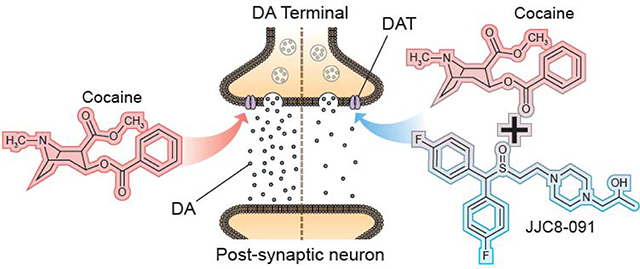
Abstract
Pharmacotherapeutics for treatment of psychostimulant use disorder are still an unmet medical goal. Recently, off label use of modafinil (MOD), an approved medication for treatment of sleep disturbances, has been tested as a therapeutic for cocaine and methamphetamine use disorder. Positive results have been found in subjects dependent on psychostimulants without concurrent abuse of other substances. Novel structural analogs of MOD have been synthesized in the search for compounds with potentially broader therapeutic efficacy than the parent drug. In the present report we review their potential efficacy as treatments for psychostimulant abuse and dependence assessed in preclinical tests. Results from these preclinical proof of concept studies reveal that some modafinil analogs do not possess typical cocaine-like neurochemical and behavioral effects. Further, they might blunt the reinforcing effects of psychostimulants in animal models, suggesting their potential efficacy as pharmacotherapeutics for treatment of psychostimulant use disorders.
Graphical Abstract
Introduction
Substance use disorders (SUD) affect a large and growing number of individuals. Psychostimulants, like cocaine, account for a large portion of this patient population. Of the 70,237 deaths in the US from drug abuse in 2017, 33% involved cocaine or other psychostimulants [1]. In spite of this growing burden, there is still lack of FDA approved medications for psychostimulant use disorder (PSUD). Recently, modafinil (MOD), a medication approved for narcolepsy and sleep disorders that shares with cocaine the ability to block the dopamine transporter (DAT), has been explored as a potential pharmacological treatment for PSUD [2]. These studies have suggested positive results, but with therapeutic efficacy limited by comorbid abuse of alcohol or other drugs [2,3]. Novel structural analogs of MOD have been synthesized in the search for compounds with potentially broader therapeutic efficacy than their parent drug. Herein, we review recent neurochemical and behavioral preclinical studies on MOD and selected MOD-analogs in animal models of PSUD.
Overview on the role of DAT in physiological and pathological conditions and as a therapeutic target for PSUD
DA neurotransmission plays a role in many vital processes including affective-, motor- and cognitive-functions [4]. DAT’s homeostatic role as the primary regulator of DA synaptic availability draws attention in psychiatric disorders linked to impaired DA function [5], including PSUD [5,6]. Pharmacological targeting of DAT can rapidly alter extracellular DA levels, an effect widely described following psychostimulant administration [7]. Further, binding affinity to DAT is predicted to play a role in psychostimulant abuse potential [6]. Unsurprisingly, compounds that compete with psychostimulants to inhibit DAT, without concomitant addictive liability, among them MOD and its R-enantiomer, have become the focus of potential agonist substitution therapies for the treatment of PSUD [8–10]. The general rationale for agonist substitution therapy for SUD is that replacing drugs of abuse with pharmacotherapeutics that exert a similar mechanism of action in the brain will facilitate abstinence, compliance, and might prevent relapse by mitigating withdrawal symptoms and craving [11,12]. Indeed, the ability of some of these compounds to elicit a mild, long-lasting stimulation of extracellular DA levels may ameliorate hypodopaminergic dysfunction described in preclinical models of addiction and in human cocaine addicts [reviewed in: 13,14]. Hypo-functionality of the dopaminergic system in the addicted brain could be a fundamental therapeutic target [14], and restoration of an impoverished DA system might thus provide potential efficacy to attenuate drug craving and relapse [13–16]. A recent systematic review and meta-analysis on studies about prescription psychostimulants for the treatment of stimulant use disorder found significant clinical beneficial effects in promoting abstinence especially in cocaine users [17]. However, none of the canonical DAT inhibitors that are FDA approved for other therapeutic endpoints such as treatment of Attention Deficit Hyperactivity Disorder (ADHD) and tested as agonist therapy for PSUD have proven fully successful, due to a combination of factors including, clinical efficacy, potential abuse liability, and significant side-effects [18].
MOD and its novel structural analogs: a new generation of atypical DAT inhibitors
Early studies suggested that all drugs that inhibit DAT would produce cocaine-like reinforcing effects with a potency directly related to DAT affinity [19]. However, several atypical DAT blockers [8,10] that inhibit the uptake of DA with high affinity, without the hallmark reinforcing properties of typical cocaine-like DAT inhibitors, have been discovered. Their effects suggest a potential ligand-specific control over DAT function. Typical and atypical inhibitors have been shown to induce/prefer different conformations of DAT and this has subsequently been linked to the unique behavioral and neurochemical effects of these DAT inhibitors [8].
Recently, interest has shifted to a new generation of atypical DAT inhibitors derived from MOD and its (R)-enantiomer ((R)-MOD) as potential medications for the treatment of PSUD [20–23]. These novel compounds display atypical mild psycho-stimulating DAT inhibition profiles without significant abuse liability. Starting from the development of structure activity relationships (SAR) derived from the design and synthesis of novel compounds with higher DAT affinity and improved solubility, we have embarked on molecular, neurochemical and behavioral studies aimed at better understanding their mechanism of action and potential therapeutic utility for the treatment of PSUD.
