Short abstract
The 6‐month infection risk was 43% lower in patients with sarcoidosis who initiated methotrexate compared to those who started azathioprine. Our findings suggest that unless contraindications exist, methotrexate should be preferred over azathioprine as the primary steroid‐sparing choice in individuals with sarcoidosis.
Keywords: azathioprine, infection, methotrexate, registry, sarcoidosis, trial emulation
ABSTRACT
Background and objective
No clinical trial has examined the risk of infection associated methotrexate and azathioprine, two advocated treatments for sarcoidosis. We aimed to compare the 6‐month risk of infection after the initiation of methotrexate or azathioprine.
Methods
We conducted a retrospective target trial emulation using Swedish pre‐existing data. We searched for eligible participants who were dispensed methotrexate or azathioprine in the Prescribed Drug Register (PDR) every day between January 2007 and June 2013. Adults were eligible if they had ≥2 ICD‐coded visits for sarcoidosis in the National Patient Register (NPR) and were dispensed ≥1 systemic corticosteroid but no methotrexate or azathioprine in the past 6 months (PDR). Within 6 months of methotrexate or azathioprine initiation, diagnosis of infectious disease was identified (visit in the NPR where infectious disease was the primary diagnosis). We estimated RR and risk differences comparing methotrexate (n = 667) to azathioprine initiations (n = 259) using targeted maximum likelihood estimation (TMLE) adjusting for demographic factors, comorbidity and sarcoidosis severity proxies.
Results
There were 43 infections in the methotrexate group (adjusted 6‐month risk 6.8%) and 29 infections in the azathioprine group (12.0%). The RR for infectious disease at 6 months associated with methotrexate compared to azathioprine initiation was 0.57 (95% CI: 0.39, 0.82) and the risk difference was −5.2% (95% CI: −8.5%, −1.8%). The RR at 9 months was attenuated to 0.77 (95% CI: 0.52, 1.14).
Conclusion
Methotrexate appears to be associated with a lower risk of infection in sarcoidosis than azathioprine, but randomized trials should confirm this finding.
Abbreviations
- ATC
Anatomical Therapeutic Chemical
- ICD
International Classification of Disease
- MPR
modified Poisson regression
- NPR
National Patient Register
- PDR
Prescribed Drug Register
- RR
risk ratio
- TMLE
targeted maximum likelihood estimation
INTRODUCTION
Sarcoidosis, a systemic granulomatous disease of unknown aetiology, is associated with a higher risk of infection. 1 , 2 The risk of infection is particularly exacerbated in patients who need corticosteroid treatment to manage symptoms and control disease progression. 2 The increase in infection risk in this patient group, especially during the first 2 years after diagnosis, 2 is perceived to be partly mediated by corticosteroid use. Changing to steroid‐sparing treatments may alleviate some of the increased risk in individuals with sarcoidosis.
Among steroid‐sparing medications indicated for controlling disease activity when corticosteroid dose is tapered, methotrexate and azathioprine are the most popular choices in Sweden. 3 Although the induction time is longer than for oral corticosteroids, they are both effective steroid‐sparing agents in sarcoidosis and their toxicity profiles are well known. 4 , 5 , 6 , 7 , 8 Choosing between the two is therefore challenging for physicians. In individuals without specific contraindications for one or the other medication, the risk of infection associated with their use in sarcoidosis could inform choice of treatment.
In the absence of safety trials, however, little is known about the risk of infection associated with methotrexate or azathioprine use. In addition, reports on adverse events from efficacy trials in sarcoidosis and other inflammatory diseases are inconclusive due to small numbers. 9 , 10 , 11 , 12 , 13 In a study on sarcoidosis, infections were about twice as common in a hospital cohort that was treated exclusively with azathioprine compared to another cohort that received methotrexate. 14 It is difficult to draw conclusions from that study because confounders were not considered, ascertainment of infection was not standardized and discontinuation of treatment varied between the two cohorts. Our objective was therefore to estimate the relative risk of infectious disease at 6 months associated with the initiation of methotrexate compared to initiation of azathioprine in patients with sarcoidosis.
METHODS
We emulated a hypothetical (termed ‘target’) trial using pre‐existing (observational) data to investigate the comparative safety of methotrexate and azathioprine in terms of infection. Target trial emulation is a recent approach to comparative safety studies. The aim is to reduce common fallacies associated with observational studies (e.g. immortal time and selection bias) by replicating features of randomized trials that protect them from these errors. 15 , 16 In line with the target trial emulation approach, 15 we compiled an explicit protocol for the target trial (Table S1 in Supplementary Information), which we then emulated using Swedish register data.
Data on medication dispensing and healthcare visits
To emulate the target trial, we obtained data on medication which had been dispensed from pharmacies across Sweden from the Prescribed Drug Register (PDR; available since July 2005). We retrieved data on morbidity from the National Patient Register (NPR), which records inpatient visits using the International Classification of Disease (ICD) codes since 1964 (nationwide since 1987) and visits to outpatient specialist (non‐primary care) clinics since 2001. Only few visits for somatic disease are either miscoded or missing from the NPR, 17 but results of examinations including microbiological analyses and imaging are not captured. Records in the NPR, PDR and other registers were linked using an individual's unique personal number assigned upon birth or immigration.
Trial emulation procedure
Each day between 1 January 2006 and 30 June 2013, we allowed for a new trial emulation to occur amounting to 2738 potential emulations (Fig. 1). An emulation was successful if at least one eligible individual (see criteria below) was dispensed methotrexate or azathioprine in the PDR that day, hereafter referred to as ‘trial start’ or ‘trial initiation’. At trial start, the assessment of participants' eligibility, the initiation of methotrexate or azathioprine and the start of follow‐up all coincided.
