titers were measured at Focus Diagnostics (Cypress, California). Briefly, for the tetanus IgG enzyme‐linked immunosorbent assay, microtiter wells were coated with tetanus toxoid with an overnight incubation at 2°C‐8°C, then blocked, dried, pouched, and stored refrigerated until use. Diluted (1:50) patient serum and assay controls were added to coated microtiter wells, and any antibodies directed against tetanus toxoid were bound to the immobilized antigen. After washing away nonbound serum proteins, horseradish peroxidase‐conjugated goat antihuman IgG was added to bind to any captured tetanus IgG molecules. After another wash step, enzyme substrate was added; the ensuing color development reaction was then stopped at a defined time by the addition of a dilute acid solution. The optical density then measured was directly proportional to the amount of tetanus IgG present in the serum specimen. Tetanus IgG level was quantified by interpolation from a standard curve. Streptococcus pneumoniae IgG was measured by 23‐plex multianalyte immunodetection (Focus Unit Code 40962). This panel measures IgG antibodies, recognizing all 23 type‐specific pneumococcal polysaccharide antigens included in the 23‐valent vaccine. Evaluation of the response to pneumococcal vaccination is best accomplished by comparing prevaccination and postvaccination antibody levels (assayed in the same test run), rather than by assessing only postvaccination antibody level. For PPV23, normal antibody response was defined as a 2‐fold or greater increase from baseline in antibodies against >70% of the 23 serotypes. 9 , 10
Safety Assessments
To assess the safety of iberdomide, AEs were continuously monitored, and physical examinations, vital signs, electrocardiograms, clinical laboratory safety testing, and concomitant medications/procedures were assessed. AEs were classified using the Medical Dictionary for Drug Regulatory Activities (versions 15.1 and 16.0).
Statistical Analyses
To assess food effects in the SAD study, analysis of variance was performed on the natural log‐transformed AUCt, AUC24, AUC∞, and Cmax using the MIXED procedure in SAS (version 9.2; SAS Institute Inc., Cary, North Carolina). The MIXED model contains terms for sequence, period, and treatment as fixed effects and subject nested within sequence as a random effect. Geometric mean ratios (fed/fasted) and their 90% confidence intervals (CIs) were calculated. For Tmax, Wilcoxon signed rank test, Hodges‐Lehmann estimate, and 90% CI were calculated for the median difference between treatments (fed‐fasted).
All statistical tests for PD parameters were conducted with a 2‐sided significance of .05. No adjustment of multiplicity was considered. To compare PD parameters before and after iberdomide treatment, a Wilcoxon signed rank test was used to compare PD raw values and percentage change from baseline at each postbaseline visit. For PD parameters and assessments of antibody responses to vaccinations, analysis of covariance, with the baseline value as a covariate and treatment (iberdomide dose and placebo) as a factor, was performed using the MIXED procedure in SAS (SAS Enterprise Guide version 4.1; SAS Institute Inc., Cary, North Carolina) to provide the estimates and standard errors of least‐squares means of on‐treatment PD values. Least‐squares means were compared between iberdomide dose regimens and placebo. To compare LPS‐ and anti‐CD3‐stimulated cytokine production between iberdomide treatment and placebo, a mixed‐model repeated‐measures (MMRM) analysis was performed for the 0.3‐ and 1‐mg dosing cohorts, separately. The MMRM included treatment, visit and treatment‐by‐visit interaction as fixed effects and covariates of baseline and baseline‐by‐visit interaction.
Results
Subjects
In the SAD study (n = 65), most subjects were men (63 men and 2 women); mean age was 31.5 years (range, 19‐52 years), and mean BMI was 25.9 kg/m2 (range, 20.2‐31.7 kg/m2). In the MAD study (n = 34), most subjects were men (32 men and 2 women); mean age was 35.1 years (range, 22‐55 years), and mean BMI was 26.4 kg/m2 (range, 19.2‐30.8 kg/m2). In both studies, baseline characteristics were generally similar for the iberdomide and placebo treatment groups.
Pharmacokinetics
The mean concentration‐time profile for iberdomide in the SAD study is shown in Figure 1. No subjects at the iberdomide 0.03‐mg dose had detectable drug concentrations. Most subjects at the iberdomide 0.1‐mg dose had detectable iberdomide plasma concentrations from 1.5 up to 8 hours postdose. Most subjects in the remaining cohorts had quantifiable iberdomide plasma concentrations up to 24 or 48 hours. PK parameters are shown in Table 1. The mean Cmax and AUC of iberdomide increased in a dose‐proportional manner over the dose range (0.1‐6 mg) after a single dose. Following oral dosing, the median Tmax was observed 2.5‐4 hours postdose. The half‐life of iberdomide ranged from approximately 9 to 13 hours. Coadministration with food did not affect the oral bioavailability of iberdomide (Table 2). The concentration‐time curves for iberdomide in the fasted and fed conditions are shown in Supplemental Figure 1.
Figure 1.
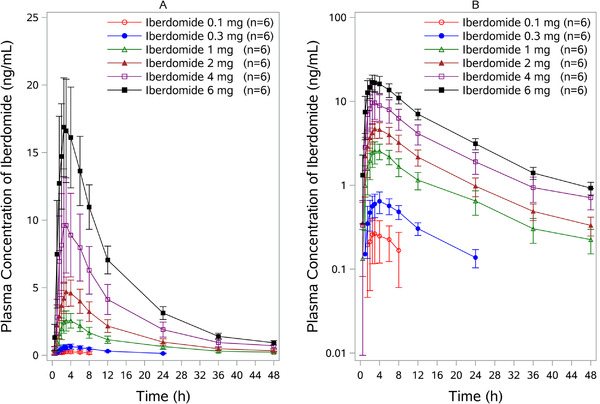
Iberdomide plasma concentration‐time profile following administration of single oral dose in healthy subjects. Both linear (A) and semilogarithmic (B) scales are shown. Results from the 0.03‐mg dose are not included bcause all concentrations at this dose were below detectable levels. Data are mean ± SD. SD, standard deviation.
