Abstract
Vilaprisan is a novel selective progesterone receptor modulator for the long‐term treatment of uterine fibroids and endometriosis. This study investigated the pharmacokinetics, safety, and tolerability of vilaprisan in healthy Chinese postmenopausal women. Twelve participants received multiple doses of vilaprisan once daily over 14 days as a 2‐mg tablet. Plasma vilaprisan concentrations were determined using liquid chromatography–tandem mass spectrometry. The main pharmacokinetic parameters of vilaprisan were assessed with noncompartmental analysis, including maximum observed concentration (Cmax), systemic exposure (area under the plasma concentration–time curve), time to reach Cmax and terminal half‐life. Safety assessments include the documentation of adverse events, measurement of clinical/anthropometric parameters and vital signs, electrocardiogram, and physical and gynecologic examination. The participants had a mean age of 53.3 (± 4.2) years and a body mass index of 23.8 ± 2.8 kg/m2. Median time to reach Cmax was 1.5 hours after both single and multiple vilaprisan administration. Mean Cmax values obtained after multiple dosing (23.3 μg/L [standard deviation (SD) = 6.73]) were 1.92‐fold (SD = 0.554) higher compared to single dosing (12.5 μg/L [SD = 3.04]). Mean area under the plasma concentration–time curve in the dosing interval increased with an accumulation factor of 2.98 (SD = 0.767) between single (91.3 μg · h/L [SD = 20.4]) and multiple dosing (276 μg · h/L [SD = 109]). The mean terminal half‐life of vilaprisan was 44.5 hours (SD = 10.3) after multiple dosing. Mild to moderate adverse events were observed similar to previous studies. Overall, daily oral administration of the therapeutic dose of 2 mg of vilaprisan over 14 days was safe and well tolerated by all participants.
Keywords: pharmacokinetics, safety, vilaprisan, Chinese, uterine fibroids, endometriosis
Gynecologic disorders such as uterine fibroids and endometriosis depend on the action of progesterone and its receptor. 1 Selective progesterone receptor modulators, which have direct effects on fibroids by blocking the progesterone receptor, inhibiting cell proliferation, and stimulating apoptosis, 2 , 3 seem to offer effective options for the treatment of these diseases. 1 Vilaprisan is a promising novel selective progesterone receptor modulators that is under development for the long‐term treatment of symptomatic uterine fibroids and endometriosis 4 , 5 , 6 , 7 (Figure 1).
Figure 1.
Structure of vilaprisan.
Uterine fibroids occur in about 30% to 40% of all women of reproductive age, 8 which is mainly characterized by heavy menstrual bleeding and pelvic pain. 9 Endometriosis is present in about 10% to 15% of all women of reproductive age, and common symptoms are chronic pelvic pain and infertility. 10
In a phase 1 study including healthy postmenopausal women, vilaprisan showed linear pharmacokinetics (PK) up to a dose of 30 mg after daily oral administration for 28 consecutive days leading to systemic exposures that are 6‐ to 30‐fold above the therapeutic dose and was well tolerated. 11 Vilaprisan is almost completely eliminated from plasma by hepatic metabolism. Cytochrome P450 (CYP) 3A4/5 has been identified as the main enzyme involved in the metabolism of vilaprisan based on in vitro studies. The role of CYP3A4/5 was subsequently confirmed by clinical drug–drug interaction studies with the potent CYP3A4 inhibitor itraconazole and the potent CYP3A4 inducer rifampicin that showed a mean increase by 6.2‐fold and a reduction by 96% of the vilaprisan exposure, respectively. 12 , 13 Biotransformation occurs mainly by oxidation reactions at the steroid skeleton, as well as reductions of the 3‐keto group, leading to a complex metabolite pattern with rather low exposure (<10%) to single metabolites in plasma. The main metabolite M‐4 (3‐hydroxy derivative) elicits only marginal pharmacologic activity at the progesterone receptor, not contributing to clinical efficacy. Previous phase 2 studies showed that the administration of 2 mg of vilaprisan daily led to effective heavy menstrual bleeding control, marked reductions in fibroid volume, and improvements in health‐related quality of life of patients in combination with a favorable tolerability, 14 , 15 and thus this dose was selected for the phase 3 studies. However, comparable data on PK and tolerability of vilaprisan in Chinese women are currently missing.