Drug Design of novel MOD analogs
Beginning with the hypothesis that DA uptake inhibitors that prefer a more occluded inward facing conformation may have an atypical behavioral profile, we identified MOD as a starting point for SAR studies [23]. Our goal was to discover an atypical DAT inhibitor with drug-like properties and a behavioral profile that would be useful as a treatment for PSUD [24–26]. One of our early leads, JJC8–016 (Table 1), showed promise in animal models of PSUD [21]. However, our subsequent synthetic campaign led to a more DAT-selective series of MOD analogues with a piperazine linker e.g., JJC8–088 and JJC8–091 (Table 1). In rodent studies, JJC8–091 reduced cocaine self-administration and cocaine-primed reinstatement of cocaine seeking [27] as well as attenuating compulsive methamphetamine self-administration in both short and long access procedures in rats [28]. In contrast, JJC8–088 was ineffective in these models.
Table 1:
Neurochemical changes evoked by MOD and its analogs. Doses were administered as noted (i.p. = intraperitoneal, i.v. = intravenous, s.c. = subcutaneous injections, or p.o. = oral). Increases/decreases in DA are marked by arrows (↑/↓) while non-significant changes are marked by =. DA dynamics were measured in the CNS (DS = dorsal striatum, NAcc = nucleus accumbens, NAC = nucleus accumbens core, NAS = nucleus accumbens shell, S = striatum, VS = ventral striatum).
| DAT Inhibitor | Dose (mg/kg) | In combination with | Neurochemical changes | SPECIES, References | ||
|---|---|---|---|---|---|---|
| Microdialysis | Voltammetry | |||||
| DA levels (↑/↓) | DAMAX (↑/↓) | DA clearance rate (↑/↓) | ||||
 |
0.5–1.0, i.v. | ↑↑ NAS, ↑ NAC | RATS, [36] | |||
| 100–300, i.p. | ↑ NAcc | RATS, [63] | ||||
| 10–55, i.p. | ↑↑ NAS | MICE, [23] | ||||
| 3–10, i.p. | ↑↑ NAS | RATS, [64] | ||||
| 1–30, i.p. | ↑↑ NAS | RATS, [38] | ||||
| 17–300, i.p. | ↑ NAC, ↑ NAS | MICE, [41] | ||||
| 0.5–56, i.p. | ↑↑ NAS | ↓ NAS | MICE, [20] | |||
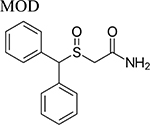 |
30–300, s.c. | ↑ NAcc | RATS, [65] | |||
| 20–60, i.v. | ↑ NAcc | RATS, [40] | ||||
| 20, i.v. | Methamphetamine | ↓ NAcc | RATS, [40] | |||
| 0–300, i.p. | ↑ NAS | MICE, [23] | ||||
| 100–300, p.o. | ↑ S | RATS, [66] | ||||
| 30–300, i.p. | ↑ DS, ↑ VS | RATS, [50] | ||||
| 10–56, i.v. | ↑ NAS | RATS, [42] | ||||
| 10–32, i.p. | Cocaine | = NAS | RATS, [42] | |||
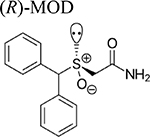 |
30–300, i.p. | ↑ NAS | MICE, [23] | |||
| x | ↑ NAcc | RATS, [22] | ||||
| 30–100, i.p. | Nicotine | ↓ NAcc | RATS, [22] | |||
| 10–32, i.v. | ↑ NAS | ↑ NAS | ↓ NAS | RATS, [45] | ||
| 5–100, i.p. | ↑ NAS | ↓ NAS | MICE, [20] | |||
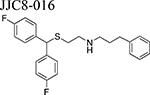 |
10–30, i.p. | = NAcc | RATS, [21] | |||
| 0.5–56, i.p. | = NAS | = NAS | ↓ NAS | MICE, [20] | ||
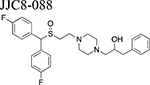 |
0.5–56, i.p. | ↑↑ NAS | ↑↑ NAS | ↓ NAS | MICE, [20] | |
| 1–56, i.p. | ↑↑ NAS | ↑↑ NAS | ↓↓ NAS | RATS, [27] | ||
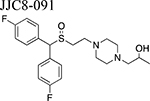 |
5–56, i.p. | ↑ NAS | = NAS | ↓ NAS | MICE, [20] | |
| 10–56, i.p. | ↑ NAS | ↑ NAS | ↓ NAS | RATS, [27] | ||
More recently, by introducing the 2,6-dimethyl substitution on the piperazine ring, some improvement in drug-like properties was realized, with RDS03–94 [29]. Nevertheless, the piperazine ring remained a metabolically labile functional group and hence a new series of analogues in which it was replaced with an amino-piperidine function was prepared[30]. These new analogues demonstrated superior metabolic stability and are currently being evaluated in rodent models of PSUD. In addition, novel heterocyclic-based analogues that may also be promising new leads for PSUD therapeutics have recently been reported [31,32].
DA Neurochemistry of MOD and lead analogs
The effects of MOD on brain neurotransmission, including norepinephrine, serotonin, acetylcholine, glutamate and GABA, have been reviewed by Mereu and colleagues [2]. In this section we will review recent studies showing how MOD and its analogues affect dopaminergic neurotransmission (see Table 1).
Brain Microdialysis Studies
Studies of changes in brain DA levels following psychostimulant administration in rodents using microdialysis procedures [33] have greatly increased our understanding of their mechanisms of action related to abuse and dependence [34–36]. Rapid and transient stimulation of DA levels in brain striatal areas, particularly in the nucleus accumbens shell (NAS) and core (NAC), were identified as indicative of abuse potential for drugs [34,35,37], including typical DAT blockers and methamphetamine [36,38,39].