Table 1.
Plasma Pharmacokinetic Parameters of Iberdomide After a Single Oral Dose
| 0.1 mg | 0.3 mg | 1 mg | 2 mg | 4 mg | 6 mg | |
|---|---|---|---|---|---|---|
| Parameter | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) |
| AUCt (ng·h/mL) | 2.3 (1.9) | 8.0 (1.7) | 40.7 (10.2) | 71.7 (15.5) | 140.7 (42.7) | 239.8 (36.7) |
| AUC∞ (ng·h/mL) | 4.9 (1.7) a | 9.8 (2.2) | 45.0 (11.6) | 78.2 (17.3) | 153.3 (46.9) | 255.1 (37.9) |
| AUC24 (ng·h/mL) | 3.7 (1.4) a | 8.0 (1.7) | 31.8 (7.8) | 58.0 (12.5) | 113.8 (34.7) | 198.6 (32.1) |
| Cmax (ng/mL) | 0.3 (0.2) | 0.7 (0.2) | 2.7 (0.6) | 4.8 (1.1) | 10.1 (3.9) | 17.7 (3.5) |
| Tmax (h) | 2.8 (2.5, 3.0) | 4.0 (2.0, 6.0) | 3.0 (2.5, 4.0) | 3.0 (3.0, 4.0) | 3.0 (2.5, 4.0) | 2.5 (2.0, 4.0) |
| t½.z (h) | 11.2 (3.4) a | 8.8 (0.7) | 13.2 (1.0) | 13.4 (2.1) | 12.1 (1.5) | 11.5 (1.7) |
| CL/F (L/h) | 22.5 (7.3) a | 31.9 (7.1) | 23.6 (7.0) | 26.6 (5.7) | 29.0 (12.1) | 24.0 (4.1) |
AUCt, area under the plasma concentrationtime curve from time 0 to the last time with detectable levels; AUC∞, area under the plasma concentrationtime curve from time 0 extrapolated to infinity; AUC24, area under the plasma concentration‐time curve from time zero to 24 hours; CL/F, apparent total plasma clearance when dosed orally; Cmax, observed maximal plasma concentration; Tmax, time to maximum concentration; t1/2.z, terminal elimination half‐life.
Data are reported above as arithmetic mean (SD), except for Tmax, which is presented as median (minimum, maximum).
n = 5.
Table 2.
Plasma Pharmacokinetic Parameters and Statistical Comparison for Single‐Dose Iberdomide in Fed and Fasted Healthy Subjects
| Parameter | 1 mg (n = 9) | Mean (SD) | Geometric Mean | Ratio of Fed/Fasted, % (90% CI of Ratio) |
|---|---|---|---|---|
| AUCt (ng·h/mL) | Fed | 36.5 (14.0) | 33.3 | 103 (94.8‐112.8) |
| Fasted | 34.9 (13.4) | 32.2 | ||
| AUC∞ (ng·h/mL) | Fed | 40.4 (14.5) | 37.5 | 104 (97.2‐112.1) |
| Fasted | 38.7 (14.6) | 35.9 | ||
| AUC24 (ng·h/mL) | Fed | NR | 26.8 | 104 (98.6‐109.5) |
| Fasted | NR | 25.8 | ||
| Cmax (ng/mL) | Fed | 2.2 (0.9) | 2.0 | 95.2 (85.6‐105.8) |
| Fasted | 2.3 (0.9) | 2.1 | ||
| t½.z (h) | Fed | 11.8 (2.1) | 11.6 | NR |
| Fasted | 13.7 (2.0) | 13.5 | ||
| CL/F (L/h) | Fed | 29.2 (14.5) | 26.7 | NR |
| Fasted | 30.7 (14.9) | 28.0 |
AUCt, area under the plasma concentrationtime curve from time 0 to the last time with detectable levels; AUC∞, area under the plasma concentrationtime curve from time 0 extrapolated to infinity; AUC24, area under the plasma concentration‐time curve from time zero to 24 hours; CI, confidence interval; Cmax, observed maximal plasma concentration; NR, not reported; SD, standard deviation.
The concentration‐time curve for iberdomide in the MAD study is shown in Figure 2. PK parameters are shown in Table 3. Steady state was reached around day 7, with approximately 2‐fold accumulation of iberdomide in plasma on multiple oral once‐daily dosing.
Figure 2.
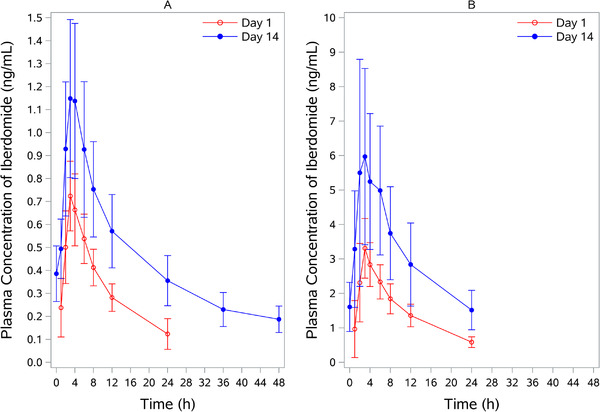
Iberdomide plasma concentration‐time profile following multiple oral doses of iberdomide in healthy subjects. (A) Iberdomide at 0.3 mg was administered once daily for 14 days. (B) Iberdomide at 1 mg was administered once daily for 28 days. Data are mean ± SD on day 1 and day 14. SD, standard deviation.
