Abstract
Background
Knowledge on peri‐implantitis bone defect characteristics and predictors is still limited.
Purpose
To describe peri‐implantitis bone defect characteristics and identify possible predictors.
Methods
Various parameters at patient‐ (age, gender, smoking, and supra‐structure), implant‐ (surface, type, connection, platform, and misfit), and site level (region, alveolar ridge position, defect characteristics, neighboring structure) were recorded retrospectively.
Results
Among 193 implants, the most prevalent defects were class Ic (25.4%), and Id (23.8%); a previously non‐described category “class Id with only one bone wall” was frequently observed (11.9%). Mean intrabony defect depth and width ranged from 4.5 to 6.2 mm and from 2.7 to 2.9 mm, respectively; mean dehiscence extent ranged from 2.8 to 7.0 mm. A total of 37.8% of the defects presented horizontal bone loss and an intrabony component; in 52.7% of the implants, total defect extent was >6 mm. Jaw region, implant position within the alveolar ridge, and implant/abutment misfit showed significant associations either to defect configuration and/or defect extent.
Conclusion
(a) Most common peri‐implantitis defects exhibited a combination of intrabony component and a buccal/oral dehiscence, while purely circumferential defects were relatively seldom; (b) implants with defects with bone dehiscence were placed more frequently closer to the lateral aspect of the ridge harboring the dehiscence; (c) implants placed in the lower anterior region had the highest risk for more severe peri‐implant bone loss; and (d) peri‐implant bone defects with only a single bone wall appropriate for regenerative procedure were relatively frequent.
Keywords: bone defects, clinical study, peri‐implant defect, peri‐implantitis
What is known:
Peri‐implant bone defect configuration is considered crucial for treatment choice and outcome. However, knowledge on bone defect characteristics and predictors is still limited.
What this study adds:
Implants with peri‐implantitis mostly exhibit a combination of intrabony and buccal/oral dehiscence defect rather than purely circumferential defect, in contrast to what previously reported.
Implants placed closer to the lateral aspect of the alveolar ridge develop frequently bone dehiscences.
Peri‐implant bone defects presenting only a single bone wall appropriate for regenerative procedure are not uncommon.
1. INTRODUCTION
Peri‐implantitis is a site‐specific pathological condition characterized by inflammation of the peri‐implant soft connective tissue and progressive loss of implant‐supporting bone. 1 , 2 With a weighted mean prevalence of 22% and an increasing number of annually placed dental implants, peri‐implantitis represents a growing problem. 3 , 4 In moderate and advanced peri‐implantitis cases, non‐surgical therapy often does not achieve complete disease resolution and surgical treatment is required. 5 , 6
Schwarz et al. established in 2007 a classification for peri‐implant bone defects, based on the pattern of bone loss around 40 implants. Specifically, a class I defect is defined by a buccal dehiscence and/or the presence of an intrabony defect, and a class II defect is characterized by horizontal bone loss. Class I defects are further subclassified (i.e., class Ia to Ie) based on the presence/absence of supporting bone walls and/or of intrabony defects at the four implant aspects (buccal‐mesial‐oral‐distal). 9 In this study, most intrabony defects (55.3%) had a circumferential configuration (i.e., class Ie). 9 In another study, based on 46 implants in total and 37 implants with an intrabony component, 40.5% of the intrabony defects presented with class Ie. 10 More recently, based on cone‐beam computed tomography (CBCT) analysis of peri‐implantitis lesions in a larger patient cohort with 158 implants, a class III configuration was added to the classification scheme, to describe defects with both intrabony and horizontal bone loss (i.e., combination of classes I and II). 11 Interestingly, circumferential defects were much more rare (i.e., only in 8.4% of the defects with an intrabony component) 11 compared to what was reported in the previous studies. 9 , 10 This difference among the later study and the former ones may be due to either the small patient cohorts (i.e., ≤40 implants) in the former studies 9 , 10 or due to the assessment method in the latter study, 11 which appears to be more prone to bias, especially in cases with thin alveolar bone. It has recently been shown, that in cases with a thin buccal bone (i.e., ≤1 mm thickness), detection of the buccal bone level at dental implants is largely inaccurate in CBCT images. 12
In this context, peri‐implant bone defect configuration is considered crucial, affecting treatment choice (i.e., resective, regenerative, or combined surgical approach) and the outcome of surgical treatment. 7 , 8 , 17 , 18 Specifically, class Ie defects have shown a better response comparing to class Ib and Ic, after a regenerative approach including grafting plus a collagen membrane; that is, after 6 and 12 months of healing, mean probing pocket depth reduction and clinical attachment level gain was up to 1 to 1.5 mm higher in class Ie compared to other defect classes, while the worst outcome was observed at implants with class Ib and Ic defects at the lateral aspect of the alveolar ridge harboring the bone dehiscence 7 ; similar findings were reported in other recent studies. 8 , 17 Considering the importance of defect configuration for the outcome of surgical peri‐implantitis treatment, it is interesting to know the prevalence of the various defect configurations and of any factors possibly contributing to the one or other configuration. The aim of the present study was (a) to assess characteristics and frequency distribution of peri‐implant bone defects (i.e., configuration and extent) in a large patient sample, based on intra‐operative recordings, and (b) to investigate a possible association of peri‐implant bone defect characteristics to patient‐, implant‐, and site‐related variables.
2. MATERIALS AND METHODS
2.1. Patient population and eligibility criteria
A retrospective case‐series was conducted in two centers (Faculty of Odontology, University of Malmö, Malmö, Sweden, and University Clinic of Dentistry, Medical University of Vienna, Vienna, Austria). The dental journals of patients, who had received surgical intervention due to peri‐implantitis, were screened backward from March 2020 until a convenience sample of 100 patients was achieved based on the following eligibility criteria: (a) detailed intra‐operative documentation of the peri‐implant bone defect configuration and extent; (b) availability of radiographs prior to surgical intervention allowing judgment of presence of and distance to neighboring structures. Ethical approval was obtained from the Ethical committee of the Medical University of Vienna (Vienna, Austria; EK‐Nr. 1276/2018) and from the Swedish Ethical Review Authority (Lund, Sweden; Dnr. 2020‐01019); reporting complies with the STROBE guidelines (Appendix 1).
