Abstract
The coronavirus disease 19 (COVID-19) has turned out to be a pandemic in short period of time due to the high transmissibility of its causative agent, severe acute respiratory syndrome coronavirus 2. Various reports have suggested the promising link between overexpression of angiotensin converting enzyme 2 (ACE2) and COVID-19 pathogenesis. The severity of COVID-19 pathophysiology is greatly depended on several comorbidities, like hypertension, diabetes mellitus (DM), respiratory and cardiovascular disease, out of which DM has emerged as a major risk factor. The current review focuses on the link among the expression of ACE2, use of ACE inhibitors (ACEIs) and angiotensin II type 1 receptor blockers (ARBs), and risk of COVID-19 pathogenesis in DM. The review also emphasizes on synergistic detrimental effect of DM and COVID-19 on the immune system in provoking uncontrolled cytokine storm which eventually leads to lethal consequences. Finally, several possible therapeutic strategies have been highlighted to reduce the excess of risk associated with COVID-19 in people with DM.
Keywords: COVID-19, Diabetes Mellitus, Angiotensin converting enzyme 2 (ACE2), Cytokine storm
Abbreviations: ACE, angiotensin converting enzyme; ACEI, angiotensin converting enzyme inhibitor; AGEs, advanced glycation end products; ARDS, acute respiratory distress syndrome, CF, cystic fibrosis; Ang (1–7), angiotensin (1–7); Ang II, angiotensin II; ARB, angiotensin II type 1 receptor blocker; AT1R, angiotensin type 1 receptor; CCL, chemokine (C-C motif) ligand; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 19; CRP, C-reactive protein; CXCL10, C-X-C motif chemokine 10; DAMPs, damage-associated molecular patterns; DM, diabetes mellitus; DPP4, dipeptidyl peptidase IV; EG, envelope glycoprotein; GLP-1, FFA, free fatty acid glucagon-like peptide-1; GM-CSF, granulocyte–macrophage colony-stimulating factor; HAG, heamagglutinin-acetylesterase glycoprotein; HMGB-1, high mobility group box protein 1 ICU, intensive care unit; IKKβ, Ikβ kinase β; IL, interleukin; INF, interferon; JNK, c-Jun N-terminal kinase; LDL, low density lipoprotein; MAPK, mitogen-activated protein kinase; MasR, Mas receptor; MCP-1, monocyte chemoattractant protein-1; MERS, Middle Eastern respiratory syndrome; MERS-CoV, Middle Eastern respiratory syndrome coronavirus; MG, membrane glycoprotein; NCP, nucleocapsid phosphoprotein; NE, neutrophil elastase; NETs, neutrophil extracellular traps; NF-κβ, nuclear factor-κβ; PAD4, peptidylarginine deiminase type 4; PAMPs, pathogen-associated molecular patterns; PKC, Protein kinase C; PLC, phospholipase C; RAGE, receptor for advanced glycation end products; RAS, renin angiotensin system; SARS, severe acute respiratory syndrome; ROS, reactive oxygen species; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SG, spike glycoprotein; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TGR, triglyceride; Th, T helper; TLR, Toll-like receptors; TLR, toll like receptor; TMPRSS2, transmembrane protease, serine 2; TNF, tumour necrosis factor; Treg, T regulatory; UTI, urinary tract infection
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped, single stranded, positive sense RNA bearing novel coronavirus of genus Batacoronavirus, family Conornaviridae which also involves other two coronaviruses such as severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle Eastern respiratory syndrome coronavirus (MERS-CoV). An outbreak of severe acute respiratory syndrome (SARS) which was triggered by SARS-CoV appeared from mammals such as bat and palm civet [1], [2] in southern China in 2002–2003 and killed approximately 800 people. After a decade, MERS-CoV brought about an epidemic of Middle Eastern respiratory syndrome (MERS) in Soudi Arabia and caused 858 deaths [3]. In December 2019, a zoonotic outbreak of coronavirus disease 19 (COVID-19) which is caused by SARS-CoV-2 emerged in Wuhan, Hubei province, China [4], [5], [6]. Like, SARS and MERS, COVID-19 is expected as an air borne disease and spreads human to human through microdroplets which are released during exhalation, talking, and coughing [7]. As it spread worldwide rapidly, WHO announced COVID-19 as a pandemic. COVID-19 had been spread across the world, affected more than 100 million people, and caused a fatality more than 2 million individuals as of 1 February 2021 [8]. The high pathogenicity and virulence of SARS-CoV-2 may be gained due to antigenic drift and/or antigenic shift during the transfection of hosts’ cells. The usual indications of COVID-19 are fever, dry cough, tiredness, complication in breathing and sometimes pneumonia and multi-organ failure [9]. The severity of clinical manifestation is escalated with comorbidities of diabetes mellitus (DM), hypertension, cardiovascular diseases, and pulmonary disease.
DM, characterised by higher blood sugar resulting from impaired insulin secretion and/or insulin resistance, is a metabolic disorder which is associated with the higher risk of acquiring infectious diseases [10], [11], [12] due to impaired function of immune system [13]. Hyperglycemia and diabetes mellitus have been proved as independent prognosticator of morbidity and fatality in patients with SARS [14] as well as MERS [15]. Likewise, DM has emerged as a major risk factor which increases severe illness and death of patients with COVID-19 [16], [17], [18]. Some studies suggest that pre-existing DM is linked with the higher risk of acquiring COVID-19; nonetheless, a meta-analysis has shown that pre-existing DM does not rise the risk of SARS-COV-2 infection [19]. However, the outcome of COVID-19 may be exacerbated due to prior incidence of uncontrolled DM. The present review sheds light on the probable molecular mechanism of severity’s escalation of pathophysiology in COVID-19 patients associated with DM. The review emphasises on the relationship between the expression of virus’ cell surface receptors like ACE2 and TRPRSS2 and the risk of SARS-CoV-2 infection. This review also aims to provide possible molecular mechanisms of abnormal cytokine storm which is generated in COVID-19 patients associated with dysregulation of glucose metabolism and potential therapeutics thereof. In addition, a number of probable therapeutics has been discussed to reduce the elevated risk associated with COVID-19 in subjects with DM.
2. Prevalence of DM comorbidity in COVID-19 patients
The epidemiological and clinical manifestations of patients with COVID-19 have shown that the comorbidities including DM, hypertension, and cardiovascular disease, and other complications are very common in the patients [16], [17], [20], [21], [22], [23], [24], [25], [26]. Among these comorbidities, DM has emerged as a critical risk factor to the COVID-19 patients. A retrospective and multicentre cohort study has reported that 36 (19%) patients showed DM as second highest comorbidity among 191 patients with COVID-19, of whom 17 (31%) patients with DM died due to COVID-19 in hospital [16]. In a clinical investigation of 1099 patients with COVID-19, 173 patients were severely infected who exhibited comorbidities of DM (16.2%), hypertension (23.7%), and cardiovascular disease (5.8%) [17]. A retrospective study of 138 COVID-19 positive patients has demonstrated that 36 severely affected patients had hypertension (58.3%), cardiovascular disease (25.0%), and diabetes mellitus (22.2%) [20]. Another investigation has done on 58 severe COVID-19 patients who suffered from hypertension (37.9%) and diabetes mellitus (13.8%) [21]. The risk of diabetes in respect to poor early outcomes, like requirement of admission to ICU, mechanical ventilation, and death within 14 days after providing critical facilities was studied in 450 COVID-19 hospitalized patients. The study reported significantly higher requirement of ICU (42.1% vs 29.8%), mechanical ventilation and greater proportion of death in patients with diabetes compared to non-diabetes [27]. Surprisingly, the case-fatality rate study of a subsample with 355 patients who died due to SARS-CoV-2 infection reported that 126 (35.5%) patients had diabetes which was found as highest comorbid disease in the sample [28]. In addition, COVID-19 patients with DM showed higher percentage of severe abnormalities [16], [17], [20], [21], [22], [23], [24], [26], [28], [29], [30], [31]. The numbers of COVID-19 patients along with comorbidity of DM are summarized in Table 1 .
