Abstract
Previous studies investigating associations between white matter alterations and duration of temporal lobe epilepsy (TLE) have shown differing results, and were typically limited to univariate analyses of tracts in isolation. In this study, we apply a multivariate measure (the Mahalanobis distance), which captures the distinct ways white matter may differ in individual patients, and relate this to epilepsy duration. Diffusion MRI, from a cohort of 94 subjects (28 healthy controls, 33 left‐TLE and 33 right‐TLE), was used to assess the association between tract fractional anisotropy (FA) and epilepsy duration. Using ten white matter tracts, we analysed associations using the traditional univariate analysis (z‐scores) and a complementary multivariate approach (Mahalanobis distance), incorporating multiple white matter tracts into a single unified analysis. For patients with right‐TLE, FA was not significantly associated with epilepsy duration for any tract studied in isolation. For patients with left‐TLE, the FA of two limbic tracts (ipsilateral fornix, contralateral cingulum gyrus) were significantly negatively associated with epilepsy duration (Bonferonni corrected p < .05). Using a multivariate approach we found significant ipsilateral positive associations with duration in both left, and right‐TLE cohorts (left‐TLE: Spearman's ρ = 0.487, right‐TLE: Spearman's ρ = 0.422). Extrapolating our multivariate results to duration equals zero (i.e., at onset) we found no significant difference between patients and controls. Associations using the multivariate approach were more robust than univariate methods. The multivariate Mahalanobis distance measure provides non‐overlapping and more robust results than traditional univariate analyses. Future studies should consider adopting both frameworks into their analysis in order to ascertain a more complete understanding of epilepsy progression, regardless of laterality.
Keywords: diffusion weighted MRI, duration, epilepsy, Limbic system, temporal lobe epilepsy
Previous studies investigating associations between white matter alterations and duration of temporal lobe epilepsy (TLE) have shown differing results, and were typically limited to univariate analyses of tracts in isolation. In this study, we apply a multivariate measure to capture the distinct ways white matter may differ in individual patients, and relate this to epilepsy duration.
Abbreviations
- ATR
anterior thalamic radiation
- CG
cingulum gyrus
- CH
cingulum hippocampus
- F
fornix
- FA
fractional anisotropy
- MRI
magnetic resonance imaging
- ROI
region of interest
- TLE
temporal lobe epilepsy
- UF
uncinate fasciculus
1. INTRODUCTION
Epilepsy affects over 50 million people worldwide, with around 60% of patients presenting with focal seizures, most commonly being of temporal lobe origin (Téllez‐Zenteno & Hernández‐Ronquillo, 2012). Temporal lobe epilepsy (TLE) can have varying aetiologies, laterality and onset at different ages. This heterogeneity makes it challenging to study the onset and progression of TLE. Evidence that epilepsy may be associated with progressive cerebral damage has been reported in experimental and human longitudinal studies (Pitkänen & Sutula, 2002; Liu et al., 2003; Galovic et al., 2019). An improved understanding of the progressive nature of epilepsy would be beneficial as this may assist in measuring where a patient may be in their disease progression, and help identify early onset pre‐symptomatic biomarkers of epilepsy risk.
While longitudinal data have advantages, these are difficult to obtain. Cross‐sectional data can infer epilepsy progression at a group level by analysing associations between neuroimaging properties and duration of epilepsy. Indeed, several studies have investigated the relationship between grey matter properties and duration (Bernhardt et al., 2009; Bonilha et al., 2006; Keller et al., 2002; Seidenberg et al., 2005; Tasch et al., 1999; Whelan et al., 2018). In contrast, the relationship between subcortical limbic white matter and epilepsy duration is less well understood, with only a handful of studies reporting partially conflicting results (Table 1). Studies included in Table 1 investigated associations between white matter FA and duration in temporal lobe epilepsy patients and consistently analysed similar limbic white matter structures. Studies omitted from Table 1 are those which focused on alternative diffusion measures, white matter tracts, or whose cohorts contained adolescents or patients with other epilepsy syndromes (Andrade et al., 2014; Arfanakis et al., 2002; Ashraf‐Ganjouei et al., 2019; Govindan et al., 2008; Gross et al., 2006; Park et al., 2018; Slinger et al., 2016).
TABLE 1.
Summary of the literature exploring associations between white matter alterations and epilepsy duration: Studies included investigated associations between white matter FA and duration in temporal lobe epilepsy patients and consistently analysed similar limbic white matter structures
| Study | Subjects | Reconstruction method | Structures analysed | Type of analysis | Results |
|---|---|---|---|---|---|
| (Thivard et al., 2005) | 36 HC | ROI (manual) | Hipp | Regression analysis | No significant correlations |
| 35 TLE‐HS | |||||
| (Lin et al., 2008) | 10 HC | Tractography (manual) | Bilateral: UF, AF | Spearman correlations | No significant correlations |
| 12 TLE | |||||
| (Concha et al., 2009) | 25 HC | Tractography (atlas) & ROI (manual) | Combined: F, C, +4 other structures | Pearson correlation | TLE‐NL: F (not after controlling for age) |
| 17 TLE‐HS | |||||
| 13 TLE‐NL | |||||
| (Kemmotsu et al., 2011) | 36 HC | ROI (atlas) | Bilateral: F, CH, UF, +3 other structures | Pearson correlation | left‐TLE: CH.L, UF.L right‐TLE: no significant correlations |
| 36 TLE | |||||
| (Keller et al., 2012) | 68 HC | ROI (atlas) | Bilateral: CH, +14 other structures | Regression analysis ipsilateral/contralateral analysis |
Ipsilateral CH, +7 other significant correlations |
| 62TLE‐HS | |||||
| (Liu et al., 2012) | 21 HC | Tractography (atlas & manual) | Combined: CH, F, UF, +10 other structures | Pearson correlation |
TLE‐HS: no significant correlations TLE‐NL: dC, bCC |
| 23 TLE‐HS | |||||
| 15 TLE‐NL | |||||
| (Chiang et al., 2016) | 28 HC | ROI (atlas) |
Bilateral: Hipp, UF, C, EC. Combined Fornix |
Spearman correlation | left‐TLE: no significant correlations right‐TLE: Hipp.R, EC.R |
| 28 TLE | |||||
| TLE | |||||
| (Kreilkamp et al., 2017) | 44 HC | Tractography (atlas) |
CH, UF, SLF, ILF |
Pearson correlation | Contralateral UF |
| 68 TLE | |||||
| (Tsuda et al., 2018) | 17 HC | TBSS | Whole brain | Regression analysis | C, F, UF, +10 other significant correlations |
| 15 TLE | |||||
|
(Hatton et al., 2020) |
1,069 HC | ROI (atlas) | Bilateral CG, CH, F.ST, UF, +29 other structures, average FA | Pearson correlation |
left‐TLE‐HS: CG.L, CG.R, F.ST.L, F.ST.R, UF.L, +12 other significant correlations left‐TLE‐NL: CG.L, CG.R, +4 other significant correlations right‐TLE‐HS: CG.L, CG.R, CH.L, CH.R, F.ST.R, UF.R, +16 other significant correlations right‐TLE‐NL: CG.R, UF.R, +5 other significant correlations |
| 599 TLE‐HS | |||||
| 275 TLE‐NL | |||||
| (Kreilkamp et al., 2019) | 40 HC | Tractography (manual) & Automated fiber quantification | Bilateral: UF and CH | Spearman correlation | No significant correlations |
| 24 TLE |
Healthy control subjects are denoted by (HC), patients with hippocampal sclerosis by (HS), and non lesional patients by (NL). (.L) and (.R) denote the left and right hemisphere, respectively.
Bold indicates significance after multiple comparisons correction (where available).
Abbreviations: AF, Arcuate fasciculus; bCC, body of Corpus Callosum, C, Cingulum; CG, Cingulum Gyrus; CH, Cingulum Hippocampus; dC, dorsal Cingulum; EC, External capsule; F, Fornix; F.ST, Fornix/Stria Terminalis; Hipp, Hippocampus; ILF, Inferior longitudinal fasciculus; SLF, Superior longitudinal fasciculus; UF, Uncinate fasciculus.
In a multi‐modal analysis investigating the inter‐relationships between measures of grey matter volume, and white matter FA, Keller et al. (2012) analysed associations with epilepsy duration in a cohort of patients with TLE and hippocampal sclerosis. Widespread associations between duration and fractional anisotropy (FA) beyond the effects of natural aging were reported. Significant correlations were found in eight white matter structures located both ipsilateral and contralateral to the epileptogenic zone, and in remote tracts beyond the temporal lobe. Investigating differences based on patient laterality, Chiang et al. (2016) correlated FA reductions in each tract with epilepsy duration. For patients with left‐TLE there were no significant correlations with duration. However, for patients with right‐TLE, significant correlations were identified in the ipsilateral hippocampus and ipsilateral external capsule prior to multiple comparison corrections.