Similarly to abused psychostimulants, MOD and its enantiomers dose-dependently increased DA efflux in the NAS and NAC [22,23,40,41], but with a slower, longer-lasting and less efficacious stimulation of DA release [23,41,42]. These reports suggest a possible dopaminergic ceiling effect for MOD and its enantiomers even at very high, but not toxic doses [20,23,41,42]. Taken together, these differences in time course and efficacy in stimulating DA levels indicate an atypical DAT blocker profile for MOD, in agreement with the low, if any, abuse liability in clinical and preclinical studies [42–44]. Further, these mild agonist activities and long duration of action fit the desired pharmacological profile for agonist substitution therapy, facilitating patient compliance and attenuating withdrawal symptoms [11,12].
Among the MOD analogs, JJC8–088, like cocaine, produced a robust, dose-dependent stimulation in NAS DA levels [20,27]. Instead, JJC8–091 elicited a blunted increase in DA levels compared to JJC8–088 or cocaine [20,27], while JJC8–016 did not significantly modify NAS DA levels at any dose tested [20,21]. JBG1–048 and JBG1–049, two bis F-MOD enantiomers, administered intravenously, produced dose-dependent increases in DA efflux similar to, but longer lasting than those elicited by (R)-MOD [45] or MOD [42]. Noticeably, among this series of analogs, molecular simulation studies showed that only JJC8–088 stabilizes DAT in an outward-facing open conformation similar to cocaine [27], in agreement with its robust cocaine-like stimulation of DA levels.
Of interest, in terms of treatment for PSUD, MOD blunted methamphetamine-induced increases in accumbens DA efflux [40]. Also, in a recent report, MOD failed to significantly affect cocaine-induced stimulation of NAS DA levels at doses that significantly potentiate cocaine-maintained self-administration behavior. The lack of dopaminergic influence on this MOD effect has been suggested to result from its facilitation of electrotonic coupling [42]. Interestingly, electrotonic coupling has been connected to the therapeutic actions of MOD on sleep disorders and cognitive impairment [2,46], effects also related to PSUD [47,48].
Fast scan cyclic voltammetry studies
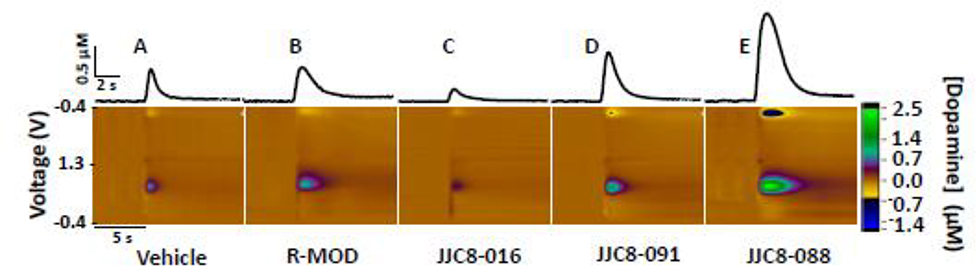
Studies of DA dynamics via FSCV procedures report the ability of cocaine to reduce DA clearance rate in brain striatal areas, as well as to enhance the maximum amplitude of DA release (DAMAX) (Figure 1) following medial forebrain bundle electrical stimulation in mice [20,49]. (R)-MOD reduced NAS DA clearance rate similarly to cocaine, but with minimal effects on DAMAX compared to cocaine [20]. However, significant effects of high doses of MOD on DAMAX and DA clearance rate were observed in the dorsal and ventral striatum in rats [50]. Interestingly, at variance with abused psychostimulants [36,37], MOD enhanced DAMAX more in the dorsal than in the ventral striatum, suggesting a potential explanation of its limited abuse liability. All the MOD analogs tested so far reduced NAS DA clearance rate in rodents [20,27,45], denoting DA uptake inhibition. A strong correlation between a compound’s DAT affinity and ability to reduce DA clearance rate (IC75) further supports these findings [20]. However, these MOD analogs differentially increased NAS DAMAX. While JJC8–088 elicited effects akin to those of cocaine, JJC8–091 and JJC8–016 did not [20,27]. Further, there was not a significant relationship between the ability of these compounds to increase DAMAX and their DAT affinity, suggesting this process may involve alternative mechanisms in addition to DAT blockade [20]. Importantly, recent results [51] reveal that pretreatment with atypical DAT blockers (JJC8–016, JJC8–091 and JHW007) before cocaine administration produce additive effects on DA clearance rate similarly to those elicited by typical DAT blockers (JJC8–088, Methylphenidate and WIN 35428) pretreatment, still suggesting their efficacy as DAT blockers. However, at variance with them, atypical DAT blockers attenuated or blunt the effects of cocaine on elicited NAS DAMAX (Keighron et al, unpublished results) in agreement with previously published microdialysis data [9]. We would hypothesize that these differences in NAS DAMAX alterations by atypical DAT blockers may play a role in their behavioral effects in combination with typical psychostimulants.
Figure 1: Effects of R-MOD and MOD analogs on DA dynamics in mice accumbens shell.
Representative FSCV color plots, redrawn from [20], demonstrating the effects of R-MOD and MOD analogs, or their vehicle, on the elicited DA peak, DAMAX and clearance rate of NAS DA from the electrode. The inset above each color plot represents the DA concentration vs time corresponding to the measurement on the color plot.