Therefore, the main objective of this study was to investigate PK and safety profiles of vilaprisan after multiple doses over 14 days as a 2‐mg tablet once daily in healthy Chinese postmenopausal women living in mainland China.
Methods
The study was conducted between June and November 2018 at one study center, Peking Union Medical College Hospital located in Beijing, China. The study protocol was reviewed and approved by the study site's Ethics Committee before the start of the study. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Council for Harmonisation guideline E6: Good Clinical Practice. All participants gave their written informed consent before entry into the study.
Participants
The study population consisted of women only because the envisaged indications are uterine fibroids and endometriosis in women. To avoid a potential impact on menstrual cycle, the PK and safety profiles of vilaprisan were evaluated in postmenopausal women. No relevant differences in PK were expected between the envisaged target population (ie, women of reproductive age) and the participants actually investigated. Eligible participants were 45 to 65 years old with a body mass index of 19 to 30 kg/m2 (inclusive). Postmenopausal state was confirmed by medical history, if applicable (natural menopause at least 12 months before first administration of vilaprisan, or surgical menopause by bilateral ovarectomy at least 3 months before first administration of vilaprisan), follicle‐stimulating hormone >40 IU/L, and estradiol ≤20 pg/mL. Participants were excluded from the study if they had diseases that may have affected the PK of vilaprisan, tumors, clinically significant medical abnormality regarding metabolic organs, migraine, and depression. Regular use of medicine and physical and gynecologic abnormalities were also exclusion criteria. A complete list of participant selection criteria is provided in the Supplemental Information.
Study Design and Treatment
This was an open‐label, single‐arm, single‐center, phase 1 study to investigate the PK, safety, and tolerability of single and multiple doses of vilaprisan in healthy Chinese postmenopausal women living in mainland China. The study was divided into 4 sections: screening (6 weeks), predose (1 day), treatment (14 days), and posttreatment (14 days). The study participants spent 15 days at the study center during the in‐house phase. The study drug (vilaprisan 2‐mg tablet) was administered orally once daily in the morning, under fasting conditions, for 14 days. The participants were not offered further treatment after they completed the study. An overview of the study design is shown in Figure 2.
Figure 2.
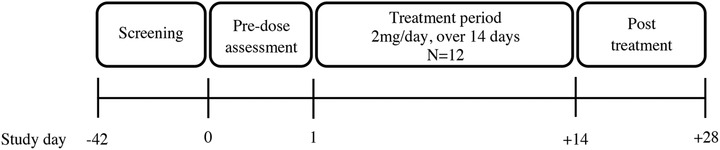
Study design overview.
Collection of Samples
Blood samples were collected to determine plasma vilaprisan concentrations before dosing and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 16, and 24 hours after the first dose of vilaprisan administered on day 1, and were collected before dosing and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 16, 24, 48, 72, 96, 168, 240, and 336 hours after the last dose of vilaprisan administered on day 14. To determine the plasma trough concentration of vilaprisan, blood samples were collected on days 2, 4, 7, 11, 12, and 13 before the morning dose.