Additional studies investigating the associations between white matter properties and epilepsy duration are listed in Table 1, with varying results. In the uncinate fasciculus, for example, there was evidence of significant correlations between the FA reduction and epilepsy duration in some, (Hatton et al., 2020; Kemmotsu et al., 2011; Kreilkamp et al., 2017; Tsuda et al., 2018) and no significant correlations in others (Chiang et al., 2016; Kreilkamp et al., 2019; Lin et al., 2008). Each study differs in the selection of tracts analysed and the method used for reconstruction. However, all studies use a univariate framework for analysis, correlating epilepsy duration with each individual tract independently.
Univariate analyses have the advantage of being clear, interpretable and simple to implement. There are, however, limitations. First, univariate analyses are susceptible to outliers within a dataset which increase the probability of inconsistent results between different studies. Second, multiple comparison corrections are required when analysing multiple white matter tracts in isolation, to mitigate the chance of a false positive (Type 1 error). However, this correction has the effect of inflating the false negative rate (Type 2 error) leading to the increased probability of overlooking genuine relationships. Third, univariate analyses do not account for the natural covariance between tracts in individuals (Cox et al., 2016; Wahl et al., 2010; Westlye et al., 2010), nor spatial colocalisation of tract segments. Accounting for this covariation is important because if multiple tracts are affected by the same process then a univariate approach does not correct for this in the statistical analysis, and can lead to erroneous conclusions (Wang et al., 2020). Furthermore, if different tracts are affected in different patients then the overall effect for each individual tract will be less than if using a multivariate approach which accounts for this (Taylor et al., 2020).
In this study, we use a multivariate measure—the Mahalanobis distance—complementing the univariate approach, by analysing the associations between white matter FA and duration of epilepsy using numerous white matter tracts simultaneously. We hypothesised that patients with a longer epilepsy duration would be associated with greater abnormalities ipsilateral to the epileptic focus. This approach has been fruitful in studies of autism and traumatic brain injury (Dean et al., 2017; Taylor et al., 2020). Applications of the Mahalanobis distance include analysing individual tracts by integrating multiple diffusion metrics into a single measure, or by pooling numerous metrics from a number of different modalities. In our study we analyse a cohort of subjects using a single diffusion metric (FA), combining multiple white matter tracts to create patient specific measures of hemispheric distance from healthy control subjects.
2. METHODS
2.1. Patients
Our cohort consists of 28 healthy controls, and 66 individuals with unilateral TLE (33 left and 33 right). The individuals with TLE were recruited from the National Hospital for Neurology and Neurosurgery epilepsy surgery programme, with diagnoses made by consultant neurologists specialising in epilepsy on the basis of clinical history, seizure semiology, and prolonged video‐EEG telemetry with ictal and interictal EEG, high resolution MRI, neuropsychological and neuropsychiatric assessments. Where applicable, tests were performed to identify group differences in categorical variables: sex, surgery outcome and evidence of hippocampal sclerosis. Two‐tailed t‐tests were conducted to check for group differences in age, age at epilepsy onset, and epilepsy duration after correspondence to normality was identified using Lilliefors tests. Epilepsy duration was estimated by subtracting the seizure onset age from the age at diffusion imaging scan. Cohort demographics and results of the statistical tests are summarised in Table 2. Written consent was acquired and the data were anonymized and exported, then analyzed under the approval of the Newcastle University Ethics Committee (2225/2017). Figure 1 summarises our processing of MRI data.
TABLE 2.
Subject demographics and clinical factors by laterality classification. Mean and standard deviations are reported: Mean(SD). Two‐tailed t‐tests were used to compare continuous variables, and two‐tailed Chi‐squared tests were used for factored variables
| Controls (1) | Left‐TLE (2) | Right‐TLE (3) | Significance | ||
|---|---|---|---|---|---|
| N | 28 | 33 | 33 | N/A | |
| Sex | |||||
| Female/Male | 16/12 | 17/16 | 24/9 |
|
|
|
| |||||
|
| |||||
| Age (years) | 38.1 (12.35) | 38.5 (10.57) | 38.3 (12.37) |
|
|
|
| |||||
|
| |||||
| Age of onset (years) | NA | 13.9 (10.85) | 15.6 (10.93) |
|
|
| Epilepsy duration (years) | NA | 25.6 (15.20) | 24.2 (13.43) |
|
|
|
Surgery outcome (ILAE 1 vs. ILAE 2+) |
NA | 18/15 | 15/18 |
|
|
|
Hippocampal sclerosis (Yes/No) |
NA | 28/5 | 20/13 |
|
|
FIGURE 1.
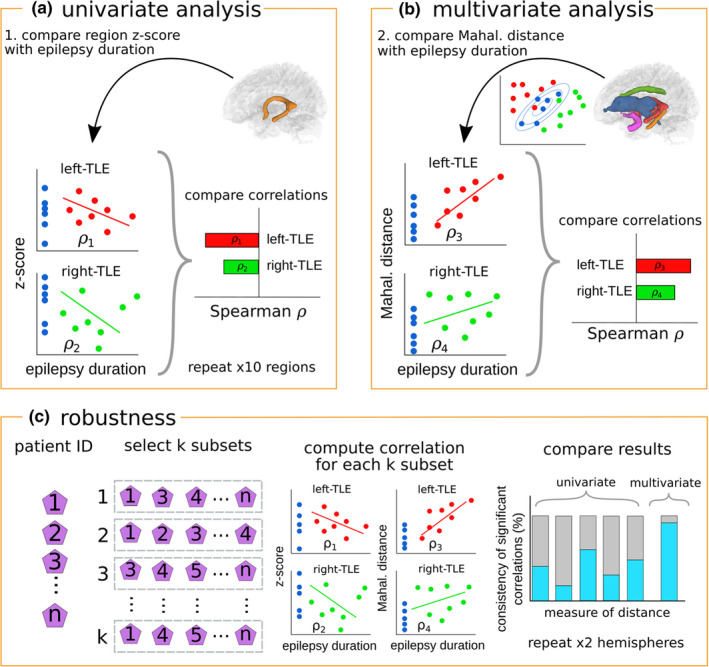
llustration of analysis pipeline. Analyses of associations with epilepsy duration through Spearman correlations. First, (a) using z‐scores derived from individual tracts, and second, (b) using Mahalanobis distances derived from all ipsilateral tracts and all contralateral tracts separately. Spearman correlations are corrected for multiple comparisons using Bonferroni corrections and assessed for significance. Finally, robustness of the results are ascertained (c). Subsamples of the patient data are selected N times and used to calculate N correlations. Proportion of samples achieving significance are reported as a measure of consistency. Note that the univariate approach results in five consistency values per hemisphere (one per tract), whereas only one consistency values is produced per hemisphere for the multivariate approach
2.2. Diffusion MRI acquisition
All subjects underwent diffusion weighted MRI acquisition on the same scanner, 3T GE Signa Excite HDx, as described previously (Sinha et al., 2020; Taylor et al., 2018; Winston et al., 2013). Diffusion MRI data were acquired using a cardiac‐triggered single‐shot spin‐echo planar imaging sequence (Wheeler‐Kingshott et al., 2002) with echo time =73 ms. Sets of 60 contiguous 2.4 mm‐thick axial slices were obtained covering the whole brain, with diffusion sensitising gradients applied in each of 52 noncollinear directions (b value of 1,200 mm2 s − 1 [δ = 21 ms, Δ = 29 ms, using full gradient strength of 40 mT m − 1]) along with 6 non‐diffusion weighted scans. The gradient directions were calculated and ordered as described elsewhere (Cook et al., 2007). The field of view was 24 cm, and the acquisition matrix size was 96 × 96, zero filled to 128 × 128 during reconstruction, giving a reconstructed voxel size of 1.875 × 1.875 × 2.4 mm. The DTI acquisition time for a total of 3,480 image slices was approximately 25 min (depending on subject heart rate).