Table 3.
Plasma Pharmacokinetic Parameters of Multiple‐Dose Iberdomide
| Iberdomide 0.3 mg Once Daily × 14 Days | Iberdomide 1 mg Once Daily x 28 Days | |||
|---|---|---|---|---|
| Parameter | Day 1 (n = 6) | Day 14 (n = 6) | Day 1 (n = 6) | Day 14 (n = 5) |
| AUCtau (ng·h/mL) | 7.51 (2.0) | NC | 35.28 (9.0) | 76.28 (31.4) |
| AUC∞ (ng·h/mL) | 9.7 (2.5) | NC | 43.44 (11.2) | NC |
| Cmax (ng/mL) | 0.72 (0.15) | 1.17 (0.33) | 3.41 (0.93) | 6.15 (2.88) |
| Tmax (h) | 3.00 (3.0, 3.0) | 4.00 (3.0, 4.0) | 3.00 (2.0, 3.0) | 3.00 (2.0, 6.0) |
| t½,z (h) | 9.74 (2.3) | 22.37 (2.7) | 9.58 (0.5) | 12.00 (0.7) |
| CL/F (L/h) | 32.66 (8.3) | 20.99 (6.0) | 24.54 (7.1) | 14.70 (5.0) |
| RA Cmax | NA | 1.61 (0.3) | NA | 1.84 (0.4) |
| RA AUCtau | NA | 1.96 (0.3) | NA | 2.22 (0.4) |
Data are reported above as mean (SD), except for Tmax, which is presented as median (minimum, maximum).
RA AUCtau = AUCtau day 14/AUCtau day 1. RA Cmax = Cmax day 14/Cmax day 1.
AUC∞, area under the plasma concentration‐time curve from time 0 extrapolated to infinity; AUCtau, area under the plasma concentration‐time curve over 1 dosing interval; CL/F, apparent total plasma clearance when does orally; Cmax, observed maximal plasma concentration; n, number of subjects; NA, not applicable; NC, not calculated; QD, once daily; t1/2,z, terminal elimination half‐life; Tmax, time to maximum concentration; RA, ratio of accumulation; SD, standard deviation.
Systemic exposure of the R‐enantiomer of iberdomide (CC‐17195) was <9% of iberdomide in the SAD study across all doses and <8% in the MAD study (1 mg) based on AUC (data not shown).
Pharmacodynamics
Effects of Iberdomide on B‐ and T‐Lymphocyte Counts in Peripheral Blood
Following multiple once‐daily doses of iberdomide ranging from 0.3 to 1 mg, there was a concentration‐dependent decrease from baseline in the number of CD19+ B lymphocytes in peripheral blood, with maximum reduction (Emax) of 92.4% and half‐maximal effective concentration (EC50) of 0.718 ng/mL (Figure 3A). Dose‐ and time‐related decreases in the mean percentage at baseline of absolute CD19+ B lymphocytes were observed following administration of iberdomide (Figure 3B). With iberdomide 0.3 mg once daily × 14 days, the maximum decrease from baseline in CD19+ B lymphocytes was 36% on day 11. With iberdomide 1 mg once daily × 28 days, decreases in CD19+ B lymphocytes were observed starting from day 2; the maximum decrease from baseline was 83%, which was observed on day 21.
Figure 3.
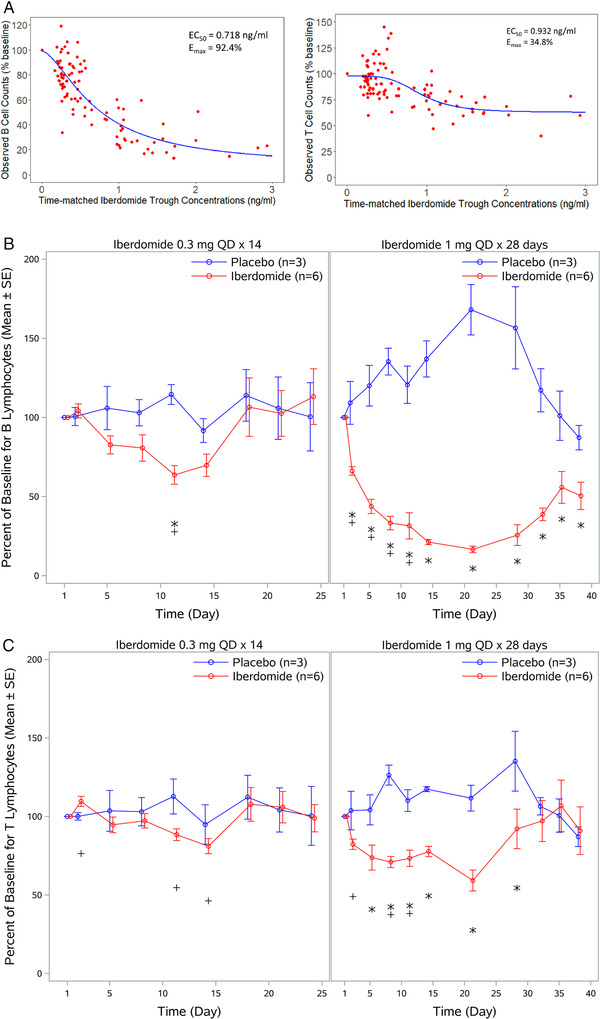
Effects of iberdomide on B‐ and T‐lymphocyte counts in peripheral blood following multiple oral doses of iberdomide. (A) Relationship between CD19+ B‐ and CD3+ T‐lymphocyte responses and iberdomide trough concentrations in humans. Line represents the predicted concentration‐effect relationship using a sigmoid PD model. The equation used was E = E0 − (Emax × Cγ)/(Cγ + EC50 γ); concentration is 0 at E0. EC50 is the concentration that reduces the cell count to 50% within the range between 100% and (100 − Emax). For B‐cell counts, Emax is 92.4 (CV%, 14.4%), EC50 is 0.718 ng/mL (CV%, 21.3%), E0 is 98.88 (CV%, 2.8%), and γ is 1.60 (CV%, 18.9%). For T‐cell counts, Emax is 34.8 (CV%, 19.2%), EC50 is 0.932 ng/mL (CV%, 13.5%), E0 is 97.5 (CV%, 1.89%), and γ is 4.24 (CV%, 50.9%). Red dots are observed data from subjects receiving 0.3 to 1 mg once‐daily iberdomide (n = 24) in the MAD study. (B) Change from baseline in CD19+ B lymphocytes in peripheral blood following multiple doses of iberdomide. (C) Change from baseline in CD3+ T‐lymphocyte count in peripheral blood following multiple doses of iberdomide. CV%, coefficient of variation; MAD, multiple ascending doses; PD, pharmacodynamics; QD, once daily. *P ≤ .05 relative to placebo; + P ≤ .05 relative to baseline.