Bioanalytical Methods
Vilaprisan was determined in human lithium heparinized plasma after addition of [13C6]vilaprisan as an internal standard followed by liquid‐liquid extraction using 1‐chlorobutane and chromatographic separation on a ACE Excel 2 C18 (Advanced Chromatography Technologies Ltd, Aberdeen, Scotland), 50 × 3.0 mm, 2‐μm analytical column using a methanol/ammonium acetate and formic acid isocratic system. For the mass spectrometric detection, a triple quadrupole mass spectrometer in negative TurboIonSpray ionization mode was applied (API 5000; Sciex, Framingham, Massachusetts). Detection was monitored using the m/z transition of 543.2 to 119.0 for vilaprisan and 549.2 to 119.0 for [13C6]vilaprisan. Calibration range was from 50.0 (lower limit of quantitation) to 50 000 ng/L (upper limit of quantitation). Accuracy and precision of vilaprisan determination in plasma are shown in Table S1. The within‐run precision (coefficient of variation [CV]) ranged between 0.99% and 8.67%, and the between‐run precision was 2.22% to 7.67%. All methods were validated according to the relevant European and US guidelines. 16 , 17
All samples were stored at –20°C and analyzed within 178 days after sample collection. The stability data indicated that the analyte is stable for this time period. The demonstrated stability is 544 days at –20°C.
Pharmacokinetic Analysis
PK parameters were assessed on the basis of concentration‐time data by a noncompartmental approach using the model‐independent (compartment‐free) method and the PK software WinNonlin (version 5.3, Certara, Princeton, New Jersey) in conjunction with the Automation Extension WinAE2.90 (Bayer AG, Leverkusen, Germany). The 2 main PK parameters for this study were maximum observed concentration (Cmax,md) and area under the concentration‐time curve during 24 hours dose interval (AUC0‐24,md) of vilaprisan after multiple dose administration on day 14. Additional PK parameters were Cmax, AUC0‐24, and time to reach Cmax (tmax) after single dosing on day 1. After multiple dosing, the following parameters were calculated: half‐life (t1/2,md), tmax,md, time of last observed concentration value above lower limit of quantitation (tlast,md), minimum observed drug concentration above lower limit of quantitation in plasma in the dose interval after multiple dosing (Cmin,md), average concentration in plasma (Cav,md), accumulation ratio calculated from AUC0‐24,md after multiple dosing and AUC0‐24 after single dosing (RAAUC), accumulation ratio calculated from Cmax,md after multiple dosing and Cmax after single dosing (RACmax), apparent oral clearance calculated from dose and AUC0‐24,md (CLmd/F). Plasma trough concentration was directly taken from observed data on Days 2, 4, 7, 11, 12, and 13. As t1/2 of vilaprisan was about 40 hours in previous studies, safety data were collected up to 14 days after last administration on Day 14. This time period corresponds to almost 7 times of the elimination half‐life (t1/2) of vilaprisan.
Safety Evaluation
The observation of safety started with signing the informed consent form (up to 6 weeks before first dose) and generally ended with the last post‐treatment visit (up to 14 days after the last administration of vilaprisan). Safety assessments included adverse events, clinical laboratory variables, physical examination, gynecological examination (inspection/palpation of the breast, inspection/palpation/transvaginal ultrasound of the genital organs, thin‐preparation cytologic test), blood pressure, heart rate, standard 12‐lead electrocardiogram (ECG). For any participant who discontinued the study prematurely for any reason after the administration of vilaprisan, series of safety examination was requested.
Statistical Analysis
No formal statistical sample size estimation has been performed for this PK and safety study. Based on prior experiences, 12 subjects were enrolled to have at least 8 PK‐evaluable participants completed. The statistical evaluation was conducted using the software package SAS release 9.2 (SAS Institute Inc., Cary, North Carolina). Descriptive statistics were used to summarize demographics, PK, and safety data. For quantitative variables, the number of observations, mean, minimum, median, and maximum were calculated. Geometric statistics such as geometric mean, geometric standard deviation (SD), and coefficient of variation (CV; both arithmetic and geometric) were provided additionally for PK data. For qualitative variables, frequency tables were used to summarize data. Medical history findings and treatment‐emergent vilaprisan‐related adverse events were summarized using Medical Dictionary for Regulatory Activities (MedDRA) terms.