2.3. Image preprocessing
Diffusion images were initially corrected for signal drift (Vos et al., 2017), followed by eddy correction using the FSL tool “eddy_correct” (Andersson & Sotiropoulos, 2016), and rotation of the b vectors using the tool “fdt_rotate_bvecs” (Jenkinson et al., 2012; Leemans & Jones, 2009). Reconstruction and registration were performed with DSI‐Studio (http://dsi‐studio.labsolver.org) using a Q‐space diffeomorphic reconstruction (QSDR) (Yeh & Tseng, 2011) with an unweighted diffusion sampling length ratio of 1.25. Diffusion maps were registered to standard space using the HCP1021 template and white matter volumetric regions of interest (ROI) were derived using atlases. The bilateral anterior thalamic radiation (ATR), cingulum in the cingulate cortex area (cingulum gyrus: CG), cingulum in the hippocampal area (cingulum hippocampus: CH), and uncinate fasciculus (UF) were defined using the JHU atlas (Hua et al., 2008). The structure of the bilateral fornix (F) was defined using the HCP842_tractography atlas native to DSI‐Studio. See Figure 2 for visual representation of tracts analysed. These structures were chosen as per their description by Catani et al. (2013). Mean FA values for each white matter region were extracted from the ten white matter structures and analysed using R (https://www.r‐project.org/).
FIGURE 2.

All white matter tracts reconstructed in DSI‐Studio. Colours correspond to each pair of homologous tracts. The anterior thalamic radiation (ATR; blue), cingulum in the cingulate cortex area (CG; green), cingulum in the hippocampal area (CH; orange), and uncinate fasciculus (UF; pink) were reconstructed using the JHU white matter atlas. The fornix (F; red) was reconstructed using the HCP842_tractography atlas native to DSI‐Studio
2.4. Statistical correction of covariates
The effects of sex and healthy aging in each tract were removed using a robust linear model (rlm function from MASS package in R (Venables & Ripley, 2002)). In place of fitting the model using an ordinary least squares (OLS) estimator, the rlm function uses an M‐estimator with Huber weightings. Standard model fitting using OLS estimators are susceptible to outliers as all data points are assigned a weight of 1. The M‐estimator using Huber weightings overcomes this limitation by assigning weights of 1 to residuals of small magnitude and progressively smaller weights to residuals of increasing magnitude. Iterated reweighted least squares is used to solve the estimator as calculation of the weights requires the residuals and calculation of the residuals requires the weights (Fox, 2016). The remaining residuals () are used throughout the paper to calculate the univariate and multivariate distances.
2.5. Univariate analysis
We quantified the univariate distance of each subject from the control distribution. Robust z‐score values were calculated to mitigate the effects of outliers in the control data. First, we selected a subject and a random subset of 24 controls from the total control population (n = 28). If the selected subject was a healthy control, we removed them from the population prior to random subset selection, to mitigate the bias of that control subject when calculating their distance. A subset of size 24 was selected as this is the maximum size in which at least 1,000 unique sub‐samples can be inferred from the population of (n‐1) controls. A z‐score distance is calculated for the subject by subtracting the mean of the sample control distribution from the subject FA residual and scaling by the sample distribution standard deviation. For each subject, the process is repeated 1,000 times to create a distribution of z‐score distances. Finally, median z‐scores for each subject are reported, providing a robust, single valued measure of univariate distance from the control distribution.
2.6. Multivariate analysis
Following the univariate analysis, we calculated subject specific distances from the control distribution, using multiple white matter tracts simultaneously. To achieve this, we used the Mahalanobis distance. An extension to the multivariate z‐score distances (Euclidean distance), the Mahalanobis distance is a measure of distance from a reference distribution in multiple dimensions whilst accounting for the covariance structure. Penalising data points that fail to adhere to the natural structure, two subjects with similar z‐scores in each individual dimension can have vastly different Mahalanobis distances (Figure S1, and (Taylor et al., 2020, Figure 1).
We derived the Mahalanobis distance from the population of healthy controls. Distances are calculated using Equation 1, where x represents a vector of subject FA residual values, µ the average tract FA residual values calculated from the healthy controls, and C a matrix representing the natural covariance structure exhibited in the healthy control population.
| (1) |
The Mahalanobis distance assumes normality in the reference distribution. Therefore, univariate assessments of normality in the control subject distribution were conducted using the Lilliefors test (R package; nortest [Gross & Ligges, 2015]) and multivariate assessments were conducted using a Mardia test (R package; MVN [Korkmaz et al., 2014]). No significant p‐values were found after Bonferroni correction, suggesting a good correspondence to normality.
Robust measures of multivariate distances for each subject were calculated by taking 1,000 random subsamples of size (24) from the control data (28), calculating a Mahalanobis distance for each sample and reporting the median value. Non‐linear shrinkage estimators of the covariance matrix were used in place of the sample covariance matrix to minimise the estimation error of the inverted matrix (Ledoit and Wolf, 2018). This technique was applied previously by (Taylor et al., 2020).
Two Mahalanobis distances are calculated per subject. One measure unifies all left hemisphere tracts into a single value and the other unifies all right hemisphere tracts. We interpret these multivariate distances as the overall abnormality associated with TLE in each hemisphere. For both patient groups, we hypothesised a positive association with duration in the ipsilateral hemisphere, that is, larger distances relate to longer duration.
2.7. Associations with duration of epilepsy
We investigated the association of the computed uni‐ and multi‐variate measures with epilepsy duration. Analysing patients with left and right TLE separately, we used Spearman correlations to quantify the association observed between the univariate and multivariate distances and epilepsy duration. A non‐parametric alternative to the Pearson correlation, the Spearman correlation is a measure of the monotonic relationship between two variables which is more robust to outliers in the dataset. Hypothesising a more negative z‐score and more positive Mahalanobis distance with a greater epilepsy duration, significant correlations were assessed using a one‐tailed test. Per patient group, ten univariate correlations were computed (one per tract) and two multivariate correlations (ipsilateral and contralateral). Reporting significance at the threshold a Bonferroni correction was applied to account for h multiple comparisons (univariate h = 10, multivariate h = 2). Significant correlation thresholds for samples of size n were approximated using a Student's t distribution with (n‐2) degrees of freedom and test statistic (t) shown in Equation 2.
| (2) |
Assessments into the effects of laterality on the correlational analysis were conducted by combining the Mahalanobis distances of all patients into a single ipsilateral and contralateral measure of distance and correlating these with epilepsy duration.
We also investigated if the ipsilateral and contralateral Mahalanobis distances calculated in patients exhibit white matter deviations from the healthy population at onset (i.e., where duration equals zero years). Using robust linear regression models of all patients' ipsilateral and contralateral Mahalanobis distances, estimates of the Mahalanobis distance at duration zero were calculated by regressing out the effects of duration and considering the intercept ( from Equation 3). Estimation of regression coefficients were obtained using the patient Mahalanobis distances only. This was done in order to assess the effects of duration within the patient groups only to see if they deviate from controls at onset. Robust z‐scores were computed using the estimated distances at duration zero as points of interest and the 56 Mahalanobis distances for control subjects as the reference distribution (56; 28 left hemisphere, 28 right hemisphere). Similar to the calculation of univariate and multivariate distances, 1,000 random subsamples of size (54) taken from the control distribution (56) were used to calculate z‐scores with the median value reported. Samples of size 54 were chosen as it is the maximum size in which at least 1,000 unique sub‐samples can be inferred from the population of 56 control distances. Given that the Mahalanobis distances, by definition, are positively skewed, the control distribution and points of interest were log transformed to ensure normality prior to calculating each z‐score.
| (3) |
2.8. Relationship with surgical outcome
Finally, we investigated associations between the z‐scores and log(Mahalanobis distances) calculated and surgical outcomes. Hypothesising that larger distances would relate to poorer outcomes, we used one‐tailed, two sample t‐tests to see if the z‐scores, and ipsilateral and contralateral Mahalanobis distances adequately separated patients who were, and were not, completely seizure free following surgery.
2.9. Robustness
To assess the robustness of the univariate and multivariate correlational analyses to outliers in the data and potentially explain some of the variability seen in the literature we used Jackknife resampling. A random subsample of 30 left‐TLE and 30 right‐TLE patients was taken and the association of each of the ten tract FA values and the ipsilateral/contralateral Mahalanobis distances with duration were calculated. Samples of size 30 were chosen as it is the maximum size in which at least 1,000 unique sub‐samples can be inferred from the population of 33 patients. Repeating 1,000 times and reporting the proportion of samples yielding significant correlations (consistency ) provides a measure of the robustness the data from each tract has to outliers in the dataset. An ideal measure would either always show a significant result ( or never show a significant result (). Where deviates far from the extremities we interpret this as being inconsistent and therefore has the potential to lead to different results depending on the sample chosen or specific methodology. This thus leads to varied reporting in the literature of (non)significant results. In order to assess the stability of our robustness analysis to cohorts of various sizes we also repeated the analysis for subsample sizes ranging from 20 to 30 patients per group.