2.2. Patient‐, implant‐, and site‐related parameters
Various parameters were extracted and calculated from the patients' dental records and radiographs. The following parameters were recorded:
At the patient level:
Age at time‐point of surgical treatment for peri‐implantitis (in years)
Gender
Smoking status at time‐point of surgical treatment for peri‐implantitis (i.e., smoker; non‐smoker)
Number of implants included herein per patient
Type of supra‐structure (i.e., single implant; multiple implants; teeth and implants combined; cross‐arch restoration)
Number of implants splinted in the supra‐structure
At the implant level:
Implant surface (i.e., modified; smooth)
Implant type (i.e., bone level; tissue level)
Connection type (i.e., internal; external)
Platform type (i.e., match; switch)
Presence of an implant/abutment misfit (i.e., presence or absence of a gap between the implant and abutment/crown)
At the site level:
Peri‐implant bone defect configuration assessed intra‐operatively according to Schwarz et al. 9
Bone defect configurations were intraoperatively recorded as classes I, II, or combinations thereof. Class I defects were further subclassified into class Ia (i.e., presence of a buccal bone dehiscence), class Ib (i.e., buccal bone dehiscence in combination with mesial and distal intrabony defects), class Ic (i.e., buccal bone dehiscence in combination with a circumferential defect), class Id (i.e., buccal and oral bone dehiscences combined with mesial and distal intrabony defects), and class Ie (i.e., circumferential defect). If defects, which fulfilled in general the above‐described characteristics for class Ic or Ie but presented with minor additional bone dehiscences buccal and/or oral, they were still classified as Ic or Ie. If defects could not be assigned to one of the above‐mentioned classes, new classes were considered later on in the analysis.
Jaw region (i.e., anterior [incisors and canines], premolars, and molars in the upper or lower jaw)
Intrabony defect depth (mm) measured from the marginal bone to the bottom of the deepest defect aspect
Horizontal bone loss height (mm) measured from the implant neck to the marginal bone
Total defect extent (mm) measured from the implant neck to the bottom of the deepest defect aspect (i.e., sum of horizontal bone loss height and intrabony defect depth, but not taking any buccal or oral bone dehiscence into account)
This parameter was further classified according to its extent into the following categories: (a) slight (i.e., 2 to 3 mm), (b) moderate (i.e., >3 to ≤6 mm), (c) advanced (i.e., >6 to ≤9 mm), and (d) severe (i.e., >9 mm).
Intrabony defect width (mm) measured from the implant surface to the bone at the height of the marginal bone level
Extent of the buccal and oral bone dehiscence (mm) measured from the implant neck to the most apical extent of the dehiscence
Presence of and distance to neighboring structures (i.e., teeth or implants) at the mesial and distal aspect of the implant
This parameter was defined as (a) “nothing” if no neighboring structure was within 5 mm, as (b) “tooth or implant too close” if the distance to the neighboring tooth or implant was <1.5 or <3 mm, respectively, or as (c) “neighboring tooth” or (d) “neighboring implant” if the tooth or implant was in a distance of 1.5 to 5 or 3 to 5 mm, respectively. A digital radiography software (Sidexis version XG 2.52, Sirona, Bensheim, Germany) was used for the measurements on periapical radiographs taken with the parallel technique. All radiographs in each center were measured by a single examiner (Vienna: C. W., Malmö: K. B.) and a calibration based on the known size of the digital sensor was performed. The above‐mentioned classes were used instead of the actual distance to minimize the effect of a measurement error due to non‐standardized radiographs.
Position of the implant in the alveolar ridge assessed at the buccal and oral aspect
Three categories applied for this parameter depending on the distance of the implant surface to the lateral aspect of the alveolar ridge; that is, as (a) “outside” if the implant surface was more buccally or orally than the lateral aspect of the alveolar ridge, as (b) “≤1 mm” if the implant surface was within 1 mm to the lateral aspect of the alveolar ridge, and as (c)” >1 mm” if the implant surface was >1 mm to lateral aspect of the alveolar ridge.
3. STATISTICAL ANALYSIS
Descriptive statistics were used and means, SD, and frequency distributions were calculated. Univariate mixed‐effects ordered logistic regression analyses were applied with total defect extent categories as ordinal primary outcome parameter and the following parameters as predictors: age, gender, smoking status, number of implants included per patient, jaw region, alveolar ridge position (buccal and oral), implant surface, implant type, connection type, platform type, implant/abutment misfit, supra‐structure, and mesial and distal aspect of the implant. Furthermore, all peri‐implant bone defect configurations, which were present more than 10‐times, were considered separately for univariate random‐effects logistic regressions analyses. The specific bone defect configuration was defined as binary primary outcome parameter and the same predictors used as listed earlier. For both approaches, a multivariate analysis was additionally performed including all parameters presenting with a P‐value <0.2 in the univariate analysis. Statistical analysis was performed with STATA/IC 16.0 for Mac and a P‐value of ≤.05 was considered as statistically significant.
4. RESULTS
4.1. Patient characteristics
Per protocol 100 patients (57 female; mean age: 64.8 years; 41% smokers) contributed with 193 implants (Table 1). In most of the cases, patients presented with a single or two implants with peri‐implantitis (i.e., in 24.4% and 33.2% of the implants, respectively), while a single patient contributed with 10 implants. Most of the implants affected by peri‐implantitis were part of a cross‐arch restorations (i.e., 45.6%; Table 1) and installed in the upper anterior and premolar region (i.e., in 31.1% and 22.8% of the implants, respectively; Table 2).
TABLE 1.
Patient and implant characteristics (n = 100)
| Age (mean ± SD [min; max]) | 64.8 ± 13.7 (28.5; 88.4) |
| Gender (female; n [%]) | 57 (57) |
| Smoking status (smoker; n [%]) a | 40 (41.2) |
| Number of implants included per patient (mean ± SD) | 1.9 ± 1.3 |
| Number of implants included per patient (n [%]) | 193 (100) |
| 1 | 47 (24.4) |
| 2 | 64 (33.2) |
| 3 | 33 (17.1) |
| 4 | 24 (12.3) |
| 5 | 15 (7.8) |
| 10 | 10 (5.2) |
| Supra‐structure (n [%]) | 193 (100) |
| Single implant | 35 (18.1) |
| Multiple implants | 70 (36.3) |
| Teeth and implants combined | 0 (0) |
| Cross‐arch restoration | 88 (45.6) |
| Number of implants splinted (mean ± SD [min; max]) | 3.5 ± 1.9 (1; 6) |
Note: max, maximum value; min, minimum value; n, number.
Three missing values.
TABLE 2.