Results
Study Participants
In total, 163 healthy postmenopausal women were enrolled into the study of which 12 participants were assigned to treatment. The most frequent reasons for screening failure are: not confirmed postmenopausal state (n = 24); clinically relevant findings in ECG (n = 20) or the gynecological examination (n = 19), criteria which in the opinion of the investigator preclude participation for the reasons of science, compliance, or safety (n = 16), systolic blood pressure <90 or ≥140 mmHg (n = 14), positive urine drug screening (n = 12) and diastolic blood pressure <60 or ≥90 mmHg (n = 10). Additionally, 16 subjects withdrew during screening period. Of the 12 participants assigned to treatment, 11 participants completed the study as planned. One participant withdrew the informed consent just after the first dose because of a family emergency, but completed the safety assessment required for the last visit.
Table 1 shows demographic data of the 12 female participants who all belonged to the Asian ethnicity. Participants had an age of 53.3 ± 4.2 years (mean ± SD) and a body mass index of 23.8 ± 2.8 kg/m2. All participants were non‐smoker and abstinent from alcohol use.
Table 1.
Demographics and Baseline Characteristics
| Variable | Total (N = 12) |
|---|---|
| Sex | |
| Female | 12 (100.0) |
| Race | |
| Asian | 12 (100.0) |
| Age, y | 53.3 ± 4.2 |
| Weight, kg | 58.1 ± 7.4 |
| Height, cm | 156.3 ± 5.5 |
| BMI, kg/m2 | 23.8 ± 2.8 |
| Alcohol breath test | |
| Negative | 12 (100.0) |
| Smoking history | |
| Never | 12 (100.0) |
| Other tobacco | |
| Never | 12 (100.0) |
| Alcohol use | |
| Abstinent | 12 (100.0) |
BMI, body mass index; SD, standard deviation.
Data are presented as N (%) or mean ± SD. Data are based on the safety analysis set.
Pharmacokinetics
Mean plasma concentration‐time profile was similar between single (first dose administration) and multiple dose administration (day 14) of vilaprisan (Figure 3). Vilaprisan was rapidly absorbed reaching peak plasma concentrations at 1.5 hours (median) both after single and after multiple drug administration. There was a subsequent rapid decline of plasma concentrations indicating a considerable tissue distribution. Following multiple dose administration, a relevant increase in trough plasma concentration of vilaprisan was observed from the second dose to 10th dose, and then remained nearly unchanged to 14th dose. It indicated that steady‐state level was achieved after 10 dose administrations (Figure 3).
Figure 3.
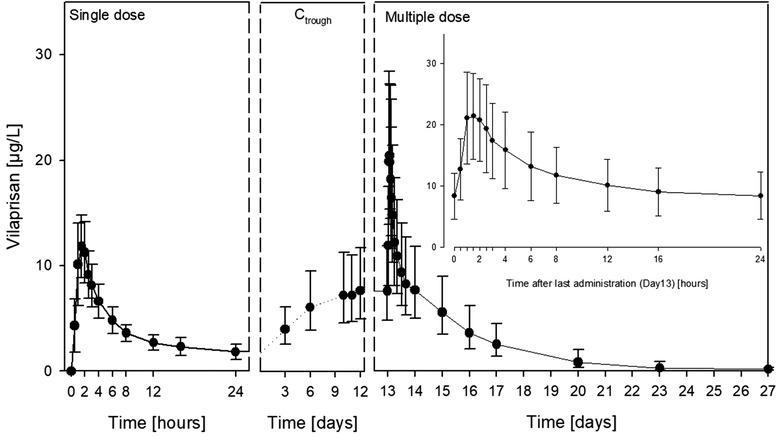
Plasma concentrations vs time curves of vilaprisan obtained after single‐ (day 1) and multiple‐dose (day 14) administrations of 2 mg of vilaprisan as well as trough concentrations obtained 3 to 12 days after first administration (N = 11). Data are presented as arithmetic mean and standard deviations. Ctrough, plasma trough concentration.