3. RESULTS
3.1. Univariate associations between FA and duration of epilepsy
Figure 3a highlights the association between epilepsy duration and z‐scores for the bilateral uncinate fasciculus in left and right‐TLE patients using Spearman correlation. In left‐TLE, lower FA, bilaterally, was associated with longer duration of epilepsy in all 10 white matter tracts (Figure 3a; upper, Figure 3b). In two tracts, this was statistically significant after multiple comparisons correction (ipsilateral fornix ⍴=−0.493, p = .002, and contralateral cingulum gyrus ⍴=−0.460, p = .004). In right‐TLE, there was no significant association between FA and duration in any tract (Figure 3a; lower panels). All ⍴ and p values are shown in Table S1.
FIGURE 3.
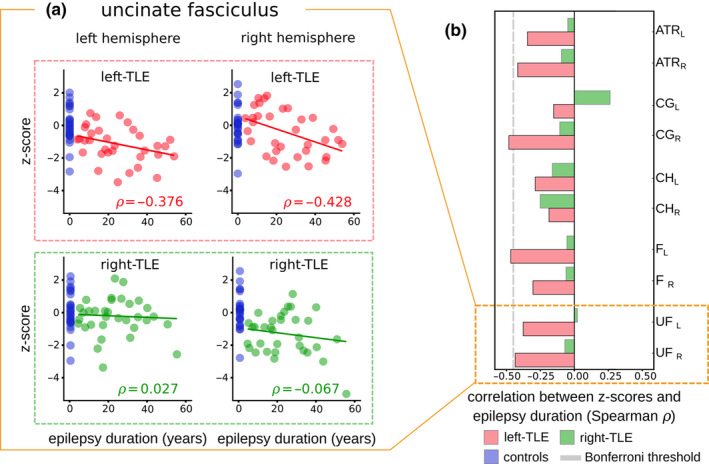
Univariate associations with epilepsy duration for all ten white matter tracts. (a) Scatter points showing individual subjects and their corresponding z‐scores and epilepsy duration values in the bilateral uncinate fasciculus. Left‐TLE patients (upper panels) are analysed independently to the right‐TLE patients (lower panels). Blue datapoints represent individual control subjects. Inset (b) summarises associations with epilepsy duration in all ten white matter tracts for both patient groups. Grey dashed line represents the significance threshold after applying the Bonferroni multiple comparisons correction (⍴ = −0.44). No significant association with duration is present for any tract in right TLE patients. ATR: Anterior thalamic radiation, CG: Cingulum gyrus, CH: Cingulum hippocampus, F: Fornix, UF: Uncinate Fasciculus. L and R correspond to the left and right hemisphere respectively
3.2.
Evaluation of associations between univariate z‐score distances and surgery outcome revealed significant results for right‐TLE patients only. After correction for multiple comparisons the contralateral uncinate fasciculus remained significant, showing that FA values which deviate the least from healthy controls relate to a better seizure free outcome (T = 2.785, p = .005).
3.3. Multivariate associations between Mahalanobis distance and duration of epilepsy
In left‐TLE patients, a significant association was present between the Mahalanobis distances and duration of epilepsy in the ipsilateral hemisphere only (⍴=0.493, p = .002), with increased distance as duration progresses (Figure 4; upper panels). In contrast to the univariate approach, right‐TLE patients showed significant association with duration ipsilaterally (⍴=0.412, p = .009) (Figure 4; lower panels). All ⍴ and p values are shown in Table S3.
FIGURE 4.

Multivariate associations with epilepsy duration for all ipsilateral and contralateral tract ROI. Scatter points show the associations between the ipsilateral and contralateral Mahalanobis distances and epilepsy duration. Left‐TLE patients (upper panels) and right‐TLE patients (lower panels) are analysed separately. Stronger correlations are observed in the ipsilateral hemisphere regardless of laterality. Mahal. Dist: Mahalanobis distance
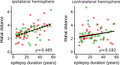
Combining all patients into a single unified analysis (Figure 5; upper panels) reveals a strong significant correlation between Mahalanobis distance and duration of epilepsy in the ipsilateral hemisphere (⍴ = 0.482, p = 1e‐05) and a weaker non‐significant correlation in the contralateral hemisphere (⍴ = 0.195, p = .058). Intercepts of the ipsilateral and contralateral progression.
FIGURE 5.
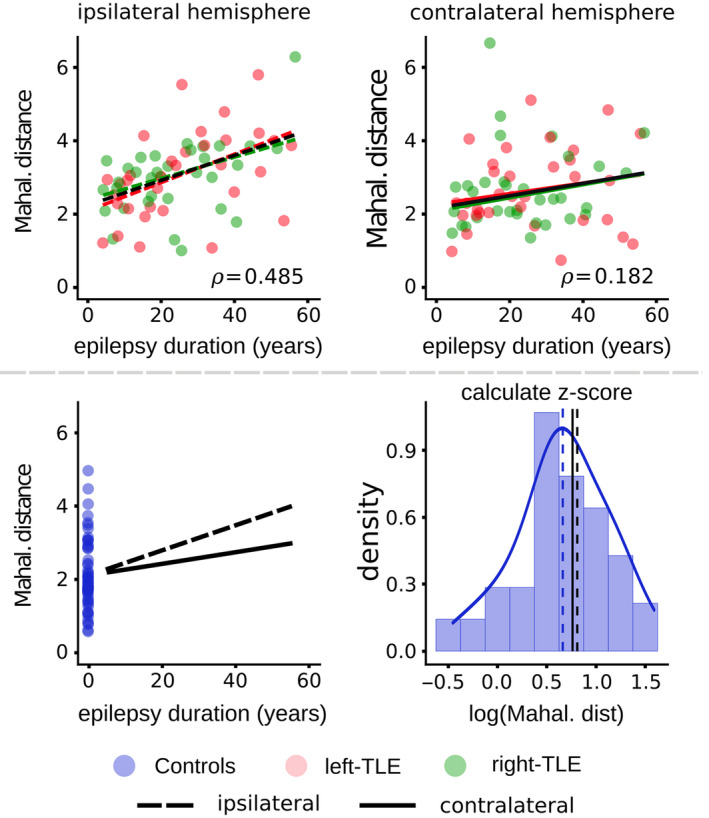
Ipsilateral and contralateral Mahalanobis distances correlated with epilepsy duration for all patients combined. Scatter points show the associations between the ipsilateral and contralateral Mahalanobis distances and epilepsy duration for all patients combined (upper panels). Spearman correlations are reported, with a stronger correlation shown ipsilaterally. (Lower panels) Ipsilateral and contralateral robust regression lines are plotted with the control distribution Mahalanobis distances shown in blue. Control subjects are treated as patients with epilepsy duration zero and act as a reference distribution of a healthy Mahalanobis distance as onset. Regressing out the effects of epilepsy duration in patients only, intercepts of the ipsilateral and contralateral regression lines are compared to the control distribution. Taking logs, z‐scores of the healthy population are calculated and used to assess if patients deviate from the healthy population at duration zero (i.e. onset). Blue dotted line represents the mean of the control distribution. The patient intercept line at duration=0 is not significantly different to the control mean. Mahal. Dist: Mahalanobis distance
( and respectively) appeared to originate from the control distribution mean () (Figure 5; lower panels). Analysis of the intercept revealed that estimates of the ipsilateral (z = 0.239, p = .811) and contralateral (z = 0.169, p = .866) Mahalanobis distances of patients at epilepsy onset (i.e., at duration equal zero; the intercept of the regression) were not significantly different from healthy controls. Analysis of patients with (TLE‐HS) and without (TLE‐NHS) hippocampal sclerosis independently revealed significant correlations between epilepsy duration and the ipsilateral Mahalanobis distance in both groups (Figure S2). No significant correlations were present between epilepsy duration and the contralateral Mahalanobis distance.
In left‐TLE patients, no significant associations with surgery outcome were present using the ipsilateral or contralateral Mahalanobis distances. However, for right‐TLE patients, the contralateral Mahalanobis distance was significantly associated with surgery outcome, surviving Bonferroni correction (T = −2.810, p = .004), with larger distances in those who did not become seizure free. No significant associations with surgery outcome were found using the ipsilateral Mahalanobis distance (Table S4).
3.4. Robustness
In patients with left‐TLE (Figure 6; upper panels), a univariate analysis of the ipsilateral fornix gives a significant association with duration 70% of the time, depending on the subsample of patients chosen (κ = 70%). Other white matter tracts are also varied such as the contralateral cingulum gyrus (κ = 50%), and contralateral uncinate fasciculus (κ = 21%). All other white matter tracts used in the univariate analyses show good consistencies with values of near 0%. The Mahalanobis approach yields very consistent results, showing a significant association with duration regardless of the subsample ipsilaterally, and never showing a significant association contralaterally (κ = 100% and κ = 0% respectively). Univariate analyses of right‐TLE patients show strong robustness to outliers with all white matter tracts showing consistencies of (κ = 0%) indicating that significant correlations are never reported. For the analysis of multivariate robustness in right‐TLE patients we see a strong robustness to outliers in the contralateral Mahalanobis distance (κ = 0%) and a relatively strong level of robustness for the ipsilateral Mahalanobis distance analysis (κ = 82%).