Frequency distribution of peri‐implant bone defect configurations in the various jaw regions
| Peri‐implant bone defect configuration | Total n (%) a | Maxilla | Mandible | ||||
|---|---|---|---|---|---|---|---|
| Anterior n (%) b | Premolar | Molar | Anterior | Premolar | Molar | ||
| Intrabony defect configuration | |||||||
| Class Ia | 2 (1.0) | 1 (50.0) | 0 | 0 | 0 | 1 (50.0) | 0 |
| Class Ib | 7 (3.6) | 0 | 1 (14.3) | 0 | 2 (28.6) | 1 (14.3) | 3 (42.8) |
| Class Ic | 49 (25.4) | 19 (38.8) | 3 (6.2) | 1 (2.1) | 11 (22.3) | 9 (18.4) | 6 (12.2) |
| Class Id | 46 (23.8) | 23 (50.0) | 9 (19.6) | 1 (2.2) | 6 (13.0) | 3 (6.5) | 4 (8.7) |
| Class Ie | 20 (10.4) | 2 (10.0) | 6 (30.0) | 1 (5.0) | 3 (15.0) | 1 (5.0) | 7 (35.0) |
| Class Id with only 1 wall | 23 (11.9) | 6 (26.1) | 9 (39.1) | 2 (8.7) | 4 (17.4) | 2 (8.7) | 0 |
| Others | 13 (6.8) | 3 (23.1) | 5 (38.5) | 0 | 4 (30.7) | 1 (7.7) | 0 |
| Horizontal bone loss configuration | |||||||
| Class II | 33 (17.1) | 6 (18.2) | 11 (33.3) | 3 (9.1) | 7 (21.2) | 5 (15.2) | 1 (3.0) |
| Total | 193 | 60 (31.1) | 44 (22.8) | 8 (4.1) | 37 (19.2) | 23 (11.9) | 21 (10.9) |
Note: The following bone defect configurations are summarized among “Others”: class Ic but one wall missing (n = 3), inverted class Ic (n = 4), intrabony defect at a single aspect (n = 2), class Ia with the bone defect/dehiscence at another aspect (n = 2), class Ia with a second bone dehiscence at another aspect (n = 1), and class Ia with an intrabony component at a single aspect (n = 1). n indicates number.
Percentages represent the percentages of the total number of implants included (n = 193).
Percentages represent the percentages per peri‐implant bone defect configuration.
4.2. Peri‐implant bone defect configuration
Peri‐implant bone defects were most often class Ic (25.4%), followed by class Id (23.8%), and pure class II defects (17.1%), while 36 defects (18.7%) did not fit the Schwarz et al 9 classification. Of those, only one specific bone defect configuration appeared frequently (i.e., 23‐times or in 11.9% of all defects): “class Id with only 1 wall”, which fulfills in general the criteria of class Id but is lacking additionally either the mesial or distal bone wall. Clinical examples of the most frequent peri‐implant bone defect configurations are presented in Figure 1. Among the remaining 13 defects (6.8%), no specific defect configuration appeared more often than four‐times: “class Ic but one wall missing” (n = 3), “inverted class Ic” (i.e., with the dehiscence at the oral aspect) (n = 4), “intrabony defect at a single aspect” (n = 2), “class Ia with the bone dehiscence at another aspect” (n = 2), “class Ia with a second bone dehiscence at another aspect” (n = 1), and “class Ia with an intrabony component at a single aspect” (n = 1). These defects configurations are summarized later on as “others”. Frequency distribution of the various peri‐implant bone defect configurations according to the jaw region is displayed in Table 2.
FIGURE 1.
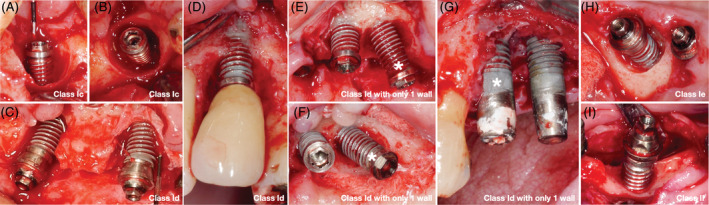
Clinical examples of the most frequent peri‐implant bone defect configurations (* indicates the corresponding implant in case of several implants with different bone defect configurations); A, buccal and, B, occlusal view of class Ic; C and D, buccal view of two cases with class Id; E and G, buccal and, F, occlusal view of two cases with class Id with only one wall; H, buccal‐occlusal view of class Ie; and, I, buccal view of class II
4.3. Extent and characteristics of peri‐implant bone defects
Defect extent and characteristics of the most prevalent peri‐implant bone defect configurations (i.e., peri‐implant bone defect configurations with a frequency >10 implants) are summarized in Table 3. Intrabony defect depth ranged from 4.5 ± 2.4 to 6.2 ± 2.4 mm, and defect width ranged from 2.7 ± 1.4 to 2.9 ± 0.7 mm. The range of the extent of the buccal bone dehiscence was from 3.3 ± 2.1 to 7.0 ± 1.9 mm, and of the oral bone dehiscence from 2.8 ± 1.3 to 6.3 ± 1.9 mm. Class Ie was most frequently combined with a class II defect (i.e., in 85% of the cases) and among the combined defects (i.e., class I and II) the horizontal bone loss height ranged from 2.3 ± 1.8 to 3.6 ± 2.0 mm, while for those defects with only class II the horizontal bone loss height averaged at 5.9 ± 2.5 mm. The total defect extent ranged from 5.9 ± 2.5 to 7.7 ± 3.0 and more than half of the implants (i.e., 52.7%) presented with advanced bone loss (i.e., with a total defect extent >6 mm). Class Ic and Ie presented the highest percentage of severe bone loss (i.e., >9 mm total defect extent; in 25% and 20% of the implants, respectively), while the total defect extent of class II defects remained ≤6 mm in about 70% of the implants (Figure 2). Furthermore, implants installed in the lower anterior followed by the upper anterior presented the highest percentage of advanced and severe peri‐implant bone loss (i.e., about 60% each of the implants with advanced or severe bone loss were installed in the anterior region; Appendix 2a).
TABLE 3.
Characteristics of the most prevalent peri‐implant bone defect configurations (ie, peri‐implant bone defect configurations with a frequency >10 implants)
| Peri‐implant bone defect configuration | Intrabony defect (mm) | Extent of the bone dehiscence (mm) | Combined with a class II defect | Horizontal bone loss (mm) | Total defect extent (mm) | ||
|---|---|---|---|---|---|---|---|
| Depth | Width | Buccal | Oral | Yes | Height | ||
| Mean ± SD (min; max) | n (%) a | Mean ± SD (min; max) | |||||
| Intrabony defect configuration | |||||||
| Class Ic |
6.2 ± 2.4 (2; 11) |
2.9 ± 0.7 (2; 4.5) |
7.0 ± 1.9 (2; 12) |
4.5 ± 1.8 (1.5; 8) |
21 (42.9) |
3.6 ± 2.0 (1; 7) |
7.7 ± 3.0 (2; 15) |
| Class Id |
6.1 ± 2.1 (1.5; 10.5) |
2.9 ± 0.6 (2; 4.5) |
6.7 ± 2.3 (2; 12.5) |
6 ± 2.2 (2; 12) |
13 (28.3) |
3 ± 1.4 (1; 6) |
6.8 ± 2.4 (3; 13) |
| Class Id with only 1 wall |
5.6 ± 2 (2; 9) |
2.7 ± 1.4 (0; 5) |
6.9 ± 2.4 (2; 12) |
6.3 ± 1.9 (2; 10) |
9 (39.1) |
2.3 ± 1.8 (1; 6) |
6.5 ± 2.6 (2; 12) |
| Class Ie |
4.5 ± 2.4 (1; 10) |
2.7 ± 0.9 (1; 5) |
3.3 ± 2.1 (1; 6) |
2.8 ± 1.3 (1; 4) |
17 (85.0) |
3.1 ± 2.2 (1; 9) |
7.2 ± 2.8 (2; 12) |
| Horizontal bone loss configuration | |||||||
| Class II | ‐ | ‐ | ‐ | ‐ | ‐ |
5.9 ± 2.5 (2; 12) |
5.9 ± 2.5 (2; 12) |
Note: max, maximum value; min, minimum value; n, number.