Summary statistics for PK parameters of vilaprisan are presented in Table 2. Median tmax values were 1.5 hours after both single (range, 1.00‐6.00 h) and multiple (range, 1.00‐2.50 h) dose administration. The arithmetic mean of the main parameter Cmax,md after multiple dose administration was 23.3 μg/L (SD = 6.73) and thus 1.92‐fold (SD = 0.554) higher than the Cmax of 12.5 μg/L (SD = 3.04) after single dose administration. After multiple dosing for 14 days, the arithmetic mean of the minimum observed plasma concentration was 8.2 μg/L (SD = 3.83), the arithmetic mean of the average concentration was 11.5 μg/L (SD = 4.55), and peak trough fluctuation was 136%.
Table 2.
Summary Statistics of Pharmacokinetic Parameters of Vilaprisan in Plasma
| Parameter | Geometric Mean | Geometric CV (%) | Arithmetic Mean | Arithmetic SD | Min | Median | Max |
|---|---|---|---|---|---|---|---|
| AUC0‐24 (μg · h/L) | 89.2 | 23.5 | 91.3 | 20.4 | 53.1 | 135 | |
| AUC0‐24,md (μg · h/L) | 258 | 39.8 | 276 | 109 | 148 | 505 | |
| Cav,md (μg/L) | 10.8 | 39.8 | 11.5 | 4.55 | 6.18 | 21.0 | |
| Cmax (μg/L) | 12.1 | 29.8 | 12.5 | 3.04 | 5.77 | 15.8 | |
| Cmax,md (μg/L) | 22.5 | 27.8 | 23.3 | 6.73 | 16.0 | 35.4 | |
| Cmin,md (μg/L) | 7.52 | 45.5 | 8.22 | 3.83 | 4.14 | 15.7 | |
| tmax (h) | 1.00 | 1.50 | 6.00 | ||||
| tmax,md (h) | 1.00 | 1.50 | 2.50 | ||||
| t1/2,md (h) | 43.4 | 22.9 | 44.5 | 10.3 | 30.8 | 66.8 | |
| tlast,md (h) | 168 | 336 | 336 | ||||
| RAAUC | 2.89 | 26.7 | 2.98 | 0.767 | 1.67 | 4.66 | |
| RACmax | 1.86 | 25.2 | 1.92 | 0.554 | 1.43 | 3.36 | |
| CLmd/F (L/h) | 7.75 | 39.8 | 8.27 | 3.10 | 3.96 | 13.5 |
AUC, area under the concentration vs time curve; AUC0‐24, AUC from time zero to 24 h after dosing; Cav,md, average concentration; Cmax, maximum observed concentration; Cmin,md, minimum observed concentration above lower limit of quantitation in the dose interval after multiple dosing; CLmd/F, apparent oral clearance calculated from dose and AUC0‐24,md; CV, coefficient of variation; md, multiple dose; RAAUC, accumulation ratio calculated as AUC0‐24,md/AUC0‐24; RACmax, accumulation ratio calculated as Cmax,md/Cmax; SD, standard deviation; t1/2,md, terminal half‐life after multiple dose; tlast,md, time of last observed concentration value above lower limit of quantitation; tmax, time to reach Cmax.
Data are presented on the pharmacokinetic analysis set (N = 11).
The arithmetic mean of AUC0‐24 was 91.3 μg · h/L (SD = 20.4) after a single dose and was elevated to 276 μg · h/L (SD = 109) after multiple dosing. The exposure to vilaprisan in the 24‐hour dosing interval increased with an arithmetic accumulation factor of 2.98 (SD = 0.767) between single and multiple dosing. After multiple daily dosing for 14 days, the arithmetic mean apparent oral clearance was 8.27 L/h (SD = 3.10).