Table 1.
COVID-19 patients with comorbidity of diabetes mellitus.
| No. of patients |
Comorbidity of diabetes mellitus |
Refs | ||
|---|---|---|---|---|
| Total | Severe/ICU care/ Non-Survivor | Non-severe/Non-ICU care/Survivor | ||
| 191 | 36 (19%) | 17 (31%) | 19 (14%) | [16] |
| 1099 | 81 (7%) | 28 (16%) | 53 (6%) | [17] |
| 138 | 14 (10%) | 8 (22%) | 6 (6%) | [20] |
| 140 | 17 (12%) | 8 (14%) | 9 (11%) | [21] |
| 201 | 22 (11%) | 16 (19%) | 6 (5%) | [22] |
| 187 | 28 (15%) | 16 (31%) | 12 (9%) | [23] |
| 1590 | 130 (8%) | 45 (21%) | 85 (6%) | [24] |
| 41 | 8 (19%) | 1 (8%) | 7 (25%) | [25] |
| 52 | 9 (17%) | 7 (22%) | 2 (10%) | [26] |
| 355 | 126 (35%) | 126 (35.5%) | NA | [28] |
| 1561 | 153 (10%) | 122 | 31 | [29] |
| 4103 | 614 (15%) | 176 (27%) | 438 (14%) | [30] |
| 7337 | 952 (13%) | NA | NA | [31] |
| 5700 | 1808 (34%) | NA | NA | [32] |
| 1082 | 235 (21%) | 65(28%) | NA | [33] |
| 135 | 12 (9%) | 9 (23%) | 3 (3%) | [34] |
| 3481 | 566 (16%) | NA | NA | [35] |
| 19,256 | 3524 (18%) | NA | NA | [36] |
| 377 | 118 (31%) | 45 (40%) | 73 (28%) | [37] |
3. Expression of angiotensin converting enzyme 2 (ACE2) in DM and severity of COVID-19 pathophysiology
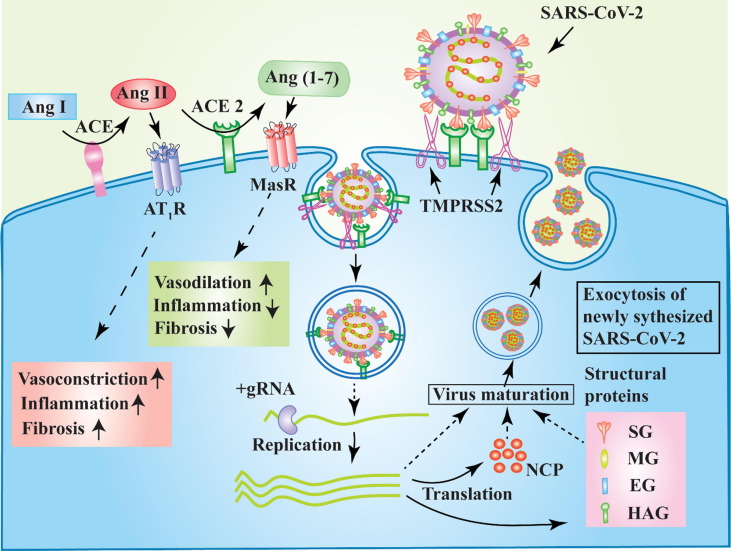
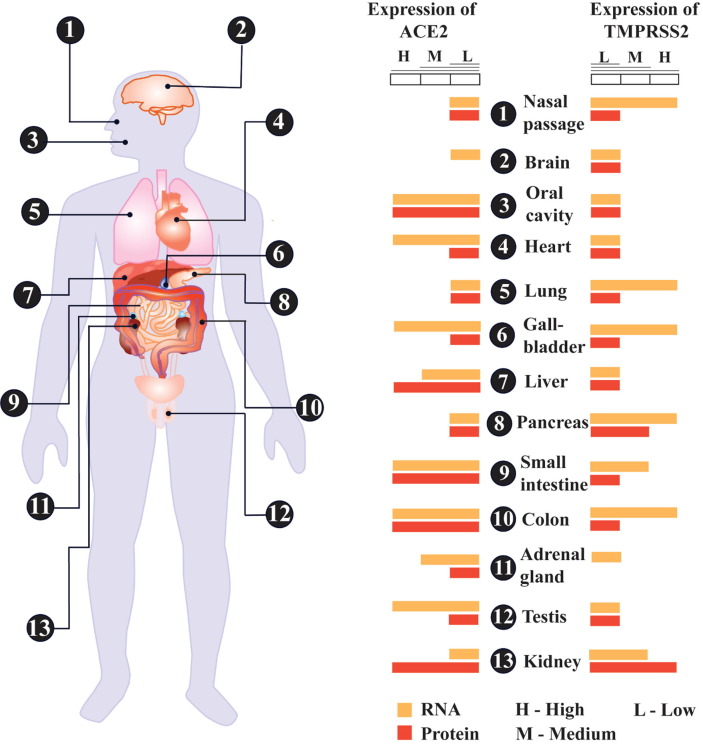
The spike glycoproteins (SG) on the surface of SARS-CoV-2 interact with the human ACE2 [38] to enter into cells of lung epithelium, pancreas, kidney, intestine and vascular system (Fig. 2). The viral SG is a trimeric integral membrane protein, and its extracellular domain S1 has a serine protease cleavage site. The SG is activated by humans’ transmembrane protease, serine 2 (TMPRSS2) is allowed to bind to ACE2, [39] permitting the entry of viral genome or entire virus (Fig. 2). TMPRSS2 is a serine protease and widely collocated with ACE2 on various human tissues (Fig. 1 ) [40].
Fig. 2.
Schematic representation of ACE2 and TMPRSS2-dependent cellular entry and multiplication of SARS-CoV-2 and function of ACE/ Ang II / AT1R and ACE2/ Ang (1–7)/MasR axis. The interaction between SG and ACE2 is primed through the action of TMPRSS2. Elevated expression of ACE2 and TMPRSS2 promotes severe infection. The binding of SG to ACE2 results in non-functional ACE2 that leads to vasoconstriction, inflammation, and tissue fibrosis through ACE/ Ang II / AT1R axis. High expression of ACE2 causes vasodilation and control inflammation and fibrosis via ACE2/ Ang (1–7)/MasR axis. NCP, nucleocapsid phosphoprotein; SG, spike glycoprotein; MG, membrane glycoprotein; EG, envelope glycoprotein and HAG, heamagglutinin-acetylesterase glycoprotein.