Behavioral Pharmacology of MOD and lead analogs
A major complication to medication development for PSUD is the lack of animal models with good predictive clinical validity. This is particularly challenging, as unlike the opioids, there are no effective pharmacotherapies that can be reverse translated into predictive animal models. Different aspects or stages in the addiction cycle have been modelled to evaluate the efficacy of MOD and its analogs as potential pharmacotherapies to treat PSUD. Intravenous drug self-administration, conditioned place preference (CPP) and intracranial self-stimulation (ICSS) are the most commonly employed models to measure potential reinforcing actions of abused drugs, while reinstatement of extinguished drug-seeking behavior has been widely used to evaluate relapse propensity after abstinence [52].
MOD and (R)-MOD studies
The therapeutic efficacy of MOD and its (R)-enantiomer assessed in PSUD models have provided mixed results (Table 2). Neither support self-administration in naïve rats and do not substitute for cocaine or nicotine in maintaining drug-seeking [42,53–55]. MOD also failed to produce place preference across a wide range of doses [55,56]. MOD and (R)-MOD have no effects on cocaine self-administration [21,55] or amphetamine-induced CPP [56], although a recent report found that MOD pretreatment enhanced cocaine self-administration [42]. Further, (R)-MOD significantly decreased cocaine-taking, but only when low cocaine doses were presented in a dose-response assessment [21]. In reinstatement tests, MOD has been reported to either reduce drug seeking primed by cocaine, methamphetamine, or drug-associated environmental cues [57–59] or have no effect [55]. Similarly, (R)-MOD, at 100 mg/kg, inhibited cocaine-induced reinstatement [21], and in another report, it significantly reduced methamphetamine self-administration during the first hour of short- (1 hr) and long- (6 hr) access procedures [28]. In contrast, other reports found that MOD substituted for cocaine in non-human primates and produced cocaine-like drug discrimination in rats [54] and mice [41].
Table 2:
Summary of the major findings of MOD and its analogs in animal models of psychostimulant reward and relapse. The doses in which various effects are observed are italicized. CPP=Conditioned Place Preference; METH=methamphetamine; IVSA=intravenous self-administration; oICSS=optical intracranial self-stimulation.
| MOD analog | Dose (mg/kg) | Major behavioral findings | Species, References |
|---|---|---|---|
| MOD | 32,64, 128 | No effect on cocaine self-administration No effect on cocaine-primed reinstatement No change in cocaine CPP |
RATS, [55] |
| 64 | No change in d-amphetamine CPP No effect on d-amphetamine locomotor activity |
RATS, [56] | |
| 10, 17, 32 | ↑ cocaine self-administration | RATS, [42] | |
| 30, 100, 300 | ↓ METH primed reinstatement ↓ cue induced reinstatement for METH IVSA |
RATS, [59] | |
| 30, 100, 300 | ↓ METH self-administration and METH primed reinstatement ↓ Context induced reinstatement when administered during abstinence ↑ Cue and ↓ METH primed reinstatement when administered during extinction ↓ Cue and METH primed reinstatement after 2 wks of abstinence and no MOD pretreatment |
RATS, [58] | |
| 300 | No effect on cocaine self-administration and ↓ cocaine reinstatement | RATS, [57] | |
| (R)-MOD | 10, 30, 100 | No effect on cocaine self-administration ↓ low dose cocaine self-administration No effect on cocaine hyperlocomotion ↓ cocaine primed reinstatement |
RATS, [21] |
| ↓ METH self-administration during the first hour of short and long access | RATS, [28] | ||
| JJC8-016 | 10, 30 | ↓ cocaine self-administration Shifted cocaine dose response curve ↓ ↓ cocaine induced hyperlocomotion |
RATS, [21] |
| ↓ short and long access METH self-administration | RATS, [58] | ||
| JJC8-088 | 3, 10, 30 | ↓ cocaine self-administration No effect on PR breakpoints |
RATS, [27] |
| ↑ oICSS | MICE, [27] | ||
| No effect on short and long access METH self-administration | RATS, [28] | ||
| JJC8-091 | 10, 30, 56 | No effect on cocaine self-administration ↓ PR breakpoints ↓ cocaine-primed reinstatement |
RATS, [27] |
| ↓ oICSS | MICE, [27] | ||
| 10, 30 56 | ↓ short access METH self-administration ↓ long access METH self-administration |
RATS, [28] | |
Unexpectedly, MOD or (R)-MOD alone significantly attenuated brain stimulation reward similar to cocaine-like psychostimulants [21,60,61].