The elimination of vilaprisan from plasma was characterized by a mean terminal half‐life of 44.5 hours (SD = 10.3) after multiple dosing.
The interindividual variability in PK parameters was low or moderate (CV, 22.9%‐45.5%).
Safety
Eleven participants (91.7%) received the scheduled cumulative oral dose of 28 mg of vilaprisan. One participant (8.3%) received 2 mg of vilaprisan and did not complete the treatment due to withdrawal by the participant because of a family emergency. As all 12 participants (100%) received at least 1 dose of the vilaprisan, they all were included in the safety analysis.
In total, for 9 participants (75.0%) treatment‐emergent adverse events (TEAEs) were reported that were considered to be related to vilaprisan administration according to the investigator (Table 3). The vilaprisan‐related TEAEs were of mild intensity for 8 subjects (66.7%) and of moderate intensity for 1 subject (8.3%), reported as moderate left ovarian cyst. The most frequently documented preferred terms were “blood triglycerides increased” (6 participants; 50.0%), “abdominal distention” (3 participants; 25.0%), and “fatigue” (2 participants; 16.7%). Most of these drug‐related TEAEs were learned from previous studies on multiple doses of vilaprisan and were predictable. All adverse events were resolved at the end of the study or were in the process of resolving. No participant experienced a serious adverse event or death, and there were no study discontinuations due to adverse events.
Table 3.
Number of Participants With Treatment‐Emergent Vilaprisan‐Related Adverse Events by Primary System Organ Class and Preferred Term
| Primary SOC PT MedDRA version 21.1 | Total N = 12 (100.0%) |
|---|---|
| Subjects with at least 1 such AE | 9 (75.0) |
| Gastrointestinal disorders | 4 (33.3) |
| Abdominal distention | 3 (25.0) |
| Abdominal pain | 1 (8.3) |
| Epigastric discomfort | 1 (8.3) |
| General disorders and administration site conditions | 2 (16.7) |
| Fatigue | 2 (16.7) |
| Investigations | 8 (66.7) |
| Alanine aminotransferase increased | 1 (8.3) |
| Aspartate aminotransferase increased | 1 (8.3) |
| Blood triglycerides increased | 6 (50.0) |
| Ultrasound of uterus abnormal | 1 (8.3) |
| Nervous system disorders | 1 (8.3) |
| Headache | 1 (8.3) |
| Reproductive system and breast disorders | 1 (8.3) |
| Cervical cyst | 1 (8.3) |
| Hydrometra | 1 (8.3) |
| Ovarian cyst | 1 (8.3) |
| Pelvic fluid collection | 1 (8.3) |
| Postmenopausal hemorrhage | 1 (8.3) |
| Skin and subcutaneous tissue disorders | 1 (8.3) |
| Eczema | 1 (8.3) |
AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; PT, preferred term; SOC, system organ class.
Data are presented as n (%). Data are based on the Safety Analysis Set. AEs are sorted in alphabetical order by primary SOC and PT.
There were no clinically relevant changes in vital signs (heart rate, blood pressure, body temperature) and ECG parameters.
In this study, transvaginal ultrasound was performed before administration and on days 14 and 28 after administration. One participant had findings at day 14 and day 28 regarded as moderate left ovarian cyst and mild endometrial echo asymmetry, and then she suffered from mild postmenopausal bleeding from day 35 for 3 days, all of these were reported as vilaprisan‐related TEAEs.
None of the result of cervical smear at day 28 were judged as clinically significant by investigators.
Discussion
This study investigated the PK properties, safety, and tolerability of vilaprisan after single and multiple doses of daily administration of 2 mg of vilaprisan in 12 Chinese postmenopausal women. The data obtained from this study will contribute to the data package needed for developing this compound for the treatment of symptomatic uterine fibroids and endometriosis.