Figure 1.
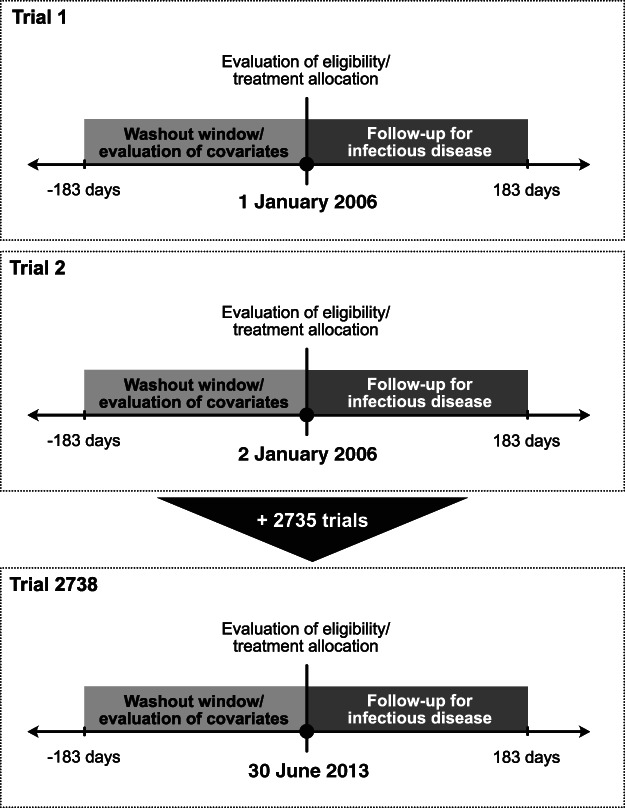
Graphical representation of the target trial emulation design including 2738 potential trial emulations nested in the Prescribed Drug Register (PDR). At each trial's initiation (i.e. time zero, depicted by a black circle, ●), eligibility criteria were evaluated (some using information collected within the previous 6 months), treatment was allocated and the 6‐month follow‐up for infectious disease started. Also, several covariates (e.g. age, sex and residential location) were evaluated at time zero some using information collected within the previous 6 months (e.g. cumulative defined daily doses of systemic corticosteroids dispensed in the PDR).
Ethical permission for this study was obtained from the Regional Ethics Review Board in Stockholm (2014/230‐31), which waived the need for informed consent.
Eligibility criteria
Eligibility was evaluated on the day each potential trial was emulated. Eligible individuals were those aged 18–85 years and who had a history of ≥2 inpatient or outpatient visits in the NPR listing an ICD code for sarcoidosis. To reduce misclassification of sarcoidosis, we excluded participants with a diagnosis of a haematopoietic or lung malignancy recorded in the Cancer Register ±6 months from their first visit for sarcoidosis in the NPR. To capture individuals who needed a steroid‐sparing treatment for sarcoidosis, we required participants to be dispensed ≥1 prescription of systemic corticosteroids in the PDR within 6 months up to the day before trial start and excluded those who were dispensed either methotrexate or azathioprine during the same 6‐month period (see Fig. 2 for study flowchart). ICD and Anatomical Therapeutic Chemical (ATC) codes used to identify morbidity and medications, respectively, are listed in Table S2 (Supplementary Information).
Figure 2.
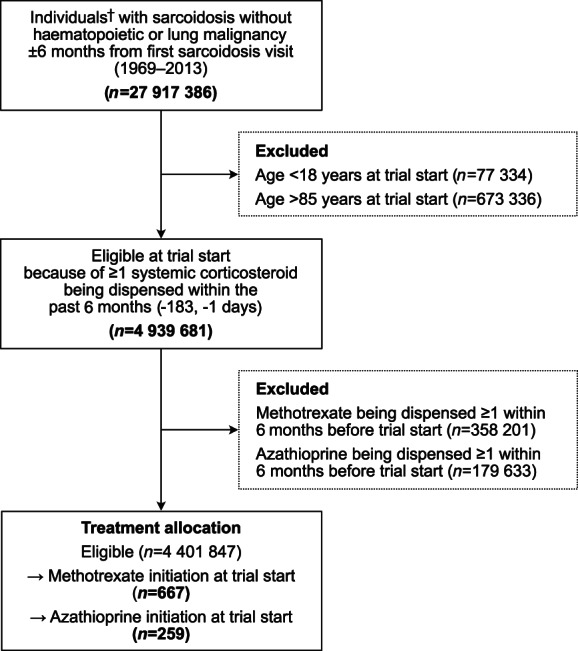
Eligible individuals for the potential 2738 target trial emulations. †Non‐unique individuals. A participant was counted once per day between 1 January 2006 and 30 June 2013 (i.e. 2738 times, as many times as the potential trials).
Treatment allocation
Eligible individuals who were dispensed methotrexate in the PDR at trial start were allocated to the methotrexate initiators group, while those who were dispensed azathioprine entered the azathioprine initiators group. Information on the administered dose was not available in the PDR. Although prescription patterns may vary by clinic, usual practice was to prescribe a weekly dose of methotrexate of up to 15 mg (followed by folic acid supplementation) or up to 150 mg azathioprine once daily. 18 An individual could appear in multiple subsequent trials if they were deemed eligible and initiated either methotrexate or azathioprine.