Fig. 1.
Schematic diagram of distribution of ACE2 and TMPRSS2 in human body. The RNA and protein expressions of ACE2 and TMPRSS2 in different organs are shown orange and red coloured bars respectively. The length of the bars indicates the level of expression. L: low expression; M: medium expression; H: high expression. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Angiotensin converting enzyme (ACE) 2, a monocarboxypeptidage, is highly expressed on oral mucosa, respiratory tract, lung, kidney, pancreas, cardiovascular system, intestine, cerebral neuron, and immune cells (Fig. 1) [40], [41], [42]. ACE2 converts angiotensin II (Ang II) to angiotensin (1–7) [Ang (1–7)] and maintains a balance between them. Ang (1–7) interacts with Mas receptor (MasR) which facilitates the functions like vasodilation and anti-inflammation which are opposite to the angiotensin type 1 receptor (AT1R) mediated functions done by Ang II [43]. Therefore, the higher expression of ACE2 improves vasodilation and mitigates inflammation and fibrosis of tissues (Fig. 2). The axis of ACE2/ Ang (1–7)/MasR counteracts the functions which are accomplished by the ACE/ Ang II / AT1R axis in the renin-angiotensin system [44].
Sufficient studies have not been done yet to understand the expression level of ACE2 in individuals with diabetes compared to without diabetes. One cross-sectional investigation identified the reduced expression of ACE2 in both the tubulointerstitium and glomeruli in patients with diabetic kidney disease compared to healthy control [45]. It has been suggested that the expression of ACE2 is decreased in patients with diabetes perhaps due to glycosylation [46]. However, one observational clinical study reported the elevated protein expression of ACE2 in alveolar tissues and bronchial epithelium of COVID-19 patients with diabetes compared to those without diabetes [47]. On the other hand, administration of renin-angiotensin system (RAS) inhibitors, like lisinopril, an ACE inhibitor (ACEI) and losartan, an angiotensin II type 1 (AT1) receptor blocker (ARB), to mice promoted the increase in cardiac expression of ACE2 by 5 folds and 3 folds, respectively [48]. In addition, another preclinical study has shown that the use of ARBs improve the mRNA or protein expression of ACE2 in tissues of experimental animals [49]. Although, the effect of ACE inhibitors on the expression and activity of ACE2 in experimental animal models are not consistent [50], use of RAS inhibitors seems to increase the expression of ACE2. That is why, a recent speculation has suggested that the patients who had comorbidities like DM, hypertension and cardiovascular disease and were treated with ACEIs and ARBs, are more prone to SARS-CoV-2 infection [51]. However, clinical investigations did not find considerable association between use of ACEIs and ARBs and risk of SARS-CoV-2 infection. According to some case-control studies involving a large number of people, previous treatment with ACEIs and ARBs did not raise the risk of SARS-COV-2 infection [52], [53]. It was also assumed that the patients taking either ACEIs or ARBs may rise the expression of ACE2 in pulmonary blood vessels and thereby are at high risk of aggravated consequences of COVID-19 [54]. However, the continuous treatment of these RAS inhibitors among hospitalised COVID-19 patients did not affect the overall outcome of COVID-19 severity in randomised clinical trials [55], [56]. In addition, the follow-up evaluation of those COVID-19 patients who participated in continuous and discontinuous ACEIs and ARBs treatment showed no significant difference in COVID-19 severity as well as blood pressure, serum potassium and serum creatinine levels [55]. According to current international society guidelines, the physicians can recommend to continue these drugs for the hospitalised COVID-19 patients if there is no clear medical conflict to continue the treatment.
3.1. Development of transient DM by SARS-CoV-2 infection
A recent in vitro and ex vivo study suggested the confirmed expression of ACE2 on the pancreatic islets particularly on β cells [57]. In other investigations, the expression ACE2 protein was found in pancreatic ductal epithelium, exocrine capillaries, and pericytes; however, no evidence of was found in α and β cells [58], [59]. The contradictory results may be obtained due to differences in antibody reactivity level, epitope properties, tissue section preparation, and immunodetection methodology sensitivity [57]. The previous report suggested that the spike glycoprotein of SARS-CoV binds to ACE2 which are situated on the surface of pancreatic islets and damage the islet mass [60]. As a consequence, insulin production is hampered significantly. In addition, the clinical study observed that more than 50% of SARS-CoV infected hospitalised patients developed diabetes. Furthermore, 5% of the patients who recovered from SARS exhibited DM even after 3 years [60]. The COVID-19 patients were newly diagnosed with diabetes after hospitalization as well; however, the overall percentage of hospitalized COVID-19 patients with newly dragonised diabetes was lower in compare to SARS. According to a meta-analysis involving 8 studies, approximately 14% of COVID-19 patients developed diabetes newly after hospitalization [61]. Furthermore, the clinical investigation reported that the severity of diabetes mellitus was also amplified in the hospital-admitted COVID-19 patients [62]. As a consequence, the doses of glucose lowering drugs were increased in patients with diabetes after SARS-CoV-2 infection and hospitalization. Among hospitalized COVID-19 individuals who had DM, 29.2% patients who were associated with previous insulin therapy were treated with elevated dose of insulin. Additionally, the new insulin therapy was initiated in 37.5% hospitalized patients who took oral anti-diabetic medicine. Moreover, the insulin dose was also elevated in COVID-19 patients with DM admitted to ICU for managing hyperglycemia [63]. However, the studies were conducted in small sample size and in single centre which warrants additional clinical evidences from multicentre cohorts. Overall, the use of unwanted or elevated dose of insulin to maintain euglycaemia of the COVID-19 patients with DM may be the consequences of more severe stress and inflammatory condition exacerbating insulin resistance.
4. Impairment of immune response in DM and COVID-19
It is not surprising that metabolism and immunity have evolved very closely and are interdependent. Numerous components of immune system are altered in metabolic disease T2DM, and the overt alteration has been seen in adipose tissues, liver, islets of pancreas, and leucocytes [64]. The immunological aberrations involve the change in the level of cytokines, altered leukocyte population, and escalation of tissue fibrosis and apoptosis [64]. In T2DM, the cellular stresses like oxidative stress, endoplasmic reticulum stress, ectopic lipid deposition in the liver, muscle and pancreas, amyloid accumulation in the pancreas and lipotoxicity and glycotoxicity, can provoke aggravated inflammatory responses [65], [66]. In COVID-19, the infection and destruction of lung epithelial cells stimulate the local immune reactions which recruit macrophages and monocytes, secrete cytokines, engage B and T lymphocytes to combat infection [67]. When patients with diabetes having the above mentioned cellular stresses are infected with SARS-CoV-2, impaired immune response may lead to serious lung and other pathogenesis and often causes death.
4.1. Exaggerated inflammatory response
Inflammation, an essential part of the innate immune response, is the immediate immune reaction to infection or injury. Thus, the production of inflammatory mediators like tumour necrosis factors (TNF)-α, interleukin (IL)-1, and IL-6 in appropriate amount is vital in response to infection. Nevertheless, overproduction of these cytokines can be harmful for own immune system and may cause acute and chronic inflammatory responses.