Following multiple doses of iberdomide ranging from 0.3 to 1 mg, concentration‐related decreases in CD3+ T‐lymphocyte count were also observed but to a lesser extent, with an Emax of 34.8% and EC50 of 0.932 ng/mL (Figure 3A). Dose‐ and time‐related changes from baseline in absolute CD3+ T‐lymphocyte count were observed following administration of iberdomide (Figure 3C). With iberdomide 0.3 mg once daily × 14 days, 12% and 19% decreases from baseline in CD3+ T‐lymphocyte count were observed on days 11 and 14, respectively. For iberdomide 1 mg once daily × 28 days, decreases were observed from day 2, with a maximum decrease in CD3+ T lymphocytes of 41% on day 21.
Effects of Iberdomide on LPS‐ and Anti‐CD3‐Stimulated Cytokine Production in Whole Blood Ex Vivo
Analysis of LPS‐stimulated IL‐1α production in whole blood showed a significant treatment‐related decrease from baseline following administration of iberdomide 1 mg once daily on day 5 of dosing (Figure 4A). No apparent treatment‐related reduction from baseline in IL‐1α production occurred during administration of iberdomide 0.3 mg once daily × 14 days. For LPS‐stimulated IL‐1β production in whole blood, a significant decrease compared with placebo was observed following administration of iberdomide 0.3 mg once daily on day 5 of dosing (Figure 4B). Treatment‐related decreases in LPS‐stimulated IL‐1β were more prominent and were significantly decreased compared with both placebo and baseline following administration of iberdomide 1 mg once daily after 2 days of dosing (Figure 4B).
Figure 4.
Effect of iberdomide on cytokine production ex vivo. (A) IL‐1α production in whole blood following lipopolysaccharide stimulation. (B) IL‐1β production in whole blood following lipopolysaccharide stimulation. (C) IL‐2 production in whole blood following anti‐CD3 stimulation. (D) IFN‐γ production in whole blood following anti‐CD3 stimulation. Data are mean percent of baseline ± SE. IL, interleukin; IFN, interferon; QD, once daily; SE, standard error. *P ≤ .05 relative to placebo; + P ≤ .05 relative to baseline.
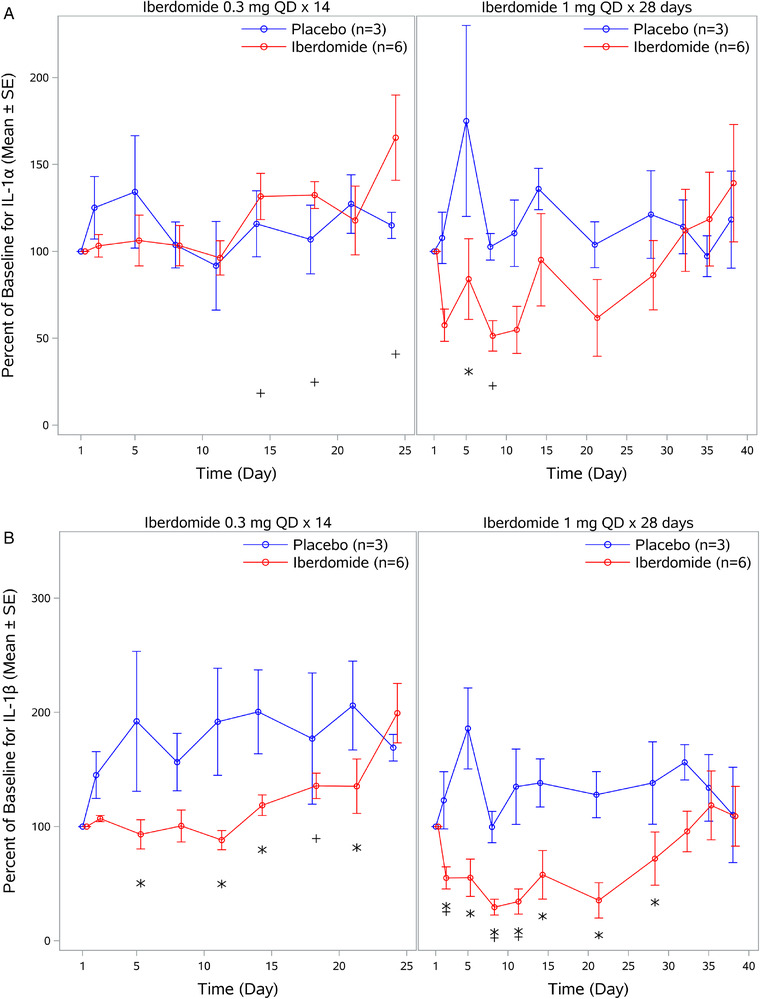
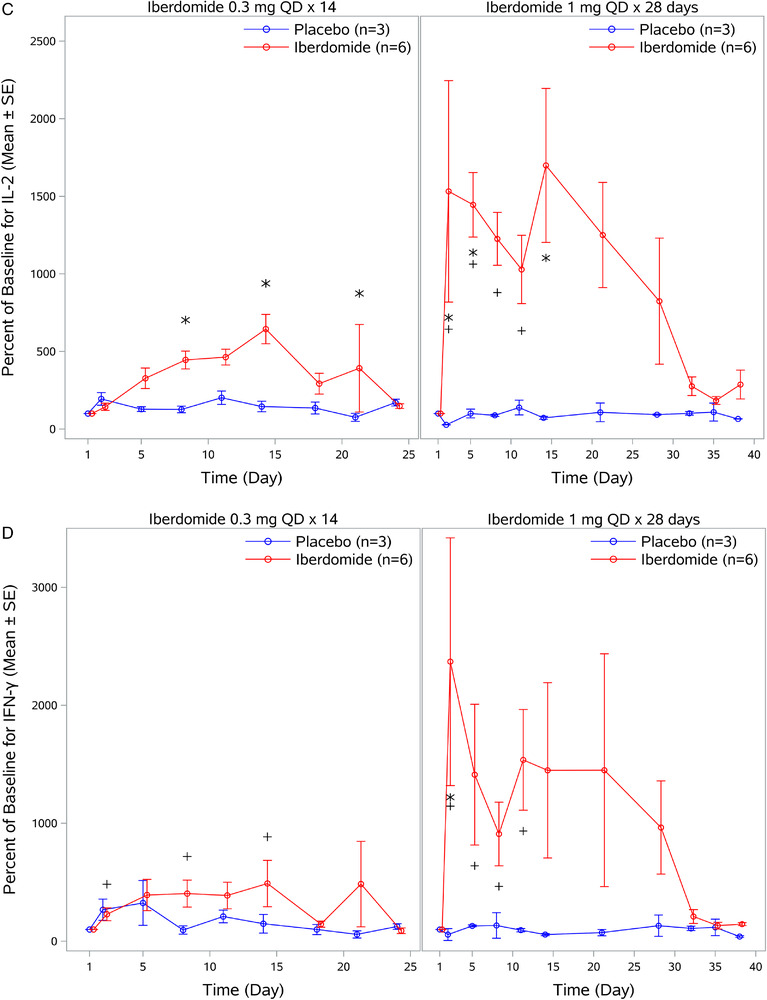