Novel MOD analogs studies
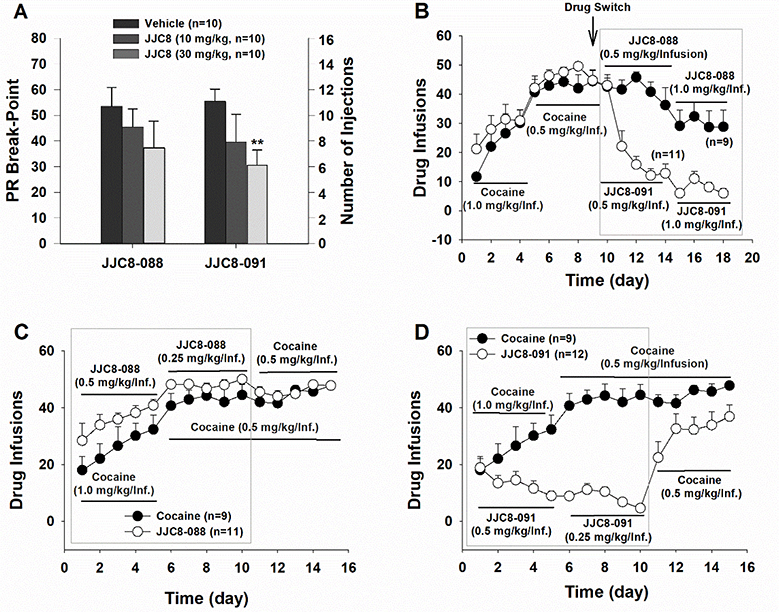
Drug-naïve rats do not self-administer JJC8–016 or JJC8–091, and both compounds fail to substitute for cocaine [21,27]. In contrast, JJC8–088 is self-administered by naïve rats and substitutes for cocaine in cocaine-experienced subjects (Table 2 and Figure 2) [27]. Systemic administration of JJC8–016 or JJC8–091 dose-dependently reduced both cocaine maintained self-administration and, following extinction, cocaine-induced reinstatement of drug-seeking behavior [21,27]. Further, JJC8–091 attenuated cocaine self-administration under progressive-ratio schedules of reinforcement [27]. Pretreatment with JJC8–088 slightly decreased cocaine self-administration under a fixed-ratio schedule but had no effect on progressive-ratio breakpoints, and reinstated drug seeking in rats following extinction from cocaine self-administration [27]. Also, JJC8–088 failed to, while JJC8–016 and JJC8–091 significantly reduced methamphetamine self-administration and escalation of its intake in rats [28]. Under brain-stimulation reward procedures in rats, JJC8–016 did not produce cocaine-like effects [21]. In optical intracranial self-stimulation procedures in mice, JJC8–091 shifted the DA-dependent stimulation-response curve downward, indicating a reduction in DA-based reward function, while JJC8–088 dose-dependently shifted this curve to the left, indicating potential cocaine-like effects [27].
Figure 2: Evaluation of the effects of JJC8–088 and JJC8–091 on self-administration or on cocaine self-administration behavior.
A: JJC8–091, but not JJC8–088, significantly lowered the number of cocaine self-administration (infusions) under the PR reinforcement schedule. B: JJC8088, but not JJC8–091, substitution sustained stable self-administration in rats that previously self-administered cocaine. C: Naive rats self-administered JJC8–088 intravenously similarly to cocaine. D: Naive rats did not self-administer JJC8–091 during the initial 10 days of self-administration training. When JJC8–091 was replaced by cocaine, animals rapidly acquired self-administration behavior for cocaine. **p < 0.01, compared to vehicle. Redrawn from [27].
Taken together, and in agreement with their dopaminergic effects, these behavioral experiments suggest that two of the novel MOD analogs, JJC8–091 and JJC8–016 produce more consistent atypical DAT inhibitor behavioral results as compared to the parent compounds. Indeed, while the parent compounds produced both cocaine-like and cocaine antagonist behavioral effects, depending on the animal model used, JJC8–091 and JJC8–016 do not act as behavioral reinforcers and consistently attenuated cocaine and methamphetamine reinforcing actions, in several behavioral paradigms. On the other hand, JJC8–088 consistently produced typical, cocaine-like psychostimulant effects in all the behavioral procedures tested, in agreement with its DA-stimulating effects.
Conclusions
Agonist substitution therapy has proven useful for the treatment of opioid and nicotine use disorders [11,12], but not fully successful for the treatment of PSUD [17]. The addictive potential of psychostimulants is positively correlated with rapid and transient stimulation in extracellular DA levels and DA neurotransmission [7,15]. Among pharmacotherapeutics tested for cocaine use disorder, MOD and (R)-MOD show an interesting pharmacodynamic profile with a slower onset, longer lasting and less efficacious alteration of DA dynamics [20–23] as compared to cocaine. This may, in part, explain why MOD analogs with atypical psychostimulant profile have lower addictive potential than abused psychostimulants. In addition, the ability of atypical DAT inhibitors to induce mild long-term increases in extracellular DA could contribute to ameliorating hypodopaminergic dysfunction in human cocaine addicts [13,14], thereby minimizing drug craving and relapse [14,16]. While some of the reviewed findings would predict that MOD and (R)-MOD potentially work as reinforcers in animals, there are only a few published reports of misuse of MOD in humans [62]. Moreover, beneficial effects of MOD on PSUD have been reported in selected psychostimulant addicted patient populations [3], confirming the difficulties to perfectly model behaviors predictive of abuse in humans or therapeutic efficacy of drugs [18].
Among the new generation of MOD analogs, neurochemical and behavioral assessments of JJC8–016 and JJC8–091 consistently produced an atypical DAT blocker pharmacological profile. Time course and pattern of dopaminergic effects were similar or improved compared to the parent drugs. Further, no cocaine-like reinforcing effects were displayed in any procedure tested, at variance with results with MOD or its (R)-enantiomer. These data demonstrate that these novel compounds have an atypical DAT inhibitor profile with minimal, if any, cocaine-like abuse potential, in contrast to JJC8–088, which cocaine-like reinforcing effects and absence of therapeutic efficacy make it an unlikely candidate for further drug development research. Collectively, compounds such as JJC8–016 and especially JJC8–091 provide proof of concept and suggest that new drugs with an atypical DAT inhibitor profile may have utility as potential pharmacotherapies for the treatment of PSUD, a fundamental and yet unmet public heath need.
Acknowledgements
We thank Lauren Brick (NIDA-IRP Visual-Media) for providing the artwork related to the Graphical Abstract.