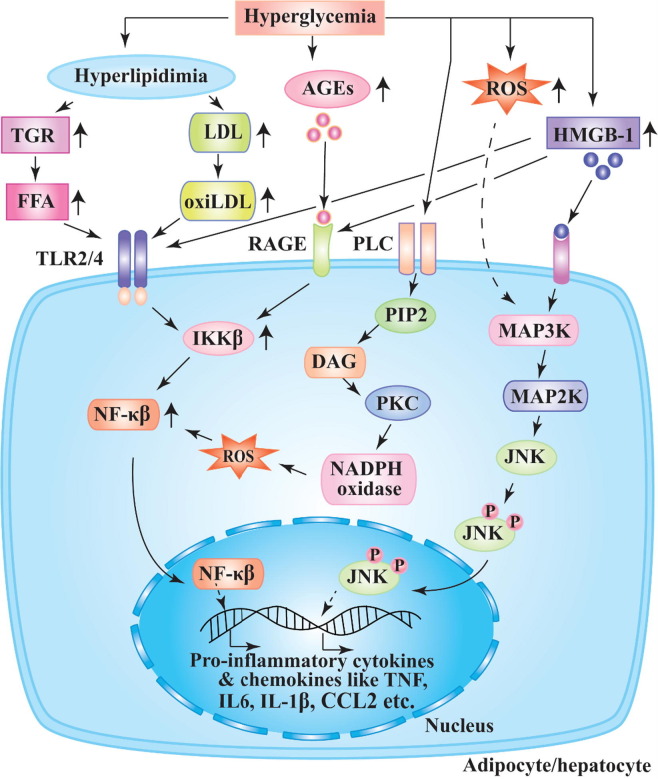
Hyperglycemia triggers numerous metabolic signalling pathways that lead to chronic inflammatory disease, secretion of cytokines, cell death and complications associated with diabetes (Fig. 3 ) [68]. The studies have revealed that hyperglycemia and DM promote elevated synthesis of diacylglycerol (DAG) which triggers activation of protein kinase C (PKC) pathway [69]. As a result, a dangerous metabolic path i.e., production of ROS via NADPH-oxidase is activated in subjects with DM. The rise in ROS generation facilitates protein glycation resulting in elevation of advanced glycation end products (AGEs) and NF-κβ which leads to hyperproduction of pro-inflammatory cytokines and chemokines (Fig. 3) [68]. The elevated productions of ROS and HMGB-1 activate MAP kinase pathway leading to the phosphorylation of JNK, which triggers the increased expression of pro-inflammatory mediators [70]. In vitro study has reported that high blood glucose induces uncontrolled secretion of INF-α, other pro-inflammatory cytokines and chemokines [71].
Fig. 3.
Proposed mechanism of pro-inflammatory cytokines and chemokines production in DM. Chronic hyperglycemia facilitates hyperlipidemia and upregulation of AGEs, HMGB-1, and ROS production. Hyperlipidemia promotes the elevation of triglyceride (TGR) and low density lipoprotein (LDL) which increase FFA and oxi-LDL activating membrane bound TLR2/4 of adipocytes and hepatocytes. The upregulation of HMGB-1 triggers the activation of TLR2/4 and RAGE which intern increases the activity of transcription factor NF-κβ through the rise of IKKβ (Ikβ kinase β). AGEs upsurges cellular level of NF-κβ by interacting RAGE. Hyperglycemia activates PLC and rises DAG which trigger PKC activation resulting in generation of NADPH oxidase and ROS. NF-κβ is upregulated in presence of ROS. HMGB-1 and ROS activate MAP kinase and thereby phosphorylate JNK. NF-κβ and activated JNK lead to increased production of pro-inflammatory cytokines and chemokines.
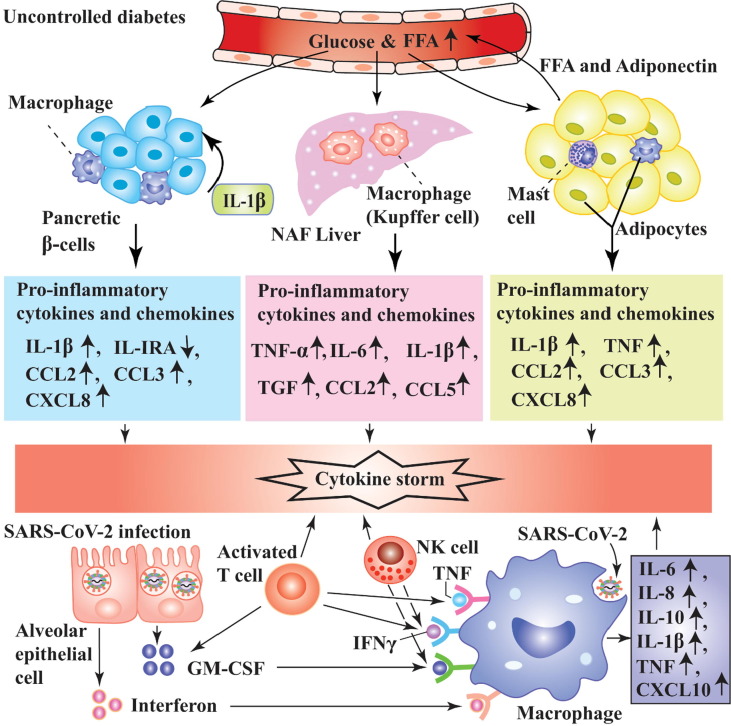
Hyperglycemia reduces the generation of anti-inflammatory factors like IL4 and IL-10 [72]. The concentrations of TNFα in patients with T1DM, IL-6 in patients with T2DM, and IL-8 in patients with T1DM and T2DM were elevated compared to subjects without diabetes [73]. In T2DM, the prospective investigation and cross-sectional data have reported that the circulatory levels of cytokines, C-reactive protein (CRP), serum amyloid A, and fibrinogen are elevated [74]. When SARS-CoV-2 infects patients with diabetes along with pre-existed inflammation, the pathogenesis of COVID-19 may reach to a new height (Fig. 4 ). The nascent report has proved that the COVID-19 patients with DM and related comorbidities secreted several folds higher inflammatory mediators. The serum levels of some pro-inflammatory factors like IL-6, serum ferritin, CRP, and D-dimer were abnormally higher in COVID-19 individuals with DM in comparison with subjects without diabetes [62]. Moreover, a considerable elevation of serum ferritin’s level activates mononuclear phagocyte system, an integral part of cytokine storm [62]. The evidence has suggested that the patients with diabetes are more prone to develop cytokine storm which eventually leads to severe complications in COVID-19 patients. On the other hand, the generation of exaggerated inflammatory factors due to SARS-CoV-2 infection may be managed by the patients without diabetes. Unfortunately, the synergistic effect of COVID-19 and DM on immune system interferes the recovery potential of body. The levels of CRP and D-dimer were several folds higher in non-survivor COVID-19 individuals with or without DM [29]. The comorbidities of DM and other diseases like hypertension, cardiovascular disease in COVID-19 trigger abnormal cytokines storms that lead to uncontrolled function in immune system. As a consequence, the mortality rate of COVID-19 patients associated with DM and/or hyperglycemia is considerably greater than patients without diabetes [75]. The possible mechanism of abnormal cytokine storm which is generated in COVID-19 patients with diabetes has been illustrated in Fig. 4.