In contrast to iberdomide‐induced decreases in LPS‐stimulated IL‐1α and IL‐1β production, iberdomide increased anti‐CD3‐stimulated IL‐2 and IFN‐γ production in whole blood (Figure 4C). With iberdomide 0.3 mg once daily × 14 days, increases in mean IL‐2 were observed compared with placebo after 8 days of dosing. With iberdomide treatment of 1 mg once daily × 28 days, increases were more prominent and observed when compared with both placebo and baseline, with a maximal increase to 1699% of baseline on day 14. Similar to observations with IL‐2, increases in IFN‐γ production from baseline were observed after anti‐CD3 stimulation following administration of iberdomide (Figure 4D). With iberdomide 0.3 mg once daily × 14 days, increases in mean IFN‐γ from baseline were observed; however, they were not statistically significant compared with placebo. In contrast, with iberdomide 1 mg once daily × 28 days, increases were more prominent and were significant from both baseline and placebo after 2 days of dosing.
Effects of Iberdomide on Response to Pneumococcal and Tetanus Toxoid Vaccination
Compared with placebo (n = 3), subjects receiving iberdomide 1 mg once daily (n = 6) had a decrease in pneumococcal antigen antibody serum levels. Fourteen days after PPV23 vaccination, a smaller percentage of subjects who received iberdomide 1 mg once daily had a normal antibody response (2‐fold increase from baseline in >70% [ie, ≥17 serotypes] of the IgG pneumococcal serotypes) compared with placebo: 60% (3 of 5 subjects) after iberdomide administration compared with 100% (3 of 3 subjects) after placebo administration (Figure 5A). On day 38, the proportion of subjects with at least a 4‐fold increase was only 20% in the iberdomide group (1 of 5 subjects) compared with 100% (3 of 3 subjects) in the placebo group. However, all subjects receiving iberdomide mounted a normal response to at least 12 of 23 PPV23 serotypes (data not shown). In the antibody recall response to tetanus toxoid vaccination, subjects treated with iberdomide 1 mg once daily showed no consistent difference compared with placebo in tetanus toxoid antibody serum levels 14 days after tetanus toxoid vaccination (study day 28) and 24 days after the vaccination (study day 38/follow‐up); see Figure 5B.
Figure 5.