Infectious disease
The outcome was diagnosis of an infectious disease within 6 months after the initiation of a trial medication. Six months was deemed enough time for adverse events associated with methotrexate and azathioprine to onset and be diagnosed. 7 , 11 , 12 Infectious disease was defined as an inpatient or outpatient visit in the NPR where the primary discharge diagnosis listed an ICD code for an infection (ICD codes listed in Table S2 in Supplementary Information). The date of hospital admission or that of the outpatient visit was considered the date of infectious disease diagnosis. A unique individual contributed only one infectious disease diagnosis per treatment episode. They could, however, contribute with more than one outcome if they were diagnosed with an infectious disease in a future treatment episode.
Other variables
We evaluated several potential confounders at the start of each trial to emulate the randomized allocation of treatment in the target trial. We retrieved data on the date of birth, sex, residential location, country of birth and civil status from the Total Population Register and attained education from the Longitudinal Integrated Database for Health Insurance and Labor Market Studies.
Using data on healthcare visits from the NPR and medication data from the PDR, we defined the following comorbidities: congestive heart disease, atrial fibrillation, acute myocardial infarction, stroke, hypertension, diabetes mellitus, dyslipidaemia, chronic obstructive pulmonary disease, asthma and autoimmune disease (excluding sarcoidosis). To approximate general health status before trial initiation, we counted visits in the NPR and searched for ≥1 non‐steroidal anti‐inflammatory drug and for an antimicrobial medication (i.e. an antibiotic, antimycobacterial, antifungal or an antiviral) being dispensed within 6 months before trial start. Lastly, we used the number of dispensed defined daily doses of systemic corticosteroids within 6 months before trial start as an indicator of sarcoidosis severity and/or regional prescribing patterns. 3 Detailed variable definitions are available in Table S2 (Supplementary Information).
Statistical analysis
Data from each trial were pooled into a single data set and were analysed according to an intention‐to‐treat scheme, thus estimating the effect of treatment initiation irrespective of adherence to treatment. We estimated the risk ratio (RR) and risk difference of infectious disease at 6 months comparing methotrexate to azathioprine initiation accounting for potentially differential loss to follow‐up (outcome missingness) due to death. We used an estimator for the RR and risk difference from the targeted maximum likelihood estimation (TMLE) framework. 19 , 20 TMLE estimators are unbiased when either the exposure or outcome models are misspecified (i.e. doubly robust) and more efficient than alternative estimators leading to narrower CI. 19 , 21
We estimated the exposure, outcome and outcome–missingness mechanisms through an ensemble (i.e. a weighted average) of machine‐learning (prediction) algorithms and conventional statistical models that was determined using cross‐validation techniques implemented in the SuperLearner package (version 2.0‐26) 22 for R. We used the following SuperLearner wrappers: logistic regression with main and/or interaction effects, a fast version of generalized linear models (speedGLM), generalized additive models and elastic net (lasso and ridge) regression models, recursive partitioning and regression trees algorithms, Kernellab's support vector machines algorithm and ranger's random forests algorithm.
The following covariates were used to estimate the exposure, outcome and outcome–missingness mechanisms: age at trial entry (continuous), age at sarcoidosis diagnosis (second visit for sarcoidosis; continuous), sex, region of residence (grouped into six healthcare regions 3 ), birth country (Nordic or non‐Nordic), education (≤9 years including missing due to very small numbers, 10–12 or ≥13 years), civil status (married/in registered partnership or other), calendar period (<2010 or ≥2010), >3 healthcare visits within 6 months before trial entry, history of comorbidity (see ‘Other variables’ section above), number of dispensed defined daily doses of systemic corticosteroids (continuous) and ≥1 non‐steroidal anti‐inflammatory drug or an antimicrobial medication being dispensed within 6 months before trial entry. 95% CI were constructed using variance of the RR or risk difference derived from their influence curve and adjusted for multiple observations per individual. 20 The number needed to harm was calculated using TMLE‐derived risks as |1/(methotrexate risk − azathioprine risk)|.
Sensitivity analysis
We conducted several sensitivity analyses to test the robustness of the RR from the main analysis. First, we excluded participants with any history of methotrexate or azathioprine being dispensed before trial start to completely avoid any carry‐over effects or confounding by indication. Second, we ascertained infectious disease at 3 and 9 months to check the impact of varying induction periods and any potential time‐varying effect of treatment initiation on infection risk. For the latter, trial emulations ran between June 2007 and March 2013 to allow for enough time to evaluate exclusion criteria and the outcome. Third, we examined definitions of infectious disease of different severity by restricting to hospitalized infections, considering infectious diseases coded as a cause of death (Cause of Death Register) or identified through antimicrobial treatment being dispensed in addition to healthcare visits. Moreover, we estimated RR for infectious disease and corresponding robust 95% CI using a modified Poisson regression model adjusted for the same covariates used in the TMLE analyses to identify any differences pertaining to TMLE's double robustness and efficiency. Lastly, we calculated the E‐value 23 to quantify the minimum strength of an association a confounder needed to have with both exposure and outcome to completely attenuate the RR observed in this study.
Data were managed and analysed with SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.6.3 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
From January 2006 to June 2013, we identified 667 episodes of methotrexate initiation (in 493 unique individuals) and 259 episodes of azathioprine initiation (in 231 unique individuals; Table 1). No individual dispensed both medications at the same time and a small proportion (<6%) started azathioprine after having a history of methotrexate use (more than 6 months before trial start) or the opposite. Participants initiated both treatments after a median 3 years from sarcoidosis diagnosis (interquartile range 1, 7).
Table 1.