Fig. 4.
Possible mechanism of exaggerated inflammatory response in COVID-19 and DM. Several pathways are associated to induce hyperactivated inflammation COVID-19 patients with DM. In uncontrolled diabetes, hyperglycemia and high FFA provoke pancreas, adipocytes and macrophages to secrete cytokines and chemokines likes IL-1β, IL-IRA, CCL2, CCL3, IL-8, TNF, IL-6, and transforming growth factor (TGF). When SARS-CoV-2 infects lung epithelial cells, they release GM-CSF and interferon which recruit macrophages, T lymphocyte and NK cells. Upon activation these cells secrete inflammatory mediators like IL-6, IL-8, IL-1β, TNF and CXCL10. The exaggerated production of inflammatory factors in COVID-19 patients with DM leads to cytokine storm that causes lethal complications.
SARS-CoV-2 infects the lung epithelium and can cause virus mediated pyroptosis which leads to vascular seepage as reported in COVID-19 patients [76]. Pyroptosis is a highly inflammatory state of programme cell death which is observed in cytopathic virus [77] like SARS-CoV-2, activating subsequent inflammatory responses. As a result of pyroptosis, damaged epithelial cells release pathogen-associated molecular patterns (PAMPs) like viral RNA and damage-associated molecular patterns (DAMPs) such as host DNA, ATP, and ASC oligomers, which are detected by lung epithelial macrophages [67] Moreover, a wide range of pro-inflammatory mediators such as granulocyte–macrophage colony-stimulating factor (GM-CSF), Il-6, INF-γ, IL-10, monocyte chemoattractant protein-1 (MCP1) [25], CCL (chemokine (C-C motif) ligand) 2, CCL5 and C-X-C motif chemokine 10 (CXCL10) [78] is secreted into blood of adversely affected COVID-19 patients. Thus, diabetes mellitus causes rapid exacerbation of COVID-19 pathophysiology and often leads to death of comorbid patients.
4.2. Elevation of neutrophil extracellular traps (NETs) in DM and COVID-19
In the sites of infection, neutrophils are recruited at the early onset to destroy pathogens including virus, bacteria and fungi by well-known processes such as oxidative burst and phagocytosis [79]. In addition, neutrophils show another method of destroying pathogen by formation of neutrophil extracellular traps (NETs), web-like structures of decondensed chromatin and proteins which are expelled from the neutrophils [80]. Several enzymes are associated in NETosis, a cell death process of NET formation. NETosis involves neutrophil elastase (NE), which damages intracellular proteins and promotes nuclear denaturation, peptidylarginine deiminase type 4 (PAD4), which citrullinates histones to induce the decondensation and secretion of chromosomal DNA, and gasdermin D that creates holes in the neutrophils’ plasma membrane triggering the degradation of the cell boundary and the outburst of DNA and associated components [80]. NETs play a vital role in host defense by killing pathogens; however, the continuous formation of NETs during viral infection [79] facilitates the pathogenesis of several diseases such as inflammatory diseases and some proposed diseases like glomerulonephritis, chronic pulmonary disease, sepsis, and vascular diseases [81]. Thus, NETs act as double-edge swords of innate immunity in an individual [81]. Unfortunately, diabetes mellitus is associated with the exaggerated induction and constitutive NETosis. It has reported that the expression of PAD4 was abnormally high in neutrophils collected from patients with DM [82]. Additionally, the citrullinated histone H3 was elevated in wound, and the healing was delayed in human and mice with diabetes [82]. Moreover, high blood glucose and T2DM can trigger formation of NETs which leads to delay in wound healing process [82]. NETs are also associated several other complications such as acute respiratory distress syndrome (ARDS), cystic fibrosis (CF), excessive thrombosis, and cytokines storm which are very similar in the patients with severe COVID-19 [80]. The mucous secretions, which are common in CF, found in airways of patients with COVID-19. The comorbidity with DM and COVID-19 seems to facilitate the excessive NETosis which may results in thick and viscous mucous in lung alveoli due to increased NE. This deposition of mucous not only impairs ventilation but also allows the colonization pathogenic bacteria [80]. Thus, the combination of two diseases seems to exacerbate the immune-pathophysiology in the patients with DM and COVID-19.
4.3. Abnormal response of T and B lymphocytes in DM and COVID-19
CD4 + T lymphocyte have two major subsets cells, pro-inflammatory cells like T helper (Th) type 1 and Th17 and anti-inflammatory cells like Th2 and Foxp3 + T regulatory (Treg), which maintain an appropriate balance to avoid inflammatory disease in healthy individual [83]. In T2DM, however, number of Th1 and Th17 cells were elevated whereas Treg cells were decreased [83]. Th1 cells that are raised in specifically SARS-CoV and MERS, were observed to secrete some inflammatory factors such as IFN-γ, TNF-α, and IL-2 [84]. Similarly, the COVID-19 individuals exhibited elevated production of IL-2 and IFN-γ [85]. In COVID-19 patients with uncontrolled DM, intensified production of these cytokines by CD4 + T lymphocytes may lead to severe immune complications. The elevated immune response of CD4 + T cells was reported in COVID-19 convalescent individuals [85]. B lymphocytes secreted an elevated level of IL-8 and reduced level of the anti-inflammatory factor like IL-10 in patients with T2DM [86]. In addition, the researchers reported that the altered activity of Toll-like receptors (TLR) in B lymphocytes was responsible for exaggerated inflammatory response. The elevated pro-inflammatory response from T and B lymphocyte among patients with diabetes may promote additional inflammation in COVID-19, often leading to lethal complications.
5. Possible therapeutic strategies to manage COVID-19 associated with DM
Hyperglycemia increases the severity of pathogenesis in COVID-19, resulting in several lethal complications and often fatality. However, some therapeutic agents have been suggested to manage the exacerbation of COVID-19 complication in patients with diabetes mellitus. Firstly, glucagon-like peptide-1 (GLP-1) receptor agonists which manage blood sugar level by provoking insulin secretion, reducing appetite, and decreasing plasma glucagon, have shown significant anti-diabetic and anti-inflammatory effects [87]. Moreover, inflammatory response and insulin resistance can also be lowered by reducing the macrophage infiltration in lesion site through the GLP-1 dependent M1/M2 macrophage polarization [88].