Funding: This work was supported in part by the Medication Development Program (Z1A-DA000611), National Institute on Drug Abuse, Intramural Research Program, NIH, DHHS
Footnotes
Declaration of interest: AHN and JJC are inventors on the licensed US patent E-073–2013/0-US-06 -NIH0072US2 in which JJC8–088 and JJC8–091 are disclosed. The NIH owns all rights to this patent. All other authors declare that they have no interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Kariisa M, Scholl L, Wilson N, Seth P, Hoots B: Drug overdose deaths involving cocaine and psychostimulants with abuse potential—United States, 2003–2017. Morbidity and mortality weekly report 2019, 68:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mereu M, Bonci A, Newman AH, Tanda G: The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology (Berl) 2013, 229:415–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kampman KM, Lynch KG, Pettinati HM, Spratt K, Wierzbicki MR, Dackis C, O’Brien CP: A double blind, placebo controlled trial of modafinil for the treatment of cocaine dependence without co-morbid alcohol dependence. Drug Alcohol Depend 2015, 155:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD: Mesolimbic dopamine signals the value of work. Nature neuroscience 2016, 19:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salatino-Oliveira A, Rohde LA, Hutz MH: The dopamine transporter role in psychiatric phenotypes. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 2018, 177:211–231.* This review article provides a brief update on preclinical and clinical studies on DAT researchincluding DAT structure and function, as well as its implications in brain disorders.
- 6.Solinas M, Belujon P, Fernagut PO, Jaber M, Thiriet N: Dopamine and addiction: what have we learned from 40 years of research. Journal of Neural Transmission 2019, 126:481–516.* This review summarizes all the major preclinical and clinical research findings on the criticalrole of dopamine and the dopaminergic system in the physiopathology of addiction.
- 7.Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R, et al. : Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995, 52:456–463. [DOI] [PubMed] [Google Scholar]
- 8.Reith ME, Blough BE, Hong WC, Jones KT, Schmitt KC, Baumann MH, Partilla JS, Rothman RB, Katz JL: Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter. Drug and alcohol dependence 2015, 147:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanda G, Newman AH, Ebbs AL, Tronci V, Green JL, Tallarida RJ, Katz JL: Combinations of cocaine with other dopamine uptake inhibitors: assessment of additivity. The Journal of pharmacology and experimental therapeutics 2009, 330:802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanda G, Newman AH, Katz JL: Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Advances in pharmacology 2009, 57:253–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan CJ, Cao J, Newman AH, Xi ZX: Progress in agonist therapy for substance use disorders: Lessons learned from methadone and buprenorphine. Neuropharmacology 2019, 158:107609.** This paper examines agonist replacement strategies for opioid use disorder and highlights progress towards development of an agonist replacement therapy for cocaine use disorder.
- 12.Jordan CJ, Xi ZX: Discovery and development of varenicline for smoking cessation. Expert Opin Drug Discov 2018, 13:671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melis M, Spiga S, Diana M: The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol 2005, 63:101–154. [DOI] [PubMed] [Google Scholar]
- 14.Diana M: The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry 2011, 2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkow ND, Fowler JS, Wang GJ: Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol 1999, 13:337–345. [DOI] [PubMed] [Google Scholar]
- 16.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C: Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 2006, 26:6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tardelli VS, Bisaga A, Arcadepani FB, Gerra G, Levin FR, Fidalgo TM: Prescription psychostimulants for the treatment of stimulant use disorder: a systematic review and meta-analysis. Psychopharmacology (Berl) 2020, 237:2233–2255. [DOI] [PubMed] [Google Scholar]
- 18.Czoty PW, Stoops WW, Rush CR: Evaluation of the “Pipeline” for Development of Medications for Cocaine Use Disorder: A Review of Translational Preclinical, Human Laboratory, and Clinical Trial Research. Pharmacol Rev 2016, 68:533–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhar MJ, Ritz MC, Boja JW: The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 1991, 14:299–302. [DOI] [PubMed] [Google Scholar]
- 20.Keighron JD, Quarterman JC, Cao J, DeMarco EM, Coggiano MA, Gleaves A, Slack RD,Zanettini C, Newman AH, Tanda G: Effects of (R)-modafinil and modafinil analogues on dopamine dynamics assessed by voltammetry and microdialysis in the mouse nucleus accumbens shell. ACS chemical neuroscience 2019, 10:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang HY, Bi GH, Yang HJ, He Y, Xue G, Cao J, Tanda G, Gardner EL, Newman AH, Xi ZX: The Novel Modafinil Analog, JJC8–016, as a Potential Cocaine Abuse Pharmacotherapeutic. Neuropsychopharmacology 2017, 42:1871–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X-F, Bi G-H, He Y, Yang H-J, Gao J-T, Okunola-Bakare OM, Slack RD, Gardner EL, Xi Z-X, Newman AH: R-modafinil attenuates nicotine-taking and nicotine-seeking behavior in alcohol-preferring rats. Neuropsychopharmacology 2015, 40:1762–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loland CJ, Mereu M, Okunola OM, Cao J, Prisinzano TE, Mazier S, Kopajtic T, Shi L, Katz JL, Tanda G: R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biological psychiatry 2012, 72:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao J, Prisinzano TE, Okunola OM, Kopajtic T, Shook M, Katz JL, Newman AH: Structure-Activity Relationships at the Monoamine Transporters for a Novel Series of Modafinil (2-[(diphenylmethyl)sulfinyl]acetamide) Analogues. ACS Med Chem Lett 2010, 2:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao J, Slack RD, Bakare OM, Burzynski C, Rais R, Slusher BS, Kopajtic T, Bonifazi A, Ellenberger MP, Yano H, et al. : Novel and High Affinity 2-[(Diphenylmethyl)sulfinyl]acetamide (Modafinil) Analogues as Atypical Dopamine Transporter Inhibitors. Journal of Medicinal Chemistry 2016, 59:10676–10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okunola-Bakare OM, Cao J, Kopajtic T, Katz JL, Loland CJ, Shi L, Newman AH: Elucidation of structural elements for selectivity across monoamine transporters: novel 2-[(diphenylmethyl)sulfinyl]acetamide (modafinil) analogues. J Med Chem 2014, 57:1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman AH, Cao J, Keighron JD, Jordan CJ, Bi GH, Liang Y, Abramyan AM, Avelar AJ, Tschumi CW, Beckstead MJ, et al. : Translating the atypical dopamine uptake inhibitor hypothesis toward therapeutics for treatment of psychostimulant use disorders. Neuropsychopharmacology 2019, 44:1435–1444.** This study pools neurochemical, computational, and behavioral effects of atypical DAT inhibitors in rodents. Specifically, this paper highlights the potential efficacy of the MOD analog JJC8–091 as a pharmacotherapy for cocaine use disorder.