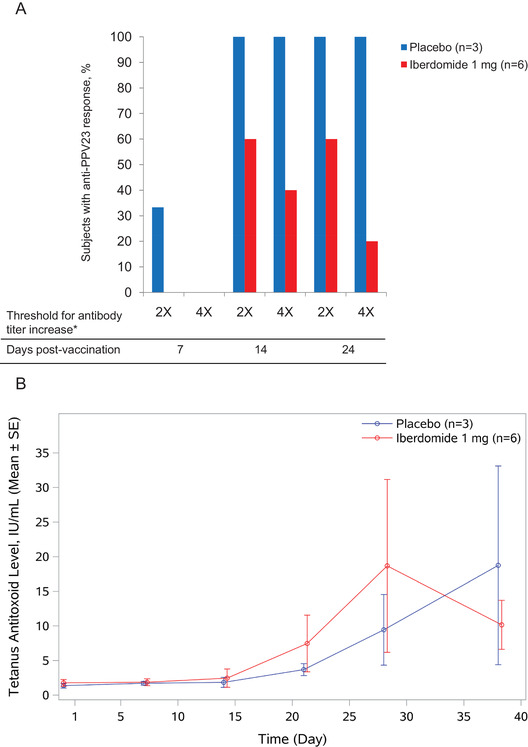
Effects of iberdomide on responses to PPV23 and tetanus toxoid vaccination. (A) Proportion of healthy subjects with IgG response to PPV23 7, 14, and 24 days postvaccination following iberdomide dosing. Vaccination occurred on day 14 during the 1‐mg once‐daily × 28 days administration of iberdomide. Response was defined as 2× (≥2‐fold of baseline in >70% of IgG pneumococcal serotypes) or 4× (≥4‐fold of baseline in >70% of serotypes). PPV23, 23‐valent pneumococcal polysaccharide vaccine. (B) Iberdomide effect on antibody recall responses to tetanus toxoid vaccination in healthy subjects. Subjects with preexisting antitetanus titer were treated with iberdomide 1 mg or placebo from baseline through day 28, with a follow‐up visit on day 38. Vaccinations were given on day 14. SE, standard error.
Safety
Iberdomide was tolerated in healthy subjects when administered as a single oral dose ranging from 0.03 to 6 mg. Of 65 subjects, 25 (38.5%) reported treatment‐emergent AEs (TEAEs); TEAEs reported in ≥2 subjects in any treatment group are shown in Supplemental Table 2. No subject experienced a serious or severe AE, and no subject discontinued the study because of AEs. Seven TEAEs, reported by 6 subjects (9.2%), were suspected to be related to iberdomide (diarrhea [n = 1], pruritus [n = 2], urticaria, T‐wave inversion on electrocardiogram, and oropharyngeal pain [n = 1 each]). No clinically significant changes or findings in clinical laboratory evaluations or vital sign measurements were observed.
In the MAD study, 23 of 34 enrolled subjects (67.6%) reported ≥1 TEAE. TEAEs reported in ≥2 patients in any treatment group are shown in Supplemental Table 3. No subject experienced a serious AE. Iberdomide 0.3 mg once daily for 14 days was tolerated in healthy subjects; however, a dose‐limiting toxicity of neutropenia was observed with iberdomide 1 mg dosed once daily for 28 days. Four subjects who received iberdomide 1 mg once daily × 28 days experienced a TEAE of neutropenia (3 subjects with grade 3, defined as ANC of 0.5‐1 × 109/L), which occurred after once‐daily dosing for approximately 3 weeks; the subjects were asymptomatic, and all cases resolved within a week after discontinuing iberdomide. As a result, dosing was discontinued for the remainder of the 1‐mg cohort prior to the day 27 dose, and the planned dose escalation to iberdomide 2 mg was stopped. Two additional cohorts were added to further understand the safety profile in which subjects received iberdomide 0.3 mg once daily × 28 days or iberdomide 1 mg once daily × 7 days, followed by a 7‐day washout, then 1 mg once daily x 7 days. No clinically significant decreases in ANC were observed with these dosing regimens. Iberdomide was tolerated in healthy subjects in these 2 dose regimens.
Discussion
Two phase 1 studies were undertaken as the first evaluations of the PK and PD effects of iberdomide in humans and to provide initial safety information for single and multiple doses. In healthy subjects, iberdomide was characterized by generally dose‐proportional PK as single or multiple doses in the dose range tested. Intersubject variability of AUC and Cmax was low in healthy subjects, with CV% most commonly in the 20s except for the low dose (0.1 mg), which may have been affected by assay sensitivity. A high‐fat meal had an insignificant impact on systemic exposure, suggesting that iberdomide can be administered regardless of food. Unlike other agents that modulate cereblon (eg, lenalidomide), iberdomide is a single stereoisomer with the S‐configuration. In vivo, iberdomide is relatively resistant to racemization, thus maintaining its S‐isomeric structure. In the current SAD study, conversion to the R‐enantiomer (CC‐17195) in humans was limited (<9%). Based on in vitro evaluation in human liver microsomes and in vivo evaluation in a human absorption, distribution, metabolism, and elimination study, biotransformation of iberdomide occurred via nonenzymatic hydrolysis, oxidation, hydrolysis of the oxidative metabolites, and a combination of these pathways. Cytochrome P450 3A4/5 is responsible for the oxidative metabolism of iberdomide in humans.