Pharmacokinetics
Vilaprisan was absorbed rapidly after single and multiple oral administrations of a 2‐mg daily dose. Peak plasma concentration occurred 1.5 hours after both single and multiple dosing of vilaprisan. The elimination of vilaprisan from plasma was 44.5 hours after multiple dosing. This is very similar to white postmenopausal women where geometric mean elimination half‐lives in the range of 37.9 to 43.2 hours were determined after multiple dosing. 11
Vilaprisan accumulated after multiple dosing resulting in a 1.92‐fold higher Cmax and 2.98‐fold higher AUC0‐24 values at steady state compared to single dose. Similar accumulations were observed in white postmenopausal women showing 1.43 and 1.56‐fold higher Cmax values, and 1.85 and 2.39‐fold higher AUC0‐24 values after multiple dosing of 1 and 5 mg vilaprisan, respectively. 11
In summary, the PK profile of vilaprisan in Chinese women is similar to the one observed in previous studies including white women.
Safety
This study showed that the multiple administration of daily‐dose 2‐mg vilaprian was safe and well tolerated in the Chinese postmenopausal women. The most frequently reported vilaprisan‐related TEAEs were blood triglycerides increased, abdominal distention, and “fatigue.” Moderate left ovarian cyst, mild endometrial echo asymmetry, and postmenopausal bleeding were observed in 1 subject. The majority of these safety findings were similar to the findings observed in previous phase 1 5 , 11 , 12 , 13 , 18 , 19 , 20 and phase 2 6 , 14 studies. Further PK, safety, and efficacy data from patients including Chinese women will be collected in the phase 3 program that is currently on partial clinical hold to allow for thorough evaluation as a precautionary measure due to preclinical findings in toxicologic long‐term studies with vilaprisan in rodents.
Conclusions
Vilaprisan was safe and well tolerated after single and multiple doses as daily 2‐mg administration for 14 days in Chinese postmenopausal women. Safety and PK profile showed that the multiple daily therapeutic dose of 2 mg of vilaprisan, which was selected for clinical development in phase 3, was also appropriate for Chinese women. Vilaprisan had a similar safety profile between this study and several previous phase 1 and phase 2 studies. Similar elimination half‐lives and accumulations after multiple dosing were observed in Chinese postmenopausal women as in white postmenopausal women.
Funding
This study was funded by Bayer AG.
Data Sharing
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supporting information
Supplementary material
Supplementary material
Acknowledgments
The authors thank all study participants and investigators for their contributions to the study, and Antonia Kohnke and Ercan Suekuer, Bayer AG, Berlin, Germany, for support with data analysis and figures. The clinical part of the study was conducted at Peking Union Medical College Hospital located in Beijing, China, and supported by Chinese National Major Project for New Drug Innovation (2019ZX09734001). Clinical laboratory analyses including hormone measurements were carried out locally. All safety laboratory and ECG determinations as well as the readouts of transvaginal ultrasound were performed locally at Peking Union Medical College Hospital (Beijing, China). PK bioanalysis was performed by Syneos Health Clinique (Québec, Canada). All the authors provided substantial contributions to the development and conduct of the study. J.J. was the principal investigator of the study. Medical writing assistance for this article was provided by M.A.R.C.O. GmbH & Co. KG, Düsseldorf, Germany, on behalf of Bayer AG, Berlin, Germany.