FIGURE 6.
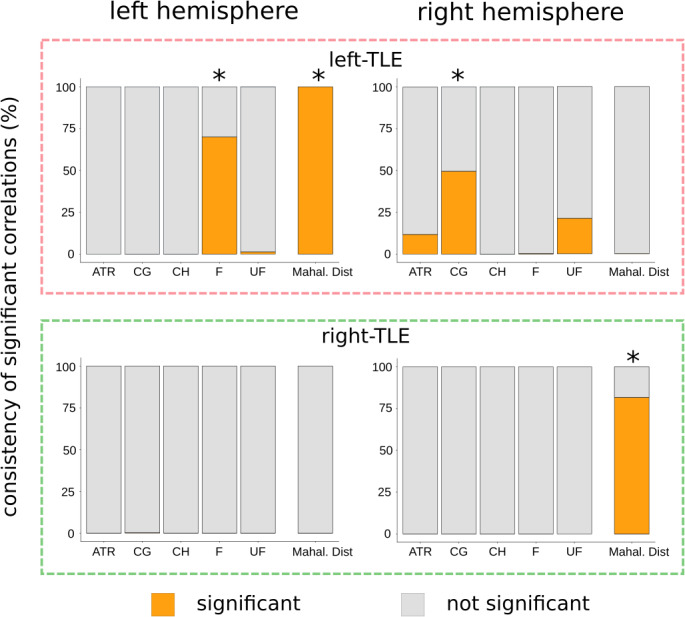
tacked bar charts depicting the robustness of the z‐score and Mahalanobis distance correlations with epilepsy duration. Each bar represents the robustness of the associations between epilepsy duration and the univariate, and Mahalanobis distances. Orange bars represent the proportion of subsamples yielding significant correlations, which we term the consistency. Grey bars represent the proportion of subsamples that do not yield significant correlations. Robust measures to outliers should have consistency values close to the range extremities (0% and 100%). Robustness of associations in left‐TLE patients (upper panels) have been computed independently to the associations for right‐TLE patients (lower panels). ATR: Anterior thalamic radiation, CG: Cingulum gyrus, CH: Cingulum hippocampus, F: Fornix, UF: Uncinate Fasciculus, Mahal. Dist: Mahalanobis distance. (*) represents measures of distance that showed significant associations with duration in Figures 3 and 4 after multiple comparisons correction
Stability of the robustness analysis over a range of other subsample sizes is reported in Figure S3 and are consistent with those in Figure 6. As expected, better performance (i.e., ability to consistently detect a significant effect) was found with larger subsample sizes. Associations with low consistency are seen over the whole subsample range. Those with high consistency values at the maximal subsample size decline rapidly as the subsample size decreases.
4. DISCUSSION
We report a multivariate analysis of FA and assessed the robustness of traditional and novel approaches to outliers. Our key findings were first, significant correlations between ipsilateral Mahalanobis distances and duration were present regardless of patient laterality, contrary to univariate findings. Second, by extrapolating our data to duration equal to zero (i.e., disease onset) we found no significant difference to controls. Third, our robustness analysis revealed that a number of univariate associations were susceptible to outliers in the dataset, whereas results obtained using multivariate Mahalanobis distances were more stable.
Previous studies have typically conducted univariate analyses, correlating the associations between white matter FA and epilepsy duration, with varied findings reported in the literature. Results of our univariate analysis add to the existing inconsistencies present in the previous literature. We showed that univariate z‐scores were significantly correlated with epilepsy duration in two white matter regions after Bonferroni correction (p < .05, h = 10), with an additional seven regions showing associations prior to correction. Significant associations were present in (1) ipsilateral fornix and (2) contralateral cingulum gyrus of left‐TLE patients. Similar associations were shown previously, with a whole brain TBSS analysis by (Tsuda et al., 2018) also showing significant associations between epilepsy duration and FA of the fornix in adult TLE patients after multiple comparisons correction. However contrary to our findings, analyses by (Concha et al., 2009; Kemmotsu et al., 2011; Liu et al., 2012; Chiang et al., 2016; Hatton et al., 2020) found no significant associations between epilepsy duration and FA of the fornix.
Keller et al. (2012) identified a significant relationship between duration and FA in the ipsilateral cingulum gyrus. No significant association with duration of epilepsy was found using the contralateral structure. A white matter ROI study by (Hatton et al., 2020) investigated associations in left and right‐TLE patients taking account of presence, or not, of hippocampal sclerosis (HS). Significant associations after multiple comparisons correction were only observed in left‐TLE patients with evidence of HS. In contrast to our univariate analyses of left TLE patients, we found no significant association with duration in right TLE for any tract.
Our multivariate assessment of associations between hemispheric Mahalanobis distances and epilepsy duration showed stronger associations in the ipsilateral hemisphere, regardless of laterality. The strength of the association between duration of epilepsy and the ipsilateral Mahalanobis distance surpassed all univariate z‐score associations. The lack of any significant univariate associations with duration in the ipsilateral hemisphere suggests that additional information is gained in a multivariate analysis, information not captured using traditional methods.
Previous studies have shown that the Mahalanobis distance provides information over and above a univariate analysis. In a study of patients with traumatic brain injury, (Taylor et al., 2020) demonstrated that the Mahalanobis distance derived from FA values of 22 white matter tracts better distinguished patients from controls (AUC = 0.82) than any individual univariate tract z‐score (AUC = 0.72), and was associated with a level of cognitive impairment. A study of patients with autism demonstrated that the Mahalanobis distance could better distinguish patients from controls (Dean et al., 2017). Interestingly, that study found that combining the FA, MD, RD and AD of 19 white matter ROIs into a single Mahalanobis distance yielded the best patient control separation, with zero overlap between the two groups. A similar approach could be used in future work applied to TLE. Together, these findings suggest that the Mahalanobis distance provides information complementary to univariate approaches in terms of assessing white matter damage.
We extrapolated our data to estimate the mean Mahalanobis distance at epilepsy duration zero, which we interpret as epilepsy onset, and suggest that white matter abnormality may not precede the onset of TLE, but progresses with the course of the condition. A longitudinal study by Liu et al. (2005) found that a cohort of patients with newly diagnosed and chronic TLE showed a reduction in hippocampal volume at baseline scan relative to healthy controls, with small reductions as time progressed. The rate of decline was comparable to those of healthy controls and thus that study concluded that initial reduction was likely attributed to a precipitating insult, with further declines attributed to healthy aging. Conversely, a study focussing on new onset seizures in children (Widjaja et al., 2012) reported no significant differences in the hippocampal volume of patients compared to controls. Conflicting results may be attributed to a number of factors, including patient selection, age, and the presence of lesions.
Diffusion MRI is sensitive to alterations in the microstructural architecture (Alexander et al., 2007; Soares et al., 2013) and therefore has the potential to reveal early deviations from healthy controls that are not captured by T1 weighted MRI. In our cross‐sectional analysis of multivariate white matter alterations, we find that patients with TLE do not deviate from controls at duration zero both ipsilateral and contralateral to the epileptogenic focus (Figure 5). The lack of difference from controls suggests that gross alterations to limbic system white matter may not be present prior to the start of the epilepsy, however, longitudinal studies of new onset patients are needed to confirm.
Assessment of framework robustness showed that association with duration obtained using univariate z‐scores were more susceptible to generate variable results than associations calculated using multivariate Mahalanobis distances. Three univariate associations between epilepsy duration and z‐scores show poor consistency when subsampling the dataset. All associations pertain to the left‐TLE patients, namely the ipsilateral fornix, contralateral cingulum gyrus, and contralateral uncinate fasciculus. Situated near the significant correlation threshold after multiple comparisons correction, it is unsurprising that these three tracts show poor consistency as small deviations from the monotonic relationship would easily alter the state of significance. As we have used a Bonferroni correction (which is dependent on the number of comparisons) it should also be noted that different consistency values would be observed if the number of tracts studied varied. Based on this and the differences in inter‐study sample sizes, it is likely that the Bonferroni correction accounts for some of the inconsistencies which exist in the literature. Consistency values associated with the correlations between epilepsy duration and Mahalanobis distances show a strong robustness to outliers. Bonferroni correction of the Mahalanobis distance analysis based on number of tracts is also not required and is a distinct advantage of the multivariate approach.