Percentages are within each peri‐implant bone defect configuration.
FIGURE 2.
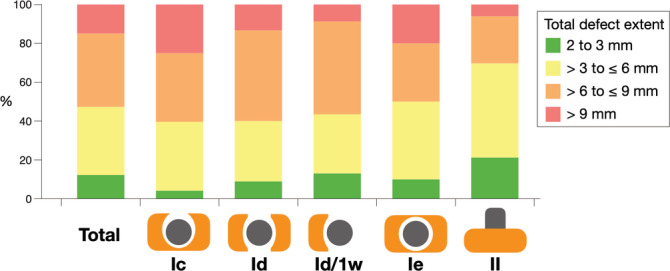
Frequency distribution of the total defect extent in total and among the most prevalent peri‐implant bone defect configurations; Id/1w, class Id with only 1 wall
Figure 3 illustrates the frequency distribution of the position of the implants in the alveolar ridge from the buccal and oral aspect. It becomes evident, that implants with circumferential defects (i.e., class Ie) were located more centrally within the alveolar ridge and had almost in 100% of the cases a distance >1 mm to the lateral aspect of the alveolar ridge, while defects with a buccal and/or oral bone dehiscence (i.e., class Ic, class Id, class Id with only 1 wall) were more often located closer to the lateral aspect of the alveolar ridge.
FIGURE 3.
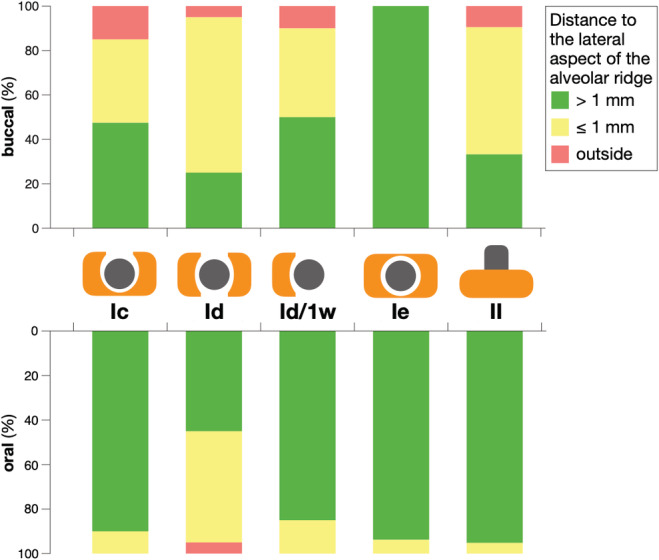
Frequency distribution of the distance of the implant surface to the lateral aspect of the alveolar ridge among the most prevalent peri‐implant bone defect configurations; Id/1w, class Id with only 1 wall
4.4. Implant characteristics
Implant characteristics of the most prevalent peri‐implant bone defect configurations are summarized in Table 4. Overall, the implants presented primarily (i.e., ≥ 85% of the cases) with modified implant surface, were bone level implants, and showed no misfit; furthermore, about 2/3 of the implants had an internal connection and showed platform match. All implants with class Id with only 1 wall defects had modified surface and were bone level type implants, while pure class II defects presented more often at smooth implants (i.e., in 27.3% of the cases). Interestingly, implants with class Id with only one wall defects, when compared to all other bone defect configurations, presented a slightly higher frequency for internal connection, platform switch, and misfit.
TABLE 4.
Implant characteristics of the most prevalent peri‐implant bone defect configurations (i.e., peri‐implant bone defect configurations with a frequency >10 implants)
| Peri‐implant bone defect configuration | Implant surface | ||||
|---|---|---|---|---|---|
| Modified/Smooth | Implant type | Connection type | Platform type | Implant/abutment misfit | |
| n (%) a | Bone level/Tissue level | Internal/External | Match/Switch | Yes/No | |
| Intrabony defect configuration | |||||
| Class Ic |
47/2 (95.9/4.1) |
42/7 (85.7/14.3) |
29/20 (59.2/40.8) |
38/11 (77.5/22.5) |
6/43 (12.2/87.8) |
| Class Id |
36/10 (78.3/21.7) |
42/4 (91.3/8.7) |
27/19 (58.7/41.3) |
28/18 (60.9/39.1) |
7/39 (15.2/84.8) |
| Class Id with only 1 wall |
23/0 (100.0/0.0) |
23/0 (100.0/0.0) |
19/4 (82.6/17.4) |
12/11 (52.2/47.8) |
6 /17 (26.1/73.9) |
| Class Ie |
18/2 (90.0/10.0) |
16/4 (80.0/20.0) |
14/6 (70.0/30.0) |
11/9 (55.0/45.0) |
2/18 (10.0/90.0) |
| Horizontal bone loss configuration | |||||
| Class II |
24/9 (72.7/27.3) |
29/4 (87.9/12.1) |
19/14 (57.6/42.4) |
24/9 (72.7/27.3) |
4/29 (12.1/87.9) |
| Total (n = 193) |
168/25 (87.1/12.9) |
169/24 (87.6/12.4) |
121/72 (62.7/37.3) |
130/63 (67.4/32.6) |
29/164 (15.0/85.0) |
Note: n indicates number.
Percentages are within each peri‐implant bone defect configuration and parameter.
Implant characteristics in relation to the total defect extent are displayed in Appendix 2b. Briefly, a somewhat larger variation (i.e., >15%) regarding the distribution of implant type and implant/abutment misfit in the four total defect extent categories was observed; that is, tissue level implants and implants with a misfit presented more often a slight peri‐implant bone loss (i.e., 2 to 3 mm).
4.5. Neighboring structure mesial and distal of the diseased implant
The data regarding the neighboring structure at the mesial and distal aspect of the implants in relation to defect configuration and the total defect extent is presented in Table 5 and Appendix 2c, respectively. Overall, ≥49% of the implants had either mesially or distally no neighboring structure within a distance of 5 mm, while it appeared only seldom that the diseased implant had been placed too close to the neighboring tooth or implant (i.e., only in about 8% to 9% of the cases each for the mesial and distal aspect). Interestingly, implants with class Id with only 1 wall defects presented a higher frequency for a tooth or implant being too close (i.e., mesial and distal in 26.1% and 17.4% of the cases, respectively) compared to all other bone defect configurations. At the same time, implants with class Id with only one wall defects had less often an implant as neighboring structure within a correct distance; regarding implants with class Ie defects, it was only once that the diseased implant was placed too close to the neighboring structure.