Demographic and clinical characteristics at trial initiation by trial arm
| Methotrexate initiation (n = 667) | Azathioprine initiation (n = 259) | |
|---|---|---|
| Unique individuals, n | 493 | 231 |
| Trials per unique individual, mean (SD) | 1.4 (0.9) | 1.1 (0.4) |
| Age at trial start, mean (SD) | 54 (13.0) | 53 (13.2) |
| Age at sarcoidosis diagnosis, mean (SD) | 49 (12.4) | 48 (14.0) |
| Female (%) | 45.3 | 42.5 |
| Region of residence (%) | ||
| Stockholm | 25.9 | 14.7 |
| Uppsala‐Örebro | 18.6 | 22.4 |
| West | 12.1 | 30.5 |
| South | 14.7 | 19.7 |
| Southeast | 16.0 | 5.4 |
| North | 12.6 | 7.3 |
| Born in non‐Nordic country † (%) | 6.7 | 10.4 |
| Completed education ‡ (%) | ||
| ≤9 years | 51.0 | 47.9 |
| 10–12 years | 19.6 | 25.5 |
| ≥13 years | 29.4 | 26.6 |
| Married or in registered partnership (%) | 52.2 | 49.8 |
| Calendar period 2006–2009 (%) | 40.0 | 54.1 |
| Years from sarcoidosis diagnosis to trial entry, median (IQR) | 3 (1, 7) | 3 (1, 7) |
| >3 Healthcare visits in the previous 6 months (%) | 45.3 | 52.1 |
| History of morbidity (%) | ||
| Congestive heart disease | 7.2 | 4.2 |
| Atrial fibrillation | 4.5 | 4.2 |
| Acute myocardial infarction | 2.5 | 2.3 |
| Stroke | 2.7 | 4.2 |
| Hypertension | 40.5 | 39.4 |
| Diabetes mellitus | 15.9 | 15.4 |
| Dyslipidaemia | 21.7 | 21.2 |
| Chronic obstructive pulmonary disease | 5.1 | 6.2 |
| Asthma | 8.2 | 6.6 |
| Autoimmune disease | 21.6 | 35.5 |
| Infectious disease within 6 months before trial start § | 7.3 | 10.0 |
| ≥1 Dispensed medication >6 months before trial start (%) | ||
| Methotrexate | 41.4 | 5.8 |
| Azathioprine | 4.5 | 21.6 |
| Medication dispensed within 6 months before trial start | ||
| Defined daily doses of systemic corticosteroids, mean (SD) | 196 (158.7) | 238 (172.2) |
| ≥1 NSAID (%) | 52.0 | 39.4 |
| ≥1 Antimicrobial ¶ (%) | 75.7 | 69.5 |
Nordic countries include Sweden, Denmark, Norway, Finland and Iceland.
Missing (<1.5% in both groups) included in ‘≤9 years’ category.
Defined as one inpatient or outpatient visit in the National Patient Register listing an ICD code for infectious disease as the primary discharge diagnosis.
Antimicrobials include antibiotic, antiviral, antimycobacterial and antifungal medications.
ICD, International Classification of Disease; IQR, interquartile range; NSAID, non‐steroidal anti‐inflammatory drug.
Mean age at trial start was about 54 years and 45% of participants were female in both the methotrexate and azathioprine groups (Table 1). Socioeconomic indicators (education, salary and civil status) were similar between the two groups. The majority of individuals in a region were dispensed methotrexate than azathioprine (>80% in Stockholm, Southeast and North) except in the West where preference for methotrexate and azathioprine was split in half (data not shown). Most comorbidities were equally distributed between the two groups, except autoimmune disease that was more prevalent in azathioprine initiators (35.5% vs 21.6%; Table 1). A slightly smaller dose of systemic corticosteroids was dispensed to methotrexate initiators within 6 months before trial entry (mean 196 defined daily doses (≈11 mg/day) vs 238 (≈13 mg/day) in the methotrexate and azathioprine groups, respectively).
Within 6 months of treatment initiation, 43 infections were identified in the methotrexate group compared to 29 infections in the azathioprine group (Table 2). Six treatment initiation episodes in each group could not be ascertained for infectious disease because of death during the 6‐month follow‐up. Five of the 43 events in the methotrexate group occurred in two unique individuals and 2 of the 29 events in the azathioprine group were attributed to one unique individual.
Table 2.
Adjusted risk difference and RR of infectious disease within 6 months associated with methotrexate compared to azathioprine initiation in sarcoidosis (infectious disease could not be evaluated in six individuals in each group who died before the end of follow‐up)
| Infectious disease at 6 months | Methotrexate initiation (n = 667) | Azathioprine initiation (n = 259) |
|---|---|---|
| Infectious disease, n (%) | 43 (6.4) | 29 (11.2) |
| Adjusted risk (95% CI), % | 6.80 (5.27, 8.62) | 11.99 (9.96, 14.25) |
| Adjusted risk difference (95% CI), % (TMLE) | −5.17 (−8.53, −1.82) | 0.00 (reference) |
| Adjusted RR (95% CI), TMLE | 0.57 (0.39, 0.82) | 1.00 (reference) |
| Adjusted RR (95% CI), MPR | 0.57 (0.34, 0.97) | 1.00 (reference) |
RR (and risk difference) from TMLE analyses and in modified Poisson models adjusted for age at trial entry and sarcoidosis diagnosis, sex, region of residence, birth country, education, civil status, calendar period, healthcare visits within 6 months before trial start, history of congestive heart disease, atrial fibrillation, acute myocardial infarction, stroke, hypertension, diabetes mellitus, dyslipidaemia, chronic obstructive pulmonary disease, asthma and autoimmune disease, dispensed defined daily doses of systemic corticosteroids, non‐steroidal anti‐inflammatory drug or an antimicrobial medication being dispensed within 6 months before trial start.