All patients studied here later underwent anterior temporal lobe surgery. This thus offered the opportunity to investigate the relationship to post‐surgical seizure‐freedom. We found significant differences between outcome groups for right TLE patients in a univariate approach (for the contralateral uncinate fasciculus) and the multivariate approach (contralateral Mahalanobis distance)—Tables S2,S4. Our finding that patients with poorer surgical outcomes were significantly further from controls than patients with seizure‐free outcomes suggests a predisposing factor to surgical treatment success. This agrees with a large number of recent studies suggesting that pre‐operative diffusion metrics may be predictive of post‐surgical outcomes (Bonilha et al., 2006; Bonilha et al., 2006; Munsell et al. 2015; Keller et al. 2017; Sinha et al. 2019; Taylor et al., 2018).
Our univariate analysis of associations between epilepsy duration and z‐score distances in ten white matter structures revealed notable differences based on patient laterality. We found stronger and more widespread correlations in left‐TLE, with two significant correlations surviving multiple comparisons correction. No significant correlations were seen in the right‐TLE patient group (Table S1). Consistent with our findings, Kemmotsu et al. (2011) found significant correlations between duration and alterations in white matter FA for patients with left‐TLE only. They reported significant Pearson correlations in the ipsilateral cingulum hippocampus (r = −0.775) and ipsilateral uncinate fasciculus (r = −0.682). In contrast, Chiang et al. (2016) found no significant correlations between white matter FA alterations and epilepsy duration in left‐TLE patients. These observed differences based on laterality could be attributed to multiple factors including the reconstruction method, the multiple comparisons correction used and the sample sizes of those studies.
Associations between epilepsy duration and the multivariate Mahalanobis distances revealed similarities regardless of laterality (Figure 4). Stronger correlations were observed using ipsilateral hemisphere white matter structures compared to the contralateral structures. Given that the Mahalanobis distance is a measure of the overall hemispheric FA alteration, the results are convincing given that univariate analyses have previously shown stronger white matter alterations in TLE patients ipsilateral to the epileptogenic zone with fewer abnormalities contralaterally (Ahmadi et al., 2009; Besson et al., 2014; Otte et al., 2012).
Our cross‐sectional analysis of epilepsy progression has limitations. First, In order to compare patients, we removed the effects of healthy ageing using regression. This procedure assumes FA alterations in all subjects follow a similar natural linear trajectory. It is likely that FA alterations in some patients are underestimated whereas others are overestimated. These residuals may have an effect of altering the magnitude of associations between FA and duration. Second, the use of cross‐sectional data only provides associations with epilepsy onset and progression, rather than giving direct causal evidence. Third, although FA is the most widely used diffusion MRI metric in the literature, it nonspecific in its measurement which can be influenced by various different factors including axonal density and myelination (Concha et al., 2010). Additionally, a limitation of the multivariate approach in this study is the loss of spatial specificity. By combining multiple white matter tracts into a single analysis, it becomes difficult to interpret which regions contribute most to the observed associations. Decomposing the Mahalanobis distance into individual variable contributions could provide additional insight for future studies looking at patient‐specific alterations. Several techniques could be used to address this limitation, with one method consisting of recomputing the Mahalanobis distance using all possible combinations of tracts and identifying significant differences between models. However, with numerous white matter tracts this would be increasingly computationally expensive. Alternatively, a statistical approach could be taken (Chinta, 2016; Garthwaite & Koch, 2016) in order to investigate the contributions of individual variables.
Collectively, our results show that the Mahalanobis distance can be used alongside the traditional univariate analyses for a more complete understanding of the progressive nature of epilepsy and its association with white matter abnormalities. More robust to outliers than the traditional univariate z‐score approach, the Mahalanobis distance is a complementary method which can be used to compare the overall epilepsy burden in a given hemisphere with clinical variables. Future studies with large cohorts and multi‐site data (Hatton et al., 2020) could confirm if the Mahalanobis distance provides consistent results when merging multiple white matter tracts into a single analysis. Additionally, future studies could focus on using robust Mahalanobis distances to explore localised changes within cohorts of patients living with epilepsy, either by combining multiple diffusion measures of individual tracts into a single analysis, or by pooling measures from different modalities.
AUTHOR CONTRIBUTIONS
TO, YW and PT designed the study. JdT, SV, GW and JD oversaw data collection. TO and PT analysed the data. TO drafted the paper. TO, JdT, SV, GW, JD, YW and PT revised the paper.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15055.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
TO was supported by the Centre for Doctoral Training in Cloud Computing for Big Data (EP/L015358/1). PNT was supported by the Wellcome Trust (105617/Z/14/Z and (210109/Z/18/Z). YW was supported by the Wellcome Trust (208940/Z/17/Z). We thank Tom Nye, and members of the CNNP lab (www.cnnp‐lab.com) for discussions. SBV was funded by the UCLH NIHR BRC. Scan acquisition and GPW were supported by the MRC (G0802012, MR/M00841X/1). The authors thank the Epilepsy Society for supporting the Epilepsy Society MRI scanner. This work was supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Owen TW, de Tisi J, Vos SB, et al. Multivariate white matter alterations are associated with epilepsy duration. Eur J Neurosci.2021;53:2788–2803. 10.1111/ejn.15055
Edited by: Associate Editor: Dr. John Foxe
DATA AVAILABILITY STATEMENT
Data and code to reproduce our analysis is available as supplementary online material.
REFERENCES
- Ahmadi, M. E. , Hagler, D. J. , McDonald, C. R. , Tecoma, E. S. , Iragui, V. J. , Dale, A. M. , & Halgren, E. (2009). Side matters: Diffusion tensor imaging tractography in left and right temporal lobe epilepsy. American Journal of Neuroradiology, 30(9), 1740–1747. 10.3174/ajnr.A1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, A. L. , Lee, J. E. , Lazar, M. , & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics: the Journal of the American Society for Experimental NeuroTherapeutics, 4(3), 316–329. 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, J. L. R. , & Sotiropoulos, S. N. (2016). An integrated approach to correction for off‐resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063–1078. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade, C. S. , Leite, C. C. , Otaduy, M. C. G. , Lyra, K. P. , Valente, K. D. R. , Yasuda, C. L. , Beltramini, G. C. , Beaulieu, C. , & Gross, D. W. (2014). Diffusion abnormalities of the corpus callosum in patients with malformations of cortical development and epilepsy. Epilepsy Research, 108(9), 1533–1542. 10.1016/j.eplepsyres.2014.08.023 [DOI] [PubMed] [Google Scholar]
- Arfanakis, K. , Hermann, B. P. , Rogers, B. P. , Carew, J. D. , Seidenberg, M. , & Meyerand, M. E. (2002). Diffusion tensor MRI in temporal lobe epilepsy. Magnetic Resonance Imaging, 20(7), 511–519. 10.1016/S0730-725X(02)00509-X [DOI] [PubMed] [Google Scholar]
- Ashraf‐Ganjouei, A. , Rahmani, F. , Aarabi, M. H. , Moghaddam, H. S. , Nazem‐Zadeh, M.‐R. , Davoodi‐Bojd, E. , & Soltanian‐Zadeh, H. (2019). White matter correlates of disease duration in patients with temporal lobe epilepsy: Updated review of literature. Neurological Sciences, 40(6), 1209–1216. 10.1007/s10072-019-03818-2 [DOI] [PubMed] [Google Scholar]