TABLE 5.
Neighboring structure mesial and distal of the diseased implant in relation to the most prevalent peri‐implant bone defect configurations (i.e., peri‐implant bone defect configurations with a frequency >10 implants)
| Peri‐implant bone defect configuration | Mesial aspect of the implant a | Distal aspect of the implant a | ||||||
|---|---|---|---|---|---|---|---|---|
| Nothing n (%) b | T/I too close | Neighboring T | Neighboring I | Nothing | T/I too close | Neighboring T | Neighboring I | |
| Intrabony defect configuration | ||||||||
| Class Ic | 26 (53.1) | 2 (4.1) | 7 (14.2) | 14 (28.6) | 28 (57.2) | 3 (6.1) | 5 (10.2) | 13 (26.5) |
| Class Id | 27 (58.7) | 1 (2.2) | 6 (13.0) | 12 (26.1) | 29 (63.0) | 3 (6.5) | 5 (10.9) | 9 (19.6) |
| Class Id with only 1 wall | 10 (43.5) | 6 (26.1) | 4 (17.4) | 3 (13) | 12 (52.2) | 4 (17.4) | 6 (26.1) | 1 (4.4) |
| Class Ie | 10 (50.0) | 1 (5.0) | 5 (25.0) | 4 (20.0) | 13 (65.0) | 0 (0.0) | 1 (5.0) | 6 (30.0) |
| Horizontal bone loss configuration | ||||||||
| Class II | 14 (42.4) | 4 (12.1) | 3 (9.1) | 12 (36.4) | 18 (54.6) | 3 (9.1) | 2 (6.1) | 10 (30.3) |
| Total (n = 193) | 95 (49.2) | 16 (8.3) | 33 (17.1) | 49 (25.4) | 113 (58.6) | 17 (8.8) | 22 (11.4) | 41 (21.2) |
Abbreviations: I, implant; n, number; T, tooth.
“Nothing” is defined as no neighboring structure within 5 mm; <1.5 mm is the cut‐off value for a tooth being too close and <3 mm is the cut‐off value for an implant being too close.
Percentages are within each peri‐implant bone defect configuration and parameter.
4.6. Association of peri‐implant bone defect configuration and extent to patient‐, implant‐, and site‐related variables
Detailed data on the association between the potential predictors and peri‐implant bone defect configuration are presented in Appendices 3a to 3f. However, only few statistically significant results were found by the univariate and multivariate regression analyses. More specifically, the jaw region showed for class Ic and class Ie, and class II defects a significant effect; class Ic defects appeared significantly less often in the upper posterior compared to all other regions and class Ie defects appeared more often in the posterior regions. Otherwise, two already above‐described tendencies showed statistical significance in the univariate regression analyses: (a) the implant surface of class Id defects was more often closer to or outside of the lateral aspect of the alveolar ridge (and this effect remained significant in the multivariate model); and (b) implants with class Id with only one wall defects were placed more often too close to the neighboring structure.
Table 6 displays the associations between the potential predictors and total defect extent. In the univariate regression analyses age, jaw region, implant type, implant/abutment misfit, and supra‐structure showed significant results. More specifically, a higher age, implants placed in the lower anterior, bone level type implants, lack of a misfit, and implants of a cross‐arch restoration showed an increased risk for presenting with more severe peri‐implant bone defects (i.e., an increased total defect extent). After combining these significant predictors together with gender (P = .079) in a multivariate regression analysis, only jaw region and implant/abutment misfit remained statistically significant.
TABLE 6.
Univariate and multivariate mixed‐effects ordered logistic regression analyses with total defect extent as ordinal primary outcome parameter
| Predictors | Univariate model | Multivariate model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||||
| Lower | Upper | Lower | Upper | ||||||
| Age | Years | 1.036 | 1.008 | 1.064 | .010 | 1.032 | 0.997 | 1.067 | .070 |
| Gender | Male | ||||||||
| Female | 1.877 | 0.929 | 3.792 | .079 | 1.990 | 0.959 | 4.131 | .065 | |
| Smoking status | Non‐smoker | ||||||||
| Smoker | 0.982 | 0.473 | 2.038 | .961 | |||||
| Implants included per patient | Count | 0.898 | 0.719 | 1.121 | .340 | ||||
| Jaw region a | Upper posterior | ||||||||
| Upper anterior | 1.671 | 0.722 | 3.870 | .231 | 2.063 | 0.809 | 5.262 | .129 | |
| Lower posterior | 0.723 | 0.286 | 1.825 | .492 | 0.949 | 0.373 | 2.414 | .912 | |
| Lower anterior | 3.407 | 1.226 | 9.466 | .019 | 3.557 | 1.211 | 10.448 | .021 | |
| Alveolar ridge position (buccal) | Outside | ||||||||
| ≤1 mm | 1.142 | 0.268 | 4.864 | .857 | |||||
| >1 mm | 1.379 | 0.304 | 6.241 | .677 | |||||
| Alveolar ridge position (oral) | Outside | ||||||||
| ≤1 mm | 1.887 | 0.017 | 214.644 | .793 | |||||
| >1 mm | 6.437 | 0.061 | 684.793 | .434 | |||||
| Implant surface | Smooth | ||||||||
| Modified | 1.034 | 0.357 | 2.991 | .952 | |||||
| Implant type | Bone level | ||||||||
| Tissue level | 0.321 | 0.117 | 0.881 | .027 | 0.402 | 0.140 | 1.150 | .089 | |
| Connection type | Internal | ||||||||
| External | 1.542 | 0.723 | 3.289 | .263 | |||||
| Platform type | Match | ||||||||
| Switch | 1.336 | 0.632 | 2.827 | .448 | |||||
| Implant/abutment misfit | No | ||||||||
| Yes | 0.358 | 0.142 | 0.904 | .030 | 0.265 | 0.102 | 0.685 | .006 | |
| Supra‐structure b | Single implant | ||||||||
| Multiple implants | 1.613 | 0.632 | 4.116 | .317 | 0.664 | 0.211 | 2.087 | .483 | |
| Cross‐arch restoration | 2.789 | 1.102 | 7.058 | .030 | 0.776 | 0.244 | 2.464 | .667 | |
| Mesial aspect of the implant c | Nothing | ||||||||
| T/I too close | 1.302 | 0.393 | 4.318 | .666 | |||||
| Neighboring T | 0.555 | 0.225 | 1.368 | .201 | |||||
| Neighboring I | 0.818 | 0.391 | 1.712 | .594 | |||||
| Distal aspect of the implant° | Nothing | ||||||||
| T/I too close | 0.929 | 0.292 | 2.961 | .901 | |||||
| Neighboring T | 0.674 | 0.237 | 1.918 | .460 | |||||
| Neighboring I | 0.952 | 0.443 | 2.047 | .900 | |||||
Note: Bold values indicate significance.