Secondly, dipeptidyl peptidase IV (DPP4) inhibitors resist the action of DPP4 which is expressed ubiquitously on the diverse tissues including immune cells [89] and degrades GLP-1 leading to impaired glucose metabolism. Beside the regulation of glucose metabolism, higher expression of DPP4 also promotes adipocyte inflammation and hepatic insulin resistance [89]. The administration of the soluble DPP4 has shown elevated secretion of pro-inflammatory mediators such as IL-6, IL-8 and MCP-1. The soluble DPP4 induces inflammation through nuclear factor (NF)-κβ pathways, and the inflammation can be prevented inhibition of DPP4 [90]. Human DPP4 knock-in mice which were developed to understand the mechanism of interaction between DPP4 and coronavirus, facilitate the proliferation of MERS-CoV in the lung upon inoculation [91]. Therefore, DPP4 is promising target for alleviating chronic T2DM and inflammation. The use of DPP4 inhibitor blockade the activity of DPP4, and as a result the function of GLP-1 will restore for long time which may lead the better glycemic control in COVID-19 patients with DM. In a multicentre, retrospective clinical trial of hospitalized COVID-19 individuals with type 2 diabetes, sitagliptin, a highly selective DPP4 inhibitor, was treated to the patients for two months and the outcome was linked to lower fatality rate [92]. The findings were consistent with a single centred comparative study of COVID-19 patients affected by type 2 diabetes. The use of DPP inhibitor reduced the mortality rate whereas treatment of other antidiabetic drugs did not show such efficacy [93]. However, a few studies reported the neutral effect of DPP4 inhibitor. The treatment of DPP4 inhibitor prior to hospitalization did not improve the primary consequence of COVID-19 patients [94]. In another study, previous use of DPP4 inhibitor neither reduce risk of hospitalization of COVID-19 patients nor improve the outcome of COVID-19 patients with diabetes when compared with SGLT inhibitors treatment [95]. However, an observational cohort study involving numerous individuals with type 2 diabetes reported a slightly higher risk of COVID-19 related mortality due to pre-prescribed DPP4 inhibitor therapy [96]. The limitation of the case-control studies is the small sample size. Therefore, large-size retrospective trials are recommended to conclude the efficacy of DPP4 inhibitor.
On the other hand, use of metformin, a popular hypoglycemic agent, provides promising outcome among the SARS-CoV-2 infected T2D patients in terms of survivability and resilience against hyperglycemia induced COVID-19 morbidity. In particular, metformin helps to alleviate diabetic condition by stimulating insulin responsiveness and metabolic control of glucose and lipid metabolism [97], [98] . As per some clinical investigations conducted among T2D patients who were hospitalized following the diagnosis of COVID-19, it was reported that individuals who used to take metformin prior to get tested positive for novel corona virus showed less severity and mortality as compared to the non-metformin users [99], [100] . The uninterrupted metformin therapy to the hospitalized patients with diabetes after they diagnosed with COVID-19, effectively promoted overall health and survivability [101] . However, some discrepancies exist among the available evidences where the efficacy profile of metformin in this regard remained unsatisfactory [102], [103] . Supportive evidence has been found from a nationwide observational investigation in England. The study clearly demonstrated a statistically reduced risk of COVID-19-associated fatality in type 2 diabetic subjects who used metformin before SARS-CoV-2 infection [96]. In addition, other glucose-lowering medications like sulfonylureas and SGLT2 inhibitors showed lower risk of fatality. Two clinical examinations depicted the reduction of death risk only in female T2D patients but not in male as an outcome of metformin use [104], [105] . Moreover, in depth molecular mechanism behind the ameliorating role of metformin in the prognosis of aggravated COVID-19 susceptibility in diabetic subjects is largely unknown and the existing study reports are found to be observational only. Furthermore, future studies are required to dissect the post COVID-19 complications if any among the metformin using survivor T2D patients.
As virus may alter the conformation of their surface proteins which are responsible for antigenicity, it is better to modulate host response to SARS-CoV-2. Thirdly, inhibitor for host serine protease like TMPRSS2 could be a good choice to curb the SARS-CoV-2 infection. Fourthly, administration of inhibitors of NF-κβ like CAPE and parthenolide, caused higher expression of pro-inflammatory cytokines in lungs and eventually increased the survival rate of SARS-CoV infected mice [78]. Therefore, inhibitors of TMPRSS2 and NF-κβ may be potential antivirals in SARS-CoV-2 treatment. Fifthly, the plasma samples of convalescent COVID-19 patients have seemingly shown encouraging result in SARS-CoV-2 infected individuals without any severe adverse effect [106]. Therefore, the convalescent plasma could be a potential boost for recovering COVID-19 patients [107]. However, the critical evaluation with control trial remains to be explored in large number of patients.
As both DM and COVID-19 exaggerate inflammatory markers in circulation of patients, the antidiabetic drugs which have anti-inflammatory property can be good players in this respect. One review has systematically elaborated the anti-inflammatory efficacy of a number of antidiabetic agents which can be used to reduce hyperactivated inflammatory response of SARS-CoV-2 infected patients [108]. Considering all, concrete validation is very much necessary in addressing particular drug to combat major challenges in COVID-19 infected diabetic patients.
Finally, some COVID-19 vaccines have shown promising results with satisfactory safety profile and considerable immune response. However, immunogenicity, safety, and efficacy of COVID-19 vaccines particularly to the patients with diabetes are needed to be optimised well.
6. Conclusion
Diabetes mellitus is one of the major comorbidities in COVID-19 patients. The pre-existing diabetes mellitus seems not to a risk factor for SARS-CoV-2 infection; however, the severity of COVID-19 pathogenesis and fatality cases are elevated due to the pre-existing uncontrolled diabetes mellitus. On the other hand, COVID-19 is associated with poor glycemic control in patients with DM. The serious pathophysiology of COVID-19 patients with DM is governed by a plethora biochemical factors including key peptides such as Ang II, cytokines, and other effector proteins which facilitate complex network pathways and eventually leads to lethal complications. As mentioned, DM is a multi-factorial metabolic disease which impairs the immune system in such a way that SARS-CoV-2 infection results in over activated and uncontrolled immune response. The cellular internalization of viral particles not only destructs the host cells but also aggravates the pro-inflammatory responses such as secretion of cytokines and chemokines in COVID-19 patients with DM. Thus, aggravation of COVID-19 pathophysiology is strongly associated with hyperactivation of immune system. The paucity of detail investigation on the interplay between DM and COVID-19 pathogenesis demands further studies to unveil the precise etiology underlying the heightened immune response in patients with diabetes.
CRediT authorship contribution statement
Sanjib Sarkar: Conceptualization, Writing - original draft, Writing - review & editing, Visualization. Dibyendu Das: Writing - review & editing. Sawlang Borsingh Wann: Supervision, Validation. Jatin Kalita: Supervision, Validation. Prasenjit Manna: Supervision, Writing - review & editing, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are thankful to the Director, CSIR-NEIST for his support, CSIR, Government of India, for providing the Senior Research Fellowship to Mr. Sanjib Sarkar (19/06/2016(ii)EU-V dtd 01-07-2017 & Sr. No. 1061630992) and Mr. Dibyendu Das (18/12/2016(I)EU-V dtd 13-02-2017 & Sr. No. 1121631073), and DBT, Government of India, for providing the Ramalingaswami Re-Entry Fellowship Grant to Dr. Manna (BT/RLF/Re-Entry/34/2013).
References
- 1.Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 2.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 3.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morawska L., Milton D.K. It is Time to Address Airborne Transmission of COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Coronavirus Disease (COVID-19) Situations Reports. (World Health Organization 2020).