MPR, modified Poisson regression; RR, risk ratio; TMLE, targeted maximum likelihood estimation.
The adjusted risk of infectious disease at 6 months was 6.80% in methotrexate initiators (95% CI: 5.27, 8.62) compared to 11.99% in azathioprine initiators (95% CI: 9.96, 14.25; Table 2). Methotrexate initiation was associated with a 43% lower risk of infectious disease at 6 months compared to azathioprine initiation (RR: 0.57 (95% CI: 0.39, 0.82); risk difference: −5.17% (95% CI: −8.53%, −1.82%)). Nineteen individuals need to initiate methotrexate (vs azathioprine) for one to develop infectious disease at 6 months.
Results of sensitivity analyses are summarized in Table 3. We observed a stronger association between methotrexate initiation and infection at 3 months (RR: 0.47 (95% CI: 0.30, 0.74)) compared to the main analysis (RR: 0.57). The association was weaker at 9 months (RR: 0.77 (95% CI: 0.52, 1.14)). Similarly, restricting to infectious diseases identified only through inpatient visits or considering antimicrobial being dispensed in addition to healthcare visits at 6 months resulted in some attenuation of the RR (0.69 and 0.87, respectively). The RR did not change markedly when we included cause of death data to define infectious disease at 6 months or when we required no history of a methotrexate or azathioprine being dispensed ever before trial start.
Table 3.
Adjusted RR comparing initiation of methotrexate and azathioprine in sensitivity analyses testing induction times, infectious disease definitions and inclusion criteria (infectious disease could not be evaluated in ≤6 individuals in each group who died before the end of follow‐up)
| Analysis | Methotrexate initiation | Azathioprine initiation |
|---|---|---|
| Infectious disease at 3 months | ||
| Treatment episodes, n | 667 | 259 |
| Infectious disease, n (%) | 23 (3.4) | 19 (7.3) |
| Adjusted RR (95% CI), TMLE | 0.47 (0.30, 0.74) | 1.00 (reference) |
| Adjusted RR (95% CI), MPR | 0.50 (0.25, 1.00) | 1.00 (reference) |
| Infectious disease at 9 months † | ||
| Treatment episodes, n | 343 | 168 |
| Infectious disease, n (%) | 36 (10.5) | 27 (16.1) |
| Adjusted RR (95% CI), TMLE | 0.77 (0.52, 1.14) | 1.00 (reference) |
| Adjusted RR (95% CI), MPR | 0.76 (0.46, 1.28) | 1.00 (reference) |
| Infectious disease at 6 months identified from inpatient visits | ||
| Treatment episodes, n | 667 | 259 |
| Infectious disease, n (%) | 29 (4.3) | 16 (6.2) |
| Adjusted RR (95% CI), TMLE | 0.69 (0.48, 1.02) | 1.00 (reference) |
| Adjusted RR (95% CI), MPR | 0.80 (0.37, 1.70) | 1.00 (reference) |
| Infectious disease at 6 months identified from inpatient/outpatient visits or ≥1 dispensed antimicrobial | ||
| Treatment episodes, n | 667 | 259 |
| Infectious disease, n (%) | 226 (33.9) | 94 (36.3) |
| Adjusted RR (95% CI), TMLE | 0.87 (0.77, 0.99) | 1.00 (reference) |
| Adjusted RR (95% CI), MPR | 0.87 (0.70, 1.07) | 1.00 (reference) |
| Infectious disease at 6 months identified from inpatient/outpatient visits or cause of death data | ||
| Treatment episodes, n | 667 | 259 |
| Infectious disease, n (%) | 45 (6.7) | 31 (12.0) |
| Adjusted RR (95% CI), TMLE | 0.56 (0.41, 0.76) | 1.00 (reference) |
| Adjusted RR (95% CI), MPR | 0.60 (0.36, 1.01) | 1.00 (reference) |
| Infectious disease at 6 months; no history of methotrexate or azathioprine being dispensed at trial start | ||
| Treatment episodes, n | 379 | 191 |
| Infectious disease, n (%) | 26 (6.9) | 23 (12.0) |
| Adjusted RR (95% CI), TMLE | 0.62 (0.39, 0.99) | 1.00 (reference) |
| Adjusted RR (95% CI), MPR | 0.64 (0.34, 1.22) | 1.00 (reference) |
RR from TMLE analyses and in modified Poisson models adjusted for age at trial entry and sarcoidosis diagnosis, sex, region of residence, birth country, education, civil status, calendar period, healthcare visits within 6 months before trial start, history of congestive heart disease, atrial fibrillation, acute myocardial infarction, stroke, hypertension, diabetes mellitus, dyslipidaemia, chronic obstructive pulmonary disease, asthma and autoimmune disease, dispensed defined daily doses of systemic corticosteroids, non‐steroidal anti‐inflammatory drug or an antimicrobial medication being dispensed within 6 months before trial start.
Trial emulation run between June 2007 and March 2013 to allow for enough time to evaluate exclusion criteria and ascertain infectious disease.
MPR, modified Poisson regression; RR, risk ratio; TMLE, targeted maximum likelihood estimation.
Replicating all analyses with modified Poisson regression yielded almost identical point estimates for the RR except for the analysis for hospitalized infection (RR: 0.80 vs 0.69 for TMLE); albeit 95% robust CI were wider than the ones from TMLE. Lastly, we found that to nullify the association we observed in the main analysis an association between an unmeasured confounder with both exposure and outcome (E‐value) of strength 2.90 on the RR scale would be needed (or of strength 1.74 for CI of the TMLE analysis to include the null value 1).