Abbreviations: CI, confidence interval; I, implant; OR, odds ratio; T, tooth.
Due to the low number of cases the premolar and molar region was pooled as “posterior” for the regression analysis.
No case with “multiple teeth + implants.”
“Nothing” is defined as no neighboring structure within 5 mm; <1.5 mm is the cut‐off value for a tooth being too close and <3 mm is the cut‐off value for an implant being too close.
5. DISCUSSION
The present study based on intra‐operatively assessed characteristics of 193 peri‐implantitis defects in 100 patients undergoing surgical treatment, showed that (a) most common peri‐implantitis defects exhibited a combination of intrabony component and a buccal/oral dehiscence (i.e., class Ic and Id), while purely circumferential defects (i.e., Ie) were relatively seldom; (b) implants with defects with bone dehiscence were placed more frequently closer to the lateral aspect of the alveolar ridge harboring the dehiscence, whereas implants with circumferential defects (i.e., class Ie) were placed more centrally within the alveolar ridge; (c) implants placed in the lower anterior region had the highest risk to show a class Ic configuration and for more severe peri‐implant bone loss (i.e., 14.5‐ and 3.5‐times higher compared to the upper posterior, respectively); and (d) peri‐implant bone defects with only a single bone wall appropriate for regenerative procedure were relatively frequent (i.e., 12% of all defects).
When introducing the classification for peri‐implant bone defects, Schwarz et al 9 reported circumferential defects (class Ie) as the most frequent bone defect configuration, occurring in more than half of the cases (i.e., 55%); the remaining defects showed a configuration comprising an intrabony component and a bone dehiscence (i.e., class Ia: 5.5%, Ib: 15%, Ic: 13%, and Id: 10%). Similarly, García‐García et al 10 reported that class Ie was the most frequent configuration among the defects exhibiting an intrabony component (i.e., in 40.5%), while about 33% were class Ic. Both of those reports, however, included a relatively low number of implants (i.e., ≤40 implants with intrabony defects). 9 , 10 In a more recent study 11 including a larger number of implants (i.e., 158 implants in 47 patients), purely circumferential defects (i.e., class Ie) were observed in only about 8% of the defects with an intrabony component, while the majority of the defects exhibited an intrabony component and a bone dehiscence, similarly to the present study. Specifically, if one considers only those defects herein with an intrabony component that could be classified according to the Schwarz et al 9 as Ia, Ib, Ic, Id, or Ie—to allow for a direct comparison to the above studies 9 , 10 —only 16.1% of the cases were class Ie, while class Ic and Id defects were the most frequent ones (i.e., in 39.5% and 37.1% of the cases, respectively), and class Ia and Ib were the most seldom ones (i.e., in <6% of the cases). Importantly, 36 of the 193 evaluated peri‐implant bone defects herein, did not fit in any of the Schwarz et al 9 classes. While some of those “non‐classifiable” defects had a quite unique configuration and could not be grouped in a reasonable way as a “new” class, one specific defect type was present more often (i.e., 23‐times or in 11.9% of all cases). These defects were related to class Id but were lacking one additional bone wall either mesially or distally (Figure 1E‐G); therefore, a new class is suggested: “class Id with only 1 wall” (i.e., Id‐m and Id‐d, when the mesial or distal bone wall is present, respectively). It appears thus important, for treatment planning or within the context of clinical studies, to have the possibility of a peri‐implant bone defect configuration presenting only a single bone wall appropriate for regenerative procedure in mind.
As outlined in the introduction, studies have indicated that peri‐implant bone defect configuration and extent influence the outcome of reconstructive therapy of peri‐implantitis., 7 , 8 , 17 , 18 although this has not been a consistent observation. 19 This may indicate that other factors, such as the difficulty to achieve effective debridement of the contaminated implant surface in deep circumferential defects can be challenging and thereby potentially limiting the outcome. Furthermore, it has been demonstrated that peri‐implantitis lesions with limited to moderate bone loss (i.e., 2 to 4 mm prior to treatment) achieve a better clinical outcome 2 years after surgical therapy compared to implants with more severe bone loss (i.e., ≥5 mm). 18 In perspective, presence of sufficient buccal and oral bone wall thickness at the timepoint of implant installation (either pristine bone or after bone augmentation) appears advantageous, not only because it shows reduced bone remodeling over time 20 , 21 and results in a stable aesthetic outcome, 22 , 23 but also in the context of peri‐implantitis by increasing the chances for a peri‐implant bone defect configuration with more favorable treatment outcomes after reconstructive interventions (i.e., class Ie). 7 , 8 , 17 Since delayed adequate therapeutic intervention most likely results in more advanced/complex peri‐implant bone defect configurations and less chances for a good treatment outcome, a timely diagnosis and treatment of peri‐implantitis lesions are imperative.
In this context, differences in the frequency distribution of purely circumferential defects and defects with an intrabony component and a bone dehiscence among studies may be explained by differences in the jaw region/anatomy in which the implants were placed and/or by the stage of disease progression. For example Schwarz et al 9 reported primarily on posterior implants (i.e., 77.5% of the included implants in that study where from the premolar or molar region), while in the present study only about 50% of the implants were posterior ones. Indeed, in the present study, Ie defects were more likely associated with posterior implants compared to anterior ones; implants harboring class Ie defects also had almost in 100% of the cases a distance >1 mm to the lateral aspect of the alveolar ridge, while implants with defects with bone dehiscence appeared placed more often closer to or beyond the lateral aspect of the alveolar ridge harboring the dehiscence. Truly, the residual alveolar ridge is wider more posteriorly comparing to more anteriorly, and this usually allows for a more central implant placement—in relation to the bucco‐oral width of the ridge—and for sufficient bone wall thickness buccally and orally to the implant after installation. It is reasonable to assume that implants placed in wider/wide ridges are more likely to develop defects with more bone walls at the event of peri‐implantitis, compared with implants placed in narrower/narrow ridges. This notion is supported by the results of a recent study, showing that implants harboring “4‐wall defects” had a wider bucco‐oral width distally and mesially to the implant, compared with implants with “2‐ and 3‐wall” defects. 8 Furthermore, it is also reasonable to assume that implants placed in “narrower/narrow” ridges will present with fewer bone walls at a “later” stage of disease, where the disease has resulted in complete resorption of the bone wall, than at an “earlier” stage of disease. Indeed, horizontal bone loss height and intrabony defect depth herein, ranged from 2.3 ± 1.8 to 5.9 ± 2.5 mm and from 4.5 ± 2.4 to 6.2 ± 2.4 mm, respectively, in the various defect classes, which indicates a rather advanced disease stage at the timepoint of treatment in this specific population. In comparison, the average horizontal bone loss height and intrabony defect depth in the Schwarz et al 9 was 1.9 ± 1.3 and 2.9 ± 1.5 mm, respectively.