Iberdomide exhibited differential immunomodulatory effects based on dose and exposure in healthy subjects. At a dose of 0.3 mg, iberdomide demonstrated primary immune‐inhibitory effects, with reductions in CD19+ B‐lymphocyte and CD3+ T‐lymphocyte counts in peripheral blood and reductions in proinflammatory cytokine IL‐1β in an LPS‐stimulated ex vivo assay. At a dose of 1 mg, iberdomide resulted in further decreases in these PD end points; however, marked increases in IL‐2 and IFN‐γ production were observed following anti‐CD3 stimulation ex vivo, suggesting iberdomide induced activation of T lymphocytes. This enhancement of T‐lymphocyte cytokine production is likely to be caused by de‐repression of IL‐2 transcription as a result of Ikaros and Aiolos degradation. 3
In addition to the dose‐ and exposure‐related immune responses, the effects of iberdomide were also time related. Though steady‐state exposure was reached within 7 days of once‐daily dosing, the further reduction in lymphocytes was observed beyond day 7. This observation is consistent with the notion that iberdomide increases the degradation of Aiolos and Ikaros, which subsequently prevents the development and differentiation of lymphocyte subsets. Although iberdomide reduced both CD19+ and CD3+ lymphocytes, reduction in B lymphocytes was more profound (Emax of 92.4%) than T lymphocytes (Emax of 34.8%).
The differential B‐ and T‐cell effects were further evaluated by exploring T‐lymphocyte‐independent and T‐lymphocyte‐dependent antibody responses using PPV23 and tetanus toxoid vaccination. Subjects receiving the 1‐mg iberdomide daily dose were potentially less likely to mount a normal response (defined as a 2‐fold increase from baseline in >70% of serotypes) to PPV23 vaccination, which is consistent with the significant reduction in B‐lymphocyte counts observed. However, patients may still benefit from PPV23 vaccination when receiving iberdomide treatment, as all subjects receiving iberdomide had a normal response to at least 12 of 23 PPV23 serotypes evaluated. Iberdomide administration did not significantly affect the responses to tetanus toxoid in subjects with preexisting titers to tetanus toxoid. A potential limitation of this assessment is the small sample size. The different effects on the antibody responses to PPV23 and tetanus toxoid are consistent with the differential effects on B‐lymphocyte reduction and T‐lymphocyte activation at the 1‐mg dose of iberdomide. Immune responses to PPV23 are primarily B‐lymphocyte mediated, 11 whereas the recall responses to tetanus toxoid likely are a mixture of T‐lymphocyte‐dependent and T‐lymphocyte‐independent antibody responses. 12
The PD effects observed in the study are likely explained by the previously described iberdomide mechanism of action. Iberdomide binding to cereblon modulates its E3 ligase activity, leading to the ubiquitination and subsequent degradation of Ikaros and Aiolos transcription factors, which regulate hematologic development and differentiation. 2 , 3 The effects of iberdomide on B and T lymphocytes observed in the study can be explained by the role of Ikaros in lymphocyte development. Ikaros is required for B‐cell development at a very early stage, so Ikaros‐null mice have a complete block in development of all B‐cell lineages, whereas the absence of Ikaros results in different effects on the T‐lymphocyte lineage. 13 Because Ikaros and Aiolos are repressors of IL‐2 transcription, 14 , 15 the observed increase in IL‐2 expression by T lymphocytes in the ex vivo stimulation assay reported here is consistent with an iberdomide‐induced reduction of Ikaros and Aiolos protein levels within T lymphocytes. Furthermore, Ikaros insufficiency in CD8+ T lymphocytes has been shown to help neighboring, non‐IL‐2‐producing cells to differentiate into IFN‐γ‐producing effectors. 16 Thus, a decrease in Ikaros expression induced by iberdomide would result in an increase in IFN‐γ production, as observed in the present study. With regard to the observed inhibition of IL‐1β production in the LPS‐stimulated blood from iberdomide‐treated subjects, this effect may be mediated by degradation of another cereblon substrate, zinc finger protein (ZFP91), which has recently been shown to regulate IL‐1β production through a noncanonical inflammasome pathway. 17 Iberdomide has been shown to be capable of inducing ZFP91 degradation within B lymphocytes in vitro. 1
Iberdomide was tolerated in healthy subjects when administered at a dose of 0.3 mg once daily for 4 weeks. However, with the iberdomide 1‐mg dose administered once daily for 3 weeks, grade 3, asymptomatic neutropenia was observed. After discontinuation of iberdomide, ANC recovered within 1 week. The protocol was amended to determine the tolerability of iberdomide 0.3 mg administered for 28 days and to determine if incorporating a 7‐day washout (iberdomide 1 mg once daily × 7 days, followed by a 7‐day washout, then 1 mg once daily × 7 days) would lessen the neutropenia observed with continuous dosing. The dosing regimen incorporating a drug holiday was tolerated, with no clinically relevant decreases in ANC observed. The effect of iberdomide on neutrophil count is consistent with the role of Ikaros, which is expressed primarily in immature stages, less so in mature neutrophils. 18
Iberdomide has potential activity in both hematologic malignancies and autoimmune disorders. The genes encoding Aiolos and Ikaros are essential for proliferation of MM cells in vitro. 19 , 20 Dysregulation of c‐Myc and IRF4 are commonly observed in MM cells, and experiments have shown that persistent degradation or downregulation of Ikaros and Aiolos with iberdomide results in downregulation of c‐Myc and IRF4, which leads to inhibition of MM cell growth and apoptosis. 7 , 8 Profound B‐lymphocyte reduction and the activation of T lymphocytes observed in the current study support the investigation in MM. A dose regimen using a high dose (1 mg instead of 0.3 mg) of iberdomide with drug holidays is recommended for MM to maximize the immunomodulatory effects and minimize neutropenia.