- 28.Tunstall BJ, Ho CP, Cao J, Vendruscolo JCM, Schmeichel BE, Slack RD, Tanda G, Gadiano AJ, Rais R, Slusher BS, et al. : Atypical dopamine transporter inhibitors attenuate compulsive-like methamphetamine self-administration in rats. Neuropharmacology 2018, 131:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slack RD, Ku TC, Cao J, Giancola JB, Bonifazi A, Loland CJ, Gadiano A, Lam J, Rais R, Slusher BS, et al. : Structure-Activity Relationships for a Series of (Bis(4-fluorophenyl)methyl)sulfinyl Alkyl Alicyclic Amines at the Dopamine Transporter: Functionalizing the Terminal Nitrogen Affects Affinity, Selectivity, and Metabolic Stability. J Med Chem 2020, 63:2343–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giancola JB, Bonifazi A, Cao J, Ku TC, Gadiano A, Lam J, Rais R, Coggiano M, Tanda G, Newman AH: Structure-Activity Relationships for a Series of (Bis(4-fluorophenyl)methyl)sulfinylethyl-aminopiperidines and -piperidine amines at the Dopamine Transporter: Bioisosteric Replacement of the Piperazine Improves Metabolic Stability. European journal of Medicinal Chemistry 2020, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalaba P, Aher NY, Ilic M, Dragacevic V, Wieder M, Miklosi AG, Zehl M, Wackerlig J, Roller A, Beryozkina T, et al. : Heterocyclic Analogues of Modafinil as Novel, Atypical Dopamine Transporter Inhibitors. J Med Chem 2017, 60:9330–9348. [DOI] [PubMed] [Google Scholar]
- 32.Kalaba P, Ilic M, Aher NY, Dragacevic V, Wieder M, Zehl M, Wackerlig J, Beyl S, Sartori SB, Ebner K, et al. : Structure-Activity Relationships of Novel Thiazole-Based Modafinil Analogues Acting at Monoamine Transporters. J Med Chem 2020, 63:391–417. [DOI] [PubMed] [Google Scholar]
- 33.Di Chiara G, Tanda G, Carboni E: Estimation of in-vivo neurotransmitter release by brain microdialysis: the issue of validity. Behav Pharmacol 1996, 7:640–657. [PubMed] [Google Scholar]
- 34.Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E: Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Annals of the New York Academy of Sciences 1999, 877:461–485. [DOI] [PubMed] [Google Scholar]
- 35.Di Chiara G, Tanda G, Cadoni C, Acquas E, Bassareo V, Carboni E: Homologies and differences in the action of drugs of abuse and a conventional reinforcer (food) on dopamine transmission: an interpretative framework of the mechanism of drug dependence. Advances in pharmacology 1998, 42:983–987. [DOI] [PubMed] [Google Scholar]
- 36.Pontieri FE, Tanda G, Di Chiara G: Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A 1995, 92:12304–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM: Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. Journal of Neuroscience 2008, 28:8821–8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohut SJ, Hiranita T, Hong S-K, Ebbs AL, Tronci V, Green J, Garcés-Ramírez L, Chun LE, Mereu M, Newman AH: Preference for distinct functional conformations of the dopamine transporter alters the relationship between subjective effects of cocaine and stimulation of mesolimbic dopamine. Biological psychiatry 2014, 76:802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munzar P, Tanda G, Justinova Z, Goldberg SR: Histamine h3 receptor antagonists potentiate methamphetamine self-administration and methamphetamine-induced accumbal dopamine release. Neuropsychopharmacology 2004, 29:705–717. [DOI] [PubMed] [Google Scholar]
- 40.Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH: Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. Journal of Pharmacology and Experimental Therapeutics 2009, 329:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mereu M, Chun LE, Prisinzano TE, Newman AH, Katz JL, Tanda G: The unique psychostimulant profile of (±)-modafinil: investigation of behavioral and neurochemical effects in mice. European Journal of Neuroscience 2017, 45:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mereu M, Hiranita T, Jordan CJ, Chun LE, Lopez JP, Coggiano MA, Quarterman JC, Bi G-H, Keighron JD, Xi Z-X, et al. : Modafinil potentiates cocaine self-administration by a dopamine-independent mechanism: possible involvement of gap junctions. Neuropsychopharmacology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR: Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology 2005, 182:186–193. [DOI] [PubMed] [Google Scholar]
- 44.Vosburg SK, Hart CL, Haney M, Rubin E, Foltin RW: Modafinil does not serve as a reinforcer in cocaine abusers. Drug and alcohol dependence 2010, 106:233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keighron JD, Giancola JB, Shaffer RJ, DeMarco EM, Coggiano MA, Slack RD, Newman AH, Tanda G: Distinct effects of (R)-modafinil and its (R)-and (S)-fluoro-analogs on mesolimbic extracellular dopamine assessed by voltammetry and microdialysis in rats. European Journal of Neuroscience 2019, 50:2045–2053.** This in vivo study analyzes the effects of R-MOD and MOD analogs on accumbens shell dopamine dynamics and related ambulatory stimulation in mice.