It is of interest whether specific patient‐, implant‐, and/or site‐related parameters predispose to a specific peri‐implant bone defect configuration and/or a more advanced lesion. Indeed, in a previous study, 11 a significant association between the defect configuration and patient age and between the amount of vertical bone loss and smoking status, type of supra‐structure, and proximity to the neighboring structures has been described. Specifically, peri‐implant bone defects with intrabony components and a bone dehiscence but without horizontal bone loss were observed more often with higher age, while heavy smoking, fixed restorations, and implant proximity were significantly associated with a larger total defect extent. 11 Herein, the only predictor that appeared somehow consistently having a relevant effect on both defect configuration and extent was jaw region. Thus, implants placed in the lower anterior region had the highest risk to show a class Ic defect and the highest risk for more severe peri‐implant bone loss, for example, 14.5‐ and 3.5‐times higher, respectively compared to the upper posterior. Although the width of keratinized mucosa was not registered in this study, the finding that implants in the lower anterior developed a more complex defect configuration and more severe disease may be, at least partly, also related to the frequent lack of adequate width or total lack of keratinized mucosa ‐ i.e. in addition to the impact of the narrow alveolar ridge, discussed above. It has indeed been shown previously 11 that only a limited number of implants (i.e., <15%) with peri‐implantitis lesions in the lower anterior presented ≥2 mm of keratinized mucosa. Lack of keratinized mucosa has been discussed as risk factor for peri‐implant biological complications, because it is associated with plaque accumulation, and it appears more relevant for patients with a low compliance. 13 , 14 , 15 , 16
Surprisingly, presence of an implant/abutment misfit was associated herein with a reduced total defect extent. It can only be hypothesized that although the presence of a misfit may initiate a biological complication and result in some extent of peri‐implant marginal bone loss, once a certain distance between the prosthetic gap and the marginal bone has been established, the misfit per se does not necessarily further drive disease progression, and other factors are probably more important. Indeed, a relatively recent review 24 concluded that the notion that prosthetic misfit leads to a higher rate of biological complications cannot be confirmed due to currently scarce information on this topic.
In perspective, this is a retrospective study with inherent limitations, which in turn may have compromised parts of the analysis. For example, despite that this study included 193 implants—the largest sample reported upon until now on this topic—some of the groups presented relatively small numbers; for example, implants with a machined surface or tissue level type implants were <15% of the total sample. Thus, lack of significance of tissue level implants to present a reduced risk for more severe peri‐implant bone defects may, at least partly, be due to the low sample size of this type of implants. Furthermore, the peri‐apical radiographs used herein for measuring the distance to the neighboring structures have not been taken under standardized conditions. Thus, classes of distances between the implant and the neighboring structures were used instead of the actual distance to minimize the effect of a measurement error due to non‐standardized radiographs. Lastly, the majority of included implants has been placed elsewhere and there was no information on aspects related to the surgical procedure, for example, implant installation in connection with bone augmentation, type of augmentation material, immediate implant installation, implant misplacement, overheating during implant site preparation, overcompression of the (thick) cortical crestal bone, and so forth, which have been suggested to possibly affect the incidence and/or progression of peri‐implantitis. 25 Therefore, no assessment of an association of these factors with defect configuration and extent was possible herein.
In conclusion, based on the current analysis, implants with peri‐implantitis exhibited a combination of intrabony and buccal/oral dehiscence defects rather than purely circumferential defects, in contrast to what reported previously. Furthermore, implants placed closer to the lateral aspect of the alveolar ridge developed frequently bone dehiscences, and implants placed in the lower anterior region had the highest risk for more severe peri‐implant bone loss. Finally, the possibility of having a peri‐implant bone defect configuration presenting only a single bone wall appropriate for regenerative procedure should be considered.
CONFLICT OF INTEREST
The authors declare no conflict of interest. The study has been self‐supported by the Division of Conservative Dentistry and Periodontology, University Clinic of Dentistry, Medical University of Vienna, Vienna, Austria, and the Department of Periodontology, Faculty of Odontology, University of Malmö, Malmö, Sweden.
Supporting information
Appendix1. STROBE Statement—cross‐sectional studies.
Appendix2a. Frequency distribution of the total defect extent# in the various jaw regions.
Appendix2b. Implant characteristics in relation to the total defect extent#.
Appendix2c. Neighboring structure mesial and distal of the diseased implant in relation to the total defect extent#.
Appendix3a. Univariate random‐effects logistic regression analyses of the most prevalent peri‐implant bone defect configurations (ie, peri‐implant bone defect configurations with a frequency >10 implants).
Appendix3b. Multivariate random‐effects logistic regression analysis of the class Ic defects.
Appendix3c. Multivariate random‐effects logistic regression analysis of the class Id defects.
Appendix3d. Multivariate random‐effects logistic regression analysis of the class Id with only 1 wall defects.
Appendix3e. Multivariate random‐effects logistic regression analysis of the class Ie defects.
Appendix3f. Multivariate random‐effects logistic regression analysis of the class II defects.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Michael Müller (Division of Conservative Dentistry and Periodontology, University Clinic of Dentistry, Medical University of Vienna, Vienna, Austria) for his contribution in data collection.
