Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the causative agent of the ongoing COVID‐19 pandemic. It is responsible for more than 1 million deaths worldwide already [1]. Because preventive and anti‐viral treatment options are still limited, COVID‐19 convalescent plasma (CPP) has been suggested as a potential therapy [2, 3, 4].
‘Convalescent’ implies that anti‐SARS‐CoV‐2 antibodies are present in plasma collected from individuals recovered from COVID‐19. However, the dose and nature of antibodies required to effectively interfere with a SARS‐CoV‐2 infection is unclear. Most ongoing observational studies and prospective clinical trials currently focus on neutralizing antibodies (nAbs) that interfere with viral binding to host cells, but non‐neutralizing antibodies might mediate a therapeutic effect as well. These and other unknowns highlight the importance of testing CCP efficacy in randomized trials. This commentary consequently does not claim to provide evidence on how to select potent CCP, but does want to provide an opinion‐based discussion on how to investigate CCP potency.
The antibody level in CCP varies greatly between donors. Therefore, it is required to measure antibody titer and/or to assess the neutralization potency of CCP. The current gold standard for the latter is in vitro viral neutralization like in the plaque reduction neutralization test (PRNT) or microneutralization (MN) assay. Both measure the ability of nAbs to prevent infection in vitro calculated either as a reduction in the formation of plaques or as the inhibition of viral infectivity in a cell monolayer, respectively [5, 6]. These assays utilize live SARS‐CoV‐2 virus and, hence, require a biosafety level 3 (BSL‐3) facility. In addition, it is time‐consuming (5–7 days). Furthermore, the output data cannot be compared among laboratories because different assay readouts (e.g. virus concentration or % inhibition) and protocols are currently being used. In addition, an international standard is not yet available. Blood establishments may choose to partner with a virology laboratory that can perform viral neutralization on donor samples. Alternatively, other assays are available using pseudoviruses (i.e. a recombinant virus expressing a SARS‐CoV‐2 protein) that require lower biosafety levels [7].
Anti‐SARS‐CoV‐2 antibody titers can also be measured using immunoassays such as enzyme‐linked (ELISA) and chemiluminescent immunoassays (CLIA) which are based on biochemical detection of antibody binding to viral proteins. Recently, the FDA suggested that all putative CCP donations should be tested in the Ortho VITROS SARS‐CoV‐2 IgG CLIA‐based test and donations with a signal to cut‐off of 12 or higher to be qualified as a high titer plasma [8]. In contrast, European blood establishments are using a variety of commercial immunoassays (Table 1), making it more difficult to compare data across the region. Sensitivities and specificities of the commercial assays presented in Table 1 can differ from those provided by the respective manufacturers. Thresholds, sensitivities and specificities may change depending on sample size, the timing post‐symptom onset and the seroprevalence in the population [9, 10].
Table 1.
Frequently used serological antibody assays for evaluation of CCP.
| Assay | Platform | Antigen | Company | Sensitivity (% [95% CI]) | Sample size | Specificity (% [95% CI]) | Sample size | References |
|---|---|---|---|---|---|---|---|---|
| Wantai total Ig | ELISA | S‐RBD | Beijing wantai biological | 99.0 (97.0–100) a | 186/187 | 99.0 (96.0–100) | 145/146 | [22] |
| Ortho IgG | CLIA | S‐RBD | Ortho clinical diagnostics | 93.3 (88.2–96.3) b | 140/150 | 100 (99.4–100) | 600/600 | [23] |
| EUROimmun IgG | ELISA | S1 | Euroimmun | 81.0 (71.0–89.0) a | 61/75 | 99.0 (97.0–100) | 156/157 | [22] |
| Abbott IgG | CLIA | N | Abbott | 92.6 (90.0–94.7) c | 500/540 | 99.9 (99.4–100) | 994/995 | [24] |
| Elecsys IgG | CLIA | N | Roche | 99.5 (97.0–100.0) d | 184/185 | 99.8 (99.7–99.9) | 10 432/10 453 | [25] |
Sample collection:
2–3 weeks upon respiratory infection.
13–73 days post‐symptom onset.
≥20 days post‐symptom onset.
≥14 days after PCR confirmation.
Immunoassays allow the detection of total or isotype‐specific antibody binding the spike (S), receptor binding domain of spike (RBD) or nucleocapsid (N) proteins. In our opinion, immunoassays for IgG targeting RBD are most likely to be relevant because (i) most potent neutralizing antibodies are directed towards RBD, (ii) IgG is efficiently transported across the epithelial lung barrier [11] and (iii) IgG has a longer half‐life. Finally, immunoassays are compatible with BSL‐1 facilities, do not require sophisticated technology and may be emulated on robots to increase throughput.