- 46.Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A: Electrical coupling: novel mechanism for sleep-wake control. Sleep 2007, 30:1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahoney JJ, Jackson BJ, Kalechstein AD, De La Garza R, Chang LC, Newton TF: Acute modafinil exposure reduces daytime sleepiness in abstinent methamphetamine-dependent volunteers. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 2012, 15:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valentino RJ, Volkow ND: Drugs, sleep, and the addicted brain. Neuropsychopharmacology 2019.** This review article provides neurobiological insights about the interconnectivity of sleep dysfunction and substance use disorder.
- 49.Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM: Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci 2006, 26:3206–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bobak MJ, Weber MW, Doellman MA, Schuweiler DR, Athens JM, Juliano SA, Garris PA: Modafinil activates phasic dopamine signaling in dorsal and ventral striata. Journal of Pharmacology and Experimental Therapeutics 2016, 359:460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keighron JD, Bonaventura J, Li Y, Cao J, DeMarco EM, Sandtner W, Michaelides M, Sitte HH, Newman AH, Tanda G: Cocaine increases the stimulation of dopamine release, at variance with atypical dopamine uptake inhibitors. New clues for the abuse liability of psychostimulants? In EB-ASPET; Orlando, FL: FASEB Journal, 33, S1: 895.13: 2019. [Google Scholar]
- 52.Xi ZX, Spiller K, Gardner EL: Mechanism-based medication development for the treatment of nicotine dependence. Acta Pharmacol Sin 2009, 30:723–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heal DJ, Buckley NW, Gosden J, Slater N, France CP, Hackett D: A preclinical evaluation of the discriminative and reinforcing properties of lisdexamfetamine in comparison to d-amfetamine, methylphenidate and modafinil. Neuropharmacology 2013, 73:348–358. [DOI] [PubMed] [Google Scholar]
- 54.Gold LH, Balster RL: Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology 1996, 126:286–292. [DOI] [PubMed] [Google Scholar]
- 55.Deroche-Gamonet V, Darnaudery M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV: Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology 2002, 161:387–395. [DOI] [PubMed] [Google Scholar]
- 56.Quisenberry AJ, Prisinzano TE, Baker LE: Modafinil alone and in combination with low dose amphetamine does not establish conditioned place preference in male Sprague-dawley rats. Experimental and Clinical Psychopharmacology 2013, 21:252–258. [DOI] [PubMed] [Google Scholar]
- 57.Mahler SV, Hensley-Simon M, Tahsili-Fahadan P, LaLumiere RT, Thomas C, Fallon RV, Kalivas PW, Aston-Jones G: Modafinil attenuates reinstatement of cocaine seeking: role for cystine-glutamate exchange and metabotropic glutamate receptors. Addict Biol 2014, 19:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reichel CM, See RE: Chronic modafinil effects on drug-seeking following methamphetamine self-administration in rats. The international journal of neuropsychopharmacology 2012, 15:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reichel CM, See RE: Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology (Berl) 2010, 210:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burrows BT, Watterson LR, Johnson MA, Olive MF: Effects of modafinil and R-modafinil on brain stimulation reward thresholds: implications for their use in the treatment of psychostimulant dependence. Journal of drug and alcohol research 2015, 4:235958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lazenka MF, Negus SS: Oral modafinil facilitates intracranial self-stimulation in rats: comparison with methylphenidate. Behavioural pharmacology 2017, 28:318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krishnan R, Chary KV: A rare case modafinil dependence. J Pharmacol Pharmacother 2015, 6:49–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferraro L, Antonelli T, O’Connor WT, Tanganelli S, Rambert FA, Fuxe K: Modafinil: an antinarcoleptic drug with a different neurochemical profile to d-amphetamine and dopamine uptake blockers. Biological psychiatry 1997, 42:1181–1183. [DOI] [PubMed] [Google Scholar]
- 64.Tanda G, Li SM, Mereu M, Thomas AM, Ebbs AL, Chun LE, Tronci V, Green JL, Zou M-F, Kopajtic TA: Relations between stimulation of mesolimbic dopamine and place conditioning in rats produced by cocaine or drugs that are tolerant to dopamine transporter conformational change. Psychopharmacology 2013, 229:307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferraro L, Tanganelli S, O’Connor WT, Antonelli T, Rambert F, Fuxe K: The vigilance promoting drug modafinil increases dopamine release in the rat nucleus accumbens via the involvement of a local GABAergic mechanism. European journal of pharmacology 1996, 306:33–39. [DOI] [PubMed] [Google Scholar]
- 66.Rowley HL, Kulkarni RS, Gosden J, Brammer RJ, Hackett D, Heal DJ: Differences in the neurochemical and behavioural profiles of lisdexamfetamine methylphenidate and modafinil revealed by simultaneous dual-probe microdialysis and locomotor activity measurements in freely-moving rats. Journal of psychopharmacology 2014, 28:254–269. [DOI] [PubMed] [Google Scholar]