Wehner C, Bertl K, Durstberger G, Arnhart C, Rausch‐Fan X, Stavropoulos A. Characteristics and frequency distribution of bone defect configurations in peri‐implantitis lesions—A series of 193 cases. Clin Implant Dent Relat Res. 2021;23:179–189. 10.1111/cid.12961
Funding information Open access provided by the Medical University of Vienna
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Berglundh T, Armitage G, Araujo MG, et al. Peri‐implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and Peri‐implant diseases and conditions. J Clin Periodontol. 2018;45(Suppl 20):S286‐S291. [DOI] [PubMed] [Google Scholar]
- 2. Schwarz F, Derks J, Monje A, Wang HL. Peri‐implantitis. J Periodontol. 2018;89(Suppl 1):S267‐S290. [DOI] [PubMed] [Google Scholar]
- 3. Derks J, Tomasi C. Peri‐implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(Suppl 16):S158‐S171. [DOI] [PubMed] [Google Scholar]
- 4. Klinge B, Klinge A, Bertl K, Stavropoulos A. Peri‐implant diseases. Eur J Oral Sci. 2018;126(Suppl 1):88‐94. [DOI] [PubMed] [Google Scholar]
- 5. Renvert S, Polyzois I, Claffey N. Surgical therapy for the control of peri‐implantitis. Clin Oral Implants Res. 2012;23(Suppl 6):84‐94. [DOI] [PubMed] [Google Scholar]
- 6. Heitz‐Mayfield LJA, Salvi GE, Mombelli A, Faddy M, Lang NP. Anti‐infective surgical therapy of peri‐implantitis. A 12‐month prospective clinical study. Clin Oral Implants Res. 2012;23(2):205‐210. [DOI] [PubMed] [Google Scholar]
- 7. Schwarz F, Sahm N, Schwarz K, Becker J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri‐implantitis. J Clin Periodontol. 2010;37(5):449‐455. [DOI] [PubMed] [Google Scholar]
- 8. Aghazadeh A, Persson RG, Renvert S. Impact of bone defect morphology on the outcome of reconstructive treatment of peri‐implantitis. Int J Implant Dent. 2020;6(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwarz F, Herten M, Sager M, Bieling K, Sculean A, Becker J. Comparison of naturally occurring and ligature‐induced peri‐implantitis bone defects in humans and dogs. Clin Oral Implants Res. 2007;18(2):161‐170. [DOI] [PubMed] [Google Scholar]
- 10. García‐García M, Mir‐Mari J, Benic GI, Figueiredo R, Valmaseda‐Castellón E. Accuracy of periapical radiography in assessing bone level in implants affected by peri‐implantitis: a cross‐sectional study. J Clin Periodontol. 2016;43(1):85‐91. [DOI] [PubMed] [Google Scholar]
- 11. Monje A, Pons R, Insua A, Nart J, Wang HL, Schwarz F. Morphology and severity of peri‐implantitis bone defects. Clin Implant Dent Relat Res. 2019;21(4):635‐643. [DOI] [PubMed] [Google Scholar]
- 12. Domic D, Bertl K, Ahmad S, Schropp L, Hellén‐Halme K, Stavropoulos A. Accuracy of cone beam computed tomography is limited at implant sites with a thin buccal bone. A laboratory study. Journal of Periodontology. in press. 2020. 10.1002/JPER.20-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin GH, Chan HL, Wang HL. The significance of keratinized mucosa on implant health: a systematic review. J Periodontol. 2013;84(12):1755‐1767. [DOI] [PubMed] [Google Scholar]
- 14. Monje A, Blasi G. Significance of keratinized mucosa/gingiva on peri‐implant and adjacent periodontal conditions in erratic maintenance compliers. J Periodontol. 2019;90(5):445‐453. [DOI] [PubMed] [Google Scholar]
- 15. Lim HC, Wiedemeier DB, Hämmerle CHF, Thoma DS. The amount of keratinized mucosa may not influence peri‐implant health in compliant patients: a retrospective 5‐year analysis. J Clin Periodontol. 2019;46(3):354‐362. [DOI] [PubMed] [Google Scholar]
- 16. Vignoletti F, Di Domenico GL, Di Martino M, Montero E, de Sanctis M. Prevalence and risk indicators of peri‐implantitis in a sample of university‐based dental patients in Italy: a cross‐sectional study. J Clin Periodontol. 2019;46(5):597‐605. [DOI] [PubMed] [Google Scholar]
- 17. Schlee M, Rathe F, Brodbeck U, Ratka C, Weigl P, Zipprich H. Treatment of Peri‐implantitis‐electrolytic cleaning versus mechanical and electrolytic cleaning‐a randomized controlled clinical trial‐six‐month results. J Clin Med. 2019;8(11). 10.3390/jcm8111909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Serino G, Turri A. Outcome of surgical treatment of peri‐implantitis: results from a 2‐year prospective clinical study in humans. Clin Oral Implants Res. 2011;22(11):1214‐1220. [DOI] [PubMed] [Google Scholar]
- 19. Roccuzzo M, Gaudioso L, Lungo M, Dalmasso P. Surgical therapy of single peri‐implantitis intrabony defects, by means of deproteinized bovine bone mineral with 10% collagen. J Clin Periodontol. 2016;43(3):311‐318. [DOI] [PubMed] [Google Scholar]
- 20. Monje A, Chappuis V, Monje F, et al. The critical Peri‐implant buccal Bone Wall thickness revisited: an experimental study in the beagle dog. Int J Oral Maxillofac Implants. 2019;34(6):1328‐1336. [DOI] [PubMed] [Google Scholar]
- 21. Raes S, Eghbali A, Chappuis V, Raes F, De Bruyn H, Cosyn J. A long‐term prospective cohort study on immediately restored single tooth implants inserted in extraction sockets and healed ridges: CBCT analyses, soft tissue alterations, aesthetic ratings, and patient‐reported outcomes. Clin Implant Dent Relat Res. 2018;20(4):522‐530. [DOI] [PubMed] [Google Scholar]
- 22. Barone A, Alfonsi F, Derchi G, et al. The effect of insertion torque on the clinical outcome of single implants: a randomized clinical trial. Clin Implant Dent Relat Res. 2016;18(3):588‐600. [DOI] [PubMed] [Google Scholar]
- 23. Chappuis V, Rahman L, Buser R, Janner SFM, Belser UC, Buser D. Effectiveness of contour augmentation with guided bone regeneration: 10‐year results. J Dent Res. 2018;97(3):266‐274. [DOI] [PubMed] [Google Scholar]
- 24. Katsoulis J, Takeichi T, Gaviria AS, Peter L, Katsoulis K. Misfit of implant prostheses and its impact on clinical outcomes. Definition, assessment and a systematic review of the literature. Eur J Oral Implantol. 2017;10(Suppl. 1):121‐138. [PubMed] [Google Scholar]
- 25. Canullo L, Schlee M, Wagner W, Covani U. On behalf of the Montegrotto Group for the Study of Peri‐implant disease. International brainstorming meeting on etiologic and risk factors of Peri‐implantitis, Montegrotto (Padua, Italy), august 2014. Int J Oral Maxillofac Implants. 2015;30:1093‐1104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix1. STROBE Statement—cross‐sectional studies.
Appendix2a. Frequency distribution of the total defect extent# in the various jaw regions.
Appendix2b. Implant characteristics in relation to the total defect extent#.
Appendix2c. Neighboring structure mesial and distal of the diseased implant in relation to the total defect extent#.
Appendix3a. Univariate random‐effects logistic regression analyses of the most prevalent peri‐implant bone defect configurations (ie, peri‐implant bone defect configurations with a frequency >10 implants).
Appendix3b. Multivariate random‐effects logistic regression analysis of the class Ic defects.
Appendix3c. Multivariate random‐effects logistic regression analysis of the class Id defects.
Appendix3d. Multivariate random‐effects logistic regression analysis of the class Id with only 1 wall defects.
Appendix3e. Multivariate random‐effects logistic regression analysis of the class Ie defects.
Appendix3f. Multivariate random‐effects logistic regression analysis of the class II defects.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