Increased expression of IKZF3 (which encodes Aiolos) and IKZF1 (which encodes Ikaros) mRNA has been observed in PBMCs of patients with SLE and has been associated with increased risk of developing SLE. 1 , 6 In whole blood from healthy volunteers, iberdomide (at concentrations of 1‐100 nM) reduced Ikaros and Aiolos protein levels in B lymphocytes, T lymphocytes, and monocytes. 1 The reduction in CD19+ B lymphocytes and the proinflammatory cytokine IL‐1β observed in the study are supportive of further investigation in SLE, although the optimal dosage of iberdomide in SLE may differ from that in MM.
Conclusion
The data provide initial evidence for the immunomodulatory effect of iberdomide in humans and suggest differential immunomodulation based on the dose administered. Overall, the PK, PD, and safety/tolerability data gathered from these studies warrant further development of iberdomide in patients with hematological malignancies and autoimmune diseases.
Conflicts of Interest
The authors received editorial support in the preparation of this article from Amy Zannikos, PharmD, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, New Jersey, sponsored by Bristol‐Myers Squibb, Summit, New Jersey. The authors, however, directed and are fully responsible for all content and editorial decisions for this manuscript. DW. was an employee of Celgene at the time of the study and may own equity. All other authors are employees of Bristol‐Myers Squibb Company and may own equity.
Funding
The authors acknowledge financial support for this study from Celgene, a Bristol‐Myers Squibb Company.
Data Sharing
Celgene is committed to responsible and transparent sharing of clinical trial data with patients, health care practitioners, and independent researchers for the purpose of improving scientific and medical knowledge as well as fostering innovative treatment approaches. For more information, please visit: https://www.celgene.com/research-development/clinical-trials/clinical-trials-data-sharing.
Supporting information
Supporting Information
Supporting Information
[The copyright line for this article was changed on 5 November 2020 after original online publication.]
References
- 1. Schafer PH, Ye Y, Wu L, et al. Cereblon modulator iberdomide induces degradation of the transcription factors Ikaros and Aiolos: immunomodulation in healthy volunteers and relevance to systemic lupus erythematosus. Ann Rheum Dis. 2018;77(10):1516‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matyskiela ME, Zhang W, Man HW, et al. A cereblon modulator (CC‐220) with improved degradation of Ikaros and Aiolos. J Med Chem. 2018;61(2):535‐542. [DOI] [PubMed] [Google Scholar]
- 3. Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co‐stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164(6):811‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. John LB, Ward AC. The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol. 2011;48(9‐10):1272‐1278. [DOI] [PubMed] [Google Scholar]
- 5. Heizmann B, Kastner P, Chan S. The Ikaros family in lymphocyte development. Curr Opin Immunol. 2018;51:14‐23. [DOI] [PubMed] [Google Scholar]
- 6. Nakayama Y, Kosek J, Capone L, Hur EM, Schafer PH, Ringheim GE. Aiolos overexpression in systemic lupus erythematosus B cell subtypes and BAFF‐induced memory B cell differentiation are reduced by CC‐220 modulation of cereblon activity. J Immunol. 2017;199(7):2388‐2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bjorklund CC, Kang J, Amatangelo M, et al. Iberdomide (CC‐220) is a potent cereblon E3 ligase modulator with antitumor and immunostimulatory activities in lenalidomide‐ and pomalidomide‐resistant multiple myeloma cells with dysregulated CRBN. Leukemia. 2020;34(4):1197‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bjorklund CC, Lu L, Kang J, et al. Rate of CRL4(CRBN) substrate Ikaros and Aiolos degradation underlies differential activity of lenalidomide and pomalidomide in multiple myeloma cells by regulation of c‐Myc and IRF4. Blood Cancer J. 2015;5:e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bingham CO 3rd, Looney RJ, Deodhar A, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62(1):64‐74. [DOI] [PubMed] [Google Scholar]
- 10. Orange JS, Ballow M, Stiehm ER, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2012;130(3 Suppl):S1‐24. [DOI] [PubMed] [Google Scholar]
- 11. Mitchell R, Kelly DF, Pollard AJ, Truck J. Polysaccharide‐specific B cell responses to vaccination in humans. Hum Vaccin Immunother. 2014;10(6):1661‐1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Genova G, Roddick J, McNicholl F, Stevenson FK. Vaccination of human subjects expands both specific and bystander memory T cells but antibody production remains vaccine specific. Blood. 2006;107(7):2806‐2813. [DOI] [PubMed] [Google Scholar]
- 13. Georgopoulos K, Winandy S, Avitahl N. The role of the Ikaros gene in lymphocyte development and homeostasis. Annu Rev Immunol. 1997;15:155‐176. [DOI] [PubMed] [Google Scholar]
- 14. Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J Immunol. 2007;179(11):7305‐7315. [DOI] [PubMed] [Google Scholar]
- 15. Quintana FJ, Jin H, Burns EJ, et al. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat Immunol. 2012;13(8):770‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Brien S, Thomas RM, Wertheim GB, Zhang F, Shen H, Wells AD. Ikaros imposes a barrier to CD8+ T cell differentiation by restricting autocrine IL‐2 production. J Immunol. 2014;192(11):5118‐5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mi C, Wang Z, Li MY, Zhang ZH, Ma J, Jin X. Zinc finger protein 91 positively regulates the production of IL‐1beta in macrophages by activation of MAPKs and non‐canonical caspase‐8 inflammasome. Br J Pharmacol. 2018;175(23):4338‐4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dumortier A, Kirstetter P, Kastner P, Chan S. Ikaros regulates neutrophil differentiation. Blood. 2003;101(6):2219‐2226. [DOI] [PubMed] [Google Scholar]
- 19. Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon‐dependent destruction of Ikaros proteins. Science. 2014;343(6168):305‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