References
- 1. Möller C, Hoffmann J, Kirkland TA, et al. Investigational developments for the treatment of progesterone‐dependent diseases. Expert Opin Investig Drugs. 2008;17(4):469‐479. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Ohara N, Wang J, et al. A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab. 2006;91(4):1296‐1304. [DOI] [PubMed] [Google Scholar]
- 3. Horak P, Mara M, Dundr P, et al. Effect of a selective progesterone receptor modulator on induction of apoptosis in uterine fibroids in vivo. Int J Endocrinol. 2012;2012:436174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wagenfeld A, Bone W, Schwede W, et al. BAY 1002670: a novel, highly potent and selective progesterone receptor modulator for gynaecological therapies. Hum Reprod. 2013;28(8):2253‐2264. [DOI] [PubMed] [Google Scholar]
- 5. Schütt B, Kaiser A, Schultze‐Mosgau MH, et al. Pharmacodynamics and safety of the novel selective progesterone receptor modulator vilaprisan: a double‐blind, randomized, placebo‐controlled phase 1 trial in healthy women. Hum Reprod. 2016;31(8):1703‐1712. [DOI] [PubMed] [Google Scholar]
- 6. Bradley L, Ren X, Groettrup‐Wolfers E, et al. Results of the asteroid (assess safety and efficacy of vilaprisan in patients with uterine fibroids) 1 study: A phase 2, placebo‐controlled dose finding study. Fertil Steril. 2016;106(3):e95‐e96. [Google Scholar]
- 7. Möller C, Bone W, Cleve A, et al. Discovery of vilaprisan (BAY 1002670): a highly potent and selective progesterone receptor modulator optimized for gynecologic therapies. ChemMedChem. 2018;13(21):2271‐2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100‐107. [DOI] [PubMed] [Google Scholar]
- 9. Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344‐1355. [DOI] [PubMed] [Google Scholar]
- 10. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789‐1799. [DOI] [PubMed] [Google Scholar]
- 11. Schultze‐Mosgau MH, Schuett B, Hafner FT, et al. Pharmacokinetics and safety of the selective progesterone receptor modulator vilaprisan in healthy postmenopausal women. Int J Clin Pharmacol Ther. 2017;55(1):16‐24. [DOI] [PubMed] [Google Scholar]
- 12. Schultze‐Mosgau MH, Hoechel J, Prien O, et al. Characterization of the pharmacokinetics of vilaprisan: Bioavailability, excretion, biotransformation, and drug‐drug interaction potential. Clin Pharmacokinet. 2018;57(8):1001‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chattopadhyay N, Kanacher T, Casjens M. CYP3A4‐mediated effects of rifampicin on the pharmacokinetics of vilaprisan and its UGT1A1‐mediated effects on bilirubin glucuronidation in humans. Br J Clin Pharmacol. 2018;84:2857‐2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bradley LD, Singh SS, Simon J, et al. Vilaprisan in women with uterine fibroids: the randomized phase 2b ASTEROID 1 study. Fertil Steril. 2019;111(2):240‐248. [DOI] [PubMed] [Google Scholar]
- 15. Seitz A, Bumbuliene Z, Costa A, et al. Rationale and design of ASTEROID 2, a randomized, placebo‐ and active comparator‐controlled study to assess the efficacy and safety of vilaprisan in patients with uterine fibroids. Contem Clin Trials. 2017;55:56‐62. [DOI] [PubMed] [Google Scholar]
- 16. Guideline on Bioanalytical Method Validation , European Medicines Agency, Committee for Medicinal Products for Human Use. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf. Published 2011. Accessed June 2020.
- 17. FDA CDER. Guidance for Industry: Bioanalytical Method Validation. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf. Published 2018. Accessed June 2020.
- 18. Chattopadhyay N, Riecke K, Ligges S, et al. Effect of hepatic impairment on the pharmacokinetics of vilaprisan: an open‐label, single‐dose, parallel‐group study. Br J Clin Pharmacol. 2019;85(9):2011‐2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schultze‐Mosgau MH, Lasseter K, Marbury T, et al. Pharmacokinetics and safety of the novel selective progesterone receptor modulator vilaprisan in participants with renal impairment [published online ahead of print, March 30, 2020]. J Clin Pharmacol. 10.1002/jcph.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schütt B, Schultze‐Mosgau MH, Draeger C, et al. Effect of the novel selective progesterone receptor modulator vilaprisan on ovarian activity in healthy women. J Clin Pharmacol. 2018;58(2):228‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material