DISCUSSION
No comparative safety trials contrasting infection risk in individuals treated with methotrexate compared to azathioprine exist in either sarcoidosis or other inflammatory diseases. In this target trial emulation using Swedish nationwide register data, we found that compared to azathioprine, initiation of methotrexate was associated with a 43% lower risk of infectious disease at 6 months in sarcoidosis (RR: 0.57). The favourable effect of methotrexate on infection risk was stronger at 3 months (RR: 0.47), but attenuated at 9 months (RR: 0.77).
The observed lower risk of infection associated with methotrexate is in line with descriptive data suggesting that infectious diseases might be less common in patients with sarcoidosis treated with methotrexate than azathioprine. 14 It remains unknown which pharmacodynamic mechanisms render methotrexate more advantageous regarding infection risk in sarcoidosis or other inflammatory diseases. 24 , 25 , 26 One may hypothesize that azathioprine is a more potent immunosuppressant and/or elicits its effect earlier than methotrexate does, which could explain the stronger association in favour of methotrexate at 3 months and the attenuation observed at 9 months after the initiation of treatment. Observational and experimental data in sarcoidosis and several other immune‐mediated diseases have shown mixed results in terms of safety and efficacy of these drugs. 10 , 11 , 12 , 13 , 14
This target trial emulation has several advantages over a traditional pharmacoepidemiological investigation. Similar to a randomized target trial, a well‐defined research question was determined in advance and guided identification of necessary data and their analysis. In addition, mimicking features of the target trial and being explicit about study design and data analysis decisions, we minimized immortal time bias and selection bias. 27 Specifically, pre‐defined inclusion and exclusion criteria in this emulation allowed us to obtain a group of individuals with sarcoidosis who needed a steroid‐sparing treatment and at the same time reduce confounding and selection bias originating from inclusion in the analyses of prevalent users of the treatments under comparison. Another major benefit of a trial emulation is that treatment allocation (i.e. initiation of methotrexate or azathioprine), eligibility assessment and start of follow‐up are designed to coincide thus further limiting immortal time and selection bias.
Despite the meticulous planning and conduct of the trial emulation, there are some fundamental ways in which it diverges from the target trial. One is randomization; a powerful feature of clinical trials that eliminates confounding by indication, that is, allocation of treatments that is intentionally or inadvertently influenced by factors related to the outcome (e.g. sarcoidosis severity). Although we did not randomize individuals into treatment groups, we believe that the impact of confounding by indication on our results is small. Despite the fact that methotrexate was more commonly prescribed, both methotrexate and azathioprine were equally advocated as steroid‐sparing treatments for sarcoidosis during the study period (2007–mid‐2013). 28 , 29 In addition, we adjusted for proxies of sarcoidosis severity (e.g. differences in corticosteroid dose) to improve the comparability of the two treatment groups.
Nevertheless, to minimize the possibility of residual confounding, we used TMLE that compared to standard outcome‐based estimators is both doubly robust and more efficient when combined with data‐adaptive modelling techniques. 21 Regarding double robustness, we did not observe any advantage of TMLE as point estimates from TMLE and modified Poisson regression models were similar. An exception was the analysis with the lowest power where we restricted to infections resulting in hospitalizations in which TMLE indicated a somewhat larger association (RR: 0.80 vs 0.69). TMLE's efficiency manifested in all analyses where 95% CI were considerably narrower than those estimated using Poisson models. Lastly, we believe that residual confounding of the magnitude estimated by the E‐value (2.90 on the RR scale) that could explain away our findings is very unlikely.
Another way in which our emulation deviates from a clinical trial is the lack of standardization of the dose or administration scheme of methotrexate and azathioprine. Assuming that treating physicians followed the recommended administration schemes, 18 we believe that our findings are similar to those that would have been obtained from a pragmatic clinical trial. 15 We observed, however, a preference for prescribing methotrexate (mimicking worldwide patterns 28 ) in all regions but the West, which we adjusted for in our analyses. In addition, we could not blind participants or physicians to the treatment received. A possible issue with the latter is differential misclassification of infectious disease. To reduce its impact on our findings, we restricted to infectious diseases that were classified as the primary discharge diagnosis and tested several other definitions, all of which resulted in the same conclusion.
Compared to the target trial, allowing for multiple treatment episodes per individual to improve power was at the expense of a slightly higher risk of confounding and selection bias (‘prevalent user’ bias). Indeed, individuals in the methotrexate group were more likely to be exposed to the medication in the past than those in the azathioprine group (21% vs 12%; Fig. 3). However, a sensitivity analysis in which we excluded participants who were ever dispensed the two medications yielded very similar results to the main analysis (RR: 0.62 vs 0.57, respectively) suggesting little, if any, bias was present in the main analysis.
Figure 3.
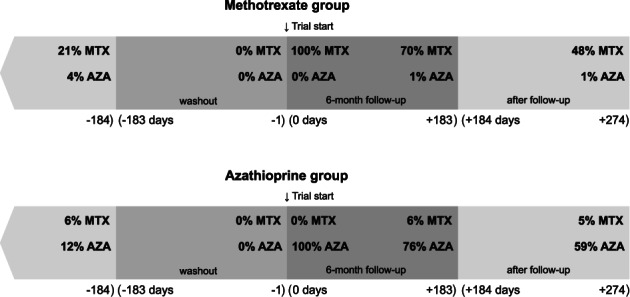
Methotrexate (MTX) and azathioprine (AZA) being dispensed before and after trial start (defined as ≥1 medication being dispensed in the specified time window). Dispensations for the time window (−∞, −184 days) were evaluated in the first episode of medication initiation in each unique individual.