- Bernhardt, B. C. , Worsley, K. J. , Kim, H. , Evans, A. C. , Bernasconi, A. , & Bernasconi, N. (2009). Longitudinal and cross‐sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology, 72(20), 1747–1754. 10.1212/01.wnl.0000345969.57574.f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson, P. , Dinkelacker, V. , Valabregue, R. , Thivard, L. , Leclerc, X. , Baulac, M. , Sammler, D. , Colliot, O. , Lehéricy, S. , Samson, S. , & Dupont, S. (2014). Structural connectivity differences in left and right temporal lobe epilepsy. NeuroImage, 100, 135–144. 10.1016/j.neuroimage.2014.04.071 [DOI] [PubMed] [Google Scholar]
- Bonilha, L. , Rorden, C. , Appenzeller, S. , Coan, A. C. , Cendes, F. , & Li, L. M. (2006). Gray matter atrophy associated with duration of temporal lobe epilepsy. NeuroImage, 32(3), 1070–1079. 10.1016/j.neuroimage.2006.05.038 [DOI] [PubMed] [Google Scholar]
- Catani, M. , Dell'Acqua, F. , Thiebaut de Schotten, M. (2013). A revised limbic system model for memory, emotion and behaviour. Neuroscience & Biobehavioral Reviews, 37(8), 1724–1737. 10.1016/j.neubiorev.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Chiang, S. , Levin, H. S. , Wilde, E. , & Haneef, Z. (2016). White matter structural connectivity changes correlate with epilepsy duration in temporal lobe epilepsy. Epilepsy Research, 120, 37–46. 10.1016/j.eplepsyres.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta, B. Directional Mahalanobis Distance and Parameter Sensitivities, 2016, SAE Technical Paper 2016–01‐0289, doi: 10.4271/2016-01-0289 [DOI]
- Concha, L. , Beaulieu, C. , Collins, D. L. , & Gross, D. W. (2009). White‐matter diffusion abnormalities in temporal‐lobe epilepsy with and without mesial temporal sclerosis. Journal of Neurology, Neurosurgery & Psychiatry, 80(3), 312–319. 10.1136/jnnp.2007.139287 [DOI] [PubMed] [Google Scholar]
- Concha, L. , Livey, D. J. , Beaulieu, C. , Wheatley, B. M. , & Gross, D. W. (2010). In vivo diffusion tensor imaging and histopathology of the fimbria‐fornix in temporal lobe epilepsy. Journal of Neuroscience, 30(3), 996–1002. 10.1523/JNEUROSCI.1619-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, P. A. , Symms, M. , Boulby, P. A. , & Alexander, D. C. (2007). Optimal acquisition orders of diffusion‐weighted MRI measurements. Journal of Magnetic Resonance Imaging, 25(5), 1051–1058. 10.1002/jmri.20905 [DOI] [PubMed] [Google Scholar]
- Cox, S. R. , Ritchie, S. J. , Tucker‐Drob, E. M. , Liewald, D. C. , Hagenaars, S. P. , Davies, G. , Wardlaw, J. M. , Gale, C. R. , Bastin, M. E. , & Deary, I. J. (2016). Ageing and brain white matter structure in 3,513 UK biobank participants. Nature Communications, 7(1), 1–13. 10.1038/ncomms13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, D. C. , Lange, N. , Travers, B. G. , Prigge, M. B. , Matsunami, N. , Kellett, K. A. , Freeman, A. , Kane, K. L. , Adluru, N. , Tromp, D. , Destiche, D. J. , Samsin, D. , Zielinski, B. A. , Fletcher, P. T. , Anderson, J. S. , Froehlich, A. L. , Leppert, M. F. , Bigler, E. D. , Lainhart, J. E. , & Alexander, A. L. (2017). Multivariate characterization of white matter heterogeneity in autism spectrum disorder. NeuroImage: Clinical, 14, 54–66. 10.1016/j.nicl.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, J. (2016). Applied Regression Analysis and Generalized Linear Models (3rd ed., pp. 1‐817). SAGE. [Google Scholar]
- Galovic, M. , van Dooren, V. Q. H. , Postma, T. S. , Vos, S. B. , Caciagli, L. , Borzì, G. , Cueva Rosillo, J. , Vuong, K. A. , de Tisi, J. , Nachev, P. , Duncan, J. S. , & Koepp, M. J. (2019). Progressive cortical thinning in patients with focal epilepsy. JAMA Neurology, 76(10), 1230–1239. 10.1001/jamaneurol.2019.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite, P. H. , & Koch, I. (2016). Evaluating the contributions of individual variables to a quadratic form. Australian & New Zealand Journal of Statistics, 58(1), 99–119. 10.1111/anzs.12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan, R. M. , Makki, M. I. , Sundaram, S. K. , Juhász, C. , & Chugani, H. T. (2008). Diffusion tensor analysis of temporal and extra‐temporal lobe tracts in temporal lobe epilepsy. Epilepsy Research, 80(1), 30–41. 10.1016/j.eplepsyres.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, D. W. , Concha, L. , & Beaulieu, C. (2006). Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia, 47(8), 1360–1363. 10.1111/j.1528-1167.2006.00603.x [DOI] [PubMed] [Google Scholar]
- Gross, J. , & Ligges, U. (2015). Nortest: Tests for Normality (version R package version 1.0‐4). https://CRAN.R‐project.org/package=nortest
- Hatton, S. N. , Huynh, K. H. , Bonilha, L. , Abela, E. , Alhusaini, S. , Altmann, A. , Alvim, M. K. M. , Balachandra, A. R. , Bartolini, E. , Bender, B. , Bernasconi, N. , Bernasconi, A. , Bernhardt, B. , Bargallo, N. , Caldairou, B. , Caligiuri, M. E. , Carr, S. J. A. , Cavalleri, G. L. , Cendes, F. , … McDonald, C. R. (2020). White matter abnormalities across different epilepsy syndromes in adults: An ENIGMA epilepsy study. Brain, 143, 2454–2473, 10.1101/2019.12.19.883405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, K. , Zhang, J. , Wakana, S. , Jiang, H. , Li, X. , Reich, D. S. , Calabresi, P. A. , Pekar, J. J. , van Zijl, P. C. M. , & Mori, S. (2008). Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract‐specific quantification. NeuroImage, 39(1), 336–347. 10.1016/j.neuroimage.2007.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. J. , Woolrich, M. W. , & Smith, S. M. (2012). FSL. NeuroImage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Keller, S. S. , Glenn, G. R. , Weber, B. , Kreilkamp, B. A. , Jensen, J. H. , Helpern, J. A. , Wagner, J. , Barker, G. J. , Richardson, M. P. , & Bonilha, L. (2017). Preoperative automated fibre quantification predicts postoperative seizure outcome in temporal lobe epilepsy. Brain, 140(1), 68–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, S. S. , Schoene‐Bake, J.‐C. , Gerdes, J. S. , Weber, B. , & Deppe, M. (2012). Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: Cross‐sectional evidence for progressive neurologic injury. PLoS One, 7(10), e46791. 10.1371/journal.pone.0046791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, S. S. , Wieshmann, U. C. , Mackay, C. E. , Denby, C. E. , Webb, J. , & Roberts, N. (2002). Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: Effects of side of seizure onset and epilepsy duration. Journal of Neurology, Neurosurgery & Psychiatry, 73(6), 648–655. 10.1136/jnnp.73.6.648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmotsu, N. , Girard, H. M. , Bernhardt, B. C. , Bonilha, L. , Lin, J. J. , Tecoma, E. S. , Iragui, V. J. , Hagler, D. J. , Halgren, E. , & McDonald, C. R. (2011). MRI analysis in temporal lobe epilepsy: Cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia, 52(12), 2257–2266. 10.1111/j.1528-1167.2011.03278.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz, S. , Goksuluk, D. , & Zararsiz, G. (2014). MVN: An R package for assessing multivariate normality. The R Journal, 6(2), 151–162. 10.32614/RJ-2014-031 [DOI] [Google Scholar]
- Kreilkamp, B. A. K. , Lucy Lisanti, G. , Glenn, R. , Wieshmann, U. C. , Das, K. , Marson, A. G. , & Keller, S. S. (2019). Comparison of manual and automated fiber quantification tractography in patients with temporal lobe epilepsy. NeuroImage: Clinical, 24, 102024. 10.1016/j.nicl.2019.102024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreilkamp, B. A. K. , Weber, B. , Richardson, M. P. , & Keller, S. S. (2017). Automated tractography in patients with temporal lobe epilepsy using TRActs constrained by UnderLying anatomy (TRACULA). NeuroImage: Clinical, 14, 67–76. 10.1016/j.nicl.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoit, O. , & Wolf, M. 2018. Analytical Nonlinear Shrinkage of Large‐Dimensional Covariance Matrices, University of Zurich, Department of Economics. doi: 10.2139/ssrn.3047302 [DOI]
- Leemans, A. , & Jones, D. K. (2009). The b‐matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine, 61(6), 1336–1349. 10.1002/mrm.21890 [DOI] [PubMed] [Google Scholar]