As viral neutralization assays are not high‐throughput and thus may become rate limiting for CCP release to patients, immunoassays may be used to select CCP donations. However, the immunoassay threshold that selects a plasma product as CCP then ideally relates reliably and reproducibly to a corresponding neutralization titer. Recently, several research groups reported on this correlation [12, 13, 14, 15]. For example, Luchsinger et al. found correlations between the Ortho IgG (r 2 = 0.75), Abbott IgG (r 2 = 0.72) and an in‐house IgG ELISA (r 2 = 0.69) with a pseudovirus neutralization assay [12]. Similar results have been observed for the EUROimmun IgG ELISA and a microneutralization or pseudotype assay [13]. Another in‐house RBD‐based IgG ELISA correlated well with virus neutralization (r 2 = 0.89) [14]. Recently, a correlation between anti‐spike EUROimmun IgA and virus neutralization (PRNT) was found, indicating that also IgA might play a role in virus neutralization [15]. These efforts are at least suggestive for correlation between certain immunoassays and viral neutralization.
The ELISA threshold and/or neutralization titer used to distinguish CCP from non‐CCP plasma remains an arbitrary choice [16]. For viral neutralization, it ranges from 1:40 to 1:320 while the FDA recommends 1:160, but without an international standard these titers are not comparable yet [8]. Note that the consequence of any threshold for an immunoassay is a shift in the balance bearing a risk of releasing poorly neutralizing CCP units on the low end, and restricting release of potentially neutralizing CCP on the high end (Fig. 1). In England, neutralizing antibody titers of 1:100 or higher were measured in 34% of donations, while using a higher cut‐off would likely have prevented a sufficient supply of CCP to fulfill trial needs [17].
Fig. 1.
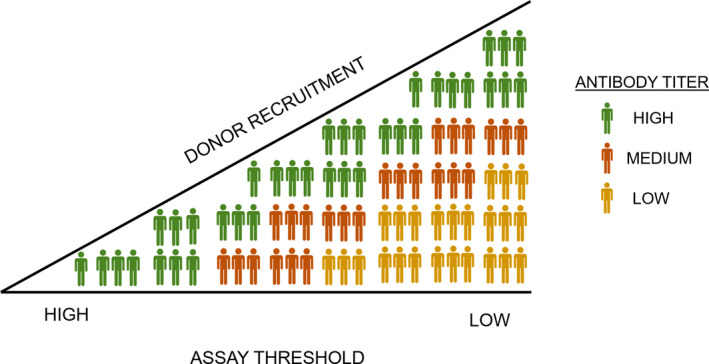
Release of neutralizing CCP units using varying assay thresholds.
Of note, unbiased screening of all donors using immunoassays without prior information on SARS‐CoV‐2 infection is not advised. As the actual number of seropositive individuals in the population is low, the positive predictive value (PPV) of any assay that is not 100% specific will unavoidably cause overrepresentation of false positives [18]. Therefore, selection of CCP should be based on laboratory confirmation of SARS‐CoV‐2 infection plus a neutralization assay or a correlating immunoassay. Observational studies from Mayo clinic and Salazar et al recently found that CCP is most effective when high amounts of anti‐SARS‐CoV‐2 IgG are present [4, 19]. In contrast, the multicentre randomized PLACID trial found no reduction in disease progression nor mortality [20] but also did not determine nAbs levels upfront. Post hoc analysis showed that the median titer of nAbs in this study was low. Together with the scientific rationale of biochemical interference with viral binding, we suggest that CCP selection is based on medium to high signal thresholds (i.e. the top 30–40% of donations containing Abs). This selection strategy may change in the future once the minimal effective dose of nAbs has been established and high‐throughput standardized assays that can reliably predict viral neutralization potency are available.
As mentioned previously, standardization or calibration of these immuno‐ and neutralization assays to allow comparison of data across studies has not yet been performed. In this context, the European Commission and the European Blood Alliance (EBA) recently launched a joint initiative to support high‐quality clinical evaluation of CCP. This SUPPORT‐E consortium (Supporting high‐quality evaluation of COVID‐19 convalescent plasma throughout Europe) [21] will investigate the relationship between (i) donor and donation parameters, (ii) antibody content and nature and (iii) clinical outcome of CCP recipients in EU cohorts. The consortium will also provide support for testing and distributes calibration standards among participating blood establishments in the EU to allow cross border standardization of assays. In addition, international standards are anticipated to be made available by the WHO in December 2020, which will facilitate such direct comparisons [18].
Although the observational studies are suggestive for CCP efficacy, hard evidence is lacking. Additional studies are required, but IgG levels obtained by ELISA seem to correlate well with virus neutralization titers. This indicates that an ELISA/CLIA assay can be used to select CCP donors, also in the light of the urgency. However, standardization of ELISAs will be essential.
Conflict of interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
This study was supported by the European Commission (SUPPORT‐E, grant number 101015756).
Acknowledgements
We thank Gaia Mori and Catherine Hartmann for the expert co‐ordination of the SUPPORT‐E project.
References
- 1. Coronavirus/COVID‐19 Worldwide Cases Live Data & Statistics. 2020. https://covidstatistics.org/ (Last accessed October 22nd 2020).