We should also note that the treatment initiation effect that we estimated corresponds to a per‐protocol effect under the assumptions of full treatment adherence. In a post hoc analysis, we found that more than 70% of methotrexate or azathioprine initiation episodes were followed by a prescription refill of the trial medication during the 6‐month follow‐up for infectious disease suggesting a relatively high and similar between‐group adherence to treatment (Fig. 3). Similar proportions of adherence were observed in other patient cohorts in which methotrexate and azathioprine are frequently used. 8 , 30 , 31 Nevertheless, lack of randomization, blinding and information on other adverse events as well as incomplete adherence remain important limitations of an emulated versus the target trial.
To conclude, results from this emulation of a target trial with pre‐existing data suggest that initiation of methotrexate as compared to azathioprine is associated with a lower risk of infectious disease at 6 months. The favourable effect of methotrexate regarding infection risk is less sustained at 9 months. Although our findings are in support of recent recommendations for the treatment of pulmonary sarcoidosis 32 that endorse methotrexate as the primary steroid‐sparing alternative in the absence of specific contraindications (i.e. severe liver, renal or bone marrow disease or prospect of pregnancy for methotrexate and thiopurine methyltransferase deficiency or use of allopurinol for azathioprine), future prospective studies are warranted before developing informed guidelines for sarcoidosis treatment.
Author contributions
Conceptualization: M.R., S.K., D.D.G., A.E., J.G., J.A., E.V.A. Data curation: M.R. Formal analysis: M.R. Methodology: M.R., D.D.G., J.A. Visualization: M.R. Funding acquisition: E.V.A. Resources: E.V.A. Supervision: E.V.A. Writing—original draft: M.R. Writing—review and editing: M.R., S.K., D.D.G., A.E., J.G., J.A., E.V.A.
Supporting information
Table S1 Protocol of the target and the emulated trial with Swedish register data to assess the 6‐month risk of infectious disease in methotrexate versus azathioprine initiators in sarcoidosis.
Table S2 Description of variable definitions used in the analyses including the ICD and ATC codes used to define diseases in the National Patient, Cancer and Prescribed Drug Registers (PDR). Dispensation data in the PDR were available starting from 1 July 2005.
Acknowledgements
The study was supported by a grant from the Swedish Heart‐Lung Foundation (Hjärt‐Lungfonden; project no. 20170412). Data linkage used in this study was funded by a grant from the Swedish Society of Medicine (Svenska Läkaresällskapet). Sarcoidosis research at Karolinska Institutet is also supported by the Swedish Research Council (Vetenskapsrådet), the Strategic Research Area in Epidemiology at Karolinska Institutet (SfoEpi) and by a regional agreement on medical training and clinical research (ALF) between Region Stockholm and Karolinska Institutet.
Rossides M, Kullberg S, Di Giuseppe D, et al. Infection risk in sarcoidosis patients treated with methotrexate compared to azathioprine: A retrospective ‘target trial’ emulated with Swedish real‐world data. Respirology. 2021;26:452–460. 10.1111/resp.14001
Associate Editor: Paul Thomas; Senior Editor: Chris Grainge
Data availability statement
The data sets used for the conduct of this study are covered by ethics and secrecy agreements and are not publicly available.
REFERENCES
- 1. Ungprasert P, Crowson CS, Matteson EL. Sarcoidosis increases risk of hospitalized infection. A population‐based study, 1976–2013. Ann. Am. Thorac. Soc. 2017; 14: 676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rossides M, Kullberg S, Eklund A, Di Giuseppe D, Grunewald J, Askling J, Arkema EV. Risk of first and recurrent serious infection in sarcoidosis: a Swedish register‐based cohort study. Eur. Respir. J. 2020; 56: 2000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rossides M, Kullberg S, Eklund A, Grunewald J, Arkema EV. Sarcoidosis diagnosis and treatment in Sweden: a register‐based assessment of variations by region and calendar period. Respir. Med. 2020; 161: 105846. [DOI] [PubMed] [Google Scholar]
- 4. Pande A, Culver DA. Knowing when to use steroids, immunosuppressants or biologics for the treatment of sarcoidosis. Expert Rev. Respir. Med. 2020; 14: 285–98. [DOI] [PubMed] [Google Scholar]
- 5. James WE, Baughman R. Treatment of sarcoidosis: grading the evidence. Expert Rev. Clin. Pharmacol. 2018; 11: 677–87. [DOI] [PubMed] [Google Scholar]
- 6. Baughman RP, Lower EE. A clinical approach to the use of methotrexate for sarcoidosis. Thorax 1999; 54: 742–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc. Diffus. Lung Dis. 2000; 17: 60–6. [PubMed] [Google Scholar]
- 8. Fang C, Zhang Q, Wang N, Jing X, Xu Z. Effectiveness and tolerability of methotrexate in pulmonary sarcoidosis: a single center real‐world study. Sarcoidosis Vasc. Diffus. Lung Dis. 2019; 36: 217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arnold MH, O'Callaghan J, McCredie M, Beller EM, Kelly DE, Brooks PM. Comparative controlled trial of low‐dose weekly methotrexate versus azathioprine in rheumatoid arthritis: 3‐year prospective study. Rheumatology 1990; 29: 120–5. [DOI] [PubMed] [Google Scholar]