- Lin, J. J. , Riley, J. D. , Juranek, J. , & Cramer, S. C. (2008). Vulnerability of the frontal‐temporal connections in temporal lobe epilepsy. Epilepsy Research, 82(2), 162–170. 10.1016/j.eplepsyres.2008.07.020 [DOI] [PubMed] [Google Scholar]
- Liu, M. , Concha, L. , Lebel, C. , Beaulieu, C. , & Gross, D. W. (2012). Mesial temporal sclerosis is linked with more widespread white matter changes in temporal lobe epilepsy. NeuroImage: Clinical, 1(1), 99–105. 10.1016/j.nicl.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, R. S. N. , Lemieux, L. , Bell, G. S. , Hammers, A. , Sisodiya, S. M. , Bartlett, P. A. , Shorvon, S. D. , Sander, J. W. A. S. , & Duncan, J. S. (2003). Progressive neocortical damage in epilepsy. Annals of Neurology, 53(3), 312–324. 10.1002/ana.10463 [DOI] [PubMed] [Google Scholar]
- Liu, R. S. N. , Lemieux, L. , Bell, G. S. , Sisodiya, S. M. , Bartlett, P. A. , Shorvon, S. D. , Sander, J. W. A. S. , & Duncan, J. S. (2005). Cerebral damage in epilepsy: A population‐based longitudinal quantitative MRI study. Epilepsia, 46(9), 1482–1494. 10.1111/j.1528-1167.2005.51603.x [DOI] [PubMed] [Google Scholar]
- Munsell, B. C. , Wee, C. Y. , Keller, S. S. , Weber, B. , Elger, C. , da Silva, L. A. , Nesland, T. , Styner, M. , Shen, D. , & Bonilha, L. (2015). Evaluation of machine learning algorithms for treatment outcome prediction in patients with epilepsy based on structural connectome data. Neuroimage, 118, 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte, W. M. , van Eijsden, P. , Sander, J. W. , Duncan, J. S. , Dijkhuizen, R. M. , & Braun, K. P. J. (2012). A meta‐analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia, 53(4), 659–667. 10.1111/j.1528-1167.2012.03426.x [DOI] [PubMed] [Google Scholar]
- Park, K. M. , Lee, B. I. , Shin, K. J. , Ha, S. Y. , Park, J. , Kim, T. H. , Mun, C. W. , & Kim, S. E. (2018). Progressive topological disorganization of brain network in focal epilepsy. Acta Neurologica Scandinavica, 137(4), 425–431. 10.1111/ane.12899 [DOI] [PubMed] [Google Scholar]
- Pitkänen, A. , & Sutula, T. P. (2002). Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal‐lobe epilepsy. The Lancet Neurology, 1(3), 173–181. 10.1016/S1474-4422(02)00073-X [DOI] [PubMed] [Google Scholar]
- Seidenberg, M. , Kelly, K. G. , Parrish, J. , Geary, E. , Dow, C. , Rutecki, P. , & Hermann, B. (2005). Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia, 46(3), 420–430. 10.1111/j.0013-9580.2005.27004.x [DOI] [PubMed] [Google Scholar]
- Sinha, N. , Wang, Y. , Silva, N. M. , Miserocchi, A. , McEvoy, A. W. , de Tisi, J. , Vos, S. B. , Winston, G. P. , Duncan, J. S. , & Taylor, P. N. (2019). Node abnormality predicts seizure outcome and relates to long‐term relapse after epilepsy surgery. bioRxiv, 747725. [Google Scholar]
- Sinha, N. , Wang, Y. , da Silva, N. M. , Miserocchi, A. , McEvoy, A. W. , de Tisi, J. , Vos, S. B. , Winston, G. P. , Duncan, J. S. , & Taylor, P. N. (2020). Structural brain network abnormalities and the probability of seizure recurrence after epilepsy surgery. [in press]. 10.1101/747725 [DOI] [PMC free article] [PubMed]
- Slinger, G. , Sinke, M. R. T. , Braun, K. P. J. , & Otte, W. M. (2016). ‘White matter abnormalities at a regional and voxel level in focal and generalized epilepsy. A Systematic Review and Meta‐Analysis’. Neuroimage: Clinical, 12, 902–909. 10.1016/j.nicl.2016.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, J. M. , Marques, P. , Alves, V. , & Sousa, N. (2013). A hitchhiker's guide to diffusion tensor imaging. Frontiers in Neuroscience, 7. 10.3389/fnins.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasch, E. , Cendes, F. , Li, L. M. , Dubeau, F. , Andermann, F. , & Arnold, D. L. (1999). Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Annals of Neurology, 45(5), 568–576. [DOI] [PubMed] [Google Scholar]
- Taylor, P. N. , Moreira, N. , da Silva, A. , Blamire, Y. W. , & Forsyth, R. (2020). Early deviation from normal structural connectivity: A novel intrinsic severity score for mild TBI. Neurology, 94(10), e1021–e1026. 10.1212/WNL.0000000000008902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, P. N. , Sinha, N. , Wang, Y. , Vos, S. B. , de Tisi, J. , Miserocchi, A. , McEvoy, A. W. , Winston, G. P. , & Duncan, J. S. (2018). The impact of epilepsy surgery on the structural connectome and its relation to outcome. NeuroImage: Clinical, 18, 202–214. 10.1016/j.nicl.2018.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Téllez‐Zenteno, J. F. , & Hernández‐Ronquillo, L. (2012). A review of the epidemiology of temporal lobe epilepsy. Epilepsy Research and Treatment, 2012, 1–5. 10.1155/2012/630853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivard, L. , Lehéricy, S. , Krainik, A. , Adam, C. , Dormont, D. , Chiras, J. , Baulac, M. , & Dupont, S. (2005). Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. NeuroImage, 28(3), 682–690. 10.1016/j.neuroimage.2005.06.045 [DOI] [PubMed] [Google Scholar]
- Tsuda, K. , Tsuji, T. , Ishida, T. , Takahashi, S. , Yamada, S. , Ohoshi, Y. , Terada, M. , Shinosaki, K. , & Ukai, S. (2018). Widespread abnormalities in white matter integrity and their relationship with duration of illness in temporal lobe epilepsy. Epilepsia Open, 3(2), 247–254. 10.1002/epi4.12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables, W. N. , & Ripley, B. D. (2002). Modern Applied Statistics with S (4th ed.). Springer. http://www.stats.ox.ac.uk/pub/MASS4 [Google Scholar]
- Vos, S. B. , Tax, C. M. W. , Luijten, P. R. , Ourselin, S. , Leemans, A. , & Froeling, M. (2017). The importance of correcting for signal drift in diffusion MRI. Magnetic Resonance in Medicine, 77(1), 285–299. 10.1002/mrm.26124 [DOI] [PubMed] [Google Scholar]
- Wahl, M. , Li, Y.‐O. , Ng, J. , Lahue, S. C. , Cooper, S. R. , Sherr, E. H. , & Mukherjee, P. (2010). Microstructural correlations of white matter tracts in the human brain. NeuroImage, 51(2), 531–541. 10.1016/j.neuroimage.2010.02.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Ludwig, T. , Little, B. , Necus, J. H. , Winston, G. , Vos, S. B. , de Tisi, J. , Duncan, J. S. , Taylor, P. N. , & Mota, B. (2020). Independent components of human brain morphology. NeuroImage, 226, 117546. 10.1016/j.neuroimage.2020.117546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye, L. T. , Walhovd, K. B. , Dale, A. M. , Bjørnerud, A. , Due‐Tønnessen, P. , Engvig, A. , Grydeland, H. , Tamnes, C. K. , Ostby, Y. , & Fjell, A. M. (2010). Life‐span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex, 20(9), 2055–2068. 10.1093/cercor/bhp280 [DOI] [PubMed] [Google Scholar]
- Wheeler‐Kingshott, C. A. M. , Hickman, S. J. , Parker, G. J. M. , Ciccarelli, O. , Symms, M. R. , Miller, D. H. , & Barker, G. J. (2002). Investigating cervical spinal cord structure using axial diffusion tensor imaging. NeuroImage, 16(1), 93–102. 10.1006/nimg.2001.1022 [DOI] [PubMed] [Google Scholar]
- Whelan, C. D. , Altmann, A. , Botía, J. A. , Jahanshad, N. , Hibar, D. P. , Absil, J. , Alhusaini, S. , Alvim, M. K. M. , Auvinen, P. , Bartolini, E. , Bergo, F. P. G. , Bernardes, T. , Blackmon, K. , Braga, B. , Caligiuri, M. E. , Calvo, A. , Carr, S. J. , Chen, J. , Chen, S. , … Sisodiya, S. M. (2018). Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain, 141(2), 391–408. 10.1093/brain/awx341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjaja, E. , Zarei Mahmoodabadi, S. , Go, C. , Raybaud, C. , Chuang, S. , Snead, O. C. , & Smith, M. L. (2012). Reduced cortical thickness in children with new‐onset seizures. American Journal of Neuroradiology, 33(4), 673–677. 10.3174/ajnr.A2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston, G. P. , Stretton, J. , Sidhu, M. K. , Symms, M. R. , & Duncan, J. S. (2013). Progressive white matter changes following anterior temporal lobe resection for epilepsy. NeuroImage: Clinical, 4, 190–200. 10.1016/j.nicl.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, F.‐C. , & Tseng, W.‐Y. (2011). NTU‐90: A High Angular Resolution Brain Atlas Constructed by q‐Space Diffeomorphic Reconstruction. NeuroImage, 58(1), 91–99. 10.1016/j.neuroimage.2011.06.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data and code to reproduce our analysis is available as supplementary online material.