- 9.Ma R.C.W., Holt R.I.G. COVID-19 and diabetes. Diabet Med. 2020;37:723–725. doi: 10.1111/dme.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah B.R., Hux J.E. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 11.Muller L.M., Gorter K.J., Hak E., Goudzwaard W.L., Schellevis F.G., Hoepelman A.I., et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 12.Joshi N., Caputo G.M., Weitekamp M.R., Karchmer A.W. Infections in Patients with Diabetes Mellitus. N Engl J Med. 1999;341:1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 13.Hodgson K., Morris J., Bridson T., Govan B., Rush C., Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144:171–185. doi: 10.1111/imm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y., et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 15.Banik G.R., Alqahtani A.S., Booy R., Rashid H. Risk factors for severity and mortality in patients with MERS-CoV: Analysis of publicly available data from Saudi Arabia. Virol Sin. 2016;31:81–84. doi: 10.1007/s12250-015-3679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadini G.P., Morieri M.L., Longato E., Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43:867–869. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 22.Wu C., Chen X., Cai Y., Ja Xia, Zhou X., Xu S., et al. Pneumonia in Wuhan, China. JAMA. Intern Med. 2019;2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur Respir J. 2020;2000547 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seiglie J., Platt J., Cromer S.J., Bunda B., Foulkes A.S., Bassett I.V., et al. Diabetes as a Risk Factor for Poor Early Outcomes in Patients Hospitalized With COVID-19. Diabetes Care. 2020;43:2938–2944. doi: 10.2337/dc20-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onder G., Rezza G., Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 29.Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C., et al. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center. Retrospective Study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 30.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell LF, Chernyak Y, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv. 2020:2020.04.08.20057794.
- 31.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31 doi: 10.1016/j.cmet.2020.04.021. 1068 77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim M.K., Jeon J.H. The Clinical Characteristics and Outcomes of Patients with Moderate-to-Severe Coronavirus Disease 2019 Infection and Diabetes in Daegu. South Korea. Diabetes Metab J. 2020;44:602–613. doi: 10.4093/dmj.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dennis J.M., Mateen B.A., Sonabend R., Thomas N.J., Patel K.A., Hattersley A.T., et al. Type 2 Diabetes and COVID-19–Related Mortality in the Critical Care Setting: A National Cohort Study in England, March–July 2020. Diabetes Care. 2021;44:50–57. doi: 10.2337/dc20-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers L.C., Parodi S.M., Escobar G.J., Liu V.X. Characteristics of Hospitalized Adults With COVID-19 in an Integrated Health Care System in California. JAMA. 2020;323:2195–2198. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(271–80) doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou L, Niu Z, Jiang X, Zhang Z, Zheng Y, Wang Z, et al. Systemic analysis of tissue cells potentially vulnerable to SARS-CoV-2 infection by the protein-proofed single-cell RNA profiling of ACE2, TMPRSS2 and Furin proteases. bioRxiv. 2020:2020.04.06.028522.
- 41.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batlle D., Jose Soler M., Ye M. ACE2 and diabetes: ACE of ACEs? Diabetes. 2010;59:2994–2996. doi: 10.2337/db10-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel V.B., Parajuli N., Oudit G.Y. Role of angiotensin-converting enzyme 2 (ACE2) in diabetic cardiovascular complications. Clin Sci (Lond) 2014;126:471–482. doi: 10.1042/CS20130344. [DOI] [PubMed] [Google Scholar]
- 45.Mizuiri S., Hemmi H., Arita M., Ohashi Y., Tanaka Y., Miyagi M., et al. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51:613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 46.Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wijnant S.R.A., Jacobs M., Van Eeckhoutte H.P., Lapauw B., Joos G.F., Bracke K.R., et al. Expression of ACE2, the SARS-CoV-2 Receptor, in Lung Tissue of Patients With Type 2 Diabetes. Diabetes. 2020;69:2691–2699. doi: 10.2337/db20-0669. [DOI] [PubMed] [Google Scholar]
- 48.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., et al. Effect of Angiotensin-Converting Enzyme Inhibition and Angiotensin II Receptor Blockers on Cardiac Angiotensin-Converting Enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 49.Sukumaran V., Tsuchimochi H., Tatsumi E., Shirai M., Pearson J.T. Azilsartan ameliorates diabetic cardiomyopathy in young db/db mice through the modulation of ACE-2/ANG 1–7/Mas receptor cascade. Biochem Pharmacol. 2017;144:90–99. doi: 10.1016/j.bcp.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Burchill L.J., Velkoska E., Dean R.G., Griggs K., Patel S.K., Burrell L.M. Combination renin-angiotensin system blockade and angiotensin-converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci (Lond) 2012;123:649–658. doi: 10.1042/CS20120162. [DOI] [PubMed] [Google Scholar]
- 51.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mancia G, Rea F. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. 2020;382:2431-40. [DOI] [PMC free article] [PubMed]
- 54.Diaz J.H. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. 2020 doi: 10.1093/jtm/taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen J.B., Hanff T.C., William P., Sweitzer N., Rosado-Santander N.R., Medina C., et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopes R.D., Macedo A.V.S., de Barros E.S.P.G.M., Moll-Bernardes R.J., Dos Santos T.M., Mazza L., et al. Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fignani D., Licata G., Brusco N., Nigi L., Grieco G.E., Marselli L., et al. SARS-CoV-2 Receptor Angiotensin I-Converting Enzyme Type 2 (ACE2) Is Expressed in Human Pancreatic β-Cells and in the Human Pancreas Microvasculature. Front Endocrinol. 2020;11 doi: 10.3389/fendo.2020.596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kusmartseva I., Wu W., Syed F., Van Der Heide V., Jorgensen M., Joseph P., et al. Expression of SARS-CoV-2 Entry Factors in the Pancreas of Normal Organ Donors and Individuals with COVID-19. Cell Metab. 2020;32:1041–1051. doi: 10.1016/j.cmet.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coate K.C., Cha J., Shrestha S., Wang W., Gonçalves L.M., Almaça J., et al. SARS-CoV-2 Cell Entry Factors ACE2 and TMPRSS2 Are Expressed in the Microvasculature and Ducts of Human Pancreas but Are Not Enriched in β Cells. Cell Metab. 2020;32 doi: 10.1016/j.cmet.2020.11.006. 1028 40.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sathish T., Kapoor N., Cao Y., Tapp R.J., Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes, Obes Metab. 2021;23:870–874. doi: 10.1111/dom.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu L., Girgis C.M., Cheung N.W. COVID-19 and diabetes: Insulin requirements parallel illness severity in critically unwell patients. Clin Endocrinol. 2020;93:390–393. doi: 10.1111/cen.14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 65.Donath M.Y., Schumann D.M., Faulenbach M., Ellingsgaard H., Perren A., Ehses J.A. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31(Suppl 2):S161–S164. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 66.Masters S.L., Dunne A., Subramanian S.L., Hull R.L., Tannahill G.M., Sharp F.A., et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Volpe C.M.O., Villar-Delfino P.H., Dos Anjos P.M.F., Nogueira-Machado J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9:119. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia P., Inoguchi T., Kern T.S., Engerman R.L., Oates P.J., King G.L. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes. 1994;43:1122–1129. doi: 10.2337/diab.43.9.1122. [DOI] [PubMed] [Google Scholar]