- 2. Casadevall A, Joyner MJ, Pirofski LA: A randomized trial of convalescent plasma for COVID‐19‐potentially hopeful signals. JAMA 2020; 324:455–7 [DOI] [PubMed] [Google Scholar]
- 3. Chai KL, Valk SJ, Piechotta V, et al.: Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database Syst Rev 2020; 10:CD013600 [DOI] [PubMed] [Google Scholar]
- 4. Joyner MJ, Senefeld JW, Klassen SA, et al.:Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID‐19: Initial Three‐Month Experience. medRxiv 2020.
- 5. Wolfel R, Corman VM, Guggemos W, et al.: Virological assessment of hospitalized patients with COVID‐2019. Nature 2020; 581:465–9 [DOI] [PubMed] [Google Scholar]
- 6. Conzelmann C, Gilg A, Gross R, et al.: An enzyme‐based immunodetection assay to quantify SARS‐CoV‐2 infection. Antiviral Res 2020; 181:104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson CP, Grayson N, Paton R, et al.: Detection of neutralising antibodies to SARS‐CoV‐2 to determine population exposure in Scottish blood donors between March and May 2020. Euro Surveill 2020; 25(42):2000685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. U.S. Department of Health and Human Services: Investigational COVID‐19 Convalescent Plasma: Guidance for Industry. September 2020. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/investigational‐covid‐19‐convalescent‐plasma(Last accessed October 22nd 2020).
- 9. Mathur G, Mathur S: Antibody testing for COVID‐19. Am J Clin Pathol 2020; 154:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Theel ES, Harring J, Hilgart H, et al.: Performance characteristics of four high‐throughput immunoassays for detection of IgG antibodies against SARS‐CoV‐2. J Clin Microbiol 2020; 58:e01243–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim KJ, Malik AB: Protein transport across the lung epithelial barrier. Am J Physiol Lung Cell Mol Physiol 2003; 284:L247–59 [DOI] [PubMed] [Google Scholar]
- 12. Luchsinger LL, Ransegnola B, Jin D, et al.: Serological assays estimate highly variable SARS‐CoV‐2 neutralizing antibody activity in recovered COVID19 patients. J Clin Microbiol 2020;58:e02005‐20–e02020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harvala H, Watkins N, Ijaz S, et al.: Convalescent plasma therapy for the treatment of patients with COVID‐19: Assessment of methods available for antibody detection and their correlation with neutralising antibody levels. Transf Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peterhoff D, Gluck V, Vogel M, et al.: A highly specific and sensitive serological assay detects SARS‐CoV‐2 antibody levels in COVID‐19 patients that correlate with neutralization. Infection 2020;1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jahrsdorfer B, Kroschel J, Ludwig C, et al.: Independent side‐by‐side validation and comparison of four serological platforms for SARS‐CoV‐2 antibody testing. J Infect Dis 2020; 16:jiaa656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. COVID‐19 Convalescent Plasma Transfusion . 2020. https://ec.europa.eu/health/blood_tissues_organs/covid‐19_en (Last accessed October 21th 2020).
- 17. Harvala H, Mehew J, Robb ML, et al.: Convalescent plasma treatment for SARS‐CoV‐2 infection: analysis of the first 436 donors in England, 22 April to 12 May 2020. Euro Surveill 2020; 25(28):2001260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. FDA calculator to select a COVID‐19 Antibody Test for your community. 2020. https://www.cdc.gov/coronavirus/2019‐ncov/downloads/lab/fda‐calculator.pdf (Last accessed October 22nd 2020).
- 19. Salazar E, Christensen PA, Graviss EA, et al.: Treatment of Coronavirus Disease 2019 Patients with Convalescent Plasma Reveals a Signal of Significantly Decreased Mortality. Am J Pathol 2020; 190:2290–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agarwal A, Mukherjee A, Kumar G, et al.: Convalescent plasma in the management of moderate covid‐19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 2020; 371:m3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Commission supports crucial research on convalescent plasma to treat COVID‐19. September 11th 2020. https://ec.europa.eu/info/news/commission‐supports‐crucial‐research‐convalescent‐plasma‐treat‐covid‐19‐2020‐sep‐11_en (Last accessed October 22nd 2020).
- 22. GeurtsvanKessel CH, Okba NMA, Igloi Z, et al.: An evaluation of COVID‐19 serological assays informs future diagnostics and exposure assessment. Nat Commun 2020; 11:3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harritshoej LH, Gybel‐Brask M, Afzal S, et al.: Comparison of sixteen serological SARS‐CoV‐2 immunoassays in sixteen clinical laboratories. medRxiv 2020: 2020.07.30.20165373.
- 24. Ainsworth M, Andersson M, Auckland K, et al.: Performance characteristics of five immunoassays for SARS‐CoV‐2: a head‐to‐head benchmark comparison. Lancet Infect Dis 2020; 20:1390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muench P, Jochum S, Wenderoth V, et al.: Development and validation of the Elecsys anti‐SARS‐CoV‐2 immunoassay as a highly specific tool for determining past exposure to SARS‐CoV‐2. J Clin Microbiol 2020; 58:e01694–20 [DOI] [PMC free article] [PubMed] [Google Scholar]