- 10. Jeurissen MEC, Agnes AM, Van De Putte LBA, Doesburg WH, Mulder J, Rasker JJ, Kruijsen MW, Haverman JF, van Beusekom HJ, Muller WH. Methotrexate versus azathioprine in the treatment of rheumatoid arthritis. A forty‐eight–week randomized, double‐blind trial. Arthritis Rheum. 1991; 34: 961–72. [DOI] [PubMed] [Google Scholar]
- 11. Pagnoux C, Mahr A, Hamidou MA, Boffa JJ, Ruivard M, Ducroix JP, Kyndt X, Lifermann F, Papo T, Lambert M et al. Azathioprine or methotrexate maintenance for ANCA‐associated vasculitis. N. Engl. J. Med. 2008; 359: 2790–803. [DOI] [PubMed] [Google Scholar]
- 12. Schram ME, Roekevisch E, Leeflang MMG, Bos JD, Schmitt J, Spuls PI. A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J. Allergy Clin. Immunol. 2011; 128: 353–9. [DOI] [PubMed] [Google Scholar]
- 13. Heckmann JM, Rawoot A, Bateman K, Renison R, Badri M. A single‐blinded trial of methotrexate versus azathioprine as steroid‐sparing agents in generalized myasthenia gravis. BMC Neurol. 2011; 11: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vorselaars ADM, Wuyts WA, Vorselaars VMM, Zanen P, Deneer VHM, Veltkamp M, Thomeer M, van Moorsel CHM, Grutters JC. Methotrexate vs azathioprine in second‐line therapy of sarcoidosis. Chest 2013; 144: 805–12. [DOI] [PubMed] [Google Scholar]
- 15. Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol. 2016; 183: 758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernán MA, Robins JM. Target Trial Emulation. Causal Inference: What if. Boca Raton, FL, Chapman & Hall/CRC, 2020. [Google Scholar]
- 17. Socialstyrelsen . Kvalitet och innehåll i patientregistret. Utskrivningar från slutenvården 1964–2007 och besök i specialiserad öppenvård (exklusive primärvårdsbesök) 1997–2007 [Quality and contents of the National Patient Register]. Swedish National Board of Health and Welfare, Stockholm, 2009. [Accessed 4 Feb 2019.] Available from URL: https://www.socialstyrelsen.se
- 18. Svensk Lungmedicinsk Förening . Vårdprogram för sarkoidos [Sarcoidosis care recommendations]. Göteborg: Mediahuset AB, 2018. [Accessed 11 Mar 2019.] Available from URL: https://slmf.se/vardprogram
- 19. van der Laan MJ, Rose S. Targeted Learning in Data Science. Cham, Springer International Publishing, 2018. [Google Scholar]
- 20. Gruber S, van der Laan MJ. tmle: an R package for targeted maximum likelihood estimation. J. Stat. Softw. 2012; 51: 1–35.23504300 [Google Scholar]
- 21. Schuler MS, Rose S. Targeted maximum likelihood estimation for causal inference in observational studies. Am. J. Epidemiol. 2017; 185: 65–73. [DOI] [PubMed] [Google Scholar]
- 22. Polley E, LeDell E, Kennedy C, van der Laan M. SuperLearner: Super Learner Prediction. R package version 2.0‐26. 2019. [Accessed 16 Sep 2019.] Available from URL: https://CRAN.R-project.org/package=SuperLearner
- 23. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann. Intern. Med. 2017; 167: 268–74. [DOI] [PubMed] [Google Scholar]
- 24. Broen JCA, van Laar JM. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat. Rev. Rheumatol. 2020; 16: 167–78. [DOI] [PubMed] [Google Scholar]
- 25. Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020; 16: 145–54. [DOI] [PubMed] [Google Scholar]
- 26. Maksimovic V, Pavlovic‐Popovic Z, Vukmirovic S, Cvejic J, Mooranian A, Al‐Salami H, Mikov M, Golocorbin‐Kon S. Molecular mechanism of action and pharmacokinetic properties of methotrexate. Mol. Biol. Rep. 2020; 47: 4699–708. [DOI] [PubMed] [Google Scholar]
- 27. Hernán MA, Sauer BC, Hernández‐Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self‐inflicted injuries in observational analyses. J. Clin. Epidemiol. 2016; 79: 70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schutt AC, Bullington WM, Judson MA. Pharmacotherapy for pulmonary sarcoidosis: a Delphi consensus study. Respir. Med. 2010; 104: 717–23. [DOI] [PubMed] [Google Scholar]
- 29. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence‐based recommendations. Expert Rev. Clin. Immunol. 2012; 8: 95–103. [DOI] [PubMed] [Google Scholar]
- 30. Hope HF, Hyrich KL, Anderson J, Bluett J, Sergeant JC, Barton A, Cordingley L, SMM V; RAMS Co‐Investigators . The predictors of and reasons for non‐adherence in an observational cohort of patients with rheumatoid arthritis commencing methotrexate. Rheumatology 2019; 59: 213–23. [DOI] [PubMed] [Google Scholar]
- 31. Chan W, Chen A, Tiao D, Selinger C, Leong R. Medication adherence in inflammatory bowel disease. Intest. Res. 2017; 15: 434–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rahaghi FF, Baughman RP, Saketkoo LA, Sweiss NJ, Barney JB, Birring SS, Costabel U, Crouser ED, Drent M, Gerke AK et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur. Respir. Rev. 2020; 29: 190146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Protocol of the target and the emulated trial with Swedish register data to assess the 6‐month risk of infectious disease in methotrexate versus azathioprine initiators in sarcoidosis.
Table S2 Description of variable definitions used in the analyses including the ICD and ATC codes used to define diseases in the National Patient, Cancer and Prescribed Drug Registers (PDR). Dispensation data in the PDR were available starting from 1 July 2005.
Data Availability Statement
The data sets used for the conduct of this study are covered by ethics and secrecy agreements and are not publicly available.