- 70.Yung J.H.M., Giacca A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cell. 2020;9 doi: 10.3390/cells9030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu R., Xia C.-Q., Butfiloski E., Clare-Salzler M. Effect of high glucose on cytokine production by human peripheral blood immune cells and type I interferon signaling in monocytes: Implications for the role of hyperglycemia in the diabetes inflammatory process and host defense against infection. Clinical Immunol. 2018;195:139–148. doi: 10.1016/j.clim.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao J., Li J., Cai L., Chakrabarti S., Li X. Cytokines and diabetes research. J Diabetes Res. 2014 doi: 10.1155/2014/920613. 2014:920613- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lechleitner M., Koch T., Herold M., Dzien A., Hoppichler F. Tumour necrosis factor-alpha plasma level in patients with type 1 diabetes mellitus and its association with glycaemic control and cardiovascular risk factors. J Intern Med. 2000;248:67–76. doi: 10.1046/j.1365-2796.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- 74.Pickup J.C., Mattock M.B., Chusney G.D., Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–1292. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 75.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R., et al. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J Diabetes Sci Technol. 2020;1932296820924469 doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fink S.L., Cookson B.T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeño J.M., Fernandez-Delgado R., Fett C., et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol. 2014;88:913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schonrich G., Raftery M.J. Neutrophil Extracellular Traps Go Viral. Front Immunol. 2016;7:366. doi: 10.3389/fimmu.2016.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217 doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaplan M.J., Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong S.L., Demers M., Martinod K., Gallant M., Wang Y., Goldfine A.B., et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jagannathan-Bogdan M., McDonnell M.E., Shin H., Rehman Q., Hasturk H., Apovian C.M., et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–1172. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shin H.S., Kim Y., Kim G., Lee J.Y., Jeong I., Joh J.S., et al. Immune Responses to Middle East Respiratory Syndrome Coronavirus During the Acute and Convalescent Phases of Human Infection. Clin Infect Dis. 2019;68:984–992. doi: 10.1093/cid/ciy595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020 doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jagannathan M., McDonnell M., Liang Y., Hasturk H., Hetzel J., Rubin D., et al. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia. 2010;53:1461–1471. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rizzo M., Nikolic D., Patti A.M., Mannina C., Montalto G., McAdams B.S., et al. GLP-1 receptor agonists and reduction of cardiometabolic risk: Potential underlying mechanisms. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2814–2821. doi: 10.1016/j.bbadis.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 88.He J., Yuan G., Cheng F., Zhang J., Guo X. Mast Cell and M1 Macrophage Infiltration and Local Pro-Inflammatory Factors Were Attenuated with Incretin-Based Therapies in Obesity-Related Glomerulopathy. Metab Syndr Relat Disord. 2017;15:344–353. doi: 10.1089/met.2017.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kokic Males V. Letter to the editor in response to the article “COVID-19 and diabetes: Can DPP4 inhibition play a role?”. Diabetes Res Clin Pract. 2020 doi: 10.1016/j.diabres.2020.108163. 163:108163-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wronkowitz N., Görgens S.W., Romacho T., Villalobos L.A., Sánchez-Ferrer C.F., Peiró C., et al. Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim et Biophys Acta (BBA) - Molecular Basis Dis. 1842;2014:1613–1621. doi: 10.1016/j.bbadis.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 91.Li K., Wohlford-Lenane C.L., Channappanavar R., Park J.E., Earnest J.T., Bair T.B., et al. Mouse-adapted MERS coronavirus causes lethal lung disease in human DPP4 knockin mice. Proc Natl Acad Sci U S A. 2017;114:E3119–E3128. doi: 10.1073/pnas.1619109114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Solerte S.B., D'Addio F., Trevisan R., Lovati E., Rossi A., Pastore I., et al. Sitagliptin Treatment at the Time of Hospitalization Was Associated With Reduced Mortality in Patients With Type 2 Diabetes and COVID-19: A Multicenter, Case-Control, Retrospective. Observational Study. Diabetes Care. 2020;43:2999–3006. doi: 10.2337/dc20-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mirani M., Favacchio G. Impact of Comorbidities and Glycemia at Admission and Dipeptidyl Peptidase 4 Inhibitors in Patients With Type 2 Diabetes With COVID-19: A Case Series From an Academic Hospital in Lombardy. Italy. Diabetes Care. 2020;43:3042–3049. doi: 10.2337/dc20-1340. [DOI] [PubMed] [Google Scholar]
- 94.Roussel R., Darmon P. Use of dipeptidyl peptidase-4 inhibitors and prognosis of COVID-19 in hospitalized patients with type 2 diabetes: A propensity score analysis from the CORONADO study. Diabetes Obes Metab. 2021;1–11 doi: 10.1111/dom.14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Israelsen SB, Pottegard A. Comparable COVID-19 outcomes with current use of GLP-1 receptor agonists, DPP-4 inhibitors or SGLT-2 inhibitors among patients with diabetes who tested positive for SARS-CoV-2. 2021. [DOI] [PMC free article] [PubMed]
- 96.Khunti K., Knighton P., Zaccardi F., Bakhai C., Barron E., Holman N., et al. Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: a nationwide observational study in England. Lancet Diabetes Endocrinol. 2021;9:293–303. doi: 10.1016/S2213-8587(21)00050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rena G., Hardie D.G., Pearson E.R. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs. 1999;58 Suppl 1:31-9; discussion 75–82. [DOI] [PubMed]
- 99.Li J., Wei Q., Li W.X., McCowen K.C., Xiong W., Liu J., et al. Metformin Use in Diabetes Prior to Hospitalization: Effects on Mortality in Covid-19. Endocrinepractice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2020;26:1166–1172. doi: 10.4158/EP-2020-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crouse A, Grimes T, Li P, Might M, Ovalle F, Shalev A. Metformin Use is Associated with Reduced Mortality in a Diverse Population wth COVID-19 and Diabetes. medRxiv. 2020:2020.07.29.20164020. [DOI] [PMC free article] [PubMed]
- 101.Luo P., Qiu L., Liu Y., Liu X.-L., Zheng J.-L., Xue H.-Y., et al. Metformin Treatment Was Associated with Decreased Mortality in COVID-19 Patients with Diabetes in a Retrospective Analysis. Am J Trop Med Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Z., Jiang N., Liu L., Yin X., Yang H., Tan X., et al. Association of metformin with mortality or ARDS in patients with COVID-19 and type 2 diabetes: A retrospective cohort study. Diabetes Res Clin Pract. 2020;173 doi: 10.1016/j.diabres.2020.108619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang J., Cooper J.M., Gokhale K., Acosta-Mena D., Dhalla S., Byne N., et al. Association of metformin with susceptibility to COVID-19 in people with Type 2 diabetes. J Clin Endocrinol Metab. 2021 doi: 10.1210/clinem/dgab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bramante CT, Ingraham NE, Murray TA, Marmor S, Hovertsen S, Gronski J, et al. Observational Study of Metformin and Risk of Mortality in Patients Hospitalized with Covid-19. medRxiv. 2020:2020.06.19.20135095.
- 105.Bramante C.T., Ingraham N.E., Murray T.A., Marmor S., Hovertsen S., Gronski J., et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy longevity. 2021;2:e34–e41. doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv. 2020:2020.03.16.20036145.
- 107.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Katsiki N., Ferrannini E. Anti-inflammatory properties of antidiabetic drugs: A “promised land” in the COVID-19 era? J Diabetes Complicats. 2020;34 doi: 10.1016/j.jdiacomp.2020.107723. [DOI] [PMC free article] [PubMed] [Google Scholar